Abstract
Procedures for quality control (QC) in a laboratory that concentrates on cytokine and soluble marker measurements in biological fluids are outlined. Intra-assay, interassay, and interlaboratory experiences are presented. Plasma and serum β2-microglobulin (β2M) and neopterin test data are presented in greatest detail, along with substantial tumor necrosis factor alpha (TNF-α), gamma interferon, soluble interleukin-2 receptor-α (sIL-2Rα), sTNF-RII, IL-4, and IL-6 data. Recommended QC procedures for cytokine and soluble-marker testing include replicate testing of two or more reference samples provided by the kit manufacturer, replicate testing of in-house frozen reference QC samples that represent normal and abnormal analyte contents, retesting 15 to 20% of randomly selected samples, and comparing normal reference ranges each year. Also, eight cytokines and soluble markers were evaluated in human immunodeficiency virus (HIV)-seronegative and HIV-seropositive individuals stratified on the basis of CD4 T-cell numbers. Levels of some but not all cytokines in serum increased in HIV infection. There was a tendency for cytokines to increase with more advanced disease, defined by reduced CD4 T-cell numbers. Cytokine changes did not relate closely to CD4 level, indicating that separate information was provided by the measurements of TNF-α, sTNF-RII, sIL-2Rα, β2M, and neopterin. Serum IL-4 and TNF-α levels were not increased. The quality of laboratory data can impact on clinical relevance. Interlaboratory comparisons revealed substantial differences at some sites and documented the need for external proficiency-testing quality assurance programs.
Cytokine levels and changes in biological fluids are now recognized as potential and useful markers of ongoing clinical disorders, indicating their stage and severity and disease prognosis (1, 10, 13, 42). Initial evidence of immune activation in human immunodeficiency virus (HIV) infection included increases in several phenotypic antigens on circulating lymphocytes as well as increases in levels of soluble products of cytokine activity in plasma (2, 3, 15, 38, 39, 43, 47). β2-microglobulin (β2M) increases were reported early in the characterization of AIDS (21, 35) but were not perceived as related to disease course until several years later (11). β2M represents the activity of several cytokines throughout the body (19, 20) and is a relatively nonspecific marker of immune activation. Neopterin, on the other hand, which is induced by gamma interferon (IFN-γ) activation of monocytes, was found at elevated levels in HIV infection and was related to prognosis (11, 23, 28, 48).
The measurement of the levels of cytokines and/or soluble markers of immune activation can provide reliable information regarding the disease diagnosis, disease stage, prognosis, and the evaluation of therapy. However, difficulties and inaccuracy have been reported, and a number of factors have been shown to affect the validity and the quality of such measurements (5, 17, 29, 30, 46, 49). Immunoassays are the most widely used technique for these measurements, although pitfalls and limitations are known (24, 36, 37). Differences in levels of measured analytes for identical samples in the range of 10- to 100-fold have been reported (26, 31, 32). Thus, a number of studies, including international collaborative studies organized by the World Health Organization for standardization of cytokine measurements, have been conducted (4, 7, 16, 31–33, 40). Variations in results have been shown to be due at least in part to differences in the standards used in the assays (16, 26, 30–33, 40) or in sample collection, processing, and storage (9, 27, 41, 45).
In our early work with the assessment of neopterin and β2M concentrations in plasma and in subsequent testing of cytokines and the products of cytokine activity, we have had large numbers of samples available to test but limited funds. As a consequence, we looked for a means to conserve costly reagents but, at the same time, to ensure consistency and accuracy of testing. Initial testing showed good agreement between duplicate samples. Thus, we chose to do single determinations rather than duplicates but also to randomly retest approximately 15% of samples. This approach had the advantage of providing representative duplicate measurements and a check on comparability between analytic runs. As an additional quality control (QC) procedure, we established a method of preparing a large number of frozen aliquots from sizable pools of plasma or serum, one each representing normal levels and abnormally elevated levels of the cytokines and soluble markers of activation. Aliquots of these reference standards were required to be included in each analysis of cytokines or activation markers. Upon repeated testing, we were able to establish the validity of runs and the comparability of reagents and technical performance. These QC procedures are now routinely used for testing neopterin, β2M, soluble tumor necrosis factor receptors I and II (sTNF-RI and -RII), soluble interleukin 2 receptor-alpha (sIL-2Rα), soluble CD8 antigen, the cytokines TNF-α, IFN-γ, IL-1, IL-2, IL-4, IL-6, IL-10, and IL-12, and chemokines.
