Abstract
We investigated effects of the severe acute respiratory syndrome coronavirus 2 (SARV-CoV-2) booster vaccination on human immunodeficiency virus (HIV) reservoir size, immune markers, and host immune responses in people with HIV receiving antiretroviral therapy. Our data suggest that the SARS-CoV-2 booster vaccine is not likely to replenish the persistent HIV reservoir nor provide an immunologic environment to facilitate active HIV expression/replication.
Keywords: booster vaccine, HIV, HIV reservoirs, immune markers and responses, SARS-CoV-2
The clinical outcomes of coronavirus diseases 2019 (COVID-19) in people with human immunodeficiency virus (PWH) are variable. Early data involving small sample sizes showed no increased risk [1], but a recent meta-analysis demonstrated an increased risk of COVID-19 mortality in PWH [2]. Nonetheless, administration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines elicits strong immunogenicity in PWH with minimal adverse effects [3]. Despite both human immunodeficiency virus (HIV) and SARS-CoV-2 being positive-sense, single-stranded ribonucleic acid (RNA) viruses, one major difference between these 2 viruses is that most individuals infected with SARS-CoV-2 are able to clear the virus, whereas HIV persists in PWH despite years of antiretroviral therapy (ART) that allows near complete virologic suppression and control. In this regard, the persistent HIV reservoir in the CD4+ T-cell compartment of the vast majority of PWH receiving ART is one of the major impediments to the eradication of the virus [4]. Previous studies have shown that vaccination against common pathogens, such as influenza, may modulate immunologic and virologic parameters in PWH receiving ART [5]. Given that one of the proposed mechanisms of HIV persistence is antigen-mediated clonal expansion and homeostatic proliferation of an existing pool of CD4+ T cells carrying intact HIV provirus, it is of interest to investigate whether the SARV-CoV-2 booster vaccination modulates immunologic and virologic parameters in PWH receiving ART.
In the present study, we set out to investigate the effects of the SARV-CoV-2 booster vaccine on HIV reservoir size, immune markers, and host immune responses to HIV and SARS-CoV-2 in a cohort of HIV-positive individuals receiving ART after the SARS-CoV-2 booster vaccination.
METHODS
Study Participants
Our study cohort comprised 9 PWH who had previously received a 2-dose series of either the Moderna or Pfizer-BioNTech mRNA-based SARS-CoV-2 vaccines. Subsequently, the study participants received their homologous booster dose between November 2021 and January 2022 and had blood drawn 2–4 weeks prior and 10–14 days postvaccination. Given the booster was administered in the same interval to all 9 study participants, we chose this time frame to study the effect of the SARS-CoV-2 vaccination on HIV reservoirs and immune parameters.
Patient Consent Statement
All participants provided written informed consent. Blood was collected in accordance with protocols approved by the Institutional Review Board of the National Institutes of Health.
Measurements of Antibody Response
Plasma levels of total immunoglobulin (Ig) against SARS-Co-V2 were determined using the ProcartaPlex Human Coronavirus Ig Total Panel 11-Plex assay (Thermo Fisher Scientific) and the xMAP INTELLIFLEX system (Luminex) according to the manufacturers’ instructions.
Quantitation of Human Immunodeficiency Virus Reservoirs
The dynamics of viral reservoirs carrying total HIV deoxyribonucleic acid (DNA), intact HIV proviral DNA, and cell-associated HIV RNA in the CD4+ T cells of study participants was assessed as described in Supplementary Data.
Examination of Immune Parameters
Peripheral blood mononuclear cells were isolated from blood by Ficoll-Hypaque density gradient centrifugation. Expression of surface markers for immune activation and exhaustion was assessed by flow cytometry (Supplementary Data). Data were acquired on an Aurora cytometer using the SpectroFlo software (Cytek Biosciences) and analyzed using FlowJo version 10.7.1.
Immune Responses to Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2
Frequencies of polyfunctional (IFN-γ+TNF-α+MIP-1β+) HIV Gag-specific CD8+ T cells were determined by the intracellular cytokine staining assay (see Supplementary Data). Levels of SARS-CoV-2-specific CD4+ T cells were determined by the activation induced marker (AIM) assay (see Supplementary Data). Data were acquired on an Aurora cytometer using the SpectroFlo Software (Cytek Biosciences) and analyzed using FlowJo version 10.7.1.
