Abstract
Background
Monoclonal antibody (mAb) treatment is associated with decreased risk of hospitalization and death in high-risk outpatients with mild to moderate coronavirus disease 2019 (COVID-19) caused by early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. Bebtelovimab exhibits in vitro activity against the Omicron variant and its sublineages; however, clinical data are lacking.
Methods
A retrospective cohort study was conducted comparing bebtelovimab-treated patients with propensity score–adjusted and matched nontreated control groups. Participants included high-risk outpatients eligible for bebtelovimab treatment under Emergency Use Authorization with a positive SARS-CoV-2 test from March 30 to May 28, 2022. Treated patients received single-dose intravenous treatment with bebtelovimab. The primary outcome was hospitalization or death over 28 days.
Results
Before matching/statistical adjustment, mAb-treated patients were, on average, 10 years older than nontreated patients (61.6 vs 51.3 years) and had higher prevalence of obstructive sleep apnea, hypertension, chronic kidney disease, cancer, organ or cell transplant, and immunocompromised status (standardized mean differences ≥0.20). The adjusted odds ratio (OR) of hospitalization or death comparing 1006 treated with 2023 nontreated patients was 0.50 (95% CI, 0.31–0.80). Among 930 treated and 930 propensity score–matched nontreated patients, the incidence of hospitalization or death was 3.1% vs 5.5%, respectively (conditional OR, 0.53; 95% CI, 0.32–0.86). The lower odds ratio of hospitalization or death associated with bebtelovimab treatment was most evident in older patients, those with immunocompromised status, and fully vaccinated patients.
Conclusions
Monoclonal antibody treatment with bebtelovimab among COVID-19 outpatients is associated with lower odds of hospitalization or death, particularly among immunocompromised and older patients.
Keywords: bebtelovimab, death, hospitalization, immunosuppression, Omicron SARS-CoV-2 variant, propensity score matching
Monoclonal antibody (mAb) treatment has demonstrated decreased risk of hospitalization and death in at-risk outpatients with mild to moderate coronavirus disease 2019 (COVID-19) caused by early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, as compared with patients who did not receive treatment [1–4]. As SARS-CoV-2 variants evolve and emerge, the US Food and Drug Administration (FDA) Emergency Use Authorizations (EUAs) for mAb products change. Decisions for EUA modifications are often based on in vitro potency of mAbs alone, as randomized controlled trials and real-world clinical data are not available in real time. At the time of this report, bebtelovimab is the only mAb authorized for treatment of COVID-19 and is expected to maintain neutralizing activity against Omicron and its sublineages [5].
There is an absence of clinical data for use of bebtelovimab and for any mAb product for use in patients infected with the Omicron variant and its sublineages. Due to this lack of clinical data, the National Institutes of Health (NIH) positions bebtelovimab as an alternative therapy for nonhospitalized adults with COVID-19 [6]. However, first-line therapies are plagued by drug–drug interactions (nirmaltrevir/ritonavir) and logistical challenges thwarting accessibility (3-day course of intravenous remdesivir); therefore, determining the clinical effectiveness of bebtelovimab is important for public health. Additionally, there is a critical need for ongoing evaluation of individual mAb products as new variants emerge to test clinical effectiveness and determine patient populations who optimally benefit from treatment. Therefore, we assessed the real-world effectiveness of bebtelovimab treatment for outpatients with mild to moderate COVID-19 during the Omicron variant era within a large US health care system. We examined the association of bebtelovimab treatment overall with 28-day incidence of hospitalization or death and stratified results by age, body mass index, immunocompromised status, and COVID-19 vaccination status.
METHODS
This was a retrospective cohort study of outpatients with COVID-19 who had at least 1 risk factor for progression to severe disease and were eligible for mAb treatment with bebtelovimab per the EUA. Patients treated with bebtelovimab were compared with nontreated control patients. All treated patients verbally consented to treatment with bebtelovimab and reviewed the FDA EUA Fact Sheet before treatment. Bebtelovimab treatment assignment occurred via a central management system overseen by a multidisciplinary COVID-19 Therapeutics Committee [7].
Patient Consent
The Quality Improvement Review Committee and Institutional Review Board at the University of Pittsburgh provided ethical review and approval of the study as an exempt protocol that did not require patient written consent, and all data remained deidentified for this analysis.
Data Sources
Health-related data captured in the electronic health record (EHR) and ancillary clinical systems were aggregated and harmonized in a Clinical Data Warehouse (CDW) [1, 8]. For treated patients and nontreated control patients, sociodemographic data, medical history, and billing charges were accessed for all outpatient and in-hospital encounters with diagnoses and procedures coded based on the International Classification of Diseases, Ninth and Tenth revisions (ICD-9 and ICD-10, respectively) [9, 10]. Race was self-declared and classified as Black vs all others based on overall low minority prevalence. Death identification at 28-day used hospital discharge disposition of “Ceased to Breathe” sourced from the inpatient Medical Record System along with deaths after discharge identified with the Death Master File from the Social Security Administration 2022 National Technical Information Service [11, 12]. A description of definitions for variables used in the analysis, as captured in the EHR, is provided below and in Supplement Appendix A.
