Abstract
To assess cell-mediated immunity to Toxoplasma gondii, we evaluated the expression of the activation antigens CD69, CD71, and CD25 on T lymphocytes by flow cytometry after specific in vitro stimulation of whole blood from 127 T. gondii-positive and 63 T. gondii-negative patients. T lymphocytes from many seropositive individuals did not express CD69 at 24 h after T. gondii antigen stimulation, but CD71 and CD25 were easily detectable on T cells from seropositive individuals 7 days after specific activation. CD25 was mainly expressed by stimulated CD4+ T cells, and its detection on total T cells was both a sensitive (98%) and a specific (97%) indicator of prior T. gondii infection. These results make flow cytometric detection of CD25 an excellent candidate for screening cell-mediated immunity to T. gondii in vitro and an interesting tool for the diagnosis of congenital infection.
Infection with Toxoplasma gondii is an important cause of morbidity and mortality in neonates, persons infected with human immunodeficiency virus, and persons with other defects in cell-mediated immunity (6, 9). Protection against toxoplasmosis is mediated primarily by cellular defenses. The key role of T lymphocytes in resistance against T. gondii was first demonstrated by Frenkel, who showed that hamsters receiving spleen and lymphoid cells from T. gondii-infected syngeneic donors were subsequently protected from an otherwise lethal challeng (reviewed by Darcy and Santoro [6]). In humans, T-cell proliferative responses after specific stimulation with a T. gondii antigen(s) have been reported (1, 2, 16).
The standard way to quantify in vitro the cellular immune response to an antigen, determination of cellular proliferation as measured by incorporation of tritiated [3H]thymidine after contact between cultured lymphocytes and the specific antigen (5), is time-consuming and requires specialized radioactive equipment. An alternative is to measure the expression of activation antigens, such as CD69, CD25, CD71, or HLA-DR, on the lymphocyte membrane. These molecules are absent from, or present at very low levels on, the surfaces of resting cells but are induced following contact with specific antigen. CD69 expression peaks at 24 h after in vitro stimulation of T cells (12), while maximal CD25 (interleukin-2 receptor) or CD71 (transferrin receptor) expression requires 4 to 8 days of culture with mitogen or antigen (3). The percentage of T lymphocytes expressing these membrane antigens following stimulation of mononuclear cells or whole blood with specific antigen can easily be determined by flow cytometry (3, 11, 12). Using this simple method, we have evaluated the T-cell responses of individuals with and without specific antibodies to T. gondii and determined that the CD25 response at 7 days is an accurate indicator of prior infection.
MATERIALS AND METHODS
Subjects.
Blood collected from 190 individuals by using Vacutainer tubes (Becton Dickinson, Meylan, France) containing lithium heparin anticoagulant was sent to the Laboratoire de Parasitologie for diagnosis or follow-up of toxoplasmosis. T. gondii antibodies were evaluated by using a commercial enzyme-linked immunosorbent assay (Enzygnost; Behring, Rueil Malmaison, France). Specific-immunoglobulin G (IgG) titers of <25 U/ml or IgM titers of <6 (arbitrary units) were considered negative. By these criteria, 127 individuals showed serologic evidence of prior infection with T. gondii (congenital toxoplasmosis, n = 84, age [mean ± standard deviation] = 6 ± 4 years; chronic infection, n = 24, age = 35 ± 10 years; toxoplasmic retinochoroiditis, n = 12, age = 18 ± 6 years; and acute infection, n = 7, age = 29 ± 4 years). The 63 others were T. gondii negative (age = 29 ± 5 years).
In vitro activation.
