Abstract
Objectives
Coronavirus 2019 vaccine responses in rare autoimmune rheumatic diseases (RAIRDs) remain poorly understood; in particular there is little known about whether people develop effective T cell responses. We conducted an observational study to evaluate the short-term humoral and cell-mediated T cell response after the second severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in RAIRD patients compared with healthy controls (HCs).
Methods
Blood samples were collected after the second dose and anti-spike, anti-nucleocapsid antibody levels and SARS-CoV-2-specific T cell responses were measured and compared with those of HCs. Activation-induced marker and deep phenotyping assays were used to identify differences in T cells between high and no/low antibody groups, followed by multidimensional clustering.
Results
A total of 50 patients with RAIRDs were included (31 with AAV, 4 with other systemic vasculitis, 9 with SLE and 6 with myositis). The median anti-spike levels were significantly lower in RAIRD patients compared with HCs (P < 0.0001). Fifteen (33%) patients had undetectable levels and 26 (57%) had levels lower than the lowest HC. Rituximab in the last 12 months (P = 0.003) was associated with reduced immunogenicity compared with a longer pre-vaccination period. There was a significant difference in B cell percentages (P = 0.03) and spike-specific CD4+ T cells (P = 0.02) between no/low antibody vs high antibody groups. Patients in the no/low antibody group had a higher percentage of terminally differentiated (exhausted) T cells.
Conclusions
Following two doses, most RAIRD patients have lower antibody levels than the lowest HC and lower anti-spike T cells. RAIRD patients with no/low antibodies have diminished numbers and poor quality of memory T cells that lack proliferative and functional capacities.
Keywords: rare autoimmune rheumatic diseases, SARS-CoV-2, vaccination, antibody, cell mediated, T cells
Rheumatology key messages.
A total of 57% of RAIRD patients had an insufficient antibody response (lower antibody levels than the lowest healthy control) following two vaccine doses.
Patients with low or no antibodies also have significantly lower levels of memory T cells that lack both functional and proliferative capacities.
Assessment of both serological and T cell responses is necessary to fully define responses to vaccination in immunosuppressed populations.
Introduction
The rapid development of vaccines and mass vaccination since the emergence of coronavirus disease 2019 (COVID-19) has helped control the transmission and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although these vaccines have a good efficacy and safety profile in the general population [1, 2], less is known about their effects in immunocompromised patients (ICPs). There is a particular gap in the literature related to people with rare autoimmune rheumatic diseases (RAIRDs) such as systemic vasculitis, who are thought to be at increased risk of severe poor outcomes and mortality from COVID-19 compared with the general population and compared with people with RA and other inflammatory arthritis [3–6]. Successful host protection from vaccination relies on a functional immune system including humoral and cell-mediated responses, which can be diminished in RAIRDs secondary to immunosuppressive therapy [7, 8]. Previous research has identified that high disease activity and high-dose glucocorticoids are associated with an increased risk of severe COVID-19 infection [9, 10]. In particular, rituximab, a monoclonal anti-CD20 B cell–depleting agent, has been shown to increase the severity of infection [11, 12] and the risk of COVID-19-related death [9] and reduce vaccine responsiveness [13]. Additionally, the time since the last rituximab treatment has been shown to impact humoral response, with a 7–9 month period prior to vaccine being the most significant predictor of impaired response [14, 15]. B cell numbers also influence response in rituximab-treated patients, with a minimum of 0.4% of circulating lymphocytes being required for seroconversion [16]. Methotrexate and glucocorticoids have also been shown to diminish immunogenicity of SARS-CoV-2 vaccines [7, 17–19].
The effect of vaccination on cellular immunity in patients with stable disease on long-term immunosuppressive therapy is less well described. A recent study on vasculitis and autoimmune glomerulonephritis patients found T cell responses in >80% of patients even in the absence of serological responses [14]. Another study, which aimed to characterize the phenotype of the T cell response, found a higher proportion of TNF-α-producing CD4 cells in seronegative autoimmune rheumatic disease patients [20]. However, both of these studies did not provide any data on memory T cells. As we know from previous research, memory T cells mediate a faster and more potent response upon repeat encounter with antigens and thereby underpin long-lasting immunity against infection [21]. In addition, some questions remain unanswered, including the short- and medium-term immune response to vaccination and vaccine response in different types of RAIRDs.
To address these research gaps, we conducted a prospective cohort study to evaluate the humoral and cell-mediated response to SARS-CoV-2 vaccination in patients with RAIRDs compared with healthy controls (HCs). Here we present the findings of the short-term response to two doses of SARS-CoV-2 vaccination with a focus on memory T cells, which have not been well described in previous studies.
Methods
Study design and population
We conducted a prospective, single-centre longitudinal cohort study in individuals with RAIRDs recruited from Nottingham University Hospitals NHS Trust in the UK from April to June 2021. Individuals were recruited through outpatient rheumatology and renal clinics either during clinic appointments or via e-mail, letter or telephone between appointments. Eligible individuals were adults ≥18 years of age with a diagnosis of a RAIRD (vasculitis, SLE, myositis, scleroderma and SS) and eligible to receive SARS-CoV-2 vaccinations. People were not eligible if they were <18 years old, ineligible to receive SARS-CoV-2 vaccinations, unable to provide blood samples, unable to travel to the hospital for study visits, unable to consent or had low English proficiency. HCs were invited from a related study and were age- and sex-matched prior to invitation using a 1:1 ratio for comparison [22]. HCs who were invited were matched with the RAIRD group who were invited. More HCs (especially of older ages) did not wish to participate, hence the differences in numbers and ages. Based on previous similar research, a sample size of 50 per group was deemed sufficient to detect any significant differences in responses. A total of 102 RAIRD patients were identified, of whom 29 were ineligible and 21 declined to participate. A total of 52 RAIRD patients participated in the study, of whom 50 gave a blood sample 4 weeks or 3 months after their second vaccine. A total of 34 HCs agreed to participate, of which 2 were excluded, as they were taking immunosuppressants for RA, leaving 32 eligible to participate. All participants provided written informed consent.