Manufacturers of commercial kits for the measurement of these analytes provide data characterizing their performance with samples of their own selection. However, each laboratory has its own performance characteristics. In our present report, the same QC samples were used for all assays. We report here on the procedures used and on the coefficient of variation (CV) obtained in control populations and at different stages of HIV infection. In addition, intra-assay, interassay, and interlaboratory variabilities are reported.
(These data were presented in part at the 3rd International Symposium on Clinical Immunology in San Francisco, Calif., 20 to 23 July 1995.)
MATERIALS AND METHODS
Samples.
Serum and plasma samples were obtained from healthy volunteers from the University of California, Los Angeles (UCLA) community and from HIV-seronegative and HIV-seropositive subjects participating in the Multicenter AIDS Cohort Study (MACS) of the natural history of AIDS, who were recruited and monitored at UCLA at approximately 6-month intervals from 1984 (25). Blood was collected by venipuncture into 15-ml sterile Vacutainers (Becton Dickinson) containing heparin as an anticoagulant for plasma samples and without anticoagulant for serum samples. Serum and plasma samples were separated and stored at −70°C for subsequent batch testing of cytokine and soluble immune activation markers.
The general reference (normal) samples were obtained from male and female employees at UCLA with an age range of 24 to 65 years; 32% were males and 68% were females. Human Subject Protection Committee approval was obtained for all studies. The study of cytokine and soluble marker changes in HIV infection was conducted with sera from 15 subjects who were negative for HIV antibodies at the time of selection and from 56 HIV-seropositive subjects who were separated into four groups (13 to 15 subjects in each group) according to their absolute number of CD4 T cells. The defining levels of CD4 T cells were 500 to 700, 350 to 499, 200 to 349, or fewer than 200/mm3.
The in-house QC samples were prepared in our laboratory as two high-volume pools of serum or plasma (500 ml each). One pool from HIV-negative donors had levels of cytokines and soluble markers within the normal range. The other pool was prepared from HIV-seropositive samples and was distinctly abnormal, with elevated levels of many cytokines and soluble markers. The samples used for QC purposes were comparable to the study patient samples. Aliquots of 1 ml each were stored in labeled tubes in a −70°C freezer until removed and thawed for assay.
Quantitation of levels of cytokines and soluble activation markers in plasma.
β2M was quantified by microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, Ill.) and reported in milligrams per liter. Neopterin was measured by competitive radioimmunoassay kit (IMMUtest Neopterin; BRAHMS, Berlin, Germany) and expressed as nanomoles per liter. sIL-2R was measured with an enzyme immunoassay (EIA) kit (T Cell Diagnostics, Cambridge, Mass.; now available from Endogen, Woburn, Mass.) and reported in units per milliliter. sTNF-RI and sTNF-RII (1:20 dilution) were quantified in plasma with EIA kits (R&D Systems) and expressed as picograms and nanograms per milliliter, respectively. TNF-α was measured by Innotest hTNFα EIA kits (Innogenetics N.V., Antwerp, Belgium) and reported in picograms per milliliter. IFN-γ was measured with radioimmunoassay kits (Centecor; no longer available) by a Center for Interdisciplinary Research in Immunology and Disease modified protocol (increases of incubation time to overnight and of number of washings between steps to 10 times) and was reported in units per liter. IL-4 and IL-6 were measured with EIA kits (Genzyme, Cambridge, Mass.). The measurements of cytokine and activation marker levels were performed according to the manufacturer’s instructions. The reference standards for each test were provided by the manufacturer. For most of the assays the manufacturers have calibrated the kit standards to the reference standards of the NIBSC and the World Health Organization, if already available.
RESULTS
Intra-assay variation.
Intra-assay variability was evaluated with 10 replicates of two different QC plasma samples in the same run. Intra-assay variabilities of β2M, neopterin, sIL-2R, sTNF-RII, TNF-α, and IFN-γ are presented in Table 1. CVs of soluble markers are under 7.5%, except for TNF-α and IFN-γ (Table 1). Similar results were found when two or more QC samples were tested in three different wells during routine assays (data not shown). Common criteria for acceptable performance cited by a clinical laboratory improvement amendment (14) in other quantitative plasma immunology tests are the target values plus or minus 3 standard deviations, but we use 2 standard deviations in our laboratory.
TABLE 1.