Statistical Analysis
Statistical significance was determined by the 2-sided Wilcoxon matched-pairs, signed-rank test using Prism 9.3.1 (GraphPad).
RESULTS
We examined the impact of the SARS-CoV-2 booster vaccine on HIV reservoirs and immune parameters. Six participants received Moderna mRNA-1273 and 3 participants received Pfizer/BioNTech BNT162b2 COVID-19 vaccines for their first and second doses and booster shot (Table 1 and Figure 1A). Of note, 1 study participant (07) had detectable plasma viremia (108 copies/mL) before receiving the booster shot (Table 1) despite sustained virologic suppression (<40 copies/mL) before participating in the current study.
Table 1.
Characteristics of Study Participants
| Participant | Age | Gender | Race | SARS-CoV-2 Vaccine | Plasma Viremia (Copies/mL) | CD4+ T-Cell Count (Cells/mm3) | CD8+ T-Cell Count (Cells/mm3) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Preboost | Postboost | Preboost | Postboost | Preboost | Postboost | |||||
| 1 | 55 | Male | White | Moderna | <40 | <40 | 860 | 800 | 1131 | 860 |
| 2 | 27 | Female | Hispanic | Moderna | <40 | <40 | 1526 | 1323 | 604 | 540 |
| 3 | 43 | Male | Hispanic | Moderna | <40 | <40 | NA | 949 | NA | 401 |
| 4 | 40 | Male | Hispanic | Pfizer-BioNTech | <40 | <40 | NA | 597 | NA | 522 |
| 5 | 49 | Male | Hispanic | Pfizer-BioNTech | <40 | <40 | 443 | 655 | 335 | 384 |
| 6 | 53 | Male | Black | Moderna | <40 | <40 | 503 | 576 | 444 | 484 |
| 7 | 49 | Male | Hispanic | Moderna | 108 | <40 | 617 | 902 | 341 | 442 |
| 8 | 34 | Male | Hispanic | Moderna | <40 | <40 | 852 | 785 | 710 | 981 |
| 9 | 31 | Male | Mixed | Pfizer-BioNTech | <40 | <40 | 307 | 383 | 613 | 689 |
Abbreviations: NA, not available; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
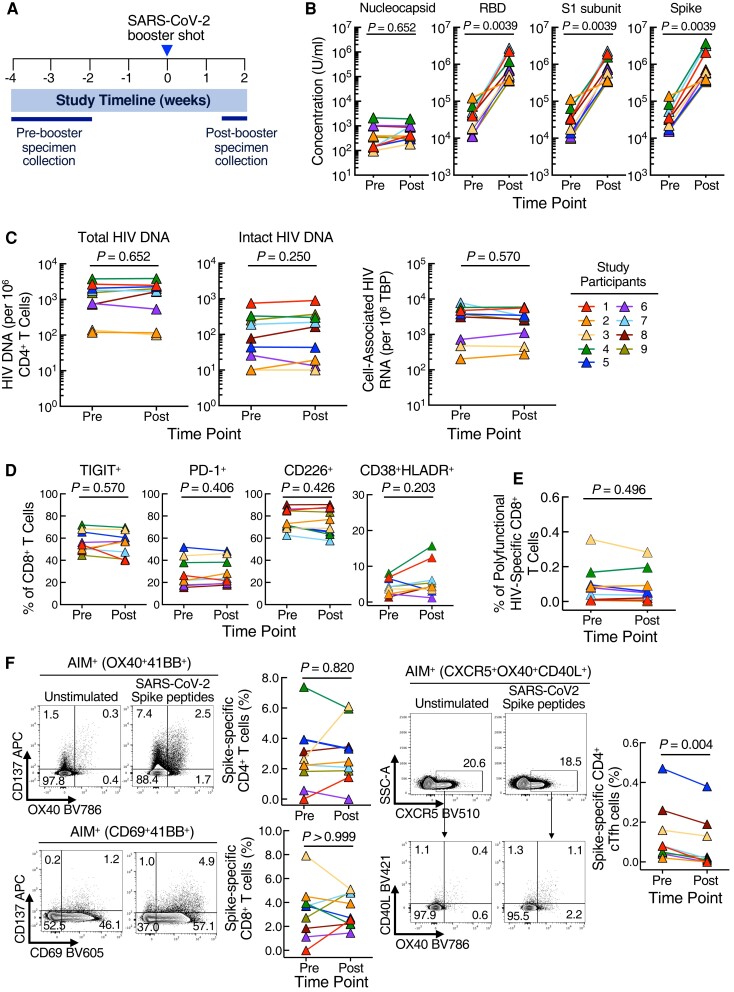
Figure 1.