Samples of 50 μl of whole blood were placed in 45- by 8.8-mm tubes (Micronic Systems, Lelystad, The Netherlands), and duplicate specimens were treated with an equal volume of soluble T. gondii antigen (final concentration, 6 μg/ml), negative-control culture supernatant, or phytohemagglutinin (10 μg/ml; Sigma, St. Quentin Fallavier, France) as a positive control. Soluble T. gondii antigen was prepared by infection of murine WEHI 164 cells (ATCC CRL 1751), at three tachyzoites/cell, with T. gondii strain RH from the peritoneal cavities of 24-h-infected OF1 mice (Iffa Credo, Saint Germain sur l’Arbresle, France). At 2 days the tachyzoites were harvested, washed, adjusted to 106/ml in phosphate-buffered saline (Biomérieux, Marcy l’Etoile, France), and disrupted by five freeze-thaw cycles. The suspension was clarified by centrifugation at 2,500 × g for 15 min and passaged through a 0.2-μm-pore-size filter. Negative-control culture supernatant was collected from uninfected WEHI 164 cells. Blood cells were collected after 24 h of culture at 37°C in the presence of antigen for determination of CD69 expression and after 7 days for determination of CD25 or CD71 expression. The 7-day cultures were supplemented at 24 h with 500 μl of RPMI 1640 medium containing 1% l-glutamine, penicillin (10,000 U/ml), streptomycin (10 mg/ml), and amphotericin B (25 mg/ml; Sigma).
Membrane staining and flow cytometry.
Excess medium was decanted from the cell suspensions, and erythrocytes were lysed with (per liter) 155 mmol of NH4Cl, 10 mmol of KHCO3, and 0.1 mmol of EDTA. The leukocytes were recovered by centrifugation, and the pellets were stained with a combination of three specific monoclonal antibodies labelled with distinguishable fluorochromes for 15 min in the dark at 4°C. For CD69 detection, a commercial preparation (Becton Dickinson, Pont de Claix, France) consisting of antibody to CD3 (Leu 4) conjugated with peridininin chlorophyll protein, anti-CD4 (Leu 3) conjugated with fluorescein isothiocyanate (FITC), and anti-CD69 (Leu 23) conjugated with phycoerythrin (PE) was used. A combination of FITC-conjugated antibodies to either CD25 or CD71 (Dako, Trappes, France) with PE–anti-CD4 (Sigma) and cyanin 5-phycoerythrin (Cy5PE)-conjugated anti-CD3 (Dako) was used to estimate CD25 and CD71 expression. The cells were then washed and resuspended in phosphate-buffered saline–0.1% bovine serum albumin–5 mM EDTA and analyzed the same day by three-color analysis with a FACScan flow cytometer (Becton Dickinson). Lysis II software was used in acquisition, with fluorescence triggering in the f13 channel to gate on CD3+ lymphocyte populations. Data were then displayed as two-color dot plots (fl1 and fl2) on the fl3 positive cells to measure the proportion of activated lymphocyte subsets that expressed CD25 or CD71 (Fig. 1). Similar results were obtained for CD69 expression after staining with specific antibodies (data not shown). Specific positivity of CD3+ or CD4+ cells expressing activation antigens was estimated by subtracting the values obtained for negative-control culture supernatant cells from those obtained for cells treated with soluble T. gondii antigen.
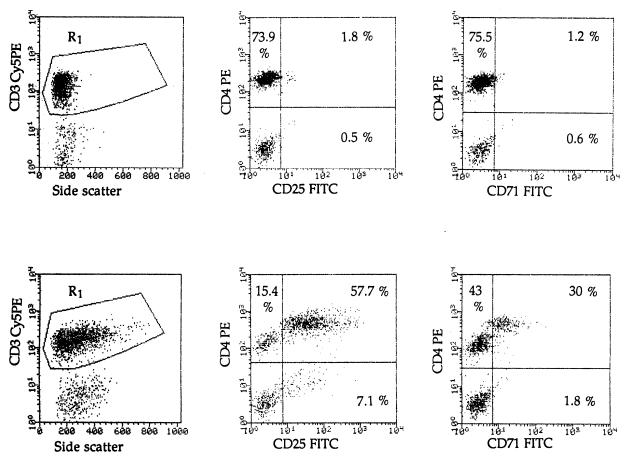
FIG. 1.
Flow cytometric analysis of CD25 and CD71 expression on T. gondii-stimulated CD4+ and CD4− T lymphocytes. Whole blood from a positive donor was incubated for 7 days with soluble T. gondii antigen (bottom row) or with negative-control culture supernatant (top row). Cells were stained, as described in Materials and Methods, with a combination of Cy5PE-conjugated anti-CD3, PE-conjugated anti-CD4, and FITC-conjugated anti-CD25 (second column) or anti-CD71 (third column) antibodies. Resting and activated CD3+ cells were gated on R1 (first column).
Statistical tools.
Differences in the percentages of cells expressing the three activation antigens were tested for significance with the Mann-Whitney U test and Student’s t test.