Intra-assay variabilitya
| CIRID control sample | Concn (CV) ofb:
|
|||||
|---|---|---|---|---|---|---|
| β2M (mg/liter) (n = 10) | NPT (nmol/liter) (n = 10) | sIL-2R (U/ml) (n = 10) | sTNF-RII (ng/ml) (n = 10) | TNF-α (pg/ml) (n = 10) | IFN-γ (U/liter) (n = 9) | |
| Normal | 0.69 ± 0.03 (4.6) | 4.5 ± 0.33 (7.4) | 282 ± 9.7 (3.4) | 1.67 ± 0.06 (3.6) | 7.36 ± 0.98 (13.0) | <50 (NA)c |
| Elevated | 2.62 ± 0.06 (2.4) | 18.7 ± 1.04 (5.6) | 765 ± 40 (5.3) | 4.57 ± 0.17 (3.9) | 38.2 ± 1.89 (4.9) | 562 ± 30 (5.3) |
All replicates were obtained from the Center for Interdisciplinary Research in Immunology and Disease (CIRID), as described in Materials and Methods.
Values are means ± standard deviations; CVs are percentages. NPT, neopterin.
<50, less than lower limit of detection. NA, not applicable.
Interassay variation.
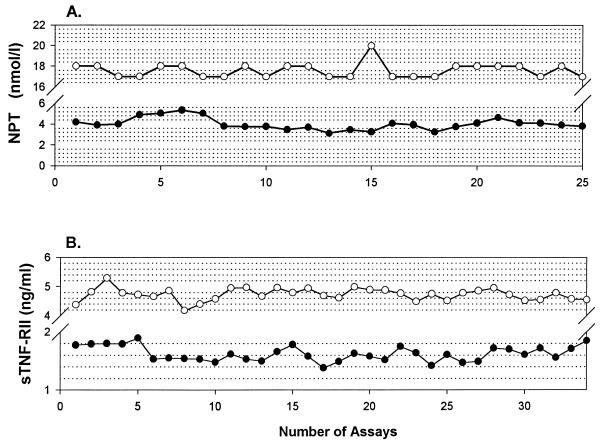
Aliquots of the two in-house QC samples were included on each assay day. The CV of serial assays for β2M, neopterin, sIL-2R, sTNF-RII, TNF-α, and IFN-γ are listed in Table 2. The CV was less than 15% for all markers except TNF-α and IFN-γ in normal control samples. Similar data are seen in repeat testing of four plasma activation markers in 15 to 20% of patient samples on a subsequent assay day (Table 3). Actual data for serial testing of the in-house QC samples for neopterin and sTNF-RII tests over an 18-month period are presented graphically in Fig. 1.
TABLE 2.
Interassay variabilitya
| CIRID control sample | Concn (CV) ofb:
|
|||||
|---|---|---|---|---|---|---|
| β2M (mg/liter) (n = 22) | NPT (nmol/liter) (n = 25) | sIL-2R (U/ml) (n = 14)c | sTNF-RII (ng/ml) (n = 30) | TNF-α (pg/ml) (n = 10) | IFN-γ (U/liter) (n = 5) | |
| Normal | 0.79 ± 0.07 (8.8) | 4.02 ± 0.58 (14.4) | 463 ± 49 (10.5) | 1.62 ± 0.14 (8.6) | 10.0 ± 1.6 (16.0) | <50 (NA)d |
| Elevated | 2.57 ± 0.16 (6.2) | 17.6 ± 0.71 (4.3) | 1690 ± 131 (7.7) | 4.72 ± 0.23 (4.3) | 32.3 ± 2.3 (7.2) | 515 ± 35 (5.3) |
All replicates were obtained from the Center for Interdisciplinary Research in Immunology and Disease (CIRID), as described in Materials and Methods.
Values are means ± standard deviations; CVs are percentages. NPT, neopterin.
Different CIRID control samples were used to test intra-assay and interassay variability for sIL-2R.
<50, less than lower limit of detection. NA, not applicable.
TABLE 3.
Comparison of original and repeat data of 15 to 20% of samplesa
| Analyte | No. of subjects | Mean concn ± SDb | Median CV (%) | Correlation coefficientc |
|---|---|---|---|---|
| β2M (O) | 98 | 2.60 ± 0.90 | 5.3 | 0.92725 |
| β2M (R) | 98 | 2.58 ± 0.89 | ||
| NPT (O)d | 117 | 10.7 ± 9.68 | 8.6 | 0.96303 |
| NPT (R) | 117 | 10.9 ± 9.88 | ||
| sIL-2R (O) | 170 | 1,071 ± 556 | 4.6 | 0.92633 |
| sIL-2R (R) | 170 | 1,064 ± 560 | ||
| sTNF-RII (O) | 273 | 3.22 ± 1.28 | 8.6 | 0.89583 |
| sTNF-RII (R) | 273 | 3.35 ± 1.32 |
The data present results of the analysis of original (O) and repeated (R) tests for each individual separately.
Data are levels of plasma activation markers. Units: milligrams per liter (β2M), nanomoles per liter (NPT), units per milliliter (sIL-2R), or nanograms per milliliter (sTNF-RII).
