Abstract
Brucella abortus and Brucella melitensis have surface lipopolysaccharides and polysaccharides carrying B. melitensis-type (M) and B. abortus-type (A) epitopes as well as common (C) epitopes present in all smooth Brucella biotypes. Crude lipopolysaccharides, hydrolytic O polysaccharides, and native hapten polysaccharides of MC or AC specificity were evaluated in indirect enzyme-linked immunosorbent assays with polyclonal, monoclonal, or protein G conjugates by using sera from cattle, sheep, and goats infected with AC, MC, or AMC Brucella biotypes. Regardless of the antigen, the levels of antibodies were lower in goats than in sheep and highest in cattle. The diagnostic performance of the assay was not affected by the absence of lipid A-core epitopes, the presence of contaminating outer membrane proteins, the AC or MC epitopic structure of the absorbed antigen, or the conjugate used. Moreover, with sera from cattle vaccinated with B. abortus S19 (AC) or from sheep and goats vaccinated with B. melitensis Rev 1 (MC), AC and MC antigens showed similar levels of reactivity. The results show that antibodies to the C epitopes largely dominate in infection, and this is consistent with the existence of multiple overlapping C epitopes (V. Weynants, D. Gilson, A. Cloeckaert, A. Tibor, P. A. Denoel, F. Godfroid, J. N. Limet, and J.-J. Letesson, Infect. Immun. 65:1939–1943, 1997) rather than with one or two C epitopes. It is concluded that, by adaptation to the corresponding antibody levels, brucellosis in cattle, sheep, and goats can be diagnosed by immunosorbent assay with a single combination of conjugate and antigen.
The members of the genus Brucella are gram-negative bacteria causing brucellosis. Brucella abortus and Brucella melitensis preferentially infect cattle and small ruminants, respectively, and are largely responsible for human brucellosis. Both species carry a smooth-type lipopolysaccharide (LPS) with O-chain variations (26), represented by biotypes 1 of B. abortus and B. melitensis, which carry the A (B. abortus-type) and M (B. melitensis-type) epitopes, respectively (9, 19, 28, 33). In addition, there is at least one common (C) epitope restricted to the smooth brucellae (19, 28, 33) and a second common epitope (C/Y) shared with Yersinia enterocolitica O:9 (19, 33). The two resulting serotypes (herein referred to as AC or MC for simplicity) are not species specific: B. abortus biotypes 4, 5, and 9 are serologically MC, B. melitensis biotype 2 is AC, and B. melitensis biotype 3 is AMC (5). The native hapten (NH), a surface polysaccharide closely related to the LPS, shows a chemical structure similar to that of the O chain, and it can be predicted to carry the same epitopes (6, 17). LPS and NH are relevant molecules in serological diagnosis (reviewed in reference 6).
Indirect enzyme-linked immunosorbent assays (iELISAs) with LPS are held to be at least as sensitive and specific as the combination of the rose bengal and complement fixation tests for brucellosis, both of which detect antibodies to the LPS (15). However, the issue of how the use of a Brucella LPS of a given serological specificity affects the detection of antibodies elicited by heterologous serotypes has not been rigorously investigated. For the rose bengal test, and depending on the antigen dilution, it has been reported that suspensions of B. melitensis biotype 1 (MC) perform better than those of B. abortus biotype 1 (AC) in identifying cattle infected with B. abortus biotype 5 (MC) (14). It is also known that, for optimal performance with sera of B. melitensis-infected goats and sheep, the standard rose bengal antigen (a B. abortus biotype 1 suspension standardized with cattle serum) has to be diluted (7, 18), and this could be due to a combination of antigen epitopic structure and titers of the antibodies of the possible specificities. It has been suggested that differences in the results of hemolysis in gel (29) and tube serum agglutination (4) tests with sera from animals infected with different Brucella biotypes are also related to the antigen specificity. Finally, the epitopic structure of Brucella NH and O-chain polysaccharide is relevant in gel precipitation tests since optimal sensitivity in identifying B. melitensis-infected animals requires polysaccharides carrying the M epitope (17). Thus, the first goal of the present study was to investigate whether the epitopic structure of the LPS O chain is relevant in the iELISA for animal brucellosis and whether the assay needs to be substantially adapted to test sera from cattle, sheep, and goats.
A second aspect to be considered in tests that detect antibodies to LPS is that this molecule contains core and lipid A epitopes in addition to the O-chain epitopes (28). Moreover, Brucella LPS preparations often contain outer membrane proteins tightly bound to lipid A (28) that evoke antibodies during infection (12, 24, 27, 31, 34). Since lengthy protocols are necessary both to achieve LPS purification and to obtain the O-chain polysaccharide and NH in a pure state (6, 11, 17), it is important to assess how the presence of epitopes other than those carried by the O chain affects the performance of the iELISA. This was the second goal of the present study.
