Abstract
In this prospective cohort of 30 vaccinated healthcare workers with mild Omicron variant infection, we evaluated viral culture, rapid antigen test (RAT), and real-time reverse-transcription polymerase chain reaction (RT-PCR) of respiratory samples at days 5, 7, 10, and 14. Viral culture was positive in 46% (11/24) and 20% (6/30) of samples at days 5 and 7, respectively. RAT and RT-PCR (Ct ≤35) showed 100% negative predictive value (NPV), with positive predictive values (PPVs) of 32% and 17%, respectively, for predicting viral culture positivity. A lower RT-PCR threshold (Ct ≤24) improved culture prediction (PPV = 39%; NPV = 100%). Vaccinated persons with mild Omicron infection are potentially transmissible up to day 7. RAT and RT-PCR might be useful tools for shortening the isolation period.
Keywords: RT-PCR, SARS-CoV-2, Omicron variant, rapid antigen test, viral culture
We evaluated the duration of viral culture positivity compared to rapid antigen test (RAT) and real-time reverse-transcription polymerase chain reaction (RT-PCR) in mild Omicron infection. Vaccinated persons are potentially transmissible up to day 7. RAT and RT-PCR are predictors of viral culture positivity.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant was first reported in South Africa in November 2021 and was quickly designated a variant of concern due to potential high transmissibility and ability to escape natural and vaccine-induced immunity [1, 2]. Omicron variant may be >10 times more transmissible than ancestral viruses and twice as contagious as the Delta variant [1].
There is scarce data on Omicron shedding, which is an important factor for determination of coronavirus disease 2019 (COVID-19) transmissibility [2]. In this study, we aimed to characterize Omicron shedding duration by comparing viral isolation, rapid antigen test (RAT), and real-time reverse-transcription polymerase chain reaction (RT-PCR) cycle threshold (Ct) values.
METHODS
Setting and Study Population
Healthy healthcare workers (HCWs) from Faculdade de Medicina da Universidade de São Paulo (FMUSP), Brazil, with mild COVID-19 diagnosed by RT-PCR or RAT within 5 days of symptom onset were invited to participate in this study. The COVID-19 vaccination campaign (with CoronaVac) at FMUSP began on 18 January 2021, and the booster dose of COVID-19 vaccine on 4 October 2021. All participants had been immunized with at least 2 doses of any COVID-19 vaccine.
Study Design
We performed a prospective cohort study with 4 time points of sample collection at days 5, 7, 10, and 14 since symptom onset. The first day of symptoms was considered day 1. At each time point, nasopharyngeal samples were collected for RAT and a different set of combined nasopharyngeal and oropharyngeal samples for RT-PCR and viral isolation was collected consecutively in a biosafety level 3 laboratory or self-collected at home. Self-collected samples were transported to the laboratory within 1 hour of collection in a refrigerated container. Demographic and clinical data were obtained at baseline, and symptom duration was monitored by telephone up to resolution of symptoms or 14 days after symptom onset, whichever was later.
SARS-CoV-2 RT-PCR
Detection of SARS-CoV-2 RNA was performed using EXTRACTA Kit FAST-DNA and RNA Viral (Loccus) and EXTRACTA 32 Loccus equipment according to the manufacturer’s instructions. The RealStar SARS-CoV-2 RT-PCR assay developed by Altona Diagnostics (Germany, 2020), which amplifies the regions of the S and E genes, was performed as previously described [3]. The diagnostic threshold of RT-PCR was a Ct value ≤35.
SARS-CoV-2 Whole-Genome Sequencing
The viral RNA, extracted as described above, was also used for whole-genome sequencing (WGS) analysis. In brief, SARS-CoV-2 complementary DNA and multiplex PCR steps were performed and the amplicons were sequenced using the MinION platform (Oxford Nanopore Technologies, United Kingdom) for lineage characterization [4]. Variant calling and consensus sequences were performed using artic minion with Nanopolish version from the ARTIC bioinformatics pipeline (https://github.com/artic-network/fieldbioinformatics). Genome regions with a depth of <20-fold were not included in final consensus sequences, and these positions were represented with N characters. Sequences with >50 times genome coverage were used to lineage classification by Pangolin version 3.1.5 (http://pangolin.cog-uk.io/)[5] and Nextclade version 1.4.0 (https://clades.nextstrain.org) and confirmed by manual genotyping. All SARS-CoV-2 viral genomes were uploaded to the GISAID platform (Supplementary Table 1).
