Abstract
White-tailed deer participate in the maintenance of the Ixodes tick life cycle and are reservoirs for some tick-borne infectious agents. Deer may be useful sentinels for tick-transmitted agents, such as ehrlichiae. In order to determine whether white-tailed deer are markers of natural transmission or are reservoirs for the human granulocytic ehrlichiosis (HGE) agent, we performed indirect immunofluorescent-antibody (IFA) tests and immunoblotting with the HGE agent and Ehrlichia chaffeensis on sera from 43 and 294 deer captured in northwest Wisconsin during 1994 and 1995, respectively, and 12 deer from southern Maryland. According to IFA testing, 47% of 1994 Wisconsin sera, 60% of 1995 Wisconsin sera, and 25% of Maryland sera contained HGE agent antibodies. All IFA-positive deer sera tested reacted with the 44-kDa band which is unique to the Ehrlichia phagocytophila group. Serologic reactions to E. chaffeensis were detected by IFA testing in 15 of 337 (4%) Wisconsin deer and in 10 of 12 (83%) Maryland deer, while 60 and 80% of E. chaffeensis IFA-positive Wisconsin and Maryland deer sera, respectively, reacted with the E. chaffeensis 28- to 29-kDa antigens by immunoblotting. A total of 4% of deer from Wisconsin and 25% of deer from Maryland were found by IFA testing to have antibodies to both the HGE agent and E. chaffeensis; 75% of these were confirmed to contain E. chaffeensis antibodies by immunoblotting. These results suggest that white-tailed deer in diverse geographical regions of the United States are naturally infected with the HGE agent, E. chaffeensis, or both and that