MATERIALS AND METHODS
Bacterial strains and cultures.
B. abortus 2308 (biotype 1 [AC], U.S. Department of Agriculture challenge strain) and B. melitensis 16 M (biotype 1 [MC], virulent) are smooth strains that have been used in previous studies (6, 17). They were grown in a Biostat fermentor (B. Braun Mesulgen AG, Leinfelden, Germany) under the conditions described previously (6). After 36 to 48 h, bacteria were inactivated with phenol (0.5%, 36°C, 48 h), harvested by tangential-flow filtration (Omega 100K filter; Filtron Technology Corp., Northborough, Mass.), and washed twice with saline.
Antigen extraction and characterization.
Crude LPS fractions (crLPS) and pure NH preparations were obtained by protocols described previously (6, 11, 17). Briefly, to obtain the crLPS fractions, wet cells were extracted with water at 100°C, the extract was precipitated with 3 volumes of cold ethanol, and the precipitate was dialyzed and freeze-dried. To obtain the pure NH preparations, the remaining supernatant was precipitated with 2 additional volumes of ethanol and the new precipitate was digested with nucleases and proteinase K and ultracentrifuged (200,000 × g, 6 h, 10°C). The supernatant fluid was chromatographed on Sephacryl S-300, and the fractions containing pure NH were pooled, dialyzed, and freeze-dried (6). To obtain the LPS hydrolytic O-polysaccharide (PS), cells were suspended in 5.0% acetic acid–10.0% NaCl and autoclaved for 30 min, and cell debris were removed by centrifugation. The extract was precipitated with 5 volumes of methanol (1.0% of methanol saturated with sodium acetate), and the precipitate was digested with lysozyme, nucleases, and proteinase K and extracted with hot phenol. After the mixture was chilled, the phenol phase was precipitated with methanol (1% methanol saturated with sodium acetate) and resuspended in water. After ultracentrifugation (100,000 × g, 18 h, 4°C), the supernatant fluid was chromatographed on Sephadex G-50 (Pharmacia, Uppsala, Sweden) and the void volume fractions were freeze-dried (11).
Detailed descriptions of the methods used in the characterization of these preparations have been published before (6, 17). The presence of LPS was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by periodate-silver staining. In addition, the LPS core marker 3-deoxy-d-manno-2-octulosonic acid (KDO) was measured and used to calculate the amount of LPS in crLPSs by using the KDO content reported for NH-free LPS preparations (6). The purity of the NH and PS fractions was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by either silver or periodate-silver staining, gel immunoprecipitation, high-performance gel filtration chromatography, and 13C nuclear magnetic resonance (6). Group 3 outer membrane proteins (Omp31 and/or Omp25 [13, 20]) contaminating the crLPS extracts were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20). The relevant features of the crLPS, PS, and NH preparations are summarized in Table 1.
TABLE 1.
Characteristics of the antigens used in the ELISA
| Strain | Preparation | % KDO | % Protein | % LPSa | Serologically relevant components and/or epitopesb |
|---|---|---|---|---|---|
| B. abortus 2308 | AC crLPS | 0.32 | 50 | 37 | Omp25; LPS (lipid A, core, and O chain [AC]) and NH (AC) |
| AC PS | 0.15 | <1 | <1 | O-chain (AC) and core epitopes | |
| AC NH | 0.03 | 8 | <1 | AC epitopes | |
| B. melitensis 16M | MC crLPS | 0.60 | 42 | 40 | Omp31 and Omp25; LPS (lipid A, core, and O chain [MC]) and NH (MC) |
| MC PS | 0.22 | <1 | <1 | O-chain (MC) and core epitopes | |
| MC NH | 0.07 | <1 | <1 | MC epitopes |
Calculated from the KDO values for fractions where the presence of LPS had been demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; values below 1% indicate that LPS was not detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining.
Dominant outer membrane proteins (identified by sodium dodecyl sulfate-polyacrylmaide gel electrophoresis) corresponded to group 3; epitopes are those of the known serotype and were confirmed by 13C nuclear magnetic resonance analysis for the NH and PS preparations.
iELISA.