Data and sample collection
A baseline questionnaire was administered to collect information on demographics, clinical factors (previous COVID-19 infection and tests), diagnosis, current and/or recent immunosuppressive medications, recent glucocorticoid use and vaccination details. Whole blood samples were collected 4 weeks after the second dose of the COVID-19 vaccine. In cases where the 4 week target could not be met due to appointment unavailability, blood samples were collected 3 months after the second dose (n = 14). Samples were taken at hospital sites and stored in accordance with the Human Tissue Authority and National Health Service guidelines. HCs had the same blood sample collections.
Patient involvement
Patients and members of the public were involved at all stages of the study design and conduct. The study proposal was peer reviewed by people with vasculitis and other RAIRDs and their feedback was incorporated into the study design. Study findings will be disseminated to patients and the public through the Vasculitis UK website and newsletters.
Antibody response
Heparinized whole blood was centrifuged to separate the plasma. Plasma was tested for nucleocapsid- and spike-specific antibodies in two separate ELISAs. Briefly, 384-well Maxisorp (Nunc) assay plates (Thermo Fisher Scientific, UK) were coated with 20 µL/well of 1 µg/mL of either Wuhan strain SARS-CoV-2 full-length spike protein or Wuhan strain SARS-CoV-2 nucleocapsid protein. Plates were sealed, incubated overnight and serially diluted, as per World Health Organization standards. Antibody titres were defined as positive if the value was >10 BAU. An antibody response was defined as sufficient if the IgG level was higher than that of the lowest HC. Further details are provided in the supplementary methods available at Rheumatology online.
T cell response
We examined the percentages of both T and B cells in 10 patients with no/low anti-spike IgG and 10 patients with high anti-spike IgG. Cryopreserved peripheral blood mononuclear cells were thawed and stimulated with SARS CoV-2-derived peptide pools (Supplementary Table S1, available at Rheumatology online). An activation-induced marker assay was used to identify total CD4+ and CD8+ T cells to spike and nucleocapsid and a deep phenotyping assay was used to determine cytokine responses and memory T cells (Supplementary Tables S2 and S3, available at Rheumatology online). Data analysis for flow cytometry was performed using Kaluza version 2.2 (Beckman Coulter, Indianapolis, IN, USA) and further multidimensional clustering analysis (FlowSOM) was then utilized to characterize the major phenotype of cells. Further details are provided in the supplementary methods.
Statistical analysis
Antibody responses were compared between individuals with RAIRDs and HCs using Stata version 14 (StataCorp, College Station, TX, USA). Differences between demographic and clinical characteristics and humoral immunogenicity were tested for significance using the chi-squared test. For outcome variables with low frequencies (<5), we used Fisher’s exact test. All other outcome variables were incorporated into the multivariable logistic regression analysis to determine the influence of RAIRDs on the magnitude of response to the second dose of the vaccine. It has previously been suggested that age, sex and rituximab can influence antibody levels [18, 23, 24] and hence we adjusted for these as a priori confounders during the analysis. A 5% α level was used to determine the significance level. Only patients with complete outcome data were included in the models. Missing data were assumed as missing at random and no imputations were performed.
Study outcomes
The primary outcomes were the antibody and T cell responses to two doses of SARS-CoV-2 vaccination. Secondary outcomes included a comprehensive analysis of T cell activation, cytokine production and generation of memory T cells.
Ethical approval
The study was approved by the West Midlands–Black Country Research Ethics Committee (REC reference 21/WM/0097). The controls were obtained from a related study (REC reference 21/NW/0048).
Results
Patient characteristics
The demographics and clinical characteristics of the patients with RAIRDs (n = 50) and HCs (n = 32) are shown in Table 1. The median age of the RAIRD cohort was 53 years (IQR 42–61). The majority were female [n = 35 (70%)] and White [n = 45 (90%)]. The HC group was also predominantly female [n = 23 (72%)] and White [n = 25 (78%)] and had a median age of 51 years (IQR 42–62). The most common RAIRD was ANCA-associated vasculitis [n = 31 (62%)], followed by SLE [n = 9 (18%)], myositis [n = 6 (12%)] and other systemic vasculitis [n = 4 (8%)]. A total of 17 patients were taking glucocorticoids daily, of which 7 (14%) were on high doses (≥10 mg/day prednisolone equivalent). A total of 22 (44%) patients had rituximab in the 12 months prior to the first vaccination and 40 (80%) patients had a prior history of rituximab. One of these patients was taking a different anti-CD20 drug due to rituximab allergy. A total of 10 (20%) patients were currently taking immunosuppressive medications other than steroids and rituximab. Five (6%) patients had hypogammaglobulinemia (IgG <5.3 g/l) and four of these patients had recently received Ig replacement therapy, thus their data were excluded from the antibody analysis. Half of the RAIRD cohort received the Pfizer-BioNTech vaccine and the other half received the Oxford-AstraZeneca vaccine.
Table 1.