SARS-CoV-2 Rapid Antigen Test
The Hotgen Coronavirus 2019-nCoV antigen test (China), which is a rapid chromatographic immunoassay for the detection of SARS-CoV-2 nucleocapsid (N) antigen in respiratory specimens, was employed. The test was performed according to the manufacturer’s instructions. After collection, the nasopharyngeal swab was immediately immersed in the buffer tube and homogenized, and 4–5 drops of the solution were placed in the cassette within 15 minutes of sample collection.
SARS-CoV-2 Viral Culture
To isolate SARS-CoV-2, Vero cells (CCL-81) were seeded in 24-well plates in a concentration of 2 × 105 cells/mL in Dulbecco’s minimal essential medium (DMEM) supplemented with 5% inactivated fetal bovine serum (FBS) and incubated overnight at 37°C. The next day, the supernatant was discarded, and 200 µL of a homogenized respiratory sample was added per well in the culture plate and incubated for 1 hour for adsorption. Following incubation, 1 mL DMEM containing 2% FBS and 1% penicillin-streptomycin-amphotericin B were added per well. The plates were incubated in a humidified 37°C incubator at 5% carbon dioxide. Cultures were observed daily to verify cytopathic effect (CPE) for 3 days. Two hundred microliters of supernatant was collected and inoculated into a new cell culture 2 more times. The supernatant was collected after the third passage, and virus replication was evaluated through CPE and confirmed by RT-PCR with a lower Ct value on viral isolation compared to the patient’s sample Ct value [6].
Data Analysis
Demographic and clinical characteristics were presented as median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. The comparison of RT-PCR Ct values of participants with and without a vaccine booster dose, the first 2 doses of CoronaVac, and previous SARS-CoV-2 infection was performed using the Mann–Whitney test. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of RAT and RT-PCR for prediction of positive viral culture were calculated. A receiver operating characteristic (ROC) curve of sensitivity and 1-specificity of Ct values for prediction of positive viral culture was plotted for the identification of an optimized RT-PCR Ct value threshold. A mean of E and S genes Ct values was calculated for each patient at each time point for calculation of the Ct values reported. Stata version 13.0 software was used for statistical analyses.
Ethical Considerations
This study was approved by FMUSP's Ethics Committee (CAAE:42708721.0.0000.0068). Informed consent was obtained from all participants for respiratory samples and clinical data collection.
RESULTS
We included 30 HCWs with mild COVID-19 between 11 and 24 January 2022. The median age was 30 (IQR, 25–36) years, 16 (53%) were male, and 22 (74%) were White. Most participants (n = 25 [83%]) had received 2 doses of CoronaVac. Twenty-six (87%) HCWs had received a booster dose of a COVID-19 vaccine, mainly BNT162b2 (n = 23/26 [92%]), and were infected a median of 93 (IQR, 72–100) days since the booster dose. Ten (33%) participants had COVID-19 previously with a median reinfection interval of 579 (IQR, 437–601) days. The most prevalent symptoms at diagnosis were coryza (n = 27 [90%]), sore throat (n = 25 [83%]), and cough (n = 21 [70%]), with a median duration of symptoms of 6 (IQR, 9–14) days (Supplementary Table 2). Four (13%) participants remained symptomatic with dry cough for 20–32 days.
We obtained respiratory samples from 24 participants at day 5 and from all participants as of day 7. All HCWs were infected by SARS-CoV-2 sublineage BA.1 (Supplementary Table 1).
