Summary
The electrocatalytic behavior of carbon materials is related to the oxygen-containing groups, in that the type of groups determines the activity of carbon materials. Among them, phenanthrenequinone (PQ) moieties functionalized carbon materials are critical species in the catalysis field. Here, we prepare PQ moieties functionalized carbon materials through electrochemical oxidation. We provide the synthesis details of the PQ groups, an approach for the detection of PQ groups, and a performance evaluation for electrocatalytic oxygen evolution reaction (OER) under acidic conditions.
For complete details on the use and execution of this protocol, please refer to Lu et al. (2022b).
Subject areas: Energy, Chemistry, Material sciences
Graphical abstract

Highlights
-
•
Detailed steps for the preparation of PQ moieties functionalized graphite flakes (GPs).
-
•
Safe PQ preparation approach enabling control of oxidation degree
-
•
Electrochemical measurements of PQ-containing GP as an OER anode in acid
-
•
Detailed approach to detect and verify the PQ groups
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The electrocatalytic behavior of carbon materials is related to the oxygen-containing groups, in that the type of groups determines the activity of carbon materials. Among them, phenanthrenequinone (PQ) moieties functionalized carbon materials are critical species in the catalysis field. Here, we prepare PQ moieties functionalized carbon materials through electrochemical oxidation. We provide the synthesis details of the PQ groups, an approach for the detection of PQ groups, and a performance evaluation for electrocatalytic oxygen evolution reaction (OER) under acidic conditions.
Before you begin
Pure graphitic carbon materials are not suitable catalysts for electrocatalysis because of the uniform electron distribution in the sp2 conjugated carbon (Hu and Dai, 2019). In this regard, O doping can well redistribute the valence electron of carbon materials, thereby endowing carbon materials with adjustable electrocatalytic activity (Han et al., 2020; Lei et al., 2020). Quinone-based carbon materials are a class of potentially low-cost, sustainable, and high-energy density electroactive materials with a large specific capacity, high redox reactivity, and excellent electrochemical reversibility (Han et al., 2019). Moreover, the electrochemical properties of quinones can easily be tailored through molecular structure engineering. Thus, quinone moieties functionalized carbon materials and their derivatives have wide application prospects in electrocatalytic water splitting (Lu et al., 2022a), batteries, supercapacitors (Han et al., 2019), electrosynthesis of hydrogen peroxide (Han et al., 2020), and organic synthesis (Wendlandt and Stahl, 2015). However, the traditional preparation methods of quinone-based carbon materials are often complicated, environmentally unfriendly, and uncontrollable (Han et al., 2020). Therefore, the development of a green, non-toxic, simple, and controllable preparation strategy is crucial for facilitating the applications of quinone-based carbon materials. Fortunately, the electrochemical oxidation strategy can meet the above requirements (Gurzęda et al., 2016). Using electrons as oxidants, the degree of oxidation and the types of generated oxygen-containing functional groups can be precisely controlled by the applied potential and oxidation time. Here, the applied potential, duration of oxidation, and the type of electrolyte are crucial for the synthesis of PQ moieties on carbon materials.
Preparation of graphite flakes
Timing: 15 min
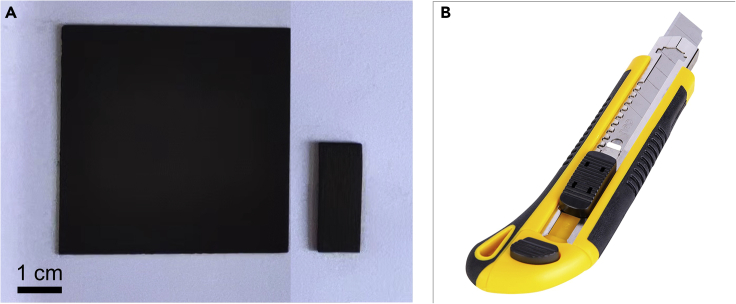
Carefully cut the 5 cm × 5 cm commercial high-purity graphite flakes (GPs) (see Figure 1A) into 2.5 cm × 1 cm pieces with a utility knife (Figure 1B).