P < 0.001.
NPT, neopterin.
FIG. 1.
Interassay variations in concentrations of neopterin (NPT) (A) and sTNF-RII (B) in normal (filled circles) and abnormal high (open circles) in-house QC preparations. Each point represents the mean value of triplicate testing.
Normal population values tested in different years.
Volunteer healthy donors, men and women from 24 to 65 years of age, from the UCLA community are tested as normal reference controls every year. The values obtained in three recent years were assembled and compared (Table 4). Because the reference populations were not identical from year to year, we did not expect to obtain identical values. These data provide reference ranges with which to judge the changes in reagents and technical staff performance from year to year.
TABLE 4.
Reference range data from normal adults for three consecutive years (1995 to 1997)
| Analyte and yr | No. of subjects | Concn (mean ± SD) | Rangea |
|---|---|---|---|
| Neopterin (nmol/liter) | |||
| 1995 | 32 | 5.34 ± 2.09 | 3.68–6.78 |
| 1996 | 24 | 4.87 ± 1.92 | 2.80–8.49 |
| 1997 | 17 | 4.62 ± 2.93 | 2.71–5.85 |
| β2M (mg/liter) | |||
| 1995 | 33 | 1.17 ± 0.53 | 0.93–1.61 |
| 1996 | 26 | 1.16 ± 0.27 | 0.87–1.55 |
| 1997 | 21 | 1.15 ± 0.30 | 0.86–1.56 |
| sTNF-RII (ng/ml) | |||
| 1995 | 18 | 1.77 ± 0.75 | 1.02–2.50 |
| 1996 | 9 | 1.99 ± 0.86 | 1.31–3.40 |
| 1997 | 9 | 1.94 ± 0.35 | 1.34–2.35 |
10th to 90th percentiles.
Interlaboratory variation: external proficiency testing for quality assurance.
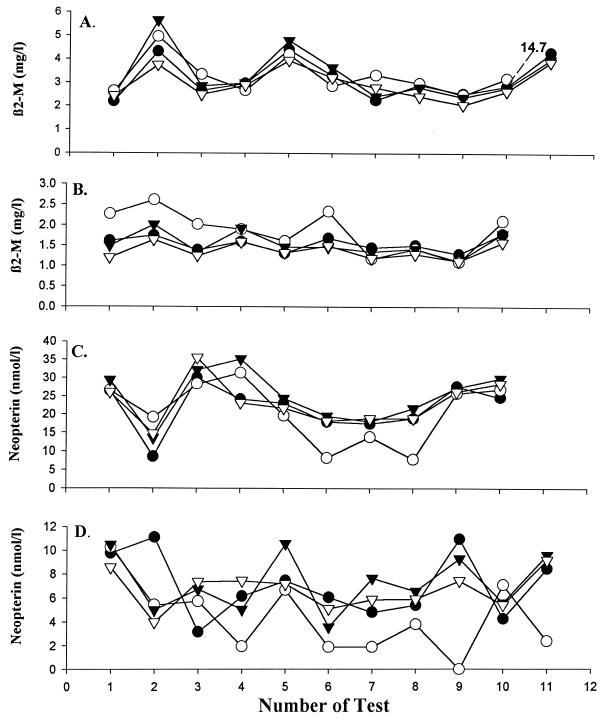
From 1989 to 1992, four laboratories participated in a QC program for two soluble activation markers (β2M and neopterin). The same kits and analytic reagent lots were used in each laboratory (Pharmacia AB, Uppsala, Sweden, supplied the β2M assay kits used for this study). The test samples were shipped frozen in dry ice overnight to each participating laboratory. The results are presented in Fig. 2. Agreement was better after the third sample. There was generally good agreement between laboratories for β2M measurements with relatively low CVs. The range of CVs for normal-level neopterin QC samples was 9 to 70% (Fig. 2D), and the range of CVs for elevated neopterin levels was 3 to 64% (Fig. 2C). Laboratory 2 reported lower levels of neopterin than the other laboratories on most dates. This laboratory was the only one using round-bottom tubes and may have been less successful in the washing step of the neopterin test.
FIG. 2.
Levels in plasma of elevated (A and C) and normal (B and D) external QA samples for β2M (A and B) and neopterin (C and D) assayed in laboratories 1 (•), 2 (○), 3 (▾), and 4 (▿), participating in an external QA program from 1989 to 1992. To avoid a change in the presentation of the other data, the outlier value of test 11 in panel A is reported without being plotted in the scale.
Cytokine and immune activation markers in plasma in HIV infection.