Selection of Patient Cohorts
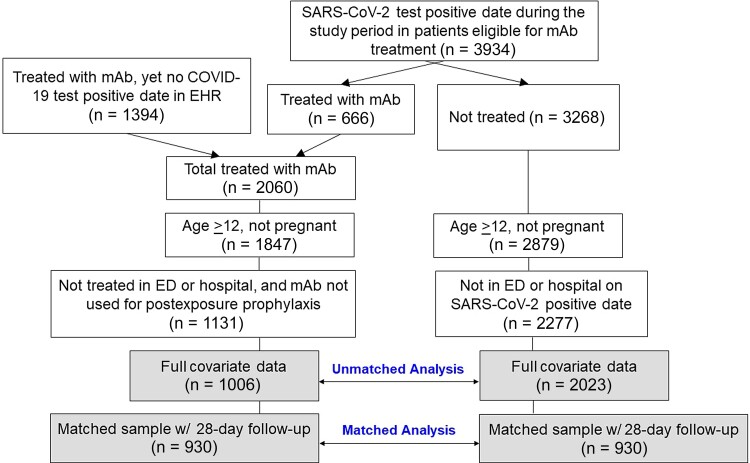
Treated patients were those 12 years of age or older who received intravenous bebtelovimab (175 mg) during the period from March 30 to May 28, 2022, in an outpatient infusion clinic for treatment of COVID-19. Patients were excluded if they were pregnant, had received mAb treatment in an emergency department or hospital setting, or had received mAb treatment for postexposure prophylaxis (Figure 1). Nontreated patients were identified as any nonpregnant patient 12 years of age or older with a positive SARS-CoV-2 polymerase chain reaction or antigen test within our health system and not treated with any mAb during the same time period. Patients had at least 1 EUA-eligible risk factor for progression to severe disease identified in the EHR on the day of the positive SARS-CoV-2 test result. Patients were excluded if they were hospitalized or in the emergency department on the day of their positive SARS-CoV-2 test result (Figure 1). After identifying patients with at least 1 health record in the EHR in the past year, both groups had complete covariate data other than for body mass index (509 missing cases, 16.7%).
Figure 1.
CONSORT diagram of selection of treated and nontreated control patients for analysis. Abbreviations: COVID-19, coronavirus disease 2019; ED, emergency department; EHR, electronic health record; mAb, monoclonal antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Outcomes
The primary outcome was the incidence of hospitalization or death at 28 days, with secondary outcomes of 28-day hospitalization, death, emergency department (ED) visit without hospitalization, and the composite outcome ED visit/hospitalization. For treated patients, the 28-day follow-up period started on the day of mAb treatment. For nontreated controls, the 28-day follow-up period started the day after the SARS-CoV-2 test positive date, as the median time from test positive result to mAb treatment (interquartile range) was 1 (1–3) day.
Covariates
In addition to specific variables used in propensity score adjustment/matching (Table 1), key covariates in prespecified subgroup analyses included (i) age (years), classified as <65 vs ≥65; (ii) body mass index (kg/m2), classified as ≤30 vs >30; (iii) immunocompromised status, classified as no vs yes; and (iv) COVID-19 vaccination status. Immunocompromised was defined from a range of conditions such as selected cancer diagnoses within the past year (eg, leukemia), selected autoimmune disorders in the past year (eg, lupus), and having an encounter in the UPMC health system within the past year and any prior history of transplant (Supplement Appendix B). Patients were classified as fully vaccinated when there was evidence in the EHR of at least 2 doses of an approved COVID-19 mRNA technology vaccine (eg, Pfizer, Moderna) or a single dose of an approved virus-based technology vaccine (eg, Johnson & Johnson). Because many patients may have been vaccinated outside of the system, the subgroup of patients with documented evidence of being fully vaccinated (51.9% of all patients) is reported, as well as all other patients with undetermined vaccination status (many of whom were likely vaccinated). Missing body mass index (16.7% of subjects) was imputed using the mean value for subjects with known values.
Table 1.