CRITICAL: Utility knives are very sharp, so use them with great care.
Note: GPs are too hard to cut with scissors, or they will damage the scissors.
Figure 1.
GPs of different sizes and the tool for cutting GPs
(A) Optical photograph of GPs with the dimension of 5 cm × 5 cm and 2.5 cm × 1 cm.
(B) Utility knife for cutting GPs.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Hydrochloric Acid (36%–38%, GR) | Aladdin | CAS: 7647-01-0 |
| Nitric Acid (65%–68%, AR) | Aladdin | CAS: 7697-37-2 |
| Sulfuric Acid (≥ 98%, GR) | Aladdin | CAS: 7664-93-9 |
| High-purity graphite flakes (Purity > 99.99%) | Beijing Jinglong Special Carbon Technology Co., Ltd. | N/A |
| Acetone (Grade: GR) | Sigma-Aldrich | CAS: 67-64-1 |
| Ethanol (Purity > 99.5%) | Aladdin | CAS: 64-17-5 |
| Deionized Water (Grade: HPLC) | Shanghai Macklin Biochemical Co., Ltd. | CAS: 7732-18-5 |
| Proton Exchange Membrane | DuPont | Nafion 117 |
| He Gas (Purity >99.999%) | Tianjin Boliming Technology Co., Ltd. | N/A |
| Other | ||
| Electrochemical Workstation | CH Instruments, Inc. | CHI 660e |
| Shimadzu GC-2014 gas chromatograph | Shimadzu | N/A |
| Stir Plate | IKA | RCT 5 digital |
| Sonicator | Branson Co., Ltd. | N/A |
| Oil Bath | Zhejiang Lichen Instrument Technology Co., Ltd. | N/A |
| Electronic Analytical Balance | Sartorius Stedim Biotech GmbH | N/A |
| H-type electrolytic cell | Home-made | N/A |
| Magneton | IKA | N/A |
| Vacuum Drying Oven | Shanghai Yiheng Co., Ltd. (Shanghai, China) | N/A |
| Pipette | Thermo Fisher Technology Co., Ltd. | N/A |
CRITICAL: Hydrochloric acid (36%–38%), nitric acid (65%–68%), and sulfuric acid (≥ 98%) are dangerous and should be operated in a fume hood with goggles on. Acetone is toxic and volatile and should be handled in a fume hood with a gas mask on.
Alternatives: We use dilute hydrochloric acid to clean the GPs, and dilute sulfuric acid can also be used to clean the GPs. Commercial high-purity GPs can also be purchased from other manufacturers, as long as the purity is ≥ 99.99%.
Optional: The resources listed above are only based on our experience. The chemicals and resources obtained from other reliable commercial sources are also feasible.
Step-by-step method details
Preparation of clean GPs
Timing: ∼15 h
-
1.Clean the GPs.
-
a.Sonicate the GPs with the size of 1 cm × 2.5 cm in 1 M dilute hydrochloric acid, acetone, ethanol, and deionized (DI) water, respectively, in a glass beaker.
-
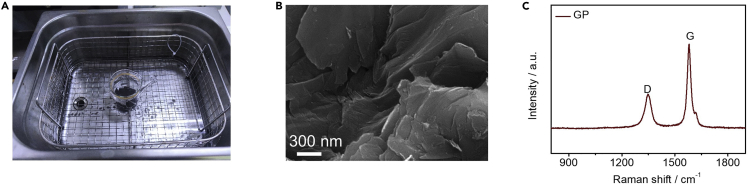
b.Hold sonication in each solution for 40 min to clean the GPs (Figure 2A).
-
c.Dry the freshly-treated GPs in a vacuum oven at 60°C for 12 h.
-
d.Characterize the morphological and structural information of the clean GPs by scanning electron microscopy (SEM) and Raman spectroscopy (Figures 2B and 2C).
-
a.
CRITICAL: Concentrated hydrochloric acid has strong acidity, so attention should be paid to safety during the dilution process, and it should be handled safely and properly in the fume hood.