RESULTS
Blood samples from 20 subjects (10 chronically infected and seropositive and 10 seronegative for T. gondii-specific antibodies) were compared for CD69, CD25, and CD71 expression on CD3+ lymphocytes after stimulation with soluble T. gondii antigen. Lymphocytes from all patients strongly expressed CD69, CD25, and CD71 after stimulation with phytohemagglutinin (data not shown). Supernatants of uninfected WEHI 164 cells used as negative controls never induced activation (data not shown). Figure 2 shows that CD25 and CD71 were expressed, respectively, by 40.60% ± 13.21% and 32.63% ± 18.65% of CD3+ lymphocytes from T. gondii-positive individuals while CD69 was induced on only 1.82% ± 2.05% of cells (P < 0.0001 in each case). None of these antigens were expressed by stimulated CD3+ lymphocytes from T. gondii-negative individuals. Since CD25 was specifically expressed on a consistently high percentage of CD3+ cells from positive donors, we chose this activation antigen as the indicator of a T. gondii-specific cellular response in a large series of donors.
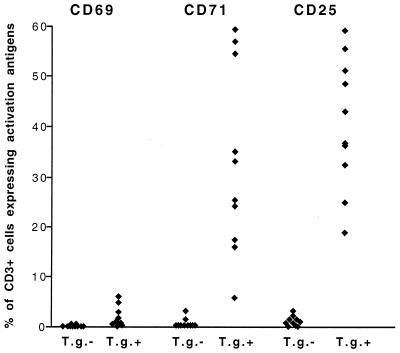
FIG. 2.
Percentages of CD3+ lymphocytes expressing CD69, CD71, and CD25 above control levels after in vitro stimulation with soluble T. gondii antigen in whole blood from 10 T. gondii-positive (T.g.+) and 10 T. gondii-negative (T.g.−) individuals. Stimulation and staining were performed as described in Materials and Methods. Each symbol represents one individual. Statistical analysis (Mann-Whitney U test) showed significantly higher percentages of CD3+ CD25+ and CD3+ CD71+ cells than of CD3+ CD69+ cells in T.g.+ individuals (P < 0.0001 in each case).
Intra-assay reproducibility was first determined with 10 replicate whole-blood samples from a single seropositive donor, separately stimulated with soluble T. gondii antigen. The optimal incubation time was determined from a kinetic curve (data not shown), and the percentages of CD3+ or CD3+ CD4+ cells expressing significant levels of CD25 fell between 55.5 and 62.6%, with coefficients of variation of 4.4 and 4.5%, respectively. Ten identical replicate samples stimulated with control supernatant produced only a small number (0.9 to 1.5%) of CD25-expressing cells.
Blood samples from 190 individuals were evaluated for expression of CD25 after stimulation with soluble T. gondii antigen (Fig. 3). A significantly greater percentage of lymphocytes from T. gondii-positive than from T. gondii-negative individuals expressed CD25 after specific stimulation (3.25 to 83.15% versus 0 to 15.95%; P < 0.0001). Two of 63 seronegative patients showed abnormally high cellular responses to antigen, while 3 of 127 seropositive individuals showed only weak specific stimulation of CD25 expression. In this population, if we adopt as the threshold a value of 3 standard deviations above the mean percentage of CD3+ lymphocytes from negative donors expressing CD25 after specific stimulation (10.1%), we obtain a specificity of 97% (95% confidence interval = 92 to 100) and a sensitivity of 98% (95% confidence interval = 95 to 100). CD4+ cells were always present in the T-cell population expressing CD25 after specific stimulation, while only 31% of positive donors had CD4− T cells which could be induced to express CD25. The ratio of CD4+ cells to CD4− cells increased after stimulation of responders by T. gondii antigen only when the CD4+ cells and not the CD4− cells showed a positive response.