Comparison of Characteristics in Treated and Nontreated Groups
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Characteristic | Treated | Nontreated | Treated | Nontreated | ||
| (n = 1006) | (n = 2023) | SMD | (n = 930) | (n = 930) | SMD | |
| Age, mean (SD), y | 61.6 (17.3) | 51.3 (20.6) | 0.53 | 61.2 (17.5) | 62.2 (18.3) | 0.05 |
| Female sex, No. (%) | 620 (61.6) | 1298 (64.2) | 0.05 | 575 (61.8) | 571 (61.4) | 0.01 |
| Black race, No. (%) | 46 (4.6) | 161 (8.0) | 0.13 | 42 (4.5) | 30 (3.2) | 0.07 |
| Area deprivation index ≥85, No. (%) | 51 (5.1) | 156 (7.7) | 0.10 | 47 (5.1) | 42 (4.5) | 0.03 |
| Body mass index, mean (SD), kg/m2 | 31.0 (6.0) | 31.3 (7.2) | 0.12 | 31.0 (5.9) | 31.6 (6.9) | 0.02 |
| History of diabetes, No. (%) | 222 (22.1) | 304 (15.0) | 0.18 | 198 (21.3) | 200 (21.5) | 0.01 |
| History of obstructive sleep apnea, No. (%) | 232 (23.1) | 291 (14.4) | 0.22 | 199 (21.4) | 198 (21.3) | 0.00 |
| History of hypertension, No. (%) | 572 (56.9) | 804 (39.7) | 0.35 | 515 (55.4) | 499 (53.7) | 0.03 |
| History of stroke, No. (%) | 88 (8.7) | 130 (6.4) | 0.09 | 82 (8.8) | 78 (8.4) | 0.02 |
| History of valvular heart disease, No. (%) | 93 (9.2) | 120 (5.9) | 0.12 | 83 (8.9) | 86 (9.2) | 0.01 |
| History of atrial fibrillation, No. (%) | 118 (11.7) | 138 (6.8) | 0.17 | 110 (11.8) | 104 (11.2) | 0.02 |
| History of congestive heart failure, No. (%) | 107 (10.6) | 121 (6.0) | 0.17 | 90 (9.7) | 86 (9.2) | 0.01 |
| History of chronic kidney disease, No. (%) | 134 (13.3) | 146 (7.2) | 0.20 | 108 (11.6) | 116 (12.5) | 0.03 |
| History of dyspnea, No. (%) | 77 (7.7) | 129 (6.4) | 0.05 | 71 (7.6) | 73 (7.8) | 0.01 |
| History of COPD, No. (%) | 224 (23.3) | 313 (15.5) | 0.17 | 199 (21.4) | 187 (20.1) | 0.03 |
| History of bronchiectasis, No. (%) | 7 (0.7) | 5 (0.2) | 0.07 | 6 (0.6) | 4 (0.4) | 0.03 |
| History of pulmonary hypertension, No. (%) | 24 (2.4) | 29 (1.4) | 0.07 | 19 (2.0) | 15 (1.6) | 0.03 |
| History of pulmonary fibrosis, No. (%) | 1 (0.1) | 6 (0.3) | 0.04 | 1 (0.1) | 0 (0.0) | 0.05 |
| History of fatty liver disease, No. (%) | 46 (4.6) | 55 (2.7) | 0.10 | 41 (4.4) | 35 (3.8) | 0.03 |
| History of cirrhosis, No. (%) | 17 (1.7) | 14 (0.7) | 0.09 | 13 (1.4) | 12 (1.3) | 0.01 |
| History of gastrostomy, No. (%) | 1 (0.1) | 2 (0.1) | 0.00 | 1 (0.1) | 1 (0.1) | 0.00 |
| History of cancer, No. (%) | 207 (20.6) | 205 (10.1) | 0.29 | 171 (18.4) | 153 (16.5) | 0.05 |
| History of chemotherapy, No. (%) | 99 (9.8) | 47 (2.3) | 0.32 | 76 (8.2) | 44 (4.7) | 0.14 |
| History of lung cancer, No. (%) | 7 (0.7) | 11 (0.5) | 0.02 | 6 (0.6) | 3 (0.3) | 0.05 |
| History of allergic rhinitis, No. (%) | 162 (16.1) | 301 (14.9) | 0.03 | 151 (16.2) | 137 (14.7) | 0.04 |
| History of rheumatoid arthritis, No. (%) | 62 (6.2) | 52 (2.6) | 0.18 | 56 (6.0) | 46 (4.9) | 0.05 |
| History of sarcoidosis, No. (%) | 9 (0.9) | 4 (0.2) | 0.09 | 6 (0.6) | 4 (0.4) | 0.03 |
| History of lupus, No. (%) | 24 (2.4) | 11 (0.5) | 0.15 | 17 (1.8) | 11 (1.2) | 0.05 |
| History of viral hepatitis, No. (%) | 20 (2.0) | 25 (1.2) | 0.06 | 18 (1.9) | 18 (1.9) | 0.00 |
| History of organ or cell transplant, No. (%) | 31 (3.1) | 3 (0.1) | 0.23 | 5 (0.5) | 3 (0.3) | 0.03 |
| History of bone marrow transplant, No. (%) | 3 (0.3) | 3 (0.1) | 0.03 | 2 (0.2) | 1 (0.1) | 0.03 |
| History of immunocompromised, No. (%) | 301 (29.9) | 227 (11.2) | 0.48 | 233 (25.1) | 194 (20.9) | 0.10 |
| Alpha blocker, No. (%) | 21 (2.1) | 22 (1.1) | 0.08 | 18 (1.9) | 17 (1.8) | 0.01 |
| ACE inhibitors, No. (%) | 170 (16.9) | 279 (13.8) | 0.08 | 158 (17.0) | 165 (17.7) | 0.02 |
| Angiotensin II receptor blocker, No. (%) | 166 (16.5) | 191 (9.4) | 0.21 | 151 (16.2) | 151 (16.2) | 0.00 |
| Beta blockers, No. (%) | 320 (31.8) | 446 (22.0) | 0.22 | 285 (30.6) | 277 (29.8) | 0.02 |