Alternatives: This step of cleaning can be omitted if ultra-pure highly oriented pyrolytic graphite (HOPG) is used.
Note: Each step of ultrasonic cleaning should be no less than 40 min.
Pause point: If a small laboratory ultrasonic cleaner is used, it is necessary to replace the hot water in the ultrasonic cleaner with cold water after continuous ultrasonication for 15 min, otherwise the hot water would damage the ultrasonic cleaner.
Figure 2.
Cleaning process of GPs and structural information of clean GPs
(A) Optical photograph of GPs in a glass beaker under ultrasonic cleaning.
(B) SEM image of GP.
(C) Raman spectrum of GP.
Preparation of the PQ moieties functionalized GPs
Timing: 36 h
We fabricate the PQ moieties functionalized GPs through a mild electrochemical cyclic voltammetry (CV) oxidation in a standard three-electrode system in 0.5 M H2SO4 with a CHI 660e electrochemical workstation. GPs prepared through this method are abbreviated to MEO-GP hereafter.
Note: 0.5 M H2SO4 solution is prepared as the electrolyte by diluting 27 mL of concentrated sulfuric acid (≥ 98%) with DI water to 1000 mL under continuous stirring.
CRITICAL: It is recommended that operators should wear a self-priming filter gas mask, and wear rubber acid and alkali-resistant clothing and gloves to strictly comply with the operating procedures and operate in the fume hood. In addition, slowly pour the concentrated sulfuric acid into DI water, stir while pouring, and naturally cool to room temperature. Do not pour DI water into concentrated sulfuric acid to avoid boiling and splashing.
Alternatives: 0.5 M H2SO4 as the electrolyte can be replaced with 1 M HClO4 or 1 M HCl.
-
2.
Seal a piece of clean GP with Teflon tape to ensure an exposed area of 1 cm × 1 cm on one side of GP for electrochemical measurement before the CV oxidation.
Note: A proper exposure of GP on the other side is also necessary to reserve the conductivity to connect with an electrode holder. The GP with a working area of 1 cm × 1 cm together with the electrode holder is used as the working electrode (Figure 3A).
Figure 3.
Experimental set-up for electrochemical oxidation
(A) Optical photograph of the working electrode.
(B) Optical photograph of the device for the three-electrode system.
-
3.
Use carbon rod and saturated calomel electrode (SCE) as the counter electrode and the reference electrode, respectively.
Note: Make sure the internal reference solution of KCl in SCE is saturated before each use. Some KCl crystals may be allowed to cope with changes in the saturation of the reference solution.
-
4.
Sweep the CV oxidation between 1 and 1.7 V vs. SCE with a sweep rate of 10 mV s−1. Accordingly, it takes 140 s for one CV cycle. The CV oxidation process lasts for 600 cycles, with a total duration of about 24 h. The electrochemical oxidation device is shown in Figure 3B.
Note: As shown in Figure 4, the oxidation degree of the GPs can be precisely controlled by adjusting the number of CV scans. The reduction peak area of the PQ groups is positively correlated with the number of activation cycles, and the larger the area, the deeper the oxidation degree (See Figure 4F).
Figure 4.
Precise control of the oxidation degree of GP
(A–E) CV scans of GP with different activation cycles at a scan rate of 1 mV s−1.
(F) The PQ groups’ reduction peak areas calculated from (A–E). The reduction peaks used here are labeled with green circles in (B–E).
CRITICAL: In the process of CV oxidation, the scanning rate is very critical and should be controlled to 10 mV s−1.
Alternatives: The MEO-GP can also be synthesized through the chronopotentiometry oxidation of high-purity GP in 0.5 M H2SO4 at a current density of 200 mA cm−2 for an accurate 1.7 h to avoid over-oxidation.
-
5.
Wash the GP after CV oxidation several times with deionized water and dry it in a vacuum drying oven at 60°C for 12 h to obtain MEO-GP.