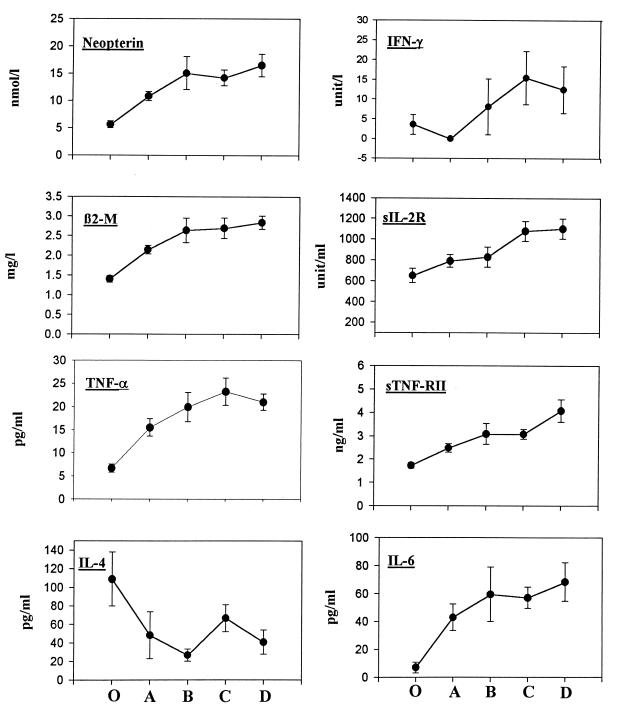
The plasma samples from 56 HIV-seropositive and 15 HIV-seronegative subjects from the MACS population were measured for levels of 10 cytokines and soluble markers. When the HIV-positive individuals were stratified by CD4 levels (Fig. 3), progressive increases in the levels of IFN-γ, TNF-α, IL-6, sIL-2R, sTNF-RII, β2M, and neopterin were generally seen. IL-4 levels, in contrast, tended to be reduced (P < 0.05). Preliminary results indicated that levels of TNF-β, IL-1β, and sTNF-RI were not significantly different between HIV-negative and HIV-positive sera. There were substantial spreads of the cytokine and soluble marker levels in each CD4 category (Fig. 3). This is consistent with data indicating that the levels of immune activation markers in plasma provide different information than CD4 T-cell levels on the pathogenic mechanisms in HIV infection (11, 12).
FIG. 3.
Cytokine and soluble marker levels in serum (means ± standard error) of 15 HIV-seronegative (○) and 56 HIV-seropositive subjects stratified by CD4+-lymphocyte levels: >500 (A), 350 to 499 (B), 200 to 349 (C), and <200 (D) lymphocytes per μl.
DISCUSSION
The procedures recommended for intralaboratory quality control of cytokine and soluble marker testing in biological fluids are outlined in Table 5.
TABLE 5.
Recommended QC procedures
| Step no. | Procedure(s) |
|---|---|
| 1 | Establish in-house reference samples that represent normal and abnormal values encountered clinically. Prepare a large number of aliquots of these reference samples for frozen storage. Include each of these reference samples in every assay. |
| 2 | Test intra-assay variability in each run. |
| 3 | If single tests (rather than duplicate tests) are used, repeat 15% of randomly selected samples on the next run to establish comparability of replicates and interassay variability. |
| 4 | Test in duplicate the reference samples provided by the manufacturer. |
| 5 | Test reference (normal) populations each year and compare summaries of yearly results. |
The procedures described here differ from those outlined by the manufacturers of reagents, in that duplicate testing was not conducted after initial experience indicated that a technologist with the reagents achieved CVs under 10% for intra-assay variability and under 10 to 15% for interassay variability, with the exception of TNF-α. The interassay variability was usually greater in the normal (lower) range, where the cytokine or soluble-marker concentrations were near the lower limit of the analyte detectability in plasma or serum and the precision of reference standard data is lower.
The procedures described here differ in some respects from those described in methodology manuals and reports. We recommend our procedures to laboratories which do many tests of this type with some frequency and with technicians of proven skill in this area. Doing duplicates (or triplicates) as well as the in-house reference sample aliquots and the manufacturer reference materials is important if testing is done infrequently. Graphic methods for assessing QC data are available (22).
External proficiency testing should facilitate the quality and comparability of laboratory performance. This proved to be important for measurements of CD4 T-cell levels in HIV-infected patients (6, 18). However, until external quality assurance (QA) proficiency testing programs are available for cytokine and soluble marker analyses, testing for clinical studies should be conducted in a single experienced laboratory with strong established internal QC procedures. It is recommended that each laboratory establish its own ranges for normal reference populations and test samples from patients with diseases characterized by abnormal levels of cytokines and/or soluble activation markers in order to be confident that abnormal levels can be detected in representative body fluids, usually plasma and serum.