Stock solutions of crLPS, NH, and PS extracts were prepared at 1 mg/ml in distilled water, sonicated briefly, and kept at −20°C. Standard 96-well polystyrene plates, either Inotech-ELISA (Bioreba, Basel, Switzerland) or Maxisorp (Nunc A/S, Roskilde, Denmark), were used (no significant differences between the brands were observed). Preliminary experiments showed that optimal antigen concentration for coating was 2.5 μg/ml for the six antigens. Two coating conditions were used: 0.06 M carbonate-bicarbonate (pH 9.6) at 37°C overnight and 10 mM phosphate-buffered saline (pH 7.2) (PBS) at 4°C overnight. Nonadsorbed material was removed with four washings with 0.05% Tween 20–PBS. To assess repeatability, batches of plates were coated simultaneously, dried, sealed, and stored at 4°C (controls with positive and negative sera showed that under these conditions plates were stable for several months). Serum dilutions were made in 0.05% Tween 20–PBS, a 100-μl aliquot was dispensed into each well, and the plates were incubated for 1 h at 37°C before being washed four times with the diluent. The following conjugates were used: (i) a polyclonal (rabbit) anti-sheep immunoglobulin G (IgG)–peroxidase conjugate (also suitable for goat IgG) of heavy- and light-chain specificity (Pierce Chemical Co., Rockford, Ill.), (ii) a polyclonal anti-bovine IgG–peroxidase conjugate of heavy- and light-chain specificity (Nordic Immunological Laboratories, Tilburg, The Netherlands), (iii) an anti-ruminant IgG1 monoclonal antibody conjugated with peroxidase (Zentral Diergeneeskunding Institut, Edelherweg, Lelystand, The Netherlands), and (iv) a recombinant protein G (reacting with the IgG of ruminants)-peroxidase conjugate (Pierce). All conjugates were diluted in 0.05% Tween–PBS (optimal dilutions were 1:1,000 to 1:2,000 for the antibodies and 0.2 μg/ml for protein G). Conjugate solution (100 μl) was added to each well, and after 1 h at 37°C, plates were washed and developed with 100 μl of 0.1% 2,2′azinobis(3-ethylbenzthiazolinesulfonic acid diammonium salt (ABTS; Sigma Chemical Co., St. Louis, Mo.)–0.05 M citrate (pH 4)–0.004% H2O2 per well for 15 min at 20°C. As controls, a negative serum and a positive serum of the appropriate animal species were used in all plates. The results were expressed as the optical density (OD) at 405 nm or as the percentage of the OD at 405 nm of the positive control serum at the dilution giving the best discrimination between infected and vaccinated animals (see below).
Sensitivity, specificity, and statistical analyses.
The sensitivity and the specificity were calculated with respect to the infected and brucella-free groups (see below) as described elsewhere (23). The receiver-operating-characteristic (ROC) analysis and the Student t test were performed with the SAS statistical package (version 6; SAS Institute Inc.).
Animals and sera.
The blood sera used were from 38 cows naturally infected with B. abortus biotype 1, 18 cows naturally infected with B. melitensis biotype 3, 83 heifers vaccinated subcutaneously with the standard dose of B. abortus S19 and bled 6 months later, and 76 cows from brucellosis-free areas. Sheep blood sera were obtained from 82 sheep naturally infected with B. melitensis biotype 1, 58 sheep naturally infected with B. melitensis biotype 3, 121 sheep from brucellosis-free areas, and 31 lambs vaccinated subcutaneously with the standard dose of B. melitensis Rev 1 and bled 4 months later. Finally, goat blood sera were from 30 goats naturally infected with B. melitensis biotype 1, 30 Brucella-free goats, and 16 young goats vaccinated subcutaneously with the standard dose of B. melitensis Rev 1 and bled 4 months later. Infections were demonstrated by bacteriological culture of cow’s milk or necropsy samples (8, 18, 22).
RESULTS
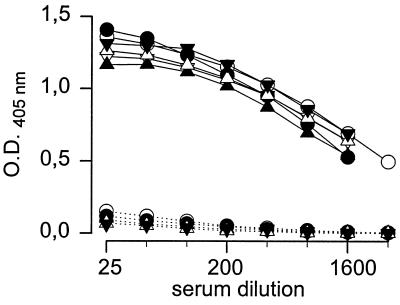
In preliminary experiments, it was confirmed (3, 18) that whereas coating in carbonate buffer at 37°C gave good results with crLPS and PS, these conditions were unsatisfactory for NH. On the other hand, binding was achieved for all antigens when they were dispensed into PBS and incubated at 4°C overnight (1). Thus, the reactivities of the antigens with sera from cattle, sheep, and goats in plates coated under those conditions were compared. The results obtained with the six antigens, the sera of the Brucella-free goats and the goats infected with B. melitensis biotype 1, and the protein G conjugate are presented in Fig. 1. It can be seen that there were only very small differences in average IgG binding to the MC or AC antigens despite the MC serological specificity of the infecting biotype. Furthermore, such small differences were not related to the serological specificity (AC or MC) of the antigens (Fig. 1) and were not constantly reproduced when different batches of coated plates were compared (not shown). Similar analyses performed with sera from sheep infected with B. melitensis biotypes 1 and 3 and Brucella-free sheep (data not shown) also showed that the IgG reactivities with the antigens were similar and independent from variations in the epitopic structure. Finally, similar results (data not shown) were also obtained with the sera of cattle infected with serologically AC B. abortus biotype 1 or serologically AMC B. melitensis biotype 3 (only the crLPSs were tested with sera from the last group). Protein G, monoclonal anti-ruminant IgG1, and polyclonal conjugates yielded the same results, even though the background reactivity with sera from Brucella-free animals was higher with the polyclonal conjugates.