Verification of the PQ groups
Timing: ∼15 min
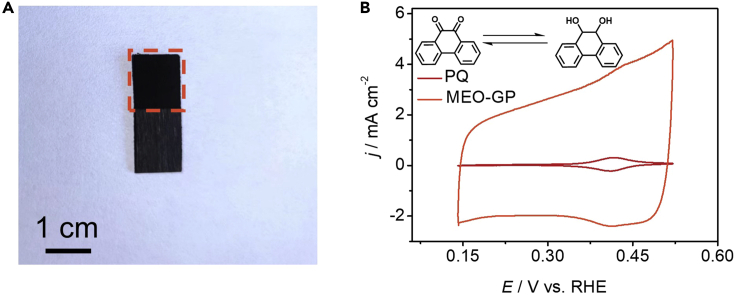
As shown in Figure 5A, the red box marks the working area of the MEO-GP, whose color is darker than that of the unexposed part. Since the quinone groups are sensitive to CV, the CV method is the easiest way to confirm the existence of the PQ groups on MEO-GP (Figure 5B).
-
6.
Measure the standard redox peaks of PQ to be around 0.16 V vs. SCE (0.4 V vs. reversible hydrogen electrode (RHE)), which is acquired through testing pure PQ molecules.
-
7.
Use MEO-GP, carbon rod, and SCE as the working electrode, counter electrode, and reference electrode, respectively.
-
8.
Conduct the CV scan by sweeping between -0.10 and 0.27 V vs. SCE with a scanning rate of 1 mV s−1 in 0.5 M H2SO4.
Note: The potential range for the detection of PQ groups by the CV method is not necessarily from -0.10 to 0.27 V vs. SCE. The potential range can be flexibly expanded or narrowed as long as a couple of characteristic redox peaks of PQ groups are included. The conversion between the RHE and the SCE is shown as follows.
| (Equation 1) |
where Emeasured is the measured potential, and ESCE is the standard electrode potential of SCE (0.241 V at 25°C).
Figure 5.
Morphological and structural information of MEO-GP
(A) Optical photograph of MEO-GP.
(B) CV scans of MEO-GP and PQ in 0.5 M H2SO4 at a scan rate of 1 mV s−1. Reproduced with permission (Lu et al., 2022b); Copyright, 2022 Elsevier Inc.
Measurements of electrocatalytic acidic oxygen evolution reaction (OER) performance of the MEO-GP
Timing: 1 h
In this section, we display the measurements of electrocatalytic acidic OER performance for MEO-GP as the anode via a conventional 3-electrodes configuration (See Figure 6).
-
9.
Prepare 0.5 M H2SO4 solution as the electrolyte.
-
10.
Use MEO-GP, carbon rod, and SCE as the working electrode, the counter electrode, and the reference electrode, respectively.
Note: The exposed area of the MEO-GP should be strictly quantified during testing so that the OER performance can be normalized during data processing.
-
11.
Employ an H-type electrolytic cell (Figure 6), with the working electrode (MEO-GP) and the counter electrode (carbon rod) locate on both sides of the proton exchange membrane, and the reference electrode (SCE) and the working electrode (MEO-GP) on the same side.
CRITICAL: The test requires additional stirring.
Note: The proton exchange membrane should be sequentially treated with 0.5 M H2SO4, 5% H2O2, and DI water in a water bath at 80°C, respectively, to remove the accumulated pollutants from the atmosphere on the membrane surface before use. The treatment for each solution last for 30 min.
-
12.
Measure the electrochemical polarization curves in the anodic direction via the linear sweep voltammetry (LSV) method at a scan rate of 10 mV s−1 in the scan range from 1.0 to 1.7 V vs. SCE.
-
13.
Evaluate the acidic oxygen evolution durability of MEO-GP by chronopotentiometry.
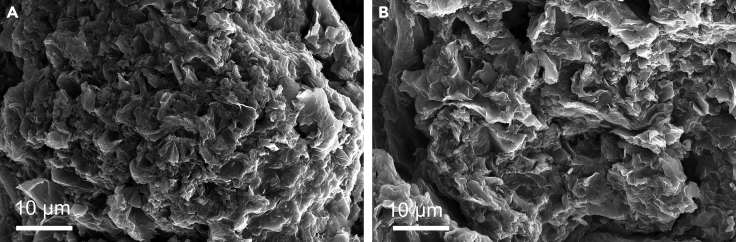
Note: We control the current density to be less than 20 mA cm−2 and monitor the variation of the applied voltage over time. There is little change in the morphology of the MEO-GP before and after the durability test (See Figure 7).