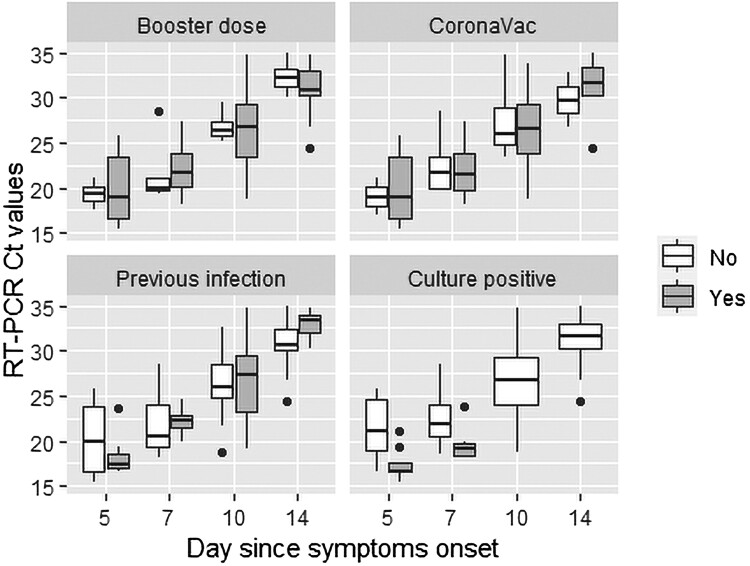
All samples were RT-PCR positive (using standard threshold of Ct ≤35) until day 7, decreasing to a positivity of 97% (n = 29) at day 10 and 57% (n = 17) at day 14. The lowest Ct levels were detected at day 5 (median Ct = 18 [IQR, 17–23]), increasing progressively until day 14 (median Ct = 32 [IQR, 30–34]), except for 7 (23%) patients. There was a substantial decrease in Ct values of 4 of these 7 patients at day 7 and 3 at day 10, with an increase in Ct values and symptom improvement at day 14 (Supplementary Table 3). Only 1 of these 7 patients had a positive viral culture at day 7, and reinfection was ruled out by WGS confirming the same Omicron sublineage in all cases. Vaccine booster dose, the first 2 doses of CoronaVac, and previous SARS-CoV-2 infection did not significantly affect Ct values on days 5–14 (Figure 1, Supplementary Table 4).
Figure 1.
Comparison of SARS-CoV-2 RT-PCR Ct values by having received a booster dose (“Booster dose”) of any COVID-19 vaccine, primary vaccination with CoronaVac (“CoronaVac”) or other vaccines, previous infection with SARS-CoV-2 (“Previous infection”), and viral culture positivity (Culture positive) on each day of symptoms.
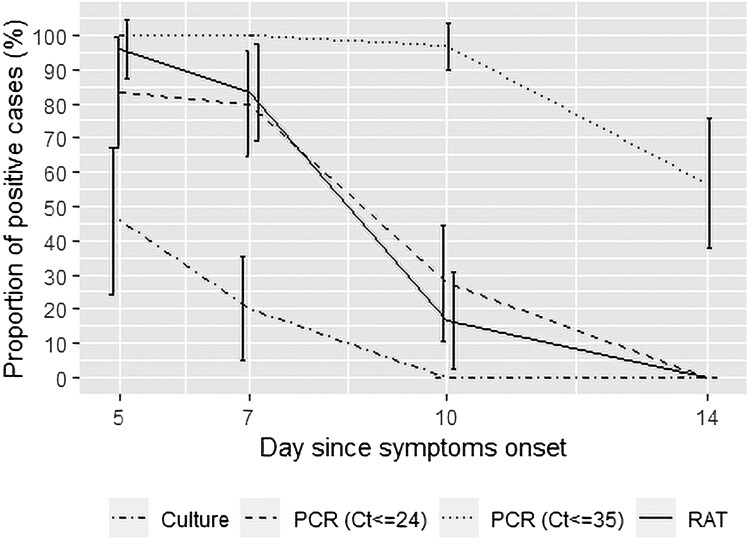
RAT was positive in 96% (n = 23/24), 83% (n = 25/30), and 17% (n = 5/30) of samples at days 5, 7, and 10, respectively. All samples were RAT negative at day 14 (Supplementary Table 5).
A positive viral culture was detected in 46% (n = 11/24) of samples at day 5, and 20% (n = 6/30) at day 7. No sample had a positive viral culture as of day 10. Among samples with positive viral culture (n = 17), all were RAT and RT-PCR positive, and the median Ct value was 18 (IQR, 17–19; range, 13–24) (Supplementary Table 4). Culture positive samples showed lower median Ct values at day 5 (17 [IQR, 16–18] vs. 21 [IQR, 18–25], P = .002) and day 7 (19 [IQR, 18–21] vs. 22 [IQR, 20–24], P = .01) (Figure 1). On the other hand, samples with negative viral culture (n = 97) were RAT and RT-PCR positive in 36 (37%) and 83 (86%) samples, respectively, and the median Ct value of the RT-PCR–positive samples was 25 (IQR, 22–30; range, 17–35) (Figure 2, Supplementary Table 5).
Figure 2.
Real-time reverse-transcription polymerase chain reaction, rapid antigen test, and viral culture positivity (with 95% confidence interval) by days since symptom onset in persons infected with severe acute respiratory syndrome coronavirus 2 Omicron variant. Abbreviations: Ct, cycle threshold; RAT, rapid antigen test; RT-PCR, real-time reverse-transcription polymerase chain reaction.