| Corticosteroids as a home medication, No. (%) | 427 (42.4) | 677 (33.5) | 0.18 | 385 (41.4) | 378 (40.6) | 0.02 |
| Statins, No. (%) | 481 (47.8) | 631 (31.2) | 0.34 | 435 (46.8) | 448 (48.2) | 0.03 |
| Antidepressants, No. (%) | 346 (34.4) | 628 (31.0) | 0.07 | 318 (34.2) | 313 (33.7) | 0.01 |
SMD values are presented as absolute values. All variables were used in the propensity score model.
Abbreviations: ACE, angiotensin-converting enzyme; COPD, chronic obstructive pulmonary disease; SMD, standardized mean difference.
Statistical Methods
Sociodemographic and clinical characteristics between mAb-treated and nontreated subjects (before and after matching) were compared using standardized mean differences (SMDs). To calculate a propensity score (for treatment) for each patient [13, 14], we fit a logistic regression model with treatment with bebtelovimab as the response variable and inclusion of explanatory variables measured at baseline (Table 1). For each clinical outcome of interest (eg, hospitalization or death), an indicator variable for treatment (yes/no) was the primary predictor of interest in an unconditional (nonmatched) logistic regression model, with inclusion of the propensity score to adjust for confounding. As a sensitivity analysis. confounding was also adjusted for in the unmatched analyses by use of inverse probability weighting. Results are presented as adjusted odds ratios (ORs). These approaches were used overall and for the prespecified subgroups of interest for the primary outcome of hospitalization or death.
In a matched cohort sensitivity analysis, nontreated control subjects were matched to treated subjects by propensity score methodology [13, 14]. Specifically, 1:1 propensity score greedy nearest neighbor matching within a caliper width of 0.20 was used to construct matched treated and nontreated groups [15]. From the matched groups, the 28-day incidence rates of patient outcomes were calculated with treated vs nontreated comparisons of association estimated by use of conditional ORs and 95% CIs [16]. The Kaplan-Meier method was used to plot survival curves for freedom from hospitalization or death by treatment status over follow-up. A third sensitivity analysis was conducted using a conditional (matched) logistic regression analysis (described above) among patients with nonmissing data on body mass index (BMI). Analyses were conducted using the SAS System (SAS, Cary, NC, USA), version 9.4. Methods and results follow The REporting of studies Conducted using Observational Routinely-Collected Health Data (RECORD) statement (Supplement Appendix C) [17].
RESULTS
Baseline Characteristics
The unmatched analysis cohort consisted of 1006 treated patients and 2023 nontreated controls (Figure 1). Of the 1006 treated patients, 930 were individually matched 1:1 to nontreated control patients. Before 1:1 propensity score matching/adjustment, the mean (SD) age of treated patients was 61.6 (17.3) years compared with 51.3 (20.6) years in nontreated controls (Table 1). Similarly, before matching, the overall risk profile was higher in treated patients compared with nontreated controls, including higher prevalence of obstructive sleep apnea, hypertension, chronic kidney disease, cancer, chemotherapy, and being immunocompromised (all SMDs ≥0.20). After 1:1 propensity score matching, treated patients were similar to nontreated patients on all variables (SMD values ≤0.07) except for a nominally higher prevalence of history of chemotherapy (8.2% vs 4.7%; SMD, 0.14) and immunocompromised status (25.1% vs 20.9%; SMD, 0.10) (Table 1). The mean (SD) propensity score probability (×100) was 39.4 (16.2) in treated patients compared with 38.4 (15.1) in matched nontreated controls.