Figure 6.
Electrocatalytic set-up of MEO-GP for acidic OER
(A) Optical photograph of acidic OER set-up with MEO-GP as the anode.
(B) Schematic diagram of water-splitting set-up.
Figure 7.
Morphology of MEO-GP before and after the durability test
(A and B) SEM images of MEO-GP (A) Before and (B) After the durability test.
-
14.
Obtain the electrochemical impedance spectroscopy (EIS) of MEO-GP at the applied potential of 1.35 V vs. SCE from 100,000 to 0.01 Hz at an amplitude of 5 mV.
-
15.
Evaluate the double-layer capacitances (Cdl) of MEO-GP by cycling the CV scans in the region of the non-Faradaic potential from 1.05 to 1.15 V vs. SCE with sweep rates of 40, 60, 80, 100, and 120 mV s−1, respectively.
Note: The CV potential range is not necessarily from 1.05 V to 1.15 V vs. SCE, as long as it is in the non-Faraday region and the absolute values of the maximum positive and negative swept currents are approximately the same.
-
16.Measure the Faradaic efficiency (FE) for MEO-GP toward acidic OER with the three-electrode configuration.
-
a.Detect the generated oxygen by a Shimadzu GC-2014 gas chromatograph (GC) and employ the thermal conductivity detector (TCD) with 5A molecular sieves column. The O2 collection device is shown in Figure 8.
-
b.Seal the chamber containing the working electrode except for the gas inlet and outlet.
-
c.Degas the entire system with He gas for 1 h to make the system free of other gases, and then close the He gas inlet before applying a specific potential.
-
d.React at a specific potential for 60 min.Note: The gases generated from the working electrode will flow through the yellow hose from the chamber to the gas outlet.
-
e.Take the first gaseous sample with a manual GC syringe at the gas sampling position, which is marked with a red circle on the yellow hose in Figure 8.
-
f.Take another two gaseous samples when the reaction time is then extended to 120 min and 180 min.Note: The whole process will take 180 min to obtain three data points.
-
g.Adopt the average of the three data points to eliminate errors.
-
h.Repeat the above steps to obtain the FE of O2 at the other applied potential.Note: The LSV curves, durability test data, and Cdl results of MEO-GP are shown in Figure 9. The EIS and FE results of MEO-GP can be found in Lu et al. (2022b).
 CRITICAL: Although MEO-GP shows excellent performance for acidic OER at a high applied potential of over 1.65 V vs. RHE, the FE of O2 will become lower than 90% due to the competitive oxidation of carbon. Upon this potential, the carbon electrode is drastically oxidized to CO and CO2. Therefore, when MEO-GP is used as the anode for the OER, the applied potential should be ≤ 1.65 V vs. RHE. Additionally, attention should also be paid when carbon electrodes are used as the anodes for other electrocatalysis.
CRITICAL: Although MEO-GP shows excellent performance for acidic OER at a high applied potential of over 1.65 V vs. RHE, the FE of O2 will become lower than 90% due to the competitive oxidation of carbon. Upon this potential, the carbon electrode is drastically oxidized to CO and CO2. Therefore, when MEO-GP is used as the anode for the OER, the applied potential should be ≤ 1.65 V vs. RHE. Additionally, attention should also be paid when carbon electrodes are used as the anodes for other electrocatalysis.
-
a.
Figure 8.
Oxygen collection device with the three-electrode system
Figure 9.
Acidic OER performances of MEO-GP
(A) LSV curves of MEO-GP and RuO2-CP for comparison.
(B) Chronopotentiometric curves of MEO-GP for 320 h and RuO2-CP for 6 h at 20 mA cm−2.