Demographic and clinical characteristics of patients with rare autoimmune rheumatic diseases and healthy age-matched controls
| Characteristics | RAIRD, n (%) (n = 50) | Controls, n (%) (n = 32) |
|---|---|---|
| Age (years) | ||
| Median (range) | 53 (22–81) | 53 (22–79) |
| 18–49 | 20 (40.0) | 14 (43.8) |
| 50–64 | 19 (38.0) | 14 (43.8) |
| ≥65 | 11 (22.0) | 4 (12.5) |
| Gender, n (%) | ||
| Female | 35 (70.0) | 23 (71.9) |
| Male | 15 (30.0) | 9 (28.1) |
| Ethnicity, n (%) | ||
| White | 45 (90.0) | 25 (78.1) |
| Non-white | 5 (10.0) | 7 (21.9) |
| Diagnosis, n (%) | ||
| ANCA-associated vasculitis | 31 (62.0) | |
| SLE | 9 (18.0) | |
| Other systemic vasculitis | 4 (8.0) | |
| Myositis | 6 (12.0) | |
| Current immunosuppression, n (%) | ||
| Methotrexate | 4 (8.0) | |
| Mycophenolate | 4 (8.0) | |
| Belimumab | 2 (4.0) | |
| Previous rituximab | 40 (80.0) | |
| ≤6 months | 17 (34.0) | |
| ≤12 months | 22 (44.0) | |
| Current glucocorticoids, n (%) | ||
| ≥10 mg/day | 7 (14.0) | |
| <10 mg/day | 10 (20.0) | |
| No steroids | 33 (66.0) | |
| Hypogammaglobulinemia, n (%) | 5 (6.0) | |
| Recent immunoglobulin therapya, n (%) | 4 (8.0) | |
| Vaccine type, n (%) | ||
| Oxford-AstraZeneca | 25 (50.0) | 8 (23.5) |
| Pfizer-BioNTech | 25 (50.0) | 26 (76.5) |
Excluded from the analysis on antibody response to vaccination.
Antibody responses
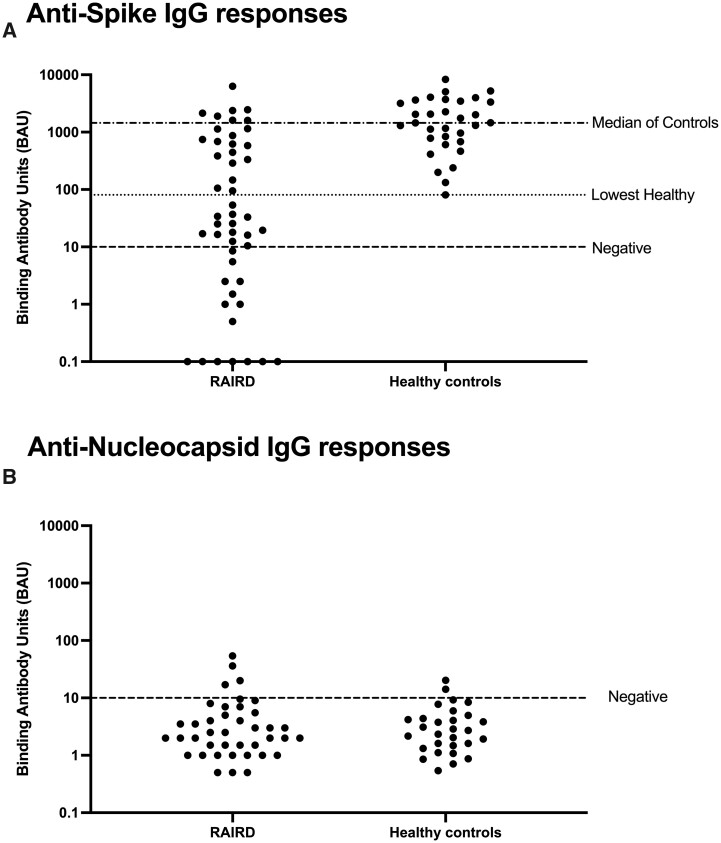
The median anti-spike IgG antibody response was significantly lower in RAIRD patients [median 34 (IQR 3–687)] compared with HCs [median 1453 (IQR 733–3405)] (χ2 = 21.2, P < 0.001). Furthermore, 15 (33%) RAIRD patients had undetectable antibodies (Supplementary Table S4, available at Rheumatology online) and only 20 (43%) patients had a sufficient antibody response (IgG higher than the lowest HC) (Fig. 1a). Both the RAIRD and HC groups had virtually undetectable anti-nucleocapsid IgG responses (Fig. 1b), which is consistent with any previous antibody response to infection no longer being detectable and the responses observed being due to vaccination only. In general, older adults, males, patients with myositis and those on immunosuppressive or steroid treatment were more likely to have insufficient antibody responses (IgG levels lower than the lowest HC) as shown in Table 2. Additionally, none of the patients who had rituximab in the 6 months prior to the first vaccine and only 3 (16%) who had rituximab in the last 12 months had a sufficient antibody response. In the univariate analyses we found a significant inverse correlation between sufficient humoral response (Table 2) and rituximab therapy in the 12 months prior to receiving the first dose of the SARS-CoV-2 vaccine (P = 0.003). There was also a strong association between the diagnosis (ANCA-associated vasculitis, other systemic vasculitis, myositis or SLE) and humoral response, however, this did not reach statistical significance. In the multivariable analyses, rituximab in the last 12 months was associated with an insufficient humoral response [OR 0.11 (95% CI 0.03, 0.48); P = 0.003]. Age, gender and ethnicity did not have an influence on the humoral response, which reached statistical significance.
Figure 1.
IgG responses to SARS-CoV-2 between RAIRD (vasculitis) patients and HCs. (A) Anti-spike IgG antibody responses in RAIRD (vasculitis) patients compared with HCs. The dashed line (negative) represents the cut-off for the assay, the dotted line (lowest healthy) shows the binding antibody units of the lowest HC and the semi-dashed line represents the median of the HCs. (B) Anti-nucleocapsid IgG responses were mainly below the limit of detection of the assay in both RAIRD patients and HCs. The dashed line (negative) represents the cut-off for the assay
Table 2.