Both RT-PCR (Ct ≤35) and RAT evidenced overall 100% sensitivity and NPV for predicting a positive viral culture. However, RAT showed an overall specificity of 63% and PPV of 32%, while RT-PCR evidenced lower specificity (14%) and PPV (17%). The analysis of the ROC curve of sensitivity and specificity of Ct values for identification of positive viral culture at days 5–7 showed that the Ct value of 24 might be a more suitable threshold (Supplementary Figure 1). The new threshold allowed an improvement in the prediction of positive viral culture (PPV = 39%) with the retention of 100% NPV (Supplementary Table 6).
DISCUSSION
Our study found that a minority of vaccinated persons infected with the SARS-CoV-2 Omicron BA.1 sublineage presented a positive viral culture as of 5 days of symptoms, and that RT-PCR and RAT may be surrogates of viral culture positivity. In addition, we demonstrated that a lower threshold of RT-PCR Ct values might provide a more suitable cutoff for predicting a positive viral culture than current cutoffs used for COVID-19 diagnosis.
The proportion of cases with viral culture positivity and the duration of viral shedding for Omicron herein reported was similar to 2 previous studies [7, 8] and lower than described in 2 other studies [9, 10]. However, those previous studies were heterogeneous regarding the COVID-19 vaccination status of the participants, which might influence SARS-CoV-2 shedding [11]. Two of those studies have also shown similar rates of culture positivity between Omicron and Delta variant infections [8, 9]. In addition, it has been described that nonsevere COVID-19 in immunocompetent patients infected with Omicron can present a positive viral culture up to day 14 in 8% of the cases [10]. Differences in viral shedding between SARS-CoV-2 lineages might be attributable to intrinsic characteristics of the variants and to host factors such as COVID-19 vaccine status or inherent immunological characteristics.
We demonstrated that RAT and RT-PCR are appropriate rule-out tests for prediction of viral culture positivity for Omicron due to their 100% NPV, although RT-PCR with the standard cutoff used for COVID-19 diagnosis may be too conservative for this purpose because of its substantially low PPV. It has been previously shown that RAT and RT-PCR have high NPV (99%–100% and 100%, respectively) and variable PPV (50%–70% and 25%–30%, respectively) for predicting viral culture positivity for Omicron, Delta, and ancestral strains [9, 12, 13]. In addition, previous studies explored the use of different cutoffs of Ct values, showing variable improvement in the PPV and maintenance of 100% NPV using lower Ct cutoffs (23.5–24.97), similar to our findings [12, 13]. However, those studies did not collect serial samples from the same group of patients and the evaluated days of symptoms were variable (mostly within 5 days of symptom onset), which may influence viral shedding assessment. Therefore, RAT and RT-PCR are suitable rule-out tests for viral culture prediction.
Extrapolating the findings from viral culture results to SARS-CoV-2 infectivity, the present study corroborates current guideline recommendations for isolation of mild COVID-19 in immunocompetent persons for 10 days since symptom onset or for 7 days with a negative RAT or RT-PCR [14], at least for vaccinated hosts infected with Omicron.
Our study has limitations. Although the small sample size might not be representative of the general population, we followed the same 30 persons in each collection time point until symptom resolution, and all the persons were vaccinated with at least 2 doses of a COVID-19 vaccine. Despite the heterogeneity of the booster vaccination status and previous SARS-CoV-2 infection in our study group, our data showed that these characteristics did not significantly affect viral shedding. In addition, viral culture possibly underestimates SARS-CoV-2 infectivity [15]. Moreover, Ct values can vary from institution to institution and between RT-PCR kits, limiting the interpretation of absolute Ct values of RT-PCR.
In conclusion, vaccinated immunocompetent persons with mild COVID-19 caused by Omicron are potentially transmissible up to day 7 since symptom onset. RAT and RT-PCR are useful rule-out tests for shortening the isolation period up to day 7, and lower cutoffs of RT-PCR Ct values can improve viral culture prediction. Thus, the routine availability of RT-PCR Ct values may improve the decision making of the COVID-19 isolation period.
Supplementary Material
Contributor Information
Alessandra Luna-Muschi, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Saidy Vásconez Noguera, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Igor C Borges, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Anderson V De Paula, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Marina Farrel Côrtes, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Carolina Larocca, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Julia Ferreira Mari, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Lara Silva Pereira Guimarães, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Pablo Munoz Torres, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Nazareno Scaccia, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Lucy S Villas-Boas, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Almir Ribeiro da Silva, Jr, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Pâmela S Andrade, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Epidemiologia, Faculdade de Saúde Pública da Universidade de São Paulo, São Paulo, Brazil.