FIG. 1.
Reactivities of sera from goats infected with B. melitensis biotype 1 (MC) (continuous lines) and Brucella-free goats (dotted lines) in the ELISA with MC crLPS (•), MC PS (▴), MC NH (▾), AC crLPS (○), AC PS (▵), and AC NH (▿) (for clarity, error bars are not shown).
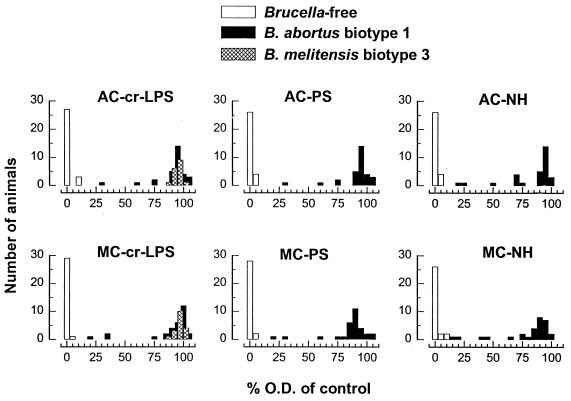
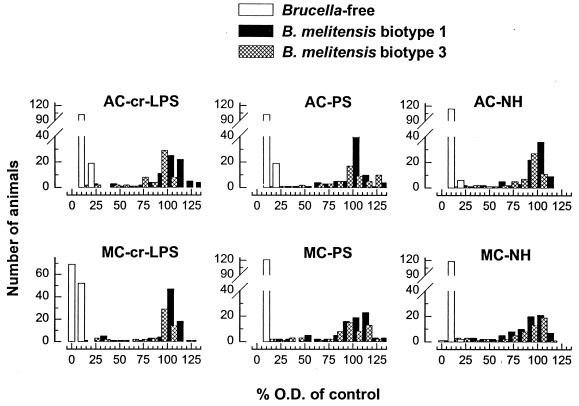
Since antigens yielding the same average reactivity could produce different diagnostic sensitivities and specificities and OD distributions, the data of the serum dilutions (1:25 for goats, 1:50 for sheep, and 1:100 for cattle) giving maximal discrimination between infected and Brucella-free animals were evaluated by ROC analysis (Table 2) and by examining the distribution of the percentage of the OD (results obtained with sera from cattle and sheep are shown in Fig. 2 and 3, respectively). For a given antigen, there were no significant differences in the sensitivities obtained with the sera of sheep infected with B. melitensis biotype 1 or biotype 3 or with the sera from cattle infected with B. abortus biotype 1 or B. melitensis biotype 3 (Table 2). Moreover, in both goats and cattle, there were no significant sensitivity differences among the six antigens. In sheep, AC or MC NHs were significantly (P ≤ 0.05) less sensitive than the other antigens, but no significant differences between the AC and MC crLPSs and PSs were observed (Table 2). The six distributions of the percentage of the OD for the sera from infected and Brucella-free cattle (Fig. 2) were very similar, with small differences in the few sera of B. abortus biotype 1-infected cattle that yielded low percentages of the OD. With these sera, the values obtained with the AC antigens were slightly higher than the values obtained with the heterologous MC preparations. However, a corresponding observation was not made for sheep (Fig. 3): whatever the infecting B. melitensis biotype, the results obtained with AC or MC antigens did not show clear differences even for those sera that produced low percentages of the OD. Figure 2 also shows that the separation between the infected and the Brucella-free cattle was lessened with the NH preparations, in particular with that of the MC type, and a corresponding observation was made with the NHs and the sheep sera (Fig. 3). No differences in the distributions of percentage of the OD obtained with goat sera and the AC and MC antigens were observed, and NH preparations also showed the lowest resolution between infected and Brucella-free populations (not shown).