Univariate and multivariate logistic regression analyses of sufficient antibody response following the second dose of vaccine in RAIRD patientsa
| Variables | Sufficient antibody responseb, n (%) (n = 20) | Insufficient antibody response, n (%) (n = 26) | Univariate analyses |
Multivariate analyses |
|
|---|---|---|---|---|---|
| OR (95% CI) or two-sided P-value | OR (95% CI) | P-value | |||
| Age (for each additional year) | 0.99 (0.95, 1.03) | 1.00 (0.95, 1.04) | 0.833 | ||
| 18–49 | 8 (50.0) | 8 (50.0) | |||
| 50–64 | 8 (42.1) | 11 (57.9) | |||
| ≥65 | 4 (36.4) | 7 (63.6) | |||
| Gender | |||||
| Female | 15 (46.7) | 17 (53.1) | 1 (reference) | 1 (reference) | |
| Male | 5 (35.7) | 9 (64.3) | 0.63 (0.17, 2.30) | 0.58 (0.14, 2.52) | 0.471 |
| Ethnicity | 0.369c | ||||
| White | 19 (46.3) | 22 (53.7) | |||
| Non-white | 1 (20.0) | 4 (80.0) | |||
| Diagnosis | 0.084c | ||||
| ANCA-associated vasculitis | 13 (46.4) | 15 (53.6) | |||
| SLE | 4 (50.0) | 4 (50.0) | |||
| Other systemic vasculitis | 3 (75.0) | 1 (25.0) | |||
| Myositis | 0 | 6 (100.0) | |||
| Current immunosuppression | |||||
| Yes | 5 (38.5) | 8 (61.5) | 0.75 (0.20, 2.78) | ||
| No | 15 (45.5) | 18 (54.6) | 1 (reference) | ||
| Current glucocorticoids | |||||
| Yes | 7 (41.2) | 10 (58.8) | 0.86 (0.26, 2.90) | ||
| No | 13 (44.8) | 16 (55.2) | 1 (reference) | ||
| Previous rituximab | |||||
| ≤6 months | |||||
| Yes | 0 | 15 (100.0) | Omitted | ||
| No | 20 (64.5) | 11 (35.5) | 1 (reference) | ||
| ≤12 months | |||||
| Yes | 3 (15.8) | 16 (84.2) | 0.11 (0.03, 0.47)d | 0.11 (0.03, 0.48) | 0.003d |
| No | 17 (63.0) | 10 (37.0) | 1 (reference) | 1 (reference) | |
| Rituximab ever | |||||
| Yes | 14 (37.8) | 23 (62.2) | 0.30 (0.65, 1.42) | ||
| No | 6 (66.7) | 3 (33.3) | 1 (reference) | ||
An antibody response was defined as sufficient if IgG levels were above that of the lowest HC.
Patients on Ig therapy were excluded from analysis on antibody response to vaccination.
P-value obtained from two-sided Fisher’s exact test.
Statistically significant P-value.
T and B cell responses in anti-spike antibody high and antibody low RAIRD patients
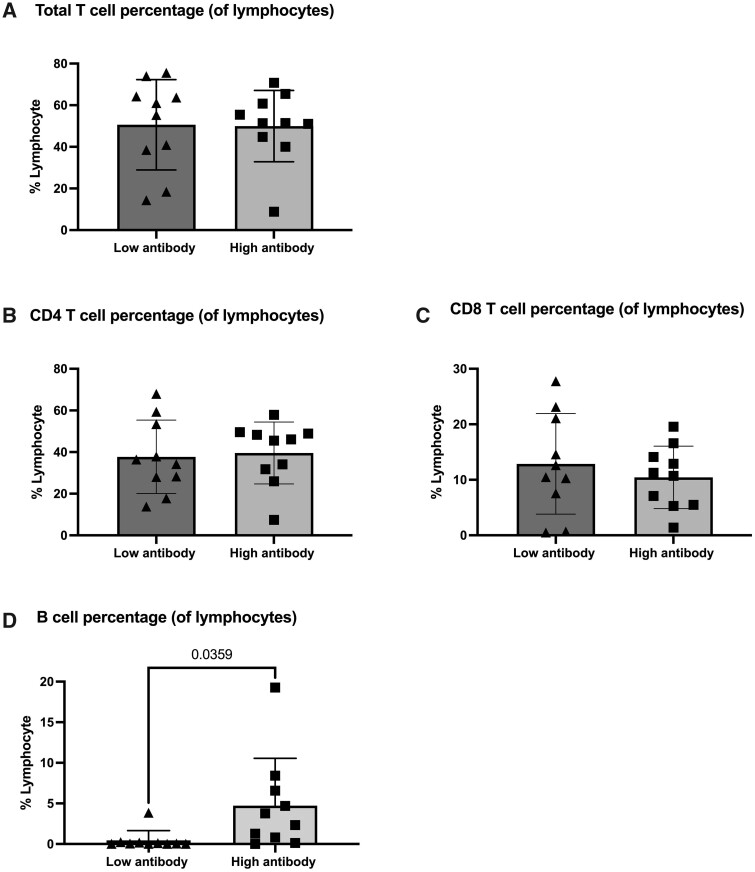
There was no significant difference in either total T cells (Fig. 2a), CD4+ T cells (Fig. 2b) or CD8+ T cells (Fig. 2c), but there was a significant difference in B cell percentages between the antibody no/low and high patients (P = 0.0359; Fig. 2d). Interestingly, two patients in the antibody high group had no detectable B cells at the time of sampling, which is likely because both had treatment with rituximab after their second vaccination and before the blood sample was collected.
Figure 2.