Outcomes
The crude overall 28-day incidence of hospitalization or death was 3.3% in treated patients compared with 3.5% in nontreated controls (Table 2). This corresponded to an unadjusted OR of 0.95. After statistical adjustment for the propensity score (ie, higher risk profile of treated patients), the estimated odds of hospitalization or death were 50% lower in treated patients compared with nontreated controls (OR, 0.50; 95% CI, 0.31–0.80). The corresponding adjusted OR for hospitalization was 0.66 (95% CI, 0.41–1.07), and there was only 1 death (0.1%) in the treated group compared with 13 deaths (0.6%) in the untreated group. Treatment with bebtelovimab was not associated with adjusted odds of ED admission with or without hospitalization. Results across outcomes were similar with the use of inverse probability weighting.
Table 2.
Twenty-Eight-Day Outcome Risks and Odds Ratios by Treatment vs Nontreatment With Bebtelovimab (Unmatched Cohort)
| Outcome | Not Treated | Treated | Unadj. OR | Adjusted Analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | PS Adj. OR | 95% CI | P Value | IPW OR |
95% CI | P Value | ||
| Hospitalization/death | 2023 | 70 (3.5) | 1006 | 33 (3.3) | 0.95 | 0.50 | 0.31–0.80 | .004 | 0.57 | 0.38–0.84 | .005 |
| Hospitalization | 2023 | 58 (2.9) | 1006 | 33 (3.3) | 1.15 | 0.66 | 0.41–1.07 | .09 | 0.72 | 0.48–1.09 | .12 |
| Death | 2023 | 13 (0.6) | 1006 | 1 (0.1) | 0.15 | 0.05 | 0.01–0.42 | .006 | 0.08 | 0.01–0.51 | .008 |
| ED admission/no hospitalization | 2023 | 65 (3.2) | 1006 | 46 (4.6) | 1.44 | 1.14 | 0.75–1.74 | .53 | 1.06 | 0.74–1.53 | .74 |
| ED admission/hospitalization | 2023 | 118 (5.8) | 1006 | 76 (7.6) | 1.32 | 0.90 | 0.65–1.25 | .52 | 0.91 | 0.68–1.20 | .49 |
Abbreviations: IPW, inverse probability weighting; OR, odds ratio; PS, propensity score adjustment as a continuous variable; Unadj, unadjusted.
Sensitivity Analyses
In the matched cohort analysis, the incidence of hospitalization or death was 3.1% in treated patients compared with 5.5% in nontreated controls, with a corresponding conditional OR of 0.53 (95% CI, 0.32–0.86) (Table 3). The divergence in freedom from death or hospitalization in the direction favoring the treated group began at about day 4 of follow-up (Supplement Figure 1). The conditional OR for hospitalization was 0.68 (95% CI, 0.41–1.12), and there was 1 death (0.1%) in the treated group compared with 11 deaths (1.2%) in the matched untreated group. Thus, results were similar in the unmatched (adjusted) and matched cohort analyses. Results for the OR of hospitalization or death were also similar for the subgroup of patients with nonmissing BMI data (OR, 0.52; 95% CI, 0.33–0.83).
Table 3.
Risks and Conditional Odds Ratios of 28-Day Outcomes by Treatment vs Nontreatment With Bebtelovimab (Matched Cohort)
| Outcome | Not Treated | Treated | OR | Conditional Matched Analysisa | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | Pairs in Analysis | Conditional OR | 95% CI | P Value | ||
| Hospitalization/death | 930 | 51 (5.5) | 930 | 29 (3.1) | 0.55 | 74 | 0.53 | 0.32–0.86 | .01 |
| Hospitalization | 930 | 41 (4.4) | 930 | 29 (3.1) | 0.70 | 66 | 0.68 | 0.41–1.12 | .13 |
| Death | 930 | 11 (1.2) | 930 | 1 (0.1) | 0.09 | 12 | 0.09 | 0.01–0.70 | .02 |
| ED admission/no hospitalization | 930 | 35 (3.8) | 930 | 41 (4.4) | 1.18 | 76 | 1.24 | 0.77–2.01 | .38 |
| ED admission/hospitalization | 930 | 72 (7.7) | 930 | 67 (7.2) | 0.93 | 127 | 0.94 | 0.66–1.35 | .75 |
The number of pairs in the analysis represents the number of matched pairs (treated vs not-treated) with discordant outcomes.
Abbreviation: OR, odds ratio.
The model includes adjustment for immunocompromised status and history of chemotherapy.
Subgroup Analyses
There was evidence that the association between treatment with bebtelovimab and odds of hospitalization or death was modified by predefined subgroups (Table 4). Specifically, among patients <65 years of age, there was no association between treatment and odds of hospitalization or death (adjusted OR, 1.03; 95% CI, 0.45–2.36), whereas there was a strong indication of lower odds in patients aged 65 and older (adjusted OR, 0.37; 95% CI, 0.21–0.63). There was no indication that the overall lower odds of hospitalization or death in treated patients was modified by obesity status.