TABLE 2.
Influence of the epitopic structure of crLPS, PS, and NH in the performance of the iELISA with sera rom infected cattle, sheep, and goats
| Simplified epitopic structure | Preparation | Value for animal host and infecting species and biotype (epitopic structure)a
|
||||
|---|---|---|---|---|---|---|
| Sheep (B. melitensis)
|
Cattle
|
Goats (B. melitensis, biotype 1 [MC]) | ||||
|
B. abortus
|
B. melitensis
|
|||||
| Biotype 1 (MC) | Biotype 3 (AMC) | Biotype 1 (AC) | Biotype 3 (AMC) | |||
| AC | crLPS | 96.34 (0.99) | 96.55 (0.99) | 100 | 100 | 100 |
| PS | 96.34 (0.99) | 96.55 (1) | 100 | NDb | 100 | |
| NH | 87.80 (0.99) | 89.65 (0.99) | 100 | ND | 100 | |
| MC | crLPS | 98.79 (0.98) | 100 | 100 | 100 | 100 |
| PS | 100 | 96.55 (1) | 100 | ND | 93.3 (0.99) | |
| NH | 96.34 (0.99) | 94.82 (1) | 93.3 (0.99) | ND | 100 | |
Figures are the optimal sensitivity (and c value) for a 100% specificity as determined by ROC analysis of iELISAs performed with peroxidase-protein G as the conjugate.
ND, not determined.
FIG. 2.
Distribution of the results of the iELISA obtained with sera from Brucella-free cattle and cattle infected with B. abortus biotype 1 and B. melitensis biotype 3 and crLPSs, PSs, and NHs of AC or MC specificity.
FIG. 3.
Distribution of the results of the iELISA obtained with sera from Brucella-free sheep and sheep infected with B. melitensis biotype 1 and B. melitensis biotype 3 and crLPSs, PSs, and NHs of AC or MC specificity.
Finally, to determine whether the epitopic structure of the antigens influenced the detection of serological responses in vaccinated animals, the six antigens were tested with sera from cattle vaccinated with B. abortus S19 (AC) and sera from sheep and goats vaccinated with B. melitensis Rev 1 (MC) and the protein G conjugate (Table 3). There were no significant differences in the percentages of sera from cattle and goats reacting with the six different antigenic preparations. For sheep sera, no significant differences were observed in the crLPSs and PSs of either MC or AC structure. However, in keeping with the lower sensitivity obtained with the AC NHs and the sera from infected sheep (Table 2), the AC NH preparation identified a significantly lower number of vaccinated sheep than the MC NH. The data also show that a higher percentage of sera from Rev 1-vaccinated than from S19-vaccinated animals were positive in the iELISA, an observation possibly reflecting the shorter postvaccination bleeding times (see Materials and Methods) and the greater stimulation caused by the Rev 1 vaccine.
TABLE 3.
Influence of the epitopic structure of crLPS, PS, and NH in the performance of the iELISA with sera from vaccinated cattle, sheep, and goats
| Simplified epitopic structure | Preparation | % of animals (n) positive in the iELISA
|
||
|---|---|---|---|---|
| Rev 1-vaccinated sheep (31) | S19-vaccinated cattle (83) | Rev 1-vaccinated goats (16) | ||
| AC | crLPS | 100 | 20.0 | 60.5 |
| PS | 87.1 | 25.0 | 75.0 | |
| NH | 80.6a | 26.6 | 62.5 | |
| MC | crLPS | 100 | 16.6 | 81.2 |
| PS | 100 | 20.0 | 50.0 | |
| NH | 100 | 20.0 | 60.4 | |
Significantly different (P < 0.05) from the values obtained with the other antigens.