T and B cell responses in anti-spike antibody high and antibody low RAIRD patients. (A) Total percentage of T lymphocytes in anti-spike antibody high and antibody no/low RAIRD patients. (B) CD4 T cell and (C) CD8 T cell percentages of lymphocytes in anti-spike antibody high and antibody no/low RAIRD patients. (D) B cell percentage of lymphocytes was significantly lower in anti-spike antibody no/low RAIRD patients compared with anti-spike antibody high RAIRD patients
T cell responses to spike and nucleocapsid peptides in antibody high and antibody no/low RAIRD patients
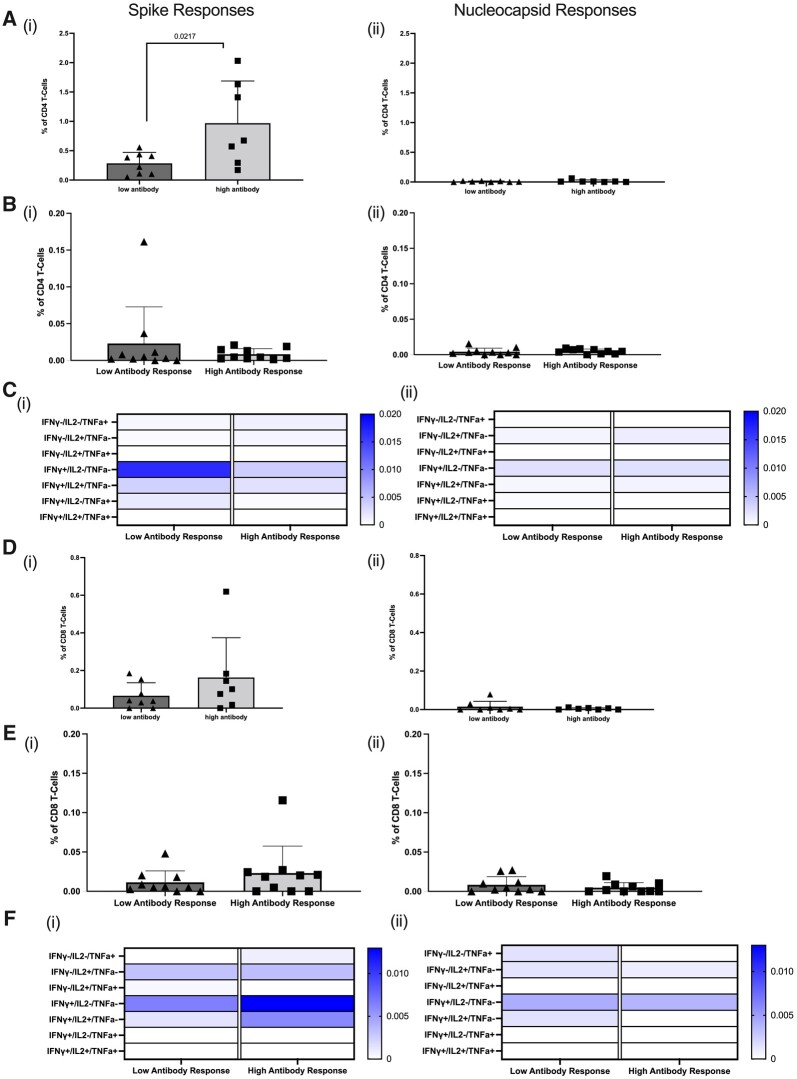
In the antibody high group there were significantly more spike-specific CD4+ T cells than in the antibody no/low group (P = 0.0217; Fig. 3ai). There were no detectable nucleocapsid-specific CD4+ T cells in either group, consistent with no residual response to any prior infection with SARS-CoV-2 if exposed at all (Fig. 3aii). In addition, there were no significant differences in total cytokine-specific CD4+ T cells to spike (Fig. 3bi) between the antibody no/low and antibody high groups and no nucleocapsid-specific cytokine-secreting CD4+ T cells (Fig. 3bii). Analysis of cytokine patterns in spike-specific CD4+ T cells showed this was mainly IFN-γ for both the antibody no/low and antibody high group, with some cells also producing TNF-α in combination with IFN-γ in the no/low group and TNF-α alone in the antibody high group (Fig. 3ci). There was no detectable cytokine production by CD4+ T cells in response to nucleocapsid (Fig. 3cii).
Figure 3.
CD4+ and CD8+ T cell responses to SARS-CoV-2 peptides in antibody high and antibody no/low RAIRD patients. (A) Spike-specific CD4+ T cells were significantly higher in the antibody high patients and there were no detectable nucleocapsid-specific CD4+ T cells. (B) No difference in total cytokine-positive spike-specific CD4+ T cells. (C) Cytokine patterns expressed by spike and nucleocapsid-specific CD4+ T cells. (D) Higher spike-specific CD8+ T cells in antibody high patients. (E) No difference in total cytokine-positive spike-specific CD8+ T cells. (F) Cytokine patterns expressed by spike and nucleocapsid-specific CD8+ T cells
We then examined CD8+ T cell responses and found no significant differences between the antibody high and antibody no/low groups for spike-specific CD8+ T cells (Fig. 3di and ei). Furthermore, there were very minimal CD8+ T cell responses to nucleocapsid (Fig. 3dii and eii). Analysis of cytokine patterns by spike-specific CD8+ T cells highlighted cytokine production in the antibody no/low group was primarily IL-2, whereas IFN-γ was the predominant cytokine in the antibody high group (Fig. 3fi). There was minimal cytokine production in response to nucleocapsid (Fig. 3fii).