While levels of cytokines in plasma may have some appeal (and are needed in a few research contexts), it is useful to remember that many cytokines cannot be accurately quantified in plasma or serum (17, 24, 36, 46, 49). Some of those that can be detected may show substantial variability because assays are at or near their limits of precise measurement. A variety of other factors could cause such effects. In contrast, measurement of the levels of immune activation markers and soluble products of cytokine activity in plasma may be preferable because they reflect the sum of lymphoid cells contributed from the entire body and are generally detectable by more precise quantitative assays.
Several points can be made about the changes in levels of cytokines and soluble markers of disease activation in plasma. Serial testing of individuals has revealed several characteristic and different patterns of cytokine and soluble marker changes in HIV disease progression (34, 44). A broad range of levels of cytokines and plasma markers of activation in plasma, representing cytokine activity throughout the body, were found in each of four major CD4 T-cell categories in HIV infection. This emphasizes the difference in disease course or activity as represented by the soluble products of activation versus the level of damage to the CD4 maintenance systems, as represented by the CD4 T-cell levels. Epidemiological studies have shown that these parameters provide different information and that combinations of the two types of measurement give more precise prognostic data than either alone (11, 12). There was no evidence of a shift from a Th1 to a Th2 pattern of cytokine expression with disease progression in these data. Viral load measurements in plasma have been shown to give good prognostic information. However, the CD4 plus cytokine–soluble-marker combinations may approximate viral load data in prognostic value (8, 12). Furthermore, in advanced disease, CD4 or activation marker levels may be prognostically superior to plasma HIV load measurements (8, 12).
Differences in results between laboratories may occur, and examples are documented here. Also, laboratories can vary in the quality of their day-to-day performance. This can be attributable to variations in reagents, to differences in technical proficiency, and to other factors. The need for proficiency testing programs is evident.
ACKNOWLEDGMENTS
We appreciate the support of the entire MACS with centers (and principal investigators) at The Johns Hopkins School of Public Health (Joseph B. Margolick and Alvaro Muñoz), Howard Brown Health Center and Northwestern University Medical School (John Phair), University of Pittsburgh (Charles Rinaldo), and UCLA (Roger Detels and Janis V. Giorgi). In particular, Roger Detels has encouraged excellence in laboratory technology contributing to epidemiological studies of HIV and AIDS since 1984. CD4 measurements were performed in the laboratory of Janis Giorgi at UCLA. We wish to acknowledge the excellent technical assistance of Hripi Nishanian and Mehran Bozorgmehri, the statistical assistance of Joanie Chung, and the assistance of Deborah Mathieson in manuscript preparation. The many professional contributions of Bo Hofmann to specific aspects of this work were noteworthy.
This work was supported by grants AI-35040, AI38858, and AI 36086.
REFERENCES
- 1.Aggarwal B B, Puri R K. Common and uncommon features of cytokines and cytokine receptors: an overview. In: Aggarwal B B, Puri R K, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995. pp. 3–24. [Google Scholar]
- 2.Aukrust P, Liabakk N B, Müller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-alpha (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection: correlations to clinical immunologic and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 3.Bass H Z, Nishanian P, Hardy W D, Mitsuyasu R T, Esmail E, Cumberland W, Fahey J L. Immune changes in HIV infection: significant correlations and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 4.Bienvenu J, Coulon L, Doche C, Gutowski M C, Grau G. Analytical performances of commercial ELISA kits for IL-2, IL-6 and TNFα. A WHO study. Eur Cytokine Netw. 1993;4:447–451. [PubMed] [Google Scholar]
- 5.Cannon J G, Nerad J L, Poutsiaka D D, Dinarello C A. Measuring circulating cytokines. J Appl Physiol. 1993;75:1897–1902. doi: 10.1152/jappl.1993.75.4.1897. [DOI] [PubMed] [Google Scholar]