(C) Cdl of MEO-GP. Reproduced with permission (Lu et al., 2022b); Copyright, 2022 Elsevier Inc.
Expected outcomes
This protocol presents a simple and controllable synthesis method for PQ moieties functionalized carbon materials. The prepared MEO-GP rich in PQ groups can be stored in a vacuum environment at room temperature for at least one month. The MEO-GP exhibits outstanding acidic OER performance. The MEO-GP shows a FE of 99% for O2 at 10 mA cm−2 with an overpotential of only 270 mV, which is 70 mV lower than that of the benchmark RuO2 (Figure 9A). Additionally, MEO-GP can continuously operate for at least 320 h with a current density of 20 mA cm−2 and an O2 FE of ∼92% (Figure 9B). MEO-GP also exhibits an electric double-layer capacitance as high as 224 mF cm−2 (Figure 9C), indicating a large catalytically active area of MEO-GP.
Limitations
Although we can purposefully fabricate PQ moieties on the surface of carbon materials, carbon materials containing only one kind of oxygen functional group cannot be obtained by the existing preparation technologies. The prepared MEO-GP will inevitably contain hydroxy, ketone, and epoxy moieties on the surface of the carbon. In addition, since the theoretical potential of carbon oxidation into CO2 is only 0.207 V vs. RHE, carbon will inevitably be oxidized into CO2 and CO when the operation potential is too large. Fortunately, the modification of PQ groups on the surface of carbon materials can not only inhibit the further oxidation of carbon but also accelerate the oxidation of water, so that the FE of O2 can still be maintained as high as 90% when applied potential is lower than 1.65 V vs. RHE (Lu et al., 2022b). Thus, MEO-GP is an excellent water oxidation electrode within certain potential windows under acidic conditions, but not a wise choice under high applied potential.
Troubleshooting
Problem 1
In the fabrication of MEO-GP, by using sulfuric acid as the electrolyte, sulfates are inevitably intercalated in the MEO-GP. The results from temperature-programmed desorption-mass spectrometry (TPD-MS) clearly suggest the existence of sulfates in the as-prepared MEO-GP (Figure 10). And the intercalated sulfate may affect the electrode behaviors in some reactions (steps 2–5).
Figure 10.
Confirmation of the intercalation of sulfates
(A) TPD-MS spectra of GP.
(B) TPD-MS spectra of MEO-GP. The obvious signal of SO2 clearly demonstrates the existence of SO42- in MEO-GP.
Potential solution
Perchloric acid can be substituted for sulfuric acid in the oxidation process. Perchloric acid is inert to many electrocatalytic reactions and therefore does not have a significant effect on electrode behaviors.
Problem 2
When MEO-GP is prepared through mild electrochemical oxidation, only one piece can be prepared at the same time, so the synthesis efficiency is low (step 2).
Potential solution
The synthesis efficiency can be promoted by using a piece of GP with a larger size (such as 5 cm × 5 cm). And the corresponding area of the counter electrode should also be enlarged to about 5 cm × 5 cm to match the area of GP as the working electrode. The production rate can be increased by 25 times then.
Problem 3
The PQ groups always co-exist with the hydroxyl, epoxy, and ketone groups, leading to interference in evaluating the performance of the PQ groups alone (steps 2–5).
Potential solution
We can further increase the electrochemical oxidation potential to destroy the PQ moieties to compare the OER performances between the samples with and without PQ moieties. The sample prepared through harsh electrochemical oxidation (EO-GP) contains the same groups with MEO-GP except for PQ moiety, but performs a poor OER activity. The specific synthesis of EO-GP is executed on a 3-electrode set-up (CHI 660e). A piece of clean GP (0.5 cm × 0.5 cm), carbon rod, and SCE are used as the working electrode, counter electrode, and reference electrode, respectively. The oxidation process lasts for 5.5 h with a constant current density of 1 A cm−2 in 0.5 M H2SO4. Finally, the EO-GP containing only OH, C-O-C, and ketone groups is obtained via flushing with deionized water and drying in a vacuum drying oven at 60°C for 12 h. For more synthesis and the activity comparison with MEO-GP details see Lu et al. (2022b).