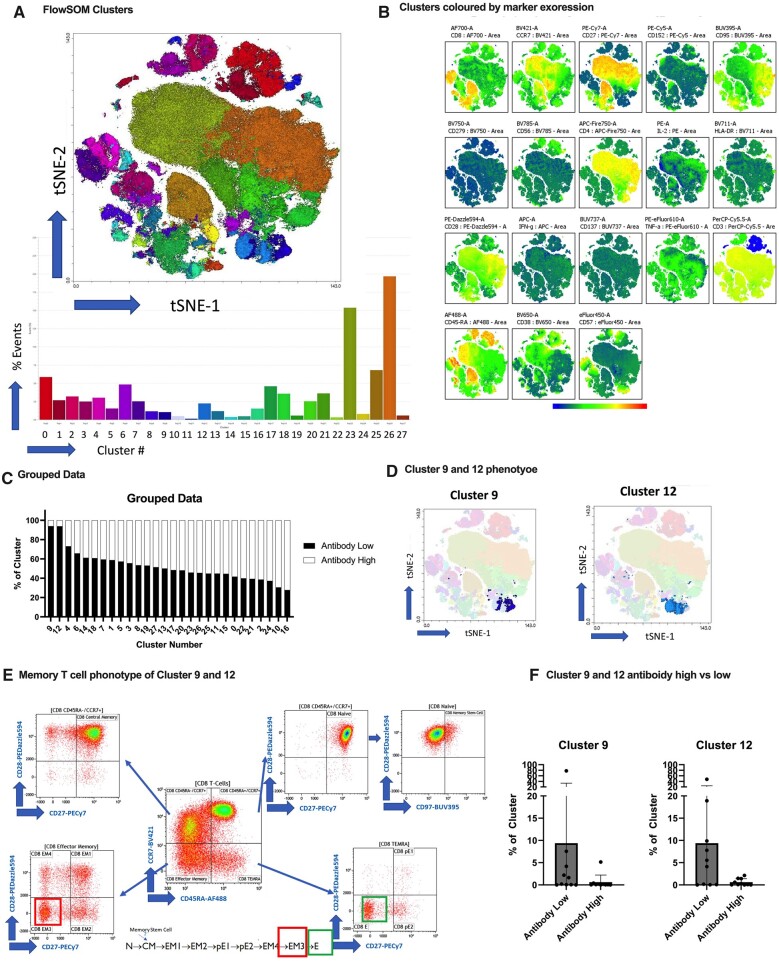
The multidimensional clustering analysis using t-distributed stochastic neighbour embedding (t-SNE) showed a total of 27 clusters with different expression markers (Fig. 4a and b). Although not statistically significant, there was a higher percentage of clusters 9 and 12 in the no/low antibody group compared with the antibody high group (Fig. 4c, d and f). These clusters are associated with two terminally differentiated populations of T cells: effector memory 3 (EM3) and effector memory T cells re-expressing CD45RA (TEMRA) (Fig. 4e).
Figure 4.
Multidimensional clustering analysis. (A) FlowSOM identified 27 clusters of major cell types from both antibody high and antibody no/low groups, which are colour-coded and displayed on a two-dimensional t-SNE plot. (B) t-SNE visualization coloured according to marker expression. (C–E) Clusters 9 and 12 were identified in the antibody no/low group and were associated with two terminally differentiated populations of T cells: EM3 and TEMRA. (F) No significant differences for clusters 9 and 12 were observed between the antibody no/low and antibody high group
Discussion
Our study highlights several important findings about the immunological effects of COVID-19 vaccination in patients with RAIRDs. We found that that antibody responses were completely undetectable in 33% of RAIRD patients and insufficient in a further 24% of RAIRD patients after two doses of SARS-CoV-2 vaccine compared with HCs. Additionally, there were no statistically significant differences in antibody response between different types of RAIRDs. However, we had small numbers, and it is notable that none of the six people with myositis had a sufficient antibody response (IgG levels greater than the lowest HC). Interestingly, five of these six (83%) individuals had a prior history of rituximab treatment, so we hypothesize that this may have contributed in part to their diminished response.
Corticosteroids are commonly used in the treatment of RAIRDs, particularly during relapses. Our results suggest that concurrent use of corticosteroids does not significantly affect humoral response in RAIRDs. However, it is important to highlight that only seven RAIRD patients were on ≥10 mg steroids/day, so our sample may have been too small to make a firm conclusion. Previous studies have revealed conflicting evidence about the role of corticosteroids in immunogenicity. Observational studies have identified a decrease in serological response to pneumococcal and hepatitis vaccines with long-term steroid use [25, 26]. The effect of corticosteroids on COVID-19 vaccines has not been thoroughly investigated. A recent study suggested that short-term use of low-dose steroids may not hinder antibody responses to COVID-19 vaccination [27]. However, this study was restricted to healthcare workers who did not have any significant comorbidities.
With the data from our cohort, we were able to demonstrate that antibody responses were significantly lower in RAIRD patients compared with HCs. This is similar to a recent Dutch study on patients with immune-mediated inflammatory disorders on concurrent immunosuppression. The authors identified that patients receiving rituximab, mycophenolate mofetil combination treatments and sphingosine 1-phosphate receptor modulators had lower rates of seroconversion following the second vaccine. These rates did improve after the third vaccine for all groups except rituximab [28]. Our results also showed that antibody response was diminished in patients receiving rituximab (anti-CD20) and the interval between the administration of rituximab and vaccination was critical in predicting response. We found that all patients who received rituximab in the last 6 months prior to the first dose had insufficient antibody responses and 89% of those who had rituximab in the 12 months prior to the first vaccination had insufficient antibody responses. These findings correlate with previous studies that also demonstrate the negative impact of B cell–depleting therapies on response to vaccines [7, 11, 24, 29–32]. Conversely, some studies have shown that even individuals with low numbers of B cells secondary to rituximab treatment were able to mount a significant antibody response to COVID-19 vaccination provided T cell–mediated immunity was intact [33, 34]. Recent literature has suggested that to maximize response, the optimal timing for vaccination in rituximab-treated patients should be at least 9 months after the last infusion [33, 35]. However, our study shows that even patients who had been treated with rituximab in the 12 months prior to the first vaccination did not mount a sufficient response.