Dynamics of immunologic and virologic parameters in study participants before and 10–14 days after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) booster vaccination. (A) Study schema. (B) Comparison of antibody titers against SARS-CoV-2 nucleocapsid protein, receptor-binding domain (RBD), S1 subunit, and Spike protein. (C) Levels of total human immunodeficiency virus (HIV) In this deoxyribonucleic acid (DNA), intact HIV proviral DNA, and cell-associated HIV ribonucleic acid (RNA) before and after SARS-CoV-2 booster vaccination. (D) Frequencies of TIGIT, PD-1, CD226, and CD38HLA-DR on CD8+ T cells before and after SARS-CoV-2 booster vaccination. (E) Effect of the SARS-CoV-2 booster vaccination on the frequency of IFN-γ+TNF-α+MIP-1β+ HIV Gag-specific CD8+ T cells in study participants. (F) Frequencies of spike-specific CD4+ and CD8+ T cells (left panel) and spike-specific CD4+ cTfh cells (right panel) before and after SARS-CoV-2 booster vaccination.
We first assessed antibody responses (Ig) to the SARS-CoV-2 booster vaccination in the study participants. As expected, the level of plasma antibodies to the nucleocapsid protein did not change after the booster vaccine (P = .65). However, the levels of plasma antibodies to the receptor-binding domain, S1 subunit, and Spike protein increased significantly (P = .003) after the administration of the booster vaccine (Figure 1B).
Previous studies have demonstrated that routine vaccination against common pathogens can modulate HIV reservoirs and phenotypic immune markers [5]. To this end, we evaluated the effect of the SARS-CoV-2 booster vaccine on the frequency of CD4+ T cells carrying total HIV DNA, intact HIV proviral DNA, and cell-associated HIV RNA. As shown in Figure 1C, no significant differences were found in the levels of these 3 viral parameters 10–14 days after the booster vaccination.
To investigate the impact of the booster vaccine on immune parameters, we performed high-dimensional flow cytometric analyses on T cells of the study participants. Intensities and frequencies of TIGIT, PD-1, CD226, and CD38/HLA-DR on CD8+ T cells remained unchanged after the booster vaccination (Figure 1D).
Finally, we evaluated T-cell responses to HIV and SARS-CoV-2 in the study participants after the booster vaccination. Frequencies of polyfunctional (IFN-γ+TNF-α+MIP-1β+) HIV Gag-specific CD8+ T cells remained unchanged between pre- and postboost time points (Figure 1E). We performed an AIM assay to measure SARS-CoV-2 Spike-specific CD4+ and CD8+ T cells after the booster vaccination (Figure 1F). No significant differences were found in the frequencies of the total Spike-specific CD4+ (P = .82) and CD8+ T cells (P > .99); however, Spike-specific circulating CD4+ T follicular helper (cTfh) cells declined after the booster vaccination (P = .004).