Table 4.
Subgroup Analyses of 28-Day Risk and Odds Ratios of Hospitalization or Death by Treatment vs Nontreatment With Bebtelovimab (Unmatched Cohort)
| Subgroup | Not Treated | Treated | Unadj. OR | Adjusted Analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | PS Adj. OR | 95% CI | P Value | IPW OR |
95% CI | P Value | ||
| Age | |||||||||||
| <65 y | 1435 | 20 (1.4) | 470 | 13 (2.8) | 2.01 | 1.03 | 0.45–2.36 | .95 | 1.09 | 0.55–2.14 | .81 |
| ≥65 y | 588 | 50 (8.5) | 536 | 20 (3.7) | 0.42 | 0.37 | 0.21–0.63 | <.001 | 0.38 | 0.22–0.62 | <.001 |
| Body mass index | |||||||||||
| ≤30 kg/m2 | 877 | 35 (4.0) | 378 | 16 (4.2) | 1.06 | 0.53 | 0.27–1.05 | .07 | 0.60 | 0.34–1.07 | .08 |
| >30 kg/m2 | 902 | 32 (3.5) | 365 | 14 (3.8) | 1.08 | 0.56 | 0.27–1.13 | .11 | 0.69 | 0.39–1.22 | .20 |
| Immunocompromised | |||||||||||
| No | 1796 | 43 (2.4) | 705 | 20 (2.8) | 1.19 | 0.85 | 0.48–1.50 | .57 | 0.92 | 0.56–1.52 | .76 |
| Yes | 227 | 27 (11.9) | 301 | 13 (4.3) | 0.33 | 0.24 | 0.11–0.50 | <.001 | 0.25 | 0.12–0.51 | <.001 |
| Vaccination status | |||||||||||
| Fully vaccinated | 957 | 40 (4.2) | 616 | 15 (2.4) | 0.57 | 0.26 | 0.13–0.51 | <.001 | 0.29 | 0.16–0.53 | <.001 |
| Not determined | 1066 | 30 (2.8) | 390 | 18 (4.6) | 1.67 | 1.07 | 0.56–2.06 | .84 | 1.14 | 0.66–1.96 | .63 |
Abbreviations: IPW, inverse probability weighting; OR, odds ratio; PS, propensity score adjustment as a continuous variable; Unadj, unadjusted.
Treatment with bebtelovimab was associated with a particularly low odds of hospitalization or death in patients with immunocompromised status (adjusted OR, 0.24; 95% CI, 0.11–0.50) and those who were fully vaccinated (adjusted OR, 0.26; 95% CI, 0.13–0.51). Results were similar with the use of inverse probability weighting. In post hoc subgroup analyses, there was an indication of substantially lower odds of hospitalization or death in immunocompromised patients who were fully vaccinated (adjusted OR, 0.19; 95% CI, 0.07–0.48), as well as immunocompromised patients with undetermined vaccination status (adjusted OR, 0.39; 95% CI, 0.12–1.29).
The absence of treatment association in patients under the age of 65 years and in those with undetermined vaccination status appeared to be driven by very low rates of hospitalization or death in the control group of nontreated patients (1.4% and 2.8%, respectively). In supplemental analyses, patients with undetermined vaccination status were less likely to have received chemotherapy (1.9% vs 7.5%) or be immunocompromised (11.8% vs 22.6%) than patients who were fully vaccinated (ie, lower overall risk).
DISCUSSION
In this analysis, treatment with bebtelovimab was associated with lower odds of hospitalization or death in propensity score–adjusted and –matched cohorts during a time period when the Omicron variant and its sublineages predominated. Patients aged 65 years and older, those with immunocompromised status, and those who were fully vaccinated had the lowest odds of hospitalization or death associated with bebtelovimab therapy, whereas OR estimates were not modified by body mass index.
Bebtelovimab was authorized for use in February 2022 as in vitro data emerged suggesting that previously approved mAbs would be ineffective at neutralizing certain Omicron sublineages, whereas bebtelovimab retained in vitro activity against all known variants [18]. This in vitro activity has since been confirmed; however, clinical data are lacking [5]. To our knowledge, this study represents the first large observational analysis of bebtelovimab treatment among a heterogeneous group of patients with COVID-19 assumed to be infected with the Omicron variant or an Omicron sublineage [19], and that includes a nontreated matched cohort. A small study of 25 solid organ transplant recipients treated with bebtelovimab suggested a possible treatment benefit with mAb therapy [20], and a larger retrospective cohort study of adult solid organ transplant recipients treated with either bebtelovimab (n = 92) or sotrovimab (n = 269) reported similar 30-day rates of hospitalization (∼3%) [21]. Our 28-day rate of hospitalization (3.3%) in bebtelovimab-treated patients is consistent with the latter study. Throughout the pandemic, some therapies with in vitro activity against SARS-CoV-2 have failed to demonstrate clinical benefit; therefore, our data are important for public health because they provide reassurance that current in vitro assessments of mAbs seemingly track with clinical assessments of effectiveness.