Problem 4
The number of PQ groups is highly correlated with the catalytic activity, and it is important to determine the amount of PQ formed per unit area or mass. However, the determination of the number of oxygen-containing functional groups on the surface of carbon materials has always been a great challenge (steps 6–8).
Potential solution
The PQ moieties are highly sensitive to CV scans with a couple of characteristic redox peaks at 0.4 V vs. RHE. We can use the area of the reduction peak to calculate the number of PQ groups. The calculation formula of the number of PQ base on reduction peak area is shown as follows.
| (Equation 2) |
where I is the current, U is the voltage, and e is the charge of an electron (1.602 × 10−19 C). The quantification of PQ moieties is performed with CV at the range of -0.10 to 0.27 V vs. SCE for 3 cycles, and the area of the reduction peak of PQ moieties in the third cycle is used to calculate the amount of PQ moieties.
Problem 5
The air tightness of the oxygen collection device (Figure 8) can seriously interfere with the FE of O2 (step 16).
Potential solution
Before each test, after the device is sealed, all the possible leaky sites are smeared with soapy water. Then the device is purged with He. And soap bubbles will generate at the site with air leakage.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Yanmei Shi (yanmeishi@tju.edu.cn).
Materials availability
This study did not generate any new unique reagents.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21901180 and 21871206).
Author contributions
Conceptualization, Y.S.; methodology, S.L.; investigation, S.L.; formal analysis, S.L.; writing – original draft, S.L.; writing – review & editing, S.L., Y.S., and B.Z.; funding acquisition, B.Z. and Y.S.; resources, B.Z. and Y.S.; supervision, B.Z. and Y.S.
Declaration of interests
The authors declare no competing interests.
Data and code availability
Data and code generated during this study are available from the lead contact upon request.
References
- Gurzęda B., Florczak P., Kempiński M., Peplińska B., Krawczyk P., Jurga S. Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid. Carbon. 2016;100:540–545. doi: 10.1016/j.carbon.2016.01.044. [DOI] [Google Scholar]
- Han C., Li H., Shi R., Zhang T., Tong J., Li J., Li B. Organic quinones towards advanced electrochemical energy storage: recent advances and challenges. J. Mater. Chem. 2019;7:23378–23415. doi: 10.1039/c9ta05252f. [DOI] [Google Scholar]
- Han G.-F., Li F., Zou W., Karamad M., Jeon J.-P., Kim S.-W., Kim S.-J., Bu Y., Fu Z., Lu Y., et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020;11:2209. doi: 10.1038/s41467-020-15782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Dai L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019;31:1804672. doi: 10.1002/adma.201804672. [DOI] [PubMed] [Google Scholar]
- Lei C., Zheng Q., Cheng F., Hou Y., Yang B., Li Z., Wen Z., Lei L., Chai G., Feng X. High-performance metal-free nanosheets array electrocatalyst for oxygen evolution reaction in acid. Adv. Funct. Mater. 2020;30:2003000. doi: 10.1002/adfm.202003000. [DOI] [Google Scholar]
- Lu S., Shi Y., Zhou W., Zhang Z., Wu F., Zhang B. Dissolution of the heteroatom dopants and formation of ortho-quinone moieties in the doped carbon materials during water electrooxidation. J. Am. Chem. Soc. 2022;144:3250–3258. doi: 10.1021/jacs.1c13374. [DOI] [PubMed] [Google Scholar]
- Lu S., Zhou W., Shi Y., Liu C., Yu Y., Zhang B. Phenanthrenequinone-like moiety functionalized carbon for electrocatalytic acidic oxygen evolution. Chem. 2022;8:1415–1426. doi: 10.1016/j.chempr.2022.01.016. [DOI] [Google Scholar]
- Wendlandt A.E., Stahl S.S. Quinone-catalyzed selective oxidation of organic molecules. Angew. Chem. Int. Ed. Engl. 2015;54:14638–14658. doi: 10.1002/anie.201505017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code generated during this study are available from the lead contact upon request.