Abstract
This scientific commentary refers to ‘Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses’ by Needham et al. (https://doi.org/10.1093/brain/awac321).
This scientific commentary refers to ‘Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses’ by Needham et al. (https://doi.org/10.1093/brain/awac321).
The coronavirus disease 19 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially associated with influenza-like respiratory illness, fever, cough, dyspnoea and malaise/myalgias. With the emergence of novel virus variants such as delta and omicron, the spectrum of clinical presentations changed over time. Besides respiratory symptoms, several neurological complications were observed in COVID-19 ranging from memory loss to stroke, autoimmune-mediated peripheral nerve damage (Guillain-Barré syndrome) and CNS inflammation (autoimmune encephalitis, acute disseminated encephalomyelitis, myelitis).1 In addition, persistent neuropsychiatric symptoms such as fatigue or cognitive impairment occurred after infection in up to one-third of patients,2 further suggesting involvement of the CNS. Although much is already known about the exaggerated host immune response in severe COVID-19 disease, little is known about the relationship between this dysregulated immune response and the extent of brain injury. In this issue of Brain, Needham and colleagues3 have provided valuable insights into this important topic by performing one of the largest studies to date. Whereas most previous studies compared people with COVID-19 to healthy controls, Needham and colleagues3 also compared their results to findings in people with influenza to determine whether the observed changes are specific to COVID-19 or also occur in other viral infections.
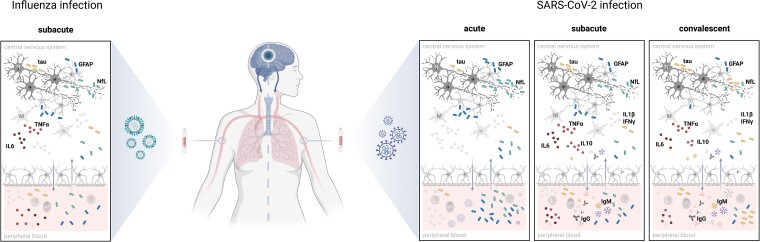
As known from other neurological diseases including traumatic brain injury, neurodegeneration and neuroinflammation, serum biomarkers can give an indication of the extent of brain injury. These include neurofilament light (NfL), an axonal structural protein; tau, a microtubule-associated protein; and the astroglial marker glial fibrillary acidic protein (GFAP).4 In hospitalized people with COVID-19, Needham and colleagues3 showed that levels of NfL and GFAP were elevated in acute (0–14 days from admission) and subacute (15–70 days from admission) COVID-19 disease in a severity-dependent manner. NfL levels remained elevated even in convalescent (>80 days from admission) people after moderate and severe COVID-19 disease compared with controls, although the same team showed in another study that serum NfL and GFAP levels eventually returned to baseline after ∼6 months.5 The magnitude of the increase of these biomarkers was comparable to that seen after severe traumatic brain injury, indicating a common specific origin in the CNS. The results presented for GFAP and NfL confirm those of previous studies.5,6 The results presented for tau are also of interest, but should be viewed with caution because levels were very low and close to the detection limit of the assay. But the most important finding of this study is that the increase in brain-injury biomarkers, and the association between high biomarker levels and a more pronounced proinflammatory cytokine profile, were not specific for COVID-19 but also occurred to a similar extent in people with severe influenza disease (Fig. 1).
Figure 1.
Severe SARS-CoV-2 and influenza infection both lead to peripheral inflammation and brain damage. Elevated levels of brain injury biomarkers—namely the axonal protein neurofilament light (NfL), the microtubule-associated protein tau and the astroglial marker glial fibrillary acidic protein (GFAP)—are found in peripheral blood at the acute time point after SARS-CoV-2 infection (0–14 days from hospital admission), with the observed increases being severity-dependent. Injury biomarkers are most likely released by the destruction of neurons (N) and astrocytes (A), which probably occurs due to the excessive proinflammatory cytokine release in peripheral blood by leucocytes (L) and as a consequence, inside the CNS via microglial cells (M). A sustained cytokine response is also found in convalescent patients (>80 days from admission), with an IgM response to various antigens dominating an IgG response, by plasma blasts (P). An increased release of tau protein up to 4 months post-admission suggests a second process. In influenza patients at the subacute time point, an immune response was detected that was similar to that seen with SARS-CoV-2 infection, accompanied by a similar release of brain injury biomarkers in the peripheral blood. Altogether these findings suggest that components of the innate and adaptive immune response may interact with cells of the CNS and may be central actors in COVID-19 disease and its post-acute sequelae. Created with BioRender.com.
The authors found a diverse autoantibody response to several neuronal and peripheral antigens that, although probably not pathogenic, may reflect a general dysregulated immune response in COVID-19. Similar findings have already been reported after COVID-19 and other viral infections.7 Moreover, the authors also described an IgG response to a pulmonary surfactant protein A (SFTPA1) synthesized by type II alveolar epithelial cells in people with COVID-19 that was independent of brain injury markers, with particularly high levels in moderate and severe disease, as well as other autoantibody responses, such as autoantibodies against anti-myelin-associated glycoprotein.