- 6.Choi S, Lagakos S W, Schooley R T, Volberding P A. CD4 lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993;118:674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- 7.De Kossodo S, Houba V, Grau G E WHO Collaborative Study Group. Assaying tumor necrosis factor concentrations in human serum. A WHO international collaborative study. J Immunol Methods. 1995;182:107–114. doi: 10.1016/0022-1759(95)00028-9. [DOI] [PubMed] [Google Scholar]
- 8.de Wolf F, Spijkerman I, Schellekens P T, Langendam M, Kuiken C, Bakker M, Roos M, Coutinho R, Miedema F, Goudsmit J. AIDS prognosis based on HIV-1 RNA, CD4+ T cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–1806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Exley A R, Cohen J. Optimal collection of blood samples for the measurement of tumor necrosis factor-alpha. Cytokine. 1990;2:353–356. doi: 10.1016/1043-4666(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 10.Fahey J L. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahey J L, Taylor J M G, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serological markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 12.Fahey J L, Taylor J M G, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 14.Federal Register. Clinical laboratory improvement amendments of 1988; final rules and notice. Fed Regist. 1992;42CFR:7188–7243. [Google Scholar]
- 15.Fuchs D, Jager H, Popescu M, Reibnegger G, Werner E R, Dierich M P, Kaboth W, Tilz G P, Wachter H. Immune activation markers to predict AIDS and survival in HIV-1 seropositives. Immunol Lett. 1990;26:75–80. doi: 10.1016/0165-2478(90)90178-s. [DOI] [PubMed] [Google Scholar]
- 16.Gaines-Das R E, Poole S. The international standard for interleukin-6. Evaluation in an international collaborative study. J Immunol Methods. 1993;160:147–153. doi: 10.1016/0022-1759(93)90172-4. [DOI] [PubMed] [Google Scholar]
- 17.Gearing A J H, Cartwright J E, Wadhwa M. Biological and immunological assays for cytokines. In: Thomson A, editor. The cytokine handbook. London, England: Academic Press; 1991. pp. 339–355. [Google Scholar]
- 18.Giorgi J V, Cheng H-L, Margolick J B, Bauer K D, Ferbas J, Waxdal M, Schmid I, Hultin L E, Jackson A L, Park L, Taylor J M G Multicenter AIDS Cohort Study Group. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 19.Hoekman K, Van-Nieuwkoop J A, Willemze R. The significance of beta-2 microglobulin in clinical medicine. Neth J Med. 1985;28:551–557. [PubMed] [Google Scholar]
- 20.Hofmann B, Bass H, Nishanian P, Faisal M, Figlin R A, Sarna G P, Fahey J L. Different lymphoid cell populations produce varied levels of neopterin, β2-microglobulin and soluble IL-2 receptor when stimulated with IL-2, interferon-gamma or tumor necrosis factor-alpha. Clin Exp Immunol. 1992;88:548–554. doi: 10.1111/j.1365-2249.1992.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann B, Wang Y X, Cumberland W G, Detels R, Bozorgmehri M, Fahey J L. Serum β2-microglobulin level increases in HIV-1 infection: relation to seroconversion, CD4 T cell fall and prognosis. AIDS. 1990;4:207–214. [PubMed] [Google Scholar]
- 22.Howanitz P J, Howanitz J H. Laboratory quality assurance. New York, N.Y: McGraw-Hill; 1987. pp. 1–54. [Google Scholar]
- 23.Huber C, Batchelor J R, Fuchs D, Hausen A, Lang A, Nierderwieser D. Immune response-associated production of neopterin: release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia S, Torre-Amione G, Mann D L. Pitfalls in measuring cytokines. Ann Intern Med. 1994;121:149–150. doi: 10.7326/0003-4819-121-2-199407150-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow R A, Ostrow D G, Detels R, Phair J P, Polk B F, Rinaldo C R., Jr The multicenter AIDS cohort study: rationale, organization and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 26.Ledur A, Fitting C, David B, Hamberger C, Cavaillon J-M. Variable estimates of cytokine levels produced by commercial ELISA kits improved results using international cytokine standards. J Immunol Methods. 1995;186:171–179. doi: 10.1016/0022-1759(95)00184-c. [DOI] [PubMed] [Google Scholar]
- 27.Leroux-Roels G, Offner F, Philippe J, Vermeulen A. Influence of blood-collecting systems on concentrations of tumor necrosis factor in serum and plasma. Clin Chem. 1988;34:2373–2374. [PubMed] [Google Scholar]
- 28.Melmed R N, Taylor J M G, Detels R, Bozorgmehri M, Fahey J L. Serum neopterin changes in HIV-1 infected subjects: indicator of significant pathology, CD4 T cell changes, and the development of AIDS. J Acquired Immune Defic Syndr. 1989;2:70–76. [PubMed] [Google Scholar]
- 29.Mire-Sluis A R. Cytokines-protein structure and biological activity: a complex relationship with implication for biological assays and standardization. Biologicals. 1993;21:131–144. doi: 10.1006/biol.1993.1062. [DOI] [PubMed] [Google Scholar]