DISCUSSION
It has long been recognized that the LPS is the major antigen of the surface of smooth brucellae (16) and the relevant molecule in classical diagnostic tests for brucellosis (15). LPS preparations of various degrees of purity have been used in many serological tests, and although specificity may be compromised in the testing of sera from vaccinated animals, no alternative antigens that could match the sensitivity achieved with LPS have been found so far (8, 12, 18, 21, 24, 25, 30, 31, 34). Studies with monoclonal antibodies have shown that the B. abortus LPS contains at least three lipid A epitopes, two core epitopes (28), and M, A, and C O-chain epitopes. Whereas the lipid A-core structure remains to be elucidated, the B. abortus biotype 1 O-chain polysaccharide has been defined by nuclear magnetic resonance analysis of PS preparations. In B. abortus biotype 1, the O-polysaccharide is an unbranched homopolymer of α-1,2-linked 4-formamido-4,6-dideoxy-d-mannose (N-formylperosamine) (26). In B. melitensis biotype 1, the O chain consists of repeating blocks of five N-formylperosamine residues, four α-1,2 linked and one α-1,3 linked (26). Obviously, the α-1,2 linkages relate to both the A and C epitope(s), and the α-1,3 linkages relate to the M epitope(s), and intermediate biotypes expressing both the A- and M-type epitopes (such as B. melitensis biotype 3) and M-type B. abortus biotypes have the expected proportions of α-1,2- and α-1,3-linked sugars (26). However, the chemical structures suggest that these epitopes are not discrete entities; in fact, some data in the literature show that the epitopic specificities of some anti-Brucella O-chain monoclonal antibodies depend on the precise dilution at which these reagents are used (9, 10, 32) and, therefore, on their intrinsic avidity. Thus, a polyclonal response should contain a large proportion of antibodies that, because of combinations of titer and avidity, should show overlapping reactivities with the overlapping stretches of different numbers of α-1,2-linked sugars present in both MC and AC polysaccharides. Moreover, antibodies evoked by epitopes constituted exclusively by α-1,2-linked sugars could show various degrees of reactivities with epitopes containing both α-1,2- and α-1,3-linked sugars, and the opposite should also be true. This interpretation is supported by the recent work of Weynants et al. (33), who have observed that PS monoclonal antibodies display intermediate degrees of reactivity with the B. abortus and B. melitensis LPS O-polysaccharides and have proposed that they correspond to overlapping epitopes in the cognate antigens.
The preceding data are relevant in the interpretation of the results of the present study. The presence or absence of lipid A epitopes (and outer membrane proteins) did not significantly affect the performance of the iELISA, as shown by the similar results obtained with the crLPSs and PSs. The fact that NHs yielded less satisfactory results suggests a reduced but relevant role of the core oligosaccharide (present in PS but absent from NH) in the immunosorbent assay. This could be due to the existence of diagnostically relevant antibodies specific for core epitopes (not detectable with NHs) or to a role of the core in polystyrene adsorption leading to a conformation of the adsorbed PS more favorable for antibody binding. Although the first possibility is suggested by the fact that infected animals produce low but significant titers of antibodies to lipid A-core epitopes (2, 28, 34), the second is also suggested by the fact that, depending on the conditions, NHs and PSs show different polystyrene binding (this work and references 2 and 18). No matter what the exact explanation is, it can be concluded that NHs are not the optimal antigens in immunosorbent assays for brucellosis. Moreover, the results of this work confirm (25) that PSs do not outperform LPSs in these assays and show that the relatively crude and technically simple-to-obtain LPS preparations used in this and previous studies (8, 18, 22) are a practical choice.
The fact that, regardless of the infecting strain or vaccine serotypes, the antigens of MC and AC epitopic structure produced similar results strongly suggests a dominance of the anti-C antibodies in natural infections and also in vaccinated animals. In fact, although they have not been tested for sheep and goat brucellosis, competitive iELISAs for cattle brucellosis based on monoclonal antibodies of C specificity as the competing reagent are sensitive assays (25). This is in apparent contradiction to our previous report that, whereas NHs and PSs of MC specificity efficiently identify cattle infected with B. abortus biotype 1, NHs and PSs of AC specificity are inefficient in detecting B. melitensis-infected sheep (17). However, these observations were made by gel immunoprecipitation, and factors other than the specificity of the antigen, such as the threshold antibody avidity of the assay (higher in immunoprecipitation than in iELISA), can account for the results (2, 3). Also, as discussed above, the O-chain structure of the LPSs of B. abortus and B. melitensis can be considered to be made of overlapping epitopes (33) to which a given antibody of a polyclonal response can bind with various avidities. Thus, the chemical analyses (26), the concept of overlapping epitopes (33), and the results of the present work make it rather unlikely that the serological diagnosis of animal brucellosis could be improved by the use of LPSs or PSs obtained from the serologically intermediate serotypes of the smooth Brucella spp. Obviously, the practical implications are that the epitopic structure of the LPS used in the iELISA is not relevant in the diagnosis and that a single antigen (and conjugate) can be used for the diagnosis of brucellosis in small ruminants and cattle.
ACKNOWLEDGMENTS
This research was supported by the Dirección General de Investigación Científica y Tecnológica (grant AGF95-1013-CO) and by the Insituto Nacional de la Investigación Agronómica (grant INIA SC-037).
REFERENCES
- 1.Abalos P, Pinochet L, Fábrega F. Prueba de ELISA para descartar la respuesta post-vacunal a Brucella abortus cepa 19. Av Cien Vet. 1993;8:138–143. [Google Scholar]
- 2.Alonso-Urmeneta, B., C. Marín, J. M. Blasco, R. Díaz, and I. Moriyón. Unpublished data.