DISCUSSION
The persistence of HIV in the CD4+ T cells of PWH receiving ART is a formidable obstacle to the eradication of the virus and/or achieving sustained virologic remission in the absence of antiretroviral drugs [4]. Although precise mechanisms of HIV persistence remain to be fully delineated, it has been shown that antigenic stimulation that leads to clonal expansion of a preexisting pool of CD4+ T cells carrying intact HIV proviral DNA could be responsible for its longevity in PWH despite years of clinically effective ART. In this regard, it has been demonstrated that routine vaccination against common pathogens, such as influenza, could modulate the degree and extent of viral expression/production in HIV-infected CD4+ T cells in vivo [5]. Recent studies on SARS-CoV-2 outcomes in PWH have shown variable findings, possibly due to multiple factors including age, race, CD4+ T-cell counts, antiretroviral drug regimens, and vaccination status [1, 6, 7]. Nonetheless, SARS-CoV-2 vaccination in PWH has been shown to be safe and effective [8, 9]. Given that the mRNA-based SARS-CoV-2 vaccines induce robust immune responses [3], we set out to investigate whether these vaccines could alter the dynamics of immunologic and virologic parameters in PWH who are receiving ART. Given that the booster vaccine was administered in the same interval to all 9 study participants, we chose this time frame to study the effects of the SARS-CoV-2 vaccination on immune and HIV reservoirs.
The antibody response to the booster vaccine was robust in all but 1 study participant. Of note, Participant 2, whose antibody responses remained largely unchanged after the booster vaccination, had been previously diagnosed with SARS-CoV-2 and had higher initial antibody levels before the vaccination. A more modest antibody response to SARS-CoV-2 booster vaccination in people previously infected with SARS-CoV-2 is also consistent with recent findings [10]. In contrast to the antibody response and reports of HIV-uninfected individuals [11], we did not observe a booster vaccine-induced increase in SARS-CoV-2-specific CD4+ and CD8+ T cells. Levels of T-cell surface markers and HIV-specific CD8+ T cells also remained unchanged after the booster vaccination. However, the frequency of SARS-CoV-2 Spike-specific CD4+ cTfh cells declined postboost. The explanation for the lack of or reduced T-cell responses to the booster vaccine is not clear and needs to be further investigated. Previous reports of 2 doses of SARS-CoV-2 mRNA vaccines in PWH have been shown to induce Spike-specific T-cell responses [12], thus it may be that repeated SARS-CoV-2 vaccination may have contributed to the unresponsiveness of these cells. Considering the relatively mild T-cell responses to SARS-CoV-2 after the booster vaccination, it is not surprising that the overall size of persistent HIV reservoir did not change over time in our study participants.
CONCLUSIONS
One of the major caveats of this study was that we could not perform HIV reservoir and immunologic analyses after the first and second doses of the SARS-CoV-2 vaccination. We were unable to bring study participants to our clinic due to the pandemic-associated restrictions imposed by the National Institutes of Health. Other caveats include a small sample size, again in part associated with pandemic restrictions, and the lack of female participants in our study. Despite these shortcomings, our data suggest that the SARS-CoV-2 booster vaccine is not likely to replenish the persistent HIV reservoir nor provide an immunologic environment that may facilitate active HIV expression/replication in PWH receiving ART.
Supplementary Material
Acknowledgments
We are grateful to the study volunteers for their participation in this study. We thank the National Institute of Allergy and Infectious Diseases HIV Outpatient Clinic staff for their assistance in the execution of this study.
Financial support. This work was funded by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
M Ali Rai, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Victoria Shi, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Brooke D Kennedy, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jesse S Justement, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Kathleen Gittens, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Genevieve McCormack, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jana Blazkova, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Susan Moir, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Tae-Wook Chun, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Sigel K, Swartz T, Golden E, et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis 2020; 71:2933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mellor MM, Bast AC, Jones NR, et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS 2021; 35:F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021; 21:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chun TW, Moir S, Fauci AS. HIV Reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 2015; 16:584–9. [DOI] [PubMed] [Google Scholar]
- 5. Gunthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis 2000; 181:522–31. [DOI] [PubMed] [Google Scholar]
- 6. Boffito M, Waters L. More evidence for worse COVID-19 outcomes in people with HIV. Lancet HIV 2021; 8:e661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyerowitz EA, Kim AY, Ard KL, et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS 2020; 34:1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35:2399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goel RR, Painter MM, Lundgreen KA, et al. Efficient recall of omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022; 185:1875–87.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS-CoV-2 omicron variant is preserved in most but not all individuals. Cell 2022; 185:1041–51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alrubayyi A, Gea-Mallorqui E, Touizer E, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun 2021; 12:5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.