- 30.Mire-Sluis A R, Gaines-Das R, Thorpe R. Immunoassays for detecting cytokines: what are they really measuring? J Immunol Methods. 1995;186:157–160. doi: 10.1016/0022-1759(95)00128-w. [DOI] [PubMed] [Google Scholar]
- 31.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. The international standard for macrophage colony stimulating factor (M-CSF)—evaluation in an international collaborative study. J Immunol Methods. 1995;179:141–151. doi: 10.1016/0022-1759(94)00306-h. [DOI] [PubMed] [Google Scholar]
- 32.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. The international standard for granulocyte-macrophage colony stimulating factor (GM-CSF)—evaluation in an international collaborative study. J Immunol Methods. 1995;179:127–135. doi: 10.1016/0022-1759(94)00273-y. [DOI] [PubMed] [Google Scholar]
- 33.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. Implications for the assay and biological activity of interleukin-8. J Immunol Methods. 1997;200:1–16. doi: 10.1016/s0022-1759(96)00157-3. [DOI] [PubMed] [Google Scholar]
- 34.Nishanian P, Taylor J M G, Manna B, Aziz N, Grosser S, Giorgi J V, Detels R, Fahey J L. Accelerated changes (inflection points) in levels of serum immune activation markers and CD4+ and CD8+ T cells prior to AIDS onset. J Acquired Immune Defic Syndr. 1998;18:162–170. doi: 10.1097/00042560-199806010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Osmond D H, Shiboski S, Bacchetti P, Winger E E, Moss A R. Immune activation markers and AIDS prognosis. AIDS. 1991;5:505–511. doi: 10.1097/00002030-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Pesce A J, Michael J G. Artifacts and limitations of enzyme immunoassays. J Immunol Methods. 1992;150:111–119. doi: 10.1016/0022-1759(92)90070-a. [DOI] [PubMed] [Google Scholar]
- 37.Petyovka N, Lyach L, Voitenok N N. Homologous ELISA for detection of oligomeric human TNF: properties of assay. J Immunol Methods. 1995;186:161–170. doi: 10.1016/0022-1759(95)00183-b. [DOI] [PubMed] [Google Scholar]
- 38.Plaeger, S., H. Z. Bass, P. Nishanian, J. Thomas, N. Aziz, R. Detels, J. King, W. Cumberland, M. Kemeny, and J. L. Fahey. The prognostic significance of HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 39.Poli G, Fauci A S. Role of cytokines in the pathogenesis of human immunodeficiency virus infection. In: Aggarwal B B, Puri R K, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995. pp. 421–450. [Google Scholar]
- 40.Poole S, Gaines-Das R E. The international standards for IL-1α and IL-1β. Evaluation in an international collaborative study. J Immunol Methods. 1991;142:1–13. doi: 10.1016/0022-1759(91)90286-o. [DOI] [PubMed] [Google Scholar]
- 41.Riches P, Gooding R, Millar B C, Rowbottom A W. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-α concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 42.Romagnani S, Del Prete G, Manetti R, Ravina A, Annunziato F, De Carli M, Mazzetti M, Piccinnì M P, D’Elios M M, Parronchi P, Sampognaro, Maggi E. Role of TH1/TH2 cytokine in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 43.Rubin L A, Kurman C C, Fitz M E, Boutin B. Soluble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–3177. [PubMed] [Google Scholar]
- 44.Salazar-Gonzalez, J. F., O. Martinez-Maza, P. Nishanian, N. Aziz, L.-P. Shen, S. Grosser, J. M. G. Taylor, R. Detels, and J. L. Fahey. Increased immune activation precedes the inflection point of CD4+ T cells and the increased serum viral load in HIV infection. J. Infect. Dis., in press. [DOI] [PubMed]
- 45.Thavasu P W, Longhurst S, Joel S P, Slevin M L, Balkwill F R. Measuring cytokine levels in blood: importance of anticoagulants, processing and storage conditions. J Immunol Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 46.Thorpe R, Wadhwa M, Bird C R, Mire-Sluis A R. Detection and measurement of cytokines. Blood Rev. 1992;6:133–148. doi: 10.1016/0268-960x(92)90025-l. [DOI] [PubMed] [Google Scholar]
- 47.Tsoukas C M, Bernard N F. Markers predicting progression of human immunodeficiency virus-related disease. Clin Microbiol Rev. 1994;7:14–28. doi: 10.1128/cmr.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wachter H, Fuchs D, Hausen A, Reibnegger G, Werner E R. Neopterin as a marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem. 1989;27:81–141. doi: 10.1016/s0065-2423(08)60182-1. [DOI] [PubMed] [Google Scholar]
- 49.Whicher J, Ingham E. Cytokine measurements in body fluids. Eur Cytokine Netw. 1990;1:239–243. [PubMed] [Google Scholar]