- 3.Alonso-Urmeneta B, Moriyón I, Díaz R, Blasco J M. Enzyme-linked immunosorbent assay with Brucella native hapten polysaccharide and smooth lipopolysaccharide. J Clin Microbiol. 1988;26:2642–2646. doi: 10.1128/jcm.26.12.2642-2646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alton G G. Standardisation of agglutinating antigens for the diagnosis of brucellosis. Res Vet Sci. 1971;12:330–337. [PubMed] [Google Scholar]
- 5.Alton G G, Jones L M, Angus R D, Verger J M. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 6.Aragón V, Díaz R, Moreno E, Moriyón I. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. 1996;178:1070–1079. doi: 10.1128/jb.178.4.1070-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasco J M, Garin-Bastuji B, Marín C, Garbier G, Fanlo J, Jiménez de Bagües M P, Cau C. Efficacy of different rose bengal and complement fixation antigens for the diagnosis of Brucella melitensis in sheep and goats. Vet Rec. 1994;134:415–420. doi: 10.1136/vr.134.16.415. [DOI] [PubMed] [Google Scholar]
- 8.Blasco J M, Marín C, Jiménez de Bagués M, Barberán M, Hernández A, Molina L, Velasco J, Díaz R, Moriyón I. Evaluation of allergic and serological tests for diagnosing Brucella melitensis infection in sheep. J Clin Microbiol. 1994;32:1835–1840. doi: 10.1128/jcm.32.8.1835-1840.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundle D R, Cherwonogrodzky J W, Gidney M A J, Meikle P J, Perry M B, Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989;57:2829–2836. doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherwonogrodzky J W. Comparison of rabbit monospecific anti-Brucella antisera with mouse anti-Brucella monoclonal antibodies. Arch Med Vet. 1995;27:131–137. [Google Scholar]
- 11.Cherwonogrodzky J W, Nielsen K H. Brucella abortus 1119-3 O-chain polysaccharide to differentiate sera from B. abortus S-19-vaccinated and field-strain-infected cattle by agar gel immunodiffusion. J Clin Microbiol. 1988;26:1120–1123. doi: 10.1128/jcm.26.6.1120-1123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloeckaert A, Kerkhofs P, Limet J N. Antibody response to Brucella outer membrane proteins in bovine brucellosis: immunoblot analysis and competitive enzyme-linked immunosorbent assay using monoclonal antibodies. J Clin Microbiol. 1992;30:3168–3174. doi: 10.1128/jcm.30.12.3168-3174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloeckaert A, Verger J M, Grayon M, Vizcaíno N. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol Lett. 1996;145:1–8. doi: 10.1111/j.1574-6968.1996.tb08547.x. [DOI] [PubMed] [Google Scholar]
- 14.Corbel M J. Comparison of Brucella abortus and B. melitensis antigens for the rose bengal plate test on the sera from cattle infected with B. abortus biovar-5. Vet Rec. 1985;117:385–386. doi: 10.1136/vr.117.15.385. [DOI] [PubMed] [Google Scholar]
- 15.Díaz R, Levieux D. Rôle respectif en sérologie de la brucellose bovine des antigènes et des immunoglobulines G1 et G2 dans les tests d’agglutination, de Coombs et au Rose de Bengale ainsi que dans le phénomène de zone. C R Acad Sci Ser D. 1972;274:1593–1596. [PubMed] [Google Scholar]
- 16.Díaz R, Jones L M, Leong D, Wilson J B. Surface antigens of smooth brucellae. J Bacteriol. 1968;96:893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz-Aparicio E, Aragón V, Marín C, Alonso B, Font M, Moreno E, Pérez-Ortiz S, Blasco J M, Díaz R, Moriyón I. Comparative analysis of Brucella serotype A and M and Yersinia enterocolitica O:9 polysaccharides for serological diagnosis of brucellosis in cattle, sheep, and goats. J Clin Microbiol. 1993;31:3136–3141. doi: 10.1128/jcm.31.12.3136-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-Aparicio E, Marín C, Alonso-Urmeneta B, Aragón V, Pérez-Ortiz S, Pardo M, Blasco J M, Díaz R, Moriyón I. Evaluation of serological tests for diagnosis of Brucella melitensis infection of goats. J Clin Microbiol. 1994;32:1159–1165. doi: 10.1128/jcm.32.5.1159-1165.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglas J T, Palmer D A. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J Clin Microbiol. 1988;26:1353–1356. doi: 10.1128/jcm.26.7.1353-1356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamazo C, Winter A J, Moriyón I, Riezu-Boj J I, Blasco J M, Díaz R. Comparative analyses of proteins extracted by hot saline or released spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect Immun. 