Abstract
Background
Organ transplant recipients (OTRs) are less protected from vaccination than immunocompetent hosts. Additional vaccine doses have shown increased immunogenicity. Few studies have assessed their clinical efficacy, particularly against Omicron variants, as most included patients from earlier phases of the pandemic, with higher base mortality rates.
Methods
We studied adult OTRs who had coronavirus disease 2019 (COVID-19) between 12/15/21 and 5/25/22. We compared clinical outcomes between those who had received 2 or ≥3 doses of an mRNA vaccine and concurrent unvaccinated controls.
Results
Among 103 OTRs, vaccination was associated with lower 90-day mortality (unvaccinated vs 2 vs ≥3 doses: 25% vs 7% vs 3%; P = .003), hospital (unvaccinated vs 2 vs ≥3 doses: 56% vs 37% vs 27%; P = .018) and intensive care unit (ICU; unvaccinated vs 2 vs ≥3 doses: 25% vs 15% vs 3%; P = .001) admission rates, and peak O2 requirements (ordinal scale Kendall’s tau b = –0.309 [lower scores, ie, O2 requirements with more vaccine doses]; P = .003). Age (age >60 years: adjusted hazard ratio [aHR], 7.73; P = .016; administration of antispike monoclonal antibody: aHR, 0.17; P = .042) and vaccination, especially with ≥3 doses (aHR, 0.105; P = .01), were independently associated with 90-day mortality. Black (P = .021) and Hispanic (P = .016) OTRs were underrepresented among the vaccinated, especially in the ≥3-dose group.
Conclusions
Despite lower mRNA vaccine efficacy in OTRs and against Omicron variants, vaccination protects this vulnerable patient population from severe COVID-19 and death. Ethnic and racial disparities in health care have been exacerbated by the COVID-19 pandemic and warrant better community outreach efforts.
Keywords: COVID-19, SARS-CoV-2, infection, Omicron, transplant, vaccines
Organ transplant recipients (OTRs) mount weaker immune responses to coronavirus disease 2019 (COVID-19) vaccination compared with immunocompetent patients and are less protected against infection and severe illness [1–3]. In some studies of OTRs, vaccination with 2 doses of an mRNA vaccine was not associated with lower risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [2, 4], critical COVID-19 [4, 5], or mortality [4, 6, 7]. However, others have found that vaccination decreased the risk of symptomatic illness [8], hospitalization [9], use of mechanical ventilation or extracorporeal membrane oxygenation (ECMO) support [10], and death [10, 11].
Among OTRs, including those with no or low antibody response after 2 doses of an mRNA vaccine, a third [12–16] and fourth [17–19] dose increase protective antibody titers. However, this “boosted” humoral immunity is less effective in vitro against Omicron variants compared with wild-type SARS-CoV-2 or Delta strains [20, 21]. Moreover, these observations may not correlate with clinical outcomes, given confounding from the increased transmissibility but attenuated virulence of Omicron strains [22]. For example, one study of vaccinated OTRs during the Omicron surge showed that seronegativity was not associated with risk of breakthrough SARS-CoV-2 infection, which, nonetheless, decreased with increasing antispike antibody titers [23]. Importantly, most of the aforementioned studies spanned earlier phases of the pandemic [2, 4, 6, 9, 11], with different circulating variants and potentially standards of care, which can affect base mortality rates [24, 25].
There is a relative paucity of recent clinical data on the efficacy of vaccination, and specifically additional mRNA vaccine doses, among OTRs using concurrent unvaccinated controls during the most recent (Omicron) phase of the pandemic. To this end, using real-world, patient-level data from our comprehensive institutional registry, we compared clinical outcomes during the current era of Omicron predominance between OTRs who developed COVID-19 after receipt of 2 or ≥3 vaccine doses and unvaccinated OTRs who had COVID-19 during the same relatively narrow time period.
METHODS
Study Design and Data Collection
We included OTRs followed at Brown University-affiliated hospitals who had polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection between 12/15/2021 (date of the first OTR case after the Omicron variant was detected in Rhode Island) and 5/25/2022. Baseline was defined as the day of symptom onset or the day of first positive SARS-CoV-2 PCR if asymptomatic. Patients were excluded if they were <18 years old, if they received <2 doses of an mRNA vaccine, or if their baseline was within <2 weeks of receiving their most recent mRNA vaccine dose. Exclusion criteria for vaccination status were determined based on the (a) low efficacy of the adenoviral vector vaccine or of “partial” vaccination with 1 dose of an mRNA vaccine (or <2 weeks from the last dose), and (b) small number of patients in each of these categories. Relevant baseline demographic and clinical information was extracted retrospectively from electronic medical records (EMRs) (Table 1). The study was approved by the Lifespan Institutional Review Board.
Table 1.
Demographic and Clinical Characteristics
| Parameter | Unvaccinated | 2 Doses | ≥3 Doses | P Value |
|---|---|---|---|---|
| No. of patients | 16 | 27 | 60 | |
| Age, y | 55 (43.5–66) | 57 (39–65) | 58 (45.8–69.3) | .344 |
| Female sex | 8 (50.0) | 11 (40.7) | 30 (50.0) | .811 |
| Ethnicity and race | ||||
| Hispanic | 4 (25.0) | 5 (18.5) | 4 (6.7) | .016 |
| Black | 3 (18.75) | 5 (18.5) | 4 (6.7) | .021 |
| White | 8 (50.0) | 17 (63.0) | 46 (76.7) | .042 |
| Other | 1 (6.25) | 0 (0) | 6 (10.0) | .430 |
| BMI, kg/m2 | 29.7 (22.9–33.3) | 27.1 (23.7–33.8) | 29.0 (25.0–32.4) | .919 |
| Smoking status | ||||
| Never | 10 (62.5) | 16 (59.3) | 38 (63.3) | .965 |
| Current or former | 6 (37.5) | 11 (40.7) | 22 (36.7) | |
| Comorbiditiesa | ||||
| Hypertension | 16 (100) | 25 (92.6) | 48 (80.0) | .034 |
| Diabetes | 4 (25.0) | 11 (40.7) | 18 (30.0) | .866 |
| Cardiac | 2 (12.5) | 4 (14.8) | 7 (11.7) | .676 |
| Chronic kidney disease | 11 (68.8) | 16 (59.3) | 27 (45.0) | .046 |
| Pulmonary | 2 (12.5) | 4 (14.8) | 6 (10.0) | .573 |
| Time since transplant, mo | 57 (34–111) | 61 (15–138) | 67 (32.3–130) | .754 |
| Transplanted organa | ||||
| Kidney | 16 (100) | 24 (88.9) | 56 (93.3) | .430 |
| Heart | 2 (12.5) | 3 (11.1) | 3 (5.0) | .152 |
| Pancreas | 1 (6.25) | 1 (3.7) | 2 (3.3) | .410 |
| Lung | 0 (0) | 0 (0) | 1 (1.7) | .922 |
| Liver | 0 (0) | 0 (0) | 1 (1.7) | .922 |
| >1 | 3 (18.8) | 1 (3.7) | 3 (5.0) | .068 |
| Maintenance immunosuppressive drug regimen | ||||
| 3-drug regimen | 12 (75.0) | 21 (77.8) | 42 (70.0) | .453 |
| 2-drug regimen | 4 (25.0) | 6 (22.2) | 18 (30.0) | .649 |
| Calcineurin/mTOR inhibitor or costimulatory blocker | ||||
| Tacrolimus | 12 (75.0) | 21 (77.8) | 51 (85.0) | .374 |
| Sirolimus | 1 (6.25) | 2 (7.4) | 8 (13.3) | .443 |
| Tacrolimus & sirolimus | 1 (6.25) | 0 (0) | 0 (0) | .010 |
| Cyclosporine | 1 (6.25) | 3 (11.1) | 1 (1.7) | .107 |
| Belatacept | 1 (6.25) | 0 (0) | 0 (0) | .010 |
| None | 0 (0) | 1 (3.7) | 0 (0) | .214 |
| Antimetabolite | ||||
| Azathioprine | 2 (12.5) | 3 (11.1) | 8 (13.3) | .983 |
| Mycophenolic acid (Myfortic) | 10 (62.5) | 14 (51.9) | 29 (48.3) | .277 |
| Mycophenolate mofetil (CellCept) | 1 (6.25) | 4 (14.8) | 10 (16.7) | .435 |
| None | 3 (18.75) | 6 (22.2) | 13 (21.7) | .974 |
| Held or decreasedb | 10 (76.9) | 17 (80.95) | 30 (63.8) | .200 |
| Prednisone | 15 (93.8) | 27 (100) | 53 (88.3) | .130 |
| Vaccine type | ||||
| BNT162b2 (Pfizer-BioNTech) | NA | 20 (74.1) | 37 (61.7) | .379 |
| mRNA-1273 (Moderna) | NA | 7 (25.9) | 23 (38.3) | |
| Mixed (Pfizer-BioNTech/Moderna) | NA | 0 | 1 (1.7) | |
| Antiviral medications | ||||
| mAb | 5 (31.3) | 19 (70.4) | 34 (56.7) | .323 |
| Remdesivir (all, hospitalized only) | 8 (50.0, 88.8) | 5 (18.5, 50) | 10 (16.7, 62.5) | .008, .185 |
| Nirmatrelvir/ritonavir (Paxlovid) | 0 | 1 (3.7) | 3 (5.0) | .590 |
| COVID-19 baseline characteristics | ||||
| Year 2021 | 3 (18.8) | 6 (22.2) | 14 (23.3) | .831 |
| Reinfection | 2 (12.5) | 2 (7.4) | 4 (6.7) | .346 |
| Days of symptoms | 4 (1–8.5) | 2.5 (1–8.5) | 3 (1–5) | .488 |
Data are presented as No. (%) for categorical variables and median (IQR) for continuous variables. All patients were coded as either female or male sex in the hospital EMR; none were listed as intersex. Ethnicity and race data were taken from the hospital EMR and may not reflect patient self-identification. Bolded P-values are those ≤ .05 (considered statistically significant).
Abbreviations: BMI, body mass index; EMR, electronic medical record; IQR, interquartile range; mAb, antispike monoclonal antibody; OTRs, organ transplant recipients.
Total will be greater than the total number of patients due to row overlap.
Percentage of OTRs who were receiving an antimetabolite at baseline.
The primary outcome was 90-day mortality given substantial delayed mortality among OTRs with COVID-19 [10, 26]. Secondary outcomes were 30-day mortality, hospitalization, intensive care unit (ICU) admission, length of stay among admitted OTRs who survived at least 90 days, and peak (worst) oxygen (O2) requirement on a modified ordinal scale: 0, outpatient only; 1, admitted to the hospital but without supplemental O2 requirement; 2, low-flow O2 requirement; 3, high-flow O2 requirement; 4, noninvasive mechanical ventilation (BiPAP, CPAP); 5, invasive mechanical ventilation.
Statistical Analyses
The normality of distribution was assessed with the Kolmogorov-Smirnoff test. Data for continuous variables are presented as median (interquartile range [IQR]) and compared with the Kruskal-Wallis test or Mann-Whitney U criterion, the latter for pairwise comparisons. Categorical variables are presented as number (%) and compared with χ2 for linear trend (Mantel-Haenszel) or the Fisher exact (for 2 × 2 comparisons) test.
Ninety-day survival was also analyzed by Kaplan-Meier curves (log-rank test for trend) and univariable and multivariable Cox regression models. The proportional hazards assumption was confirmed by visual assessment of Schoenfeld residuals and by building time-dependent variables. Factors with a P value of <.2 on univariate analyses were entered into the multivariate models and retained if the P value for variable removal was <.05.
The association between vaccination status and peak O2 requirements was assessed by calculation of the Kendall’s tau b correlation coefficient. Additional sensitivity analyses were performed by analyzing age as a continuous variable, after excluding patinets diagnosed in December 2021 (for Omicron and Delta overlap), or with history of prior COVID-19 infection, by using binary logistic instead of Cox regression, by entering variables that differed substantially between the 3 groups, and by using ordinal logistic regression instead of ranks correlation to examine the association between vaccination status and peak O2 requirement.
All analyses were performed with SPSS statistical software, version 24.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was set at a 2-tailed P value of .05, unless otherwise indicated above.
RESULTS
One hundred eleven OTRs acquired COVID-19 during the study period. Six received an adenoviral vector vaccine, and 2 were <18 years old. One hundred three OTRs met the inclusion criteria. Of these, 49 (48%) were female, 13 (13%) Hispanic, and 12 (12%) Black. Most (96, 93%) had received a kidney transplant. The median age at the time of COVID-19 diagnosis (IQR) was 58 (43–68) years. Sixteen (16%) patients were unvaccinated, 27 (26%) received 2 vaccine doses, and 60 (58%) received ≥3 COVID-19 mRNA vaccine doses (3 of whom received 4 doses), all >2 weeks from baseline.
Demographic and clinical data are summarized in Table 1 and were largely comparable. However, those who were vaccinated, especially with ≥3 doses, were less likely to have chronic kidney disease (unvaccinated vs 2 vs ≥3 doses: 69% vs 59% vs 45%; P = .046) or hypertension (unvaccinated vs 2 vs ≥3 doses: 100% vs 93% vs 80%; P = .034). Patients identified in the EMR as Black (unvaccinated vs 2 vs ≥3 doses: 19% vs 18.5% vs 7%; P = .021) or Hispanic (unvaccinated vs 2 vs ≥3 doses: 25% vs 19% vs 7%; P = .016) were underrepresented among the vaccinated, especially in the ≥3-dose group (Table 1).
Vaccinated patients were less likely to be treated with remdesivir, which at our institution is not administered to outpatients, as they were less likely to require hospital admission (unvaccinated vs 2 vs ≥3 doses: 50% vs 19% vs 17%; P = .008); this difference did not reach statistical significance among hospitalized patients, where remdesivir is administered to most OTRs, especially those requiring supplemental O2 (unvaccinated vs 2 vs ≥3 doses: 89% vs 50% vs 63%) (Table 1).
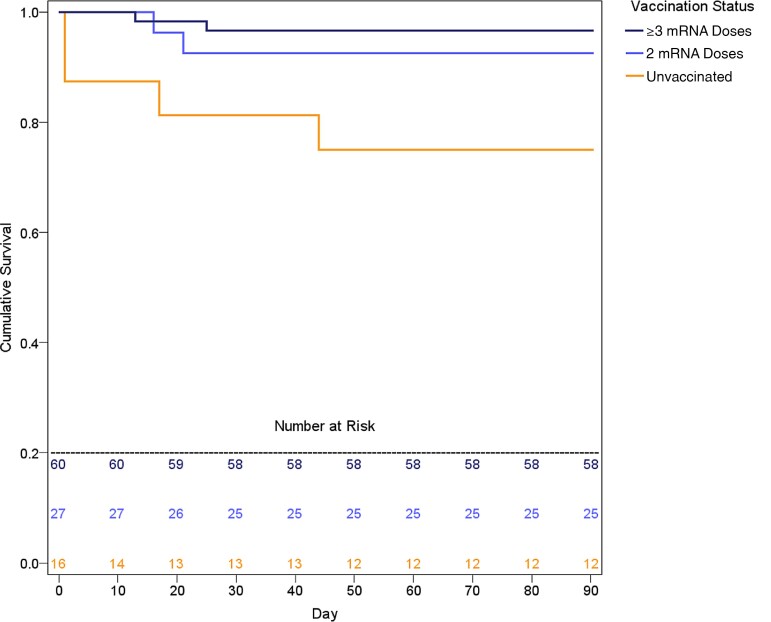
Clinical outcomes are summarized in Table 2. Vaccinated patients were less likely to be admitted to the hospital (unvaccinated vs 2 vs ≥3 doses: 56% vs 37% vs 27%; P = .018) or ICU (unvaccinated vs 2 vs ≥3 doses: 25% vs 15% vs 3%; P = .001) or to die by day 30 (unvaccinated vs 2 vs ≥3 doses: 19% vs 7% vs 3%; P = .019) or day 90 (unvaccinated vs 2 vs ≥3 doses: 25% vs 7% vs 3%; P = .003; log-rank P = .006) (Figure 1). There was no significant difference in 90-day mortality between subjects who received either 2 or ≥3 doses of BNT162b2 (Pfizer: 3/57, 5.3%) and those who received 2 or ≥3 doses of mRNA-1273 (Moderna: 1/30, 3.3%) vaccines.
Table 2.
Clinical Outcomes
| Outcome | Unvaccinated | 2 Doses | ≥3 Doses | P Value |
|---|---|---|---|---|
| No. of patients | 16 | 27 | 60 | |
| 90-d mortality | 4 (25) | 2 (7.4) | 2 (3.3) | .003 |
| 30-d mortality | 3 (18.8) | 2 (7.4) | 2 (3.3) | .019 |
| Hospitalization | 9 (56.25) | 10 (37.0) | 16 (26.7) | .018 |
| ICU admission | 4 (25) | 4 (14.8) | 2 (3.3) | .001 |
| Length of hospital stay, d | 7 (3–12) | 7.5 (1.3–11) | 3.5 (2–5.5) | .130 |
Length of hospital stay was isolated to admitted OTRs who survived at least 90 days and is presented as median (IQR). All other variables are presented as No. (%). Bolded P-values are those ≤ .05 (considered statistically significant).
Abbreviations: ICU, intensive care unit; IQR, interquartile range; OTRs, organ transplant recipients.
Figure 1.
Kaplan-Meier 90-day survival curves. Log-rank P = .006.
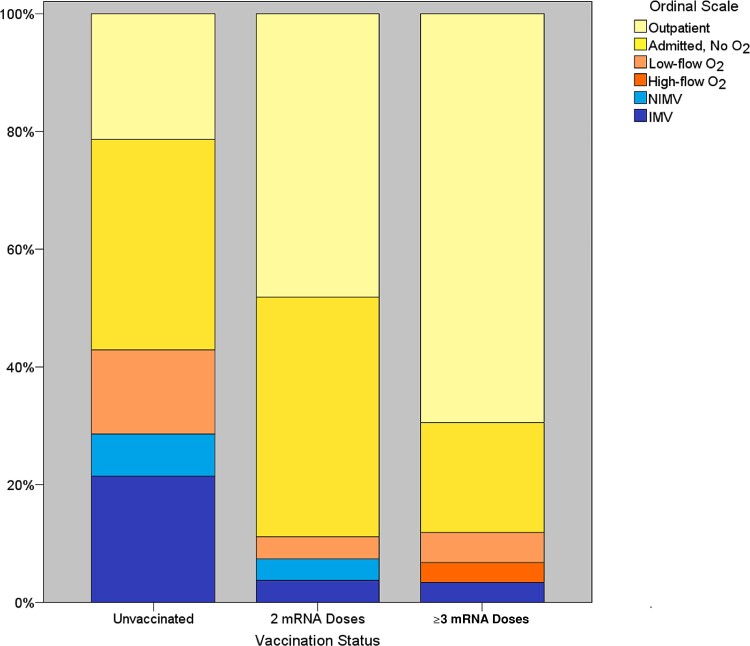
Vaccinated patients, especially those who received ≥3 doses, had decreased peak supplementary O2 requirements (ordinal scale Kendall’s tau b = –0.309 [lower scores, ie, O2 requirements with more vaccine doses]; P = .003) (Figure 2). Among hospitalized 90-day survivors, the difference in length of hospital stay did not reach statistical significance (P = .13) (Table 2).
Figure 2.
Peak O2 requirement ordinal scale value distribution by vaccination status. Kendall’s tau b = –0.309 (lower scores, ie, O2 requirements with more vaccine doses; P = .003). Abbreviations: IMV, invasive mechanical ventilation; NIMV, noninvasive mechanical ventilation.
On univariable and multivariable Cox regression analyses (Table 3), older age (>60 years) was associated with increased risk of 90-day mortality (adjusted hazard ratio [aHR], 7.73; P = .016). Administration of antispike mAb (aHR, 0.17; P = .042) and vaccination, especially with ≥3 doses (aHR, 0.105; P = .01), were associated with decreased risk of 90-day mortality. All mAbs were administered under Emergency Use Authorization (EUA), not a clinical trial. The benefits associated with vaccination, especially with ≥3 doses, did not change substantially across all sensitivity analyses (data not shown, available upon request).
Table 3.
Ninety-Day Mortality Univariable and Multivariable Cox Regression Analyses
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| mAb | 0.24 | 0.05–1.20 | .083 | 0.17 | 0.03–0.94 | .042 |
| Age >60 y | 4.02 | 0.81–19.9 | .089 | 7.73 | 1.47–40.58 | .016 |
| Vaccination | .037 | .037 | ||||
| 2 doses | 0.27 | 0.05–1.47 | .129a | 0.41 | 0.07–2.37 | .319 |
| ≥3 doses | 0.12 | 0.02–0.65 | .014a | 0.105 | 0.02–0.59 | .010 |
Bolded P-values are those ≤ .05 (considered statistically significant). Abbreviations: aHR, adjusted hazard ratio; HR, hazard ratio; ICU, intensive care unit; mAb, antispike monoclonal antibody.
Reference category: unvaccinated.
DISCUSSION
Several studies have yielded mixed results regarding vaccine clinical efficacy among OTRs, especially with 2 doses of an mRNA vaccine or 1 of an adenoviral vector vaccine, during the pre-Omicron waves of the pandemic. Aslam et al. [8] found that vaccination with 2 doses of an mRNA vaccine was protective against symptomatic COVID-19. Their findings were consistent with another report from a multistate registry, which also showed decreased efficacy among immunocompromised patients, especially OTRs, compared with immunocompetent hosts [1]. A recent population-based study among OTRs from Canada showed increasing protection with 3 > 2 > 1 COVID-19 vaccine doses, against both SARS-CoV-2 infection and severe COVID-19 [3].
On the contrary, a smaller pre-Delta/Delta (January–June 2021) study from Europe showed that kidney transplant recipients who acquired COVID-19 after 1 or 2 doses of the BNT162b2 (Pfizer) mRNA vaccine had clinical outcomes comparable to those of unvaccinated controls from the previous pandemic wave (September–December 2020) [4]. Likewise, we previously found that COVID-19 mortality during the Delta surge among vaccinated OTRs after 2 mRNA vaccine doses or 1 dose of an adenoviral vector vaccine was comparable to that of unvaccinated controls, including controls from the pre-Delta phase [7]. The above findings were consistent with 2 more studies conducted during the Delta wave, which did not show a mortality benefit from vaccination [6, 9].
In our earlier report, vaccinated patients were older than unvaccinated ones and waited longer to seek medical care [7]. After these findings and our results showing benefit from mAb administration [27], we reached out with letters to OTRs followed at our center and emphasized the importance of additional vaccine doses and early treatment of COVID-19. Notably, in the present study, symptom duration at presentation was shorter than before [7], and there were no significant differences among the 3 different vaccination status groups (Table 1), highlighting the importance of patient education.
In this study, we analyzed patient-level data from a rather homogeneous cohort of OTRs, all of whom had COVID-19 in the smallest US state, during a recent time frame without significant variations in health care resources, management protocols, or circulating SARS-CoV-2 variants. Despite lower immunologic [12–14] and clinical [1, 10] efficacy of COVID-19 vaccines among OTRs and against Omicron variants [20, 21], we observed a strong, linear mortality and morbidity benefit with mRNA vaccination. OTRs who had received ≥3 doses had the highest survival rate and lowest rates of hospitalization and critical illness (Table 2, Figures 1 and 2).
Recent analyses from the National COVID Cohort Collaborative (NC3; the largest COVID-19 database in the United States) are in agreement with our results: across the pre-Delta, Delta, and Omicron waves, vaccination, especially with 3 vaccine doses, protected OTRs from infection and serious adverse outcomes [10]. The benefits from vaccination were less prominent compared with nonimmunocompromised patients, but the relative risk attenuated significantly over time (pre-Delta > Delta > Omicron), likely indicating inferior standards of care or/and higher strain virulence earlier in the pandemic, disproportionally affecting the immunosuppressed. Nevertheless, the NC3 investigators did not provide detailed outcome results specific to the Omicron era.
To our knowledge, no other study to date has assessed the clinical efficacy of mRNA vaccination among OTRs during the Omicron surge using concurrent unvaccinated controls. Our findings and those of the above studies [3, 10] support the recommendation to administer additional vaccine doses to moderately or severely immunosuppressed patients, which was originally based on immunogenicity data alone [12–14].
Vaccination rates, especially with ≥3 doses, were significantly lower among OTRs identified as Black or Hispanic in the EMR, compared with non-Hispanic White OTRs (Table 1). Although we cannot rule out type I error due to small numbers, this was a concerning finding. Minority racial and ethnic groups in America, particularly Black, Native American and Alaskan Native, and Latina/o/x groups, are disproportionately burdened with COVID-19 infections [28, 29], hospital admissions [27], and deaths [28, 29]. One comprehensive review found that the nationwide vaccine hesitancy rates for Black (42%) and Hispanic (30%) patients were higher than the nationwide pooled hesitancy rate (26%) [30]. Several factors may be contributing to such observations, namely socioeconomic status [30, 31], geographic proximity and access to transportation, education and health literacy [32], preexisting beliefs and exposure to misinformation [30], language barriers, and medical mistrust, founded in intergenerational trauma from racism and discrimination, provider prejudice, or lack of racial concordance with providers [30, 33].
The last factor may be pertinent for our small transplant team, as we do not have any Black or Hispanic providers, and our results are discrepant from those observed in the general population of Rhode Island, where most vaccine-hesitant patients are non-Hispanic White [34]. It should be noted, however, that the underrepresentation of Black and Hispanic OTRs was most prominent in the third dose group. A third mRNA vaccine dose became available later in the pandemic as part of the primary series only for immunocompromised hosts. Therefore, it is possible that our Black or Hispanic OTRs missed that dose not because of vaccine hesitancy, but rather due to incomplete or delayed vaccine education, which highlights the importance of continued outreach to ethnic and racial minorities, especially among the immunosuppressed.
Another important finding from our study was that mAb administration to OTRs with COVID-19 was associated with better survival (Table 3), in agreement with the results of a recent meta-analysis [35]. Vaccinated patients received mAbs more frequently; however, the difference was not statistically significant (Table 2) and is unlikely to have contributed to the independent benefits observed with vaccination (Table 3). In fact, it is possible that because vaccinated patients were less frequently hypoxic or hospitalized due to COVID-19 (both of which preclude mAb use under EUA), they were more likely to receive mAbs than unvaccinated patients.
Regardless, mAbs remain our first-line outpatient treatment for OTRs with COVID-19, as they have been shown to decrease the risk of hospitalization [27] and emergency room visit and death [35]. Nirmatrelvir/ritonavir (Paxlovid), although at least as effective as mAbs, inhibits irreversibly the catabolism of calcineurin and mTOR inhibitors, which are the backbone of maintenance immunosuppression among OTRs. Therefore, its administration in this patient population requires close laboratory monitoring of immunosuppressant levels and has the potential for iatrogenic toxicities [36, 37].
Our study has several limitations, which, nonetheless, are unlikely to have affected our core conclusions. First, it is a single-center report; therefore, the results may not be generalizable, although they are well aligned with those of many pre-Omicron studies [1, 3, 8, 10]. Second, our cohort included mostly kidney transplant recipients; therefore, our conclusions may not necessarily apply to other OTRs. Third, the number of patients was relatively small, but the differences in clinical outcomes were convincing (Table 2, Figures 1 and 2). Moreover, the 3 patient groups were relatively well balanced not only in their baseline characteristics (Table 1), but also in geography and Omicron phase of the pandemic, unlike older reports [2, 4, 6, 9, 11]. Fourth, data were retrospectively collected, but all key independent and outcome variables were objective and easy to extract from EMRs. Fifth, we did not have patient-level SARS-CoV-2 sequences, and there may have been some overlap between Omicron and Delta variants early on. Nonetheless, by January 2022, >95% of SARS-CoV-2 infections in our state were caused by Omicron [38], the distribution of cases in late 2021 vs 2022 was similar across the 3 vaccination status groups (Table 1), and exclusion of OTRs from 2021 did not significantly change our conclusions.
Also, the size of our cohort did not allow us to match patients who received 2 vs ≥3 doses for time from last dose, to clarify if the benefit from the additional dose was independent of the fact that patients had higher antibody titers at the time of COVID-19, because the most recent vaccine dose was closer to infection. However, previous studies have shown that even nonresponders to 2 mRNA vaccine doses can mount an immune response to additional doses, suggesting increased immunogenicity [12–14]. Last, clinicians may have a lower threshold to admit unvaccinated OTRs to the hospital, but this should not affect mortality or peak O2 requirements.
In conclusion, mRNA vaccination protects OTRs from severe COVID-19 and death. Health care disparities in the prevention and treatment of COVID-19 call for better outreach initiatives and patient education. Every effort should be made toward high enrollment of OTRs in vaccine clinical trials, with strong representation of ethnic and racial minorities.
Acknowledgments
Financial support. A.H.L. has received research support from the Infectious Diseases Society of America Foundation (Grants for Emerging Researchers/Clinicians Mentorship Program) and the Brown Emerging Infectious Disease Scholars Program (NIH 5R25AI140490). P.A. and E.J.K. have received research support from the Summer Assistantship Program of the Warren Alpert Medical School of Brown University.
Patient consent. This retrospective study was approved by the Lifespan IRB with a waiver of informed consent.
Contributor Information
Alexis Hope Lerner, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Panos Arvanitis, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Kendra Vieira, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Elizabeth Jessica Klein, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Dimitrios Farmakiotis, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
References
- 1. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19–associated hospitalizations among immunocompromised adults—nine states, January–September 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callaghan CJ, Mumford L, Curtis RM, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation 2022; 106:436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naylor KL, Kim SJ, Smith G, et al. Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: a population-based cohort study from Canada. Am J Transplant 2022; 22:2228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reischig T, Kacer M, Vlas T, et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant 2022; 22:801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saharia KK, Anjan S, Streit J, et al. Clinical characteristics of COVID-19 in solid organ transplant recipients following COVID-19 vaccination: a multicenter case series. Transpl Infect Dis 2022; 24:e13774. [DOI] [PubMed] [Google Scholar]
- 6. Mazuecos A, Villanego F, Zarraga S, et al. Breakthrough infections following mRNA SARS-CoV-2 vaccination in kidney transplant recipients. Transplantation 2022; 106:1430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vieira K, Klein E, Lerner A, Farmakiotis D. High case fatality rate among fully vaccinated kidney transplant recipients with breakthrough COVID-19 during the Delta surge. Am J Transplant 2022; 22(Suppl 3):440. [Google Scholar]
- 8. Aslam S, Liu J, Sigler R, et al. Coronavirus disease 2019 vaccination is protective of clinical disease in solid organ transplant recipients. Transpl Infect Dis 2022; 24:e13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demir E, Dheir H, Safak S, Artan AS, Sipahi S, Turkmen A. Differences in clinical outcomes of COVID-19 among vaccinated and unvaccinated kidney transplant recipients. Vaccine 2022; 40:3313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vinson AJ, Anzalone AJ, Sun J, et al. The risk and consequences of breakthrough SARS-CoV-2 infection in solid organ transplant recipients relative to non-immunosuppressed controls. Am J Transplant 2022; 22:2418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation 2021; 105:e263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021; 174:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021; 326:1063–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odriozola A, Lamadrid-Perojo P, Cuadrado A, et al. Immune response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in liver transplant recipients. Transplantation 2022; 106:e341–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charmetant X, Espi M, Benotmane I, et al. Infection or a third dose of mRNA vaccine elicits neutralizing antibody responses against SARS-CoV-2 in kidney transplant recipients. Sci Transl Med 2022; 14:eabl6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benotmane I, Bruel T, Planas D, Fafi-Kremer S, Schwartz O, Caillard S. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int 2022; 101:1073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masset C, Benotmane I, Dantal J, et al. A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Kidney Int 2022; 101:825–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Midtvedt K, Vaage JT, Heldal K, Munthe LA, Lund-Johansen F, Asberg A. Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Jurdi A, Gassen RB, Borges TJ, et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int 2022; 101:1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benning L, Morath C, Bartenschlager M, et al. Neutralizing antibody response against the B. 1.617. 2 (Delta) and the B. 1.1. 529 (Omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am J Transplant 2022; 22:1873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cochran W, Shah P, Barker L, et al. COVID-19 Clinical outcomes in solid organ transplant recipients during the Omicron surge. Transplantation 2022; 106:e346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alejo JL, Chiang TP, Zeiser LB, et al. Incidence and severity of COVID-19 among vaccinated solid organ transplant recipients during the Omicron wave. Transplantation 2022; 106:e413–5. [DOI] [PubMed] [Google Scholar]
- 24. Dodd LE, Freidlin B, Korn EL. Platform trials - beware the noncomparable control group. N Engl J Med 2021; 384:1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant 2022; 22:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heldman MR, Kates OS, Safa K, et al. Delayed mortality among solid organ transplant recipients hospitalized for COVID-19. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein EJ, Hardesty A, Vieira K, Farmakiotis D. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: efficacy, ethnic and racial disparities. Am J Transplant 2022; 22:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omer SB, Benjamin RM, Brewer NT, et al. Promoting COVID-19 vaccine acceptance: recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. Lancet 2021; 398:2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barry K, McCarthy M, Melikian G, Almeida-Monroe V, Leonard M, De Groot AS. Responding to COVID-19 in an uninsured Hispanic/Latino community: testing, education and telehealth at a free clinic in Providence. R I Med J (2013) 2020; 103:41–6. [PubMed] [Google Scholar]
- 30. Khubchandani J, Macias Y. COVID-19 vaccination hesitancy in Hispanics and African-Americans: a review and recommendations for practice. Brain Behav Immun Health 2021; 15:100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020; 26:100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health 2021; 21:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson HS, Manning M, Mitchell J, et al. Factors associated with racial/ethnic group-based medical mistrust and perspectives on COVID-19 vaccine trial participation and vaccine uptake in the US. JAMA Netw Open 2021; 4:e2111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rogers BG, Tao J, Almonte A, et al. Statewide evaluation of COVID-19 vaccine hesitancy in Rhode Island. PLoS One 2022; 17:e0268587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang M, Li A, Wang Y, Tran C, Zhao S, Ao G. Monoclonal antibody therapy improves severity and mortality of COVID-19 in organ transplant recipients: a meta-analysis. J Infect. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mecadon K, Arvanitis P, Farmakiotis D, Rogers R. Single-center experience with nirmatrelvir/ritonavir in kidney transplant recipients on tacrolimus maintenance immunosuppression. Clin Transplant 2022; 36:e14752. [DOI] [PubMed] [Google Scholar]
- 37. Farmakiotis D. COVID-19 treatments for nonhospitalized patients. JAMA 2022; 327:2247. [DOI] [PubMed] [Google Scholar]
- 38. Singh M, Novitsky V, Carpenter-Azevedo K, et al. SARS-CoV-2 variants in Rhode Island; May 2022 update. R I Med J (2013) 2022; 105:6–11. [PMC free article] [PubMed] [Google Scholar]