This analysis shows evidence of substantially lower 28-day adjusted ORs of hospitalization and death among patients 65 years of age or older and/or immunocompromised patients treated with bebtelovimab compared with no treatment. Patients who have moderate to severe immune compromise due to a medical condition or receipt of immunosuppressive medications and/or who may not mount an adequate immune response to COVID-19 vaccination are at high risk of severe SARS-CoV-2 infection and complications. Accordingly, the NIH prioritizes these individuals for mAb treatment during times of scarcity. Our results support continued prioritization of these patients, which occurred intermittently throughout the pandemic at our health system during times of staff or drug shortages (explaining the difference in age and immunocompromised status in the unmatched cohort). Importantly, the lower odds of death or hospitalization among bebtelovimab-treated patients was statistically significant despite a nominally higher prevalence of immunocompromised patients in the treated group after matching and relatively low event rates in the nontreated cohort. These results are consistent with previous data describing an overall lower rate of hospitalization during the Omicron period as compared with the Delta time period [22].
In this study, there was no association between bebtelovimab treatment and 28-day odds of hospitalization or death in patients with unknown vaccination status, which was in contrast to patients known to be fully vaccinated, who had a much lower OR of hospitalization or death with bebtelovimab treatment. Multiple potential explanations exist for this finding. First, the 28-day incidence of hospitalization or death was only 2.8% in nontreated controls with undetermined vaccinated status, and patients with undetermined vaccinated status had a generally lower risk profile than fully vaccinated patients. Thus, the apparent absence of treatment association may simply reflect low overall risk in this subcohort of patients with undetermined vaccination status. Second, “fully vaccinated” was defined as at least 2 mRNA vaccines or a single dose of an approved virus-based technology vaccine. This definition did not consider or require receipt of a third dose of an mRNA vaccine, which is now considered the primary series for an immunocompromised patient, or receipt of vaccine booster shots (consistent with more recent definitions of “fully” vaccinated). There is evidence that vaccination alone may be insufficient to mount protection against SARS-CoV-2 among immunocompromised and/or patients with advanced age; therefore, full vaccination in this population may be less protective against disease than having no comorbidities [23]. Finally, “fully vaccinated” status may be an indicator of patients being more likely to access health care, with overlap with elderly and immunocompromised patients.
Assessment of existing and new mAb products is paramount, including continuous appraisal of selected patient populations most likely to benefit from treatment. Given the speed of SARS-CoV-2 mutations, the conduct of conventional randomized controlled trials may be impractical, thereby necessitating analyses from large observational cohorts. Nonetheless, our observational study has several limitations.
First, matching of nontreated controls used EUA-eligible risk factors only, and we were unable to determine the time from symptom onset to SARS-CoV-2 test positive result or symptom severity (whether symptomatic or asymptomatic) of patients. We postulate that many nontreated patients may have been asymptomatic (ie, due to routine SARS-CoV-2 testing or incidental findings) and thereby at low risk of hospitalization, which would tend to bias results against mAb treatment. Second, as previously stated, we were unable to determine vaccination status (including booster status) in all patients, and the definition of “fully vaccinated” has changed dramatically with updated dosing schedules and authorization of additional vaccines. Third, receipt of tixagevimab/cilgavimab was not assessed, and we were unable to assess other treatments outside of our health system for control (untreated) patients, although, 3-day remdesivir was not offered by UPMC or any other regional hospital. Fourth, Omicron and its sublineages were the dominant SARS-CoV-2 variants during the study period, yet no patient-specific genotype sampling was conducted. Fifth, our definition of “immunocompromised” is broad, with multiple qualifying conditions. Sixth, hospitalizations that may have occurred outside the UPMC system are not captured in the present analyses. Finally, we cannot rule out potential residual confounding of the estimated mAb treatment effects despite our close propensity score matching of treated patients and nontreated “mAb-eligible” controls.
CONCLUSIONS
In this cohort study of outpatients with COVID-19 during an Omicron variant period, treatment with bebtelovimab was associated with significantly lower odds of hospitalization or death. Results indicate that outpatient use of bebtelovimab should be prioritized for older adults and those who are immunocompromised.