Cellular immune responses are essential in providing long-lasting immunity and underpin vaccine efficacy. Most current vaccines rely on the delivery of spike protein, and as a consequence the generation of spike-specific T cell response, in order to maintain immune memory after antibodies have waned [36]. Previous studies have shown that in healthy individuals, two doses of vaccine are sufficient to generate a T cell response similar to that after natural infection [37, 38]. However, our study revealed that RAIRD patients with no/low antibody response had significantly fewer spike-specific CD4+ T cells, which are essential in coordinating and regulating antiviral immunity. Our results are in line with a recent study in kidney transplant recipients on immunosuppression, which also found a weak T cell response and positive SARS-CoV-2 antibodies in only 5–10% of patients following the first and second vaccine doses [39, 40]. However, this serological response improved to 36% after administration of the third dose [41]. This augmented response suggests that repeated booster strategies could provide more long-term immunity in ICPs and warrants further research.
Our study also brings to light new findings about the function of memory T cells in people with RAIRDs. We observed the importance of an IFN-γ-predominant CD8+ T cell response in RAIRD patients with high antibodies in coordinating the adaptive immune system. We also noted that this response was lower and predominantly IL-2-related in patients with no/low antibodies. This suggests that while CD8+ T cells may be activated, the main effector cytokines for sustaining the antiviral response are not produced in RAIRD patients in the absence of antibodies. We also observed increased levels of clusters 9 and 12 encoding for EM3 (CD27−CD28−) and TEMRA in RAIRD patients with no/low antibodies. These cells lack expression of CD27 and CD28, suggesting immunosenescence and incompetence to vaccination [42, 43] and therefore increased susceptibility and greater probability of more severe SARS-CoV-2 infection.
The strengths of this study include the broad inclusion criteria, patients with a variety of RAIRD diagnoses and the use of age-matched HCs, which increases the generalisability of our findings. In addition, we evaluated both the humoral and cellular responses to two doses of SARS-CoV-2 vaccines. The limitations of this study include a small sample size and demographic differences between the RAIRD and control groups, some of which were adjusted for during our analysis. Furthermore, 14 patients were unable to have a blood sample taken 4 weeks post-vaccination, and in these cases we were only able to analyse their 3 month post-vaccination sample (however, in patients with both samples, we found no significant differences in the titres of antibodies). Additionally, some of the outcome variables had low frequencies and hence we could not adjust for these as potential confounders in our multivariate analysis.
In summary, we identified that patients with RAIRDs have significantly diminished antibody and T cell responses following two doses of SARS-CoV-2 vaccines. Receipt of rituximab in the last 12 months was associated with a reduced humoral response, so, where possible, vaccination should precede treatment with rituximab, as per clinical guidance. This also justifies the need for the additional booster vaccine doses, in line with national guidelines [44], and emphasizes the importance of assessing B and T cell responses in ICPs. We also recommend that for individuals requiring maintenance rituximab, shared decision making and risk assessments should be conducted by clinicians to review the timing of rituximab with future booster doses. It also raises questions about whether additional prophylactic measures such as antivirals may be required in addition to booster vaccine doses in individuals who do not mount a sufficient antibody response. Notably, some patients with no/low antibody response also have poor memory T cells that lack both proliferative and functional capacities and so future research is important to determine the long-term immune response to additional vaccine doses.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Contributor Information
Leher Gumber, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Nancy Gomez, School of Life Sciences, University of Nottingham, Nottingham, UK.
Georgina Hopkins, School of Life Sciences, University of Nottingham, Nottingham, UK.
Davis Tucis, School of Life Sciences, University of Nottingham, Nottingham, UK.
Laura Bartlett, School of Life Sciences, University of Nottingham, Nottingham, UK.
Kieran Ayling, School of Medicine, University of Nottingham, Nottingham, UK.
Kavita Vedhara, School of Medicine, University of Nottingham, Nottingham, UK.
Graham Steers, School of Life Sciences, University of Nottingham, Nottingham, UK.
Mithun Chakravorty, Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Megan Rutter, Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK; Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK.
Hannah Jackson, School of Life Sciences, University of Nottingham, Nottingham, UK.
Patrick Tighe, School of Life Sciences, University of Nottingham, Nottingham, UK.
Alastair Ferraro, Department of Nephrology, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Sheila Power, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Marie-Josèphe Pradère, Nottingham University Hospitals NHS Trust, Nottingham, UK.
David Onion, School of Life Sciences, University of Nottingham, Nottingham, UK.
Peter C Lanyon, Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK; Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre, Nottingham, UK.
Fiona A Pearce, Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK; Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK; NIHR Nottingham Biomedical Research Centre, Nottingham, UK.
Lucy Fairclough, School of Life Sciences, University of Nottingham, Nottingham, UK.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributions
PL, FP and LF conceived this research and revised the manuscript. LG wrote the manuscript. NG, GH, DT and LB processed the blood samples and ran the cell mediated assays. KA and KV provided the healthy control samples. GS provided help with the assays. AF, MC and MR identified patients to invite to the study. HJ and PT ran the antibody assays. SP and M-J P recruited RAIRD patients and collected blood samples. DO supported the flow cytometry. All authors approved the final manuscript.
Funding
This study was funded by Vasculitis UK. M.R., a Versus Arthritis Clinical Research Fellow, is funded by Versus Arthritis (22727). F.A.P., a National Institute for Health and Care Research (NIHR) Advanced Fellow, was funded by the NIHR (NIHR300863) for this research project. The ID7000C spectral cell analyzer was funded by the Biotechnology and Biological Sciences Research Council to L.F. and D.O. (BBSRC) (BB/T017619/1). The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
Disclosure statement: F.A.P. and P.C.L. are recipients of an investigator-led research award from Vifor Pharma for another project unrelated to COVID-19 or vaccination.