1989;57:1419–1426. doi: 10.1128/iai.57.5.1419-1426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmen F, Weynants V, Scarcez T, Letesson J-J, Saman E. Cloning and sequence analysis of a newly identified Brucella abortus gene and serological evaluation of the 17-kilodalton antigen that it encodes. Clin Diagn Lab Immunol. 1995;2:263–267. doi: 10.1128/cdli.2.3.263-267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez de Bagüés M P, Marín C M, Blasco J M, Moriyón I, Gamazo C. An ELISA with Brucella lipopolysaccharide antigen for the diagnosis of B. melitensis infection in sheep and for the evaluation of serological responses following subcutaneous or conjunctival B. melitensis Rev 1 vaccination. Vet Microbiol. 1991;30:233–241. doi: 10.1016/0378-1135(92)90117-c. [DOI] [PubMed] [Google Scholar]
- 23.Jones L M, Berman D T, Moreno E, Deyoe B L, Gilsdorf M J, Huber J D, Nicoletti P. Evaluation of a radial immunodiffusion test with polysaccharide B antigen for diagnosis of bovine brucellosis. J Clin Microbiol. 1980;12:753–760. doi: 10.1128/jcm.12.6.753-760.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letesson J J, Tibor A, van Eynde G, Wansard V, Weynants V, Denoel P, Saman E. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–564. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen K H, Kelly L, Gall D, Balsevicius S, Bosse J, Nicoletti P, Kelly W. Comparisons of enzyme immunoassays for the diagnosis of bovine brucellosis. Prev Vet Med. 1996;26:7–32. [Google Scholar]
- 26.Perry M B, Bundle D R. Lipopolysaccharide antigens and carbohydrates of Brucella. In: Adams L G, editor. Advances in brucellosis research. College Station: Texas A&M University Press; 1990. pp. 76–88. [Google Scholar]
- 27.Riezu-Boj J I, Moriyón I, Blasco J M, Gamazo C, Díaz R. Antibody response to Brucella ovis outer membrane proteins in ovine brucellosis. Infect Immun. 1990;58:489–494. doi: 10.1128/iai.58.2.489-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas N, Freer E, Weintraub A, Ramirez M, Lind S, Moreno E. Immunochemical identification of Brucella abortus lipopolysaccharide epitopes. Clin Diagn Lab Immunol. 1994;1:206–213. doi: 10.1128/cdli.1.2.206-213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruckerbauer G M, García M M, Rigby C E, Robertson F J, Samagh B S, Stemshorn B W. An hemolysis-in-gel test for bovine brucellosis. Rev Dev Biol Stand. 1984;56:513–520. [PubMed] [Google Scholar]
- 30.Salih-Alj Debbarh H, Cloeckaert A, Bézard G, Dubray G, Zygmunt M S. Enzyme-linked immunosorbent assay with partially purified cytosoluble 28-kilodalton protein for serological differentiation between Brucella melitensis-infected and B. melitensis Rev.1-vaccinated sheep. Clin Diagn Lab Immunol. 1996;3:305–308. doi: 10.1128/cdli.3.3.305-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibor A, Saman E, de Wergifosse P, Cloeckaert A, Limet J N, Lettesson J-J. Molecular characterization, occurrence, and immunogenicity in infected sheep and cattle of two minor outer membrane proteins of Brucella abortus. Infect Immun. 1996;64:100–107. doi: 10.1128/iai.64.1.100-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vizcaíno N, Chordi A, Fernández-Lago L. Characterization of smooth Brucella lipopolysaccharides and polysaccharides by monoclonal antibodies. Res Microbiol. 1991;142:971–972. doi: 10.1016/0923-2508(91)90007-w. [DOI] [PubMed] [Google Scholar]
- 33.Weynants V, Gilson D, Cloeckaert A, Tibor A, Denoel P A, Godfroid F, Limet J N, Lettesson J-J. Characterization of smooth lipopolysaccharides and O polysaccharides of Brucella species by competition binding assays with monoclonal antibodies. Infect Immun. 1997;65:1939–1943. doi: 10.1128/iai.65.5.1939-1943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zygmunt M S, Cloeckaert A, Dubray G. Brucella melitensis cell envelope protein and lipopolysaccharide epitopes involved in humoral immune responses of naturally and experimentally infected sheep. J Clin Microbiol. 1994;32:2514–2522. doi: 10.1128/jcm.32.10.2514-2522.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]