Supplementary Material
Acknowledgments
We acknowledge the staff at UPMC Clinical Analytics and the UPMC Wolff Center for curating and managing the data. We acknowledge Debbie Albin, Lorraine Brock, Margherita Sciullo, Jessica Shirley, Judith Shovel, and Jill Trainor for their critical role in the daily operations of the monoclonal antibody program.
Author contributions. Dr. Kip and Dr. Marroquin take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Kip, Marroquin, McCreary. Acquisition of data: Collins, Marroquin, McCreary, Kip. Interpretation of data: all authors. Drafting of the manuscript: Kip, Marroquin. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: Kip, Marroquin, McCreary.
Reproducible research statement. Study protocol: No separate study protocol was required, a priori, as this retrospective analysis was deemed a quality improvement initiative, with ethical review and approval granted by the UPMC Quality Improvement Review Committee and Institutional Review Board.
Availability of data. Selected statistical code may be requested by contacting by Dr. Kip at kipke2@upmc.edu. The data set contains protected health information and will not be available upon request.
Contributor Information
Erin K McCreary, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Kevin E Kip, Clinical Analytics, UPMC, Pittsburgh, Pennsylvania, USA.
Kevin Collins, Clinical Analytics, UPMC, Pittsburgh, Pennsylvania, USA.
Tami E Minnier, Wolff Center, UPMC, Pittsburgh, Pennsylvania, USA.
Graham M Snyder, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Ashley Steiner, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Russell Meyers, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Tina Borneman, UPMC Corporate Pharmacy Service Center, Pittsburgh, Pennsylvania, USA.
Michelle Adam, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Lauren Thurau, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Donald M Yealy, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
David T Huang, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
J Ryan Bariola, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Mark Schmidhofer, Division of Cardiology, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Richard J Wadas, Department of Emergency Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Derek C Angus, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Paula L Kip, Wolff Center, UPMC, Pittsburgh, Pennsylvania, USA.
Oscar C Marroquin, Clinical Analytics, UPMC, Pittsburgh, Pennsylvania, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Bariola JR, McCreary EK, Wadas RJ, et al. . Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb RL, Nirula A, Chen P, et al. . Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 5. Takashita E, Kinoshita N, Yamayoshi S, et al. . Efficacy of antibodies and antiviral drugs against COVID-19 Omicron variant. N Engl J Med 2022; 386:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health . Therapeutic management of nonhospitalized adults with COVID-19. COVID-19 treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults–therapeutic-management/. Accessed July 27, 2022.
- 7. McCreary EK, Bariola JR, Minnier T, et al. . Launching a comparative effectiveness adaptive platform trial of monoclonal antibodies for COVID-19 in 21 days. Contemp Clin Trials 2022; 113:106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reitz KM, Seymour CW, Vates J, et al. . Strategies to Promote ResiliencY (SPRY): a Randomised Embedded Multifactorial Adaptative Platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients. BMJ Open 2020; 10:e037690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2011. Available at: https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed July 27, 2022.
- 10. Centers for Disease Control and Prevention . International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). 2021. Available at: https://www.cdc.gov/nchs/icd/icd10cm.htm. Accessed July 27, 2022.
- 11. US Department of Commerce . National Technical Information Service. 2021. Available at: https://www.ntis.gov/. Accessed July 27, 2022.
- 12. Social Security Administration . Social Security master file of social security number holders and applications: death information. October 13, 2021. Available at: https://www.ssa.gov/dataexchange/request_dmf.html. Accessed July 27, 2022.
- 13. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 15. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008; 27:2037–49. [DOI] [PubMed] [Google Scholar]
- 17. Benchimol EISL, Guttmann A, Harron K, et al. . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eli Lilly and Company . Fact sheet for healthcare providers. Emergency Use Authorization for bebtelovimab. Revised June 16, 2022. Available at: https://pi.lilly.com/eua/bebtelovimab-eua-factsheet-hcp.pdf. Accessed July 27, 2022.
- 19. Centers for Disease Control and Prevention (CDC) . COVID data tracker, variants and genomic surveillance. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed June 15, 2022.
- 20. Shertel T, Lange NW, Salerno DM, et al. . Bebtelovimab for treatment of COVID-19 in ambulatory solid organ transplant recipients. Transplantation 2022; 106:e463–e464. doi: 10.1097/TP.0000000000004278. Epub 2022 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yetmar ZA, Beam E, O'Horo JC, et al. . Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch. Transpl Infect Dis 2022; 24:e13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nyberg T, Ferguson NM, Nash SG, et al. . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haidar G, Agha M, Bilderback A, et al. . Prospective evaluation of coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 vaccination in the immunocompromised study (COVICS). Clin Infect Dis 2022; 75:e630–e644. doi: 10.1093/cid/ciac103. PMID: 35179197; PMCID: PMC8903515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.