References
- 1. Polack FP, Thomas SJ, Kitchin N, C4591001 Clinical Trial Group et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pablos JL, Galindo M, Carmona L, RIER investigators group et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020;79:1544–9. [DOI] [PubMed] [Google Scholar]
- 4. Akiyama S, Hamdeh S, Micic D. et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021;80:384–91. [DOI] [PubMed] [Google Scholar]
- 5. Peach E, Rutter M, Lanyon P. et al. Risk of death among people with rare autoimmune diseases compared with the general population in England during the 2020 COVID-19 pandemic. Rheumatology 2021;60:1902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freites Nuñez DD, Leon L, Mucientes A. et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:1393–9. [DOI] [PubMed] [Google Scholar]
- 7. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 8. Fagni F, Simon D, Tascilar K. et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol 2021;3:e724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strangfeld A, Schäfer M, Gianfrancesco MA, COVID-19 Global Rheumatology Alliance et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felten R, Duret P-M, Bauer E. et al. B-cell targeted therapy is associated with severe COVID-19 among patients with inflammatory arthritides: a 1-year multicentre study in 1116 successive patients receiving intravenous biologics. Ann Rheum Dis 2022;81:143–5. [DOI] [PubMed] [Google Scholar]
- 11. Mehta P, Porter JC, Chambers RC. et al. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol 2020;2:e589–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulze-Koops H, Krueger K, Vallbracht I. et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2021;80:e67. [DOI] [PubMed] [Google Scholar]
- 13. Shields AM, Venkatachalam S, Paneesha S. et al. Vaccine efficacy after rituximab exposure: first interim analysis of virtue project on behalf of West Midlands Research Consortium, UK. Blood 2021;138:196. [Google Scholar]
- 14. Prendecki M, Clarke C, Edwards H. et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moor MB, Suter-Riniker F, Horn MP. et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stefanski A-L, Rincon-Arevalo H, Schrezenmeier E. et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. Arthritis Rheumatol 2022;74:934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braun-Moscovici Y, Kaplan M, Braun M. et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 18. Simon D, Tascilar K, Schmidt K. et al. Humoral and cellular immune responses to SARS-CoV‐2 infection and vaccination in autoimmune disease patients with B cell depletion. Arthritis Rheumatol 2022;74:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geisen UM, Berner DK, Tran F. et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szebeni GJ, Gémes N, Honfi D. et al. Humoral and cellular immunogenicity and safety of five different SARS-CoV-2 vaccines in patients with autoimmune rheumatic and musculoskeletal diseases in remission or with low disease activity and in healthy controls: a single center study. Front Immunol 2022;13:846248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tungland B. Role of gut microbiota in immune homeostasis. In: Human microbiota in health and disease: From pathogenesis to therapy. London: Elsevier, 2018:135–54. [Google Scholar]
- 22. Vedhara K. Do behaviours & mood affect how well COVID19 vaccines work? [COVID-19]. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/do-behaviours-mood-affect-how-well-covid19-vaccines-work-covid-19/ (accessed 10 May 2022).
- 23. Walsh EE, Frenck RW, Falsey AR. et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bingham CO, Looney RJ, Deodhar A. et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010;62:64–74. [DOI] [PubMed] [Google Scholar]
- 25. Yıldız N, Sever L, Kasapçopur Ö. et al. Hepatitis B virus vaccination in children with steroid sensitive nephrotic syndrome: immunogenicity and safety? Vaccine 2013;31:3309–12. [DOI] [PubMed] [Google Scholar]
- 26. Fischer L, Gerstel PF, Poncet A. et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases – a longitudinal study. Arthritis Res Ther 2015;17:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang J, Ko J-H, Baek JY. et al. Effects of short-term corticosteroid use on reactogenicity and immunogenicity of the first dose of ChAdOx1 nCoV-19 vaccine. Front Immunol 2021;12:744206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wieske L, van Dam KPJ, Steenhuis M, T2B! Immunity against SARS-CoV-2 study group et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol 2022;4:e338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hua C, Barnetche T, Combe B. et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 30. Rondaan C, Furer V, Heijstek MW. et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open 2019;5:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Assen S, Holvast A, Benne CA. et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
- 32. Kearns P, Siebert S, Willicombe M. et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE Trial. SSRN Electron J Published Online First 2021. [Google Scholar]
- 33. Jyssum I, Kared H, Tran TT. et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. >Mrak D, Tobudic S, Koblischke M. et al. SARS-CoV-2 vaccination in rituximab-treated patients: b cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. [DOI] [PubMed] [Google Scholar]
- 35. Bitoun S, Henry J, Desjardins D. et al. Rituximab impairs B cell response but not T cell response to COVID‐19 vaccine in autoimmune diseases. Arthritis Rheumatol 2022;74:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tregoning JS, Flight KE, Higham SL. et al. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021;21:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skelly DT, Harding AC, Gilbert-Jaramillo J, C-MORE/PHOSP-C Group et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun 2021;12:5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022;23:186–193. [DOI] [PubMed] [Google Scholar]
- 39. Benotmane I, Gautier-Vargas G, Cognard N. et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 2021;99:1487–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chavarot N, Ouedrani A, Marion O. et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation 2021;105:e94–5. [DOI] [PubMed] [Google Scholar]
- 41. Schrezenmeier E, Rincon-Arevalo H, Stefanski A-L. et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol 2021;32:3027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romero P, Zippelius A, Kurth I. et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 2007;178:4112–9. [DOI] [PubMed] [Google Scholar]
- 43. Mojumdar K, Vajpayee M, Chauhan NK. et al. Altered T cell differentiation associated with loss of CD27 and CD28 in HIV infected Indian individuals. Cytometry B Clin Cytom 2012;82:43–53. [DOI] [PubMed] [Google Scholar]
- 44. Department of Health and Social Care. Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination. 2021. https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (accessed 4 May 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.