Juliana C Teixeira, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Camille Escadafal, FIND, the Global Alliance for Diagnostics, Geneva, Switzerland.
Vitor Falcão de Oliveira, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Tania R Tozetto-Mendoza, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Maria Cássia Mendes-Correa, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Anna S Levin, Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Ester C Sabino, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Silvia F Costa, Instituto de Medicina Tropical, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Departamento de Moléstias Infecciosas e Parasitárias, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Divisião de Moléstias Infecciosas e Parasitárias, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. F. C., A. S. L., E. C. S., and M. C. M.-C. contributed to project conceptualization and methodology. S. F. C. contributed to the acquisition of the financial support for the project leading to this publication. A. L. M. contributed to execution of research activities. A. L.-M., S. V. N., M. F. C., C. L., J. F. M., L. S. P. G., J. C. T., P. M. T., and V. F. O. contributed to respiratory sample collection and RAT performance. A. V. P., L. S. V.-B., A. R. S., and T. R. T.-M. contributed to RT-PCR analyses and viral isolation. P. A. S. contributed to the whole-genome sequencing and analysis. A. L.-M., S. V. N., I. C. B., M. F. C., and N. S. contributed to data analysis and interpretation. A. L.-M., S. V. N., I. C. B., A. V. P., M. F. C., J. F. M., L. S. P. G., J. C. T., P. M. T., N. S., C. E., and V. F. O. wrote the first draft. All authors revised the final version of the manuscript.
Acknowledgments. We thank the participants for volunteering for the study; the members of LIM-46 of the IMT-FMUSP, Brazil, for performing the SARS-CoV-2 whole genome sequencing; and FIND, the Global Alliance for Diagnostics, for donating the RATs.
Financial support. This study was supported by the Itaú Unibanco “Todos pela saúde” program.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chen J, Wang R, Gilby NB, Wei G-W. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022; 62:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021; 398:2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visseaux B, le Hingrat Q, Collin G, et al. Evaluation of the RealStar SARS-CoV-2 RT-PCR kit RUO performances and limit of detection. J Clin Virol 2020; 129:104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021; 372:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020; 5:1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basile K, McPhie K, Carter I, et al. Cell-based culture informs infectivity and safe de-isolation assessments in patients with coronavirus disease 2019. Clin Infect Dis 2021; 73:e2952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institute of Infectious Diseases Disease Control and Prevention Center, and National Center for Global Health and Medicine . Active epidemiological investigation on SARS-CoV-2 infection caused by Omicron variant (Pango lineage B.1.1.529) in Japan: preliminary report on infectious period. 2022. https://www.niid.go.jp/niid/en/2019-ncov-e/10884-covid19-66-en.html. Accessed 18 May 2022.
- 8. Boucau J, Marino C, Regan J, et al. Duration of shedding of culturable virus in SARS-CoV-2 omicron (BA.1) infection. N Engl J Med 2022; 387:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouton TC, Atarere J, Turcinovic J, et al. Viral dynamics of omicron and Delta SARS-CoV-2 variants with implications for timing of release from isolation: a longitudinal cohort study [manuscript published online ahead of print 23 June 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keske Ş, Esken GG, Vatansever C, et al. Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms [manuscript published online ahead of print 16 July 2022]. Clin Microbiol Infect 2022. doi: 10.1016/j.cmi.2022.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of Alpha and Delta variants. N Engl J Med 2022; 386:744–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korenkov M, Poopalasingam N, Madler M, et al. Evaluation of a rapid antigen test to detect SARS-CoV-2 infection and identify potentially infectious persons. J Clin Microbiol 2021; 59:e0089621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopera TJ, Alzate-Ángel JC, Díaz FJ, Rugeles MT, Aguilar-Jiménez W. The usefulness of antigen testing in predicting contagiousness in COVID-19. Microbiol Spectr 2022; 10:e0196221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed 18 May 2022.
- 15. Gniazdowski V, Paul Morris C, Wohl S, et al. Repeated coronavirus disease 2019 molecular testing: correlation of severe acute respiratory syndrome coronavirus 2 culture with molecular assays and cycle thresholds. Clin Infect Dis 2021; 73:e860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.