However, these findings need to be considered with caution since the authors of this and other studies have used protein or peptide microarrays to profile autoantibody responses in people with COVID-19. Numerous studies have clearly demonstrated that this methodological approach fails to detect pathogenic autoantibodies in neurological diseases, such as autoimmune encephalitis or neuromyelitis spectrum disorder.8 Therefore, as also discussed by Needham et al.,3 the detected antibodies are unlikely to be pathogenic, but may well reflect an overactive immune response and serve as useful biomarkers. Unfortunately, the selection of antigens did not include many known autoantibody targets in COVID-19, such as class I interferons and their receptors7 and therefore it is difficult to compare the current study to previous reports. While Needham and colleagues3 did include interferon alpha 1 as an antigen in their protein microarray analysis, they did not find increased reactivity to it in people with COVID-19. A possible explanation for this discrepancy may be the observation that class I interferon autoantibodies were detectable in 10% of COVID-19 patients with life-threatening disease, but not in those with mild or asymptomatic infection.9 In the study by Needham and colleagues,3 only 34 people with severe disease were included in the autoantibody analysis, plus 17 with moderate and 71 with mild disease. Therefore, it may be that the number of people with severe disease was too small to detect an effect in people with COVID-19 compared to healthy controls. It would be of great interest to elucidate how anti-cytokine antibodies relate to the autoantibody responses described by the authors, as also shown in other studies.10 Although some autoantibodies were detected more frequently than others, the important finding of the current study is not that of an individual disease-specific autoantibody, but rather the tendency to develop autoantibodies to a number of targets in COVID-19.
This study thus provides valuable information on the relationship between an impaired innate and adaptive immune response and biomarkers of brain injury in COVID-19. Given the socio-economic burden of protracted post-COVID-19 sequelae, and of neuropsychiatric symptoms in particular, this study is of great clinical relevance. Because many therapies used for severe COVID-19 disease successfully target inflammatory or immune processes, the findings of Needham and colleagues3 underscore the hypothesis that brain damage is a consequence of dysregulated immune responses rather than the direct result of self-reactive antibodies or the virus itself.
Of course, there are several important questions left unanswered by this work. First, as also discussed by the authors, this study does not establish causality between the immunological findings and the presence of brain injury, and it remains possible that additional factors, such as tissue damage, are responsible for the observed associations. In addition, the novel finding that serum total tau levels were significantly elevated in convalescent patients irrespective of disease severity, requires validation in an independent cohort, as some samples had concentrations below the lower limit of quantification and many samples had concentrations close to the limit of detection.
Overall, the study by Needham and colleagues3 is another hint that brain injury is a common consequence of severe viral infections, not only in severe COVID-19 but also in severe influenza, and that it occurs largely in the context of a dysregulated immune response. Further studies will show whether these biomarkers of brain injury are also released peripherally in other viral diseases with severe courses.
Contributor Information
Angelika Bauer, Clinical Department of Neurology, Medical University of Innsbruck, 6020 Innsbruck, Austria; VASCage Research Centre on Vascular Ageing and Stroke, 6020 Innsbruck, Austria.
Markus Reindl, Clinical Department of Neurology, Medical University of Innsbruck, 6020 Innsbruck, Austria.
Funding
The research of M.R. is supported by a research grant of the Euregio Science Fund and Austrian Science Funds (FWF, international project network IPN170).
Competing interests
M.R. was supported by research funds from Euroimmun and Roche (to institution). The University Hospital and Medical University of Innsbruck (Austria, employer of M.R.) receives payments for antibody assays (MOG, AQP4, and other autoantibodies) and for MOG and AQP4 antibody validation experiments organized by Euroimmun (Lübeck, Germany). A.B. is an employee of VASCage—Research Centre on Vascular Ageing and Stroke (Innsbruck, Austria).
References
- 1. Ren AL, Digby RJ, Needham EJ. Neurological update: COVID-19. J Neurol. 2021;268:4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Needham EJ, Ren AL, Digby RJ, et al. . Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain. 2022;145:4097–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12:563–574. [DOI] [PubMed] [Google Scholar]
- 5. Kanberg N, Simren J, Eden A, et al. . Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70:103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutter R, Hert L, De Marchis GM, et al. . Serum neurofilament light chain levels in the intensive care unit: Comparison between severely ill patients with and without coronavirus disease 2019. Ann Neurol. 2021;89:610–616. [DOI] [PubMed] [Google Scholar]
- 7. Casanova JL, Abel L. From rare disorders of immunity to common determinants of infection: Following the mechanistic thread. Cell. 2022;185:3086–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15:89–102. [DOI] [PubMed] [Google Scholar]
- 9. Bastard P, Rosen LB, Zhang Q, et al. . Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang EY, Mao T, Klein J, et al. . Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. [DOI] [PubMed] [Google Scholar]