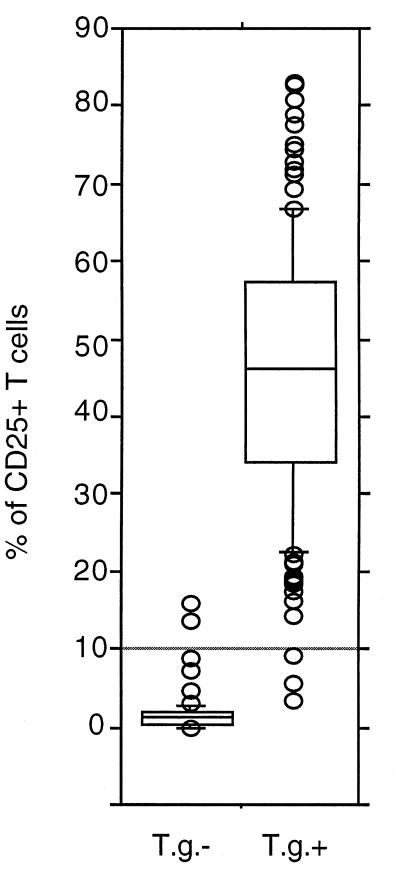
FIG. 3.
Percentages of CD3+ lymphocytes expressing CD25 above control levels after in vitro stimulation with soluble T. gondii antigen in whole blood of 190 individuals (127 positive for T. gondii-specific IgG antibodies [T.g.+] and 63 negative [T.g.−]). Stimulation and staining were performed as described in Materials and Methods. Boxes represent values between 25th and 75th percentiles and medians; bars indicate 10th and 90th percentiles. Circles outside the bars are extra values. The horizontal bar shows mean percentages + 3 standard deviations. Statistical analysis (Student’s t test) showed significantly higher percentages of CD3+ CD25+ cells in T.g.+ than in T.g.− individuals (P < 0.0001).
DISCUSSION
We describe a convenient method for evaluating cellular immune responses in toxoplasmosis patients which requires no manipulation of radioactive materials. The expression of activation antigens by T lymphocytes after specific stimulation with an antigen to which an individual has had prior exposure can be readily measured by flow cytometry and can be induced in a small sample of whole blood without separation of leukocytes. We evaluated three such antigens. CD69 expression occurs rapidly (24 h) on antigen-reactive cells, but the small number (<2%) of such cells precluded accurate identification of positive individuals. The number of CD69-positive cells did not increase over 6 days of culture (data not shown) because CD69 is a very early activation marker and its expression decreases rapidly (4). CD69 expression is also unreliable in evaluating cellular responses to tetanus toxoid (12). CD25 and CD71 were regularly induced on a much larger percentage of T lymphocytes from T. gondii-seropositive than T. gondii-seronegative subjects. These antigens also show an amplified expression in response to tetanus toxoid or influenza virus antigens (3). Both appear suitable for the evaluation of a cellular response, but the smaller range of dispersion of the percentage of cells expressing CD25 led us to choose this marker for further evaluation.
The test proved to correlate well with serological status, and samples from seronegative individuals usually showed no stimulation by our antigen preparation, in agreement with a previous report (14). Less pure antigen preparations are liable to evoke CD25 expression on lymphocytes from T. gondii-seronegative individuals (data not shown). Nevertheless, 2 of 63 negative individuals expressed CD25 on an anomalously high percentage of T cells after specific stimulation. This could reflect a superantigen effect (7) or possibly cross-reactivity to a related parasite. The three anomalously low responses in seropositive individuals all occurred in congenitally infected children aged 1 year or older. Absence of stimulation of lymphocytes by T. gondii antigen, measured by [3H]thymidine incorporation, has been described, both for acute acquired T. gondii infection (1, 8) and for congenitally infected children (10, 15, 16). In our study, most positive subjects had congenital infection. The small number of low measurements may reflect the fact that CD25 expression does not depend on cellular proliferation (3).
Detection of CD25 expression by flow cytometry is as specific as measurement of [3H]thymidine incorporation for detecting lymphocyte responses to T. gondii (1, 15, 16) and provides more information about the cellular immune response. In particular, T-lymphocyte subsets implicated in activation can be easily assessed and compared individually for their responses to stimuli. Unlike in studies with staphylococcal enterotoxin B (11), tetanus toxoid, and influenza virus (3), where stimulation induced almost identical percentages of CD4+ and CD4− activated T cells CD25 was predominantly detected on CD4+ cells. This agrees with recent studies of humans revealing that T. gondii-infected antigen-presenting cells elicited stronger CD4-mediated than CD8-mediated cell proliferation and generated CD4+ cytotoxic T lymphocytes more readily than CD8+ cytotoxic T lymphocytes (2, 13).
The test we describe is simple, uses cytometry apparatus readily available in many hospital laboratories, and requires no specific radioactive equipment. Even if the incubation periods are long (7 days), the assay itself can be performed directly on whole blood in a few short steps. CD25 can be detected earlier, but maximal expression is required for clear-cut differentiation between positive and negative patients. Only 300 μl of whole blood is needed, which is an advantage in taking samples from children, with whom sampling is always delicate. The technique allows exploration of cell-mediated immunity and could be adapted to other microbial agents. We are attempting detection of CD25 expression in whole blood from newborn children for early diagnosis of congenital infection.
ACKNOWLEDGMENTS
This work was supported by grants (UCBL JE 1947).
We thank J. Ferrandiz and C. Bernardoux for their helpful technical assistance, as well as M. Alkurdi.
REFERENCES
- 1.Anderson S E, Krahenbuhl J L, Remington J S. Longitudinal studies of lymphocyte responses to Toxoplasma antigens in humans infected with T. gondii. J Clin Lab Immunol. 1979;2:293–297. [PubMed] [Google Scholar]
- 2.Canessa A, Pistoia V, Merli A, Melioli G, Terragna A, Ferranini M. An in vitro model of toxoplasma infection in man. Interaction between CD4+ monoclonal T cells and macrophages results in killing of trophozoites. J Immunol. 1988;140:3580–3588. [PubMed] [Google Scholar]
- 3.Caruso A, Licenziati S, Corulli M, Canaris A D, De Francesco M A, Fiorentini S, Peroni L, Fallacara F, Dima F, Balsari A, Turano A. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 1997;27:71–76. doi: 10.1002/(sici)1097-0320(19970101)27:1<71::aid-cyto9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Cebrian M, Yagüe E, Rincon M, Lopez-Botet M, de Landazuri M O, Sanchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradin G, Etlinger H M, Chiller J M. Lymphocyte specific T-cell dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977;119:1048–1053. [PubMed] [Google Scholar]
- 6.Darcy F, Santoro F. Toxoplasmosis. In: Kierszenbaum F, editor. Parasitic infections and the immune system. San Diego, Calif: Academic Press Inc.; 1994. pp. 163–201. [Google Scholar]
- 7.Denkers E Y, Caspar P, Sher A. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor Vβ5-bearing CD8+ lymphocytes. J Exp Med. 1994;180:985–994. doi: 10.1084/jem.180.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson W D., Jr Chronological development of cellular immunity in human toxoplasmosis. Infect Immun. 1981;33:948–949. doi: 10.1128/iai.33.3.948-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–219. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod R, Beem M O, Estes R G. Lymphocyte anergy specific to Toxoplasma gondii antigens in a baby with congenital toxoplasmosis. J Clin Lab Immunol. 1985;17:149–153. [PubMed] [Google Scholar]
- 11.Maino V C, Suni M A, Ruitenberg J J. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–133. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- 12.Mardiney M, III, Brown M R, Fleisher T A. Measurement of T-cell CD69 expression: a rapid and efficient means to assess mitogen- or antigen-induced proliferative capacity in normals. Cytometry. 1996;26:305–310. doi: 10.1002/(SICI)1097-0320(19961215)26:4<305::AID-CYTO11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 13.Purner M B, Berens R L, Nash P B, van Linden A, Ross E, Kruse C, Krug E C, Curiel T J. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect Immun. 1996;64:4330–4338. doi: 10.1128/iai.64.10.4330-4338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purner M B, Krug E C, Nash P, Cook D R, Berens R L, Curiel T J. Cross-reactivity of human Toxoplasma-specific T cells: implications for development of a potential immunotherapeutic or vaccine. J Infect Dis. 1995;171:984–991. doi: 10.1093/infdis/171.4.984. [DOI] [PubMed] [Google Scholar]
- 15.Stray-Pedersen B. Infants potentially at risk for congenital toxoplasmosis. Am J Dis Child. 1980;134:638–642. doi: 10.1001/archpedi.1980.02130190006003. [DOI] [PubMed] [Google Scholar]
- 16.Wilson C B, Desmonts G, Couvreur J, Remington J S. Lymphocyte transformation in the diagnosis of congenital toxoplasma infection. N Engl J Med. 1983;302:785–788. doi: 10.1056/NEJM198004033021406. [DOI] [PubMed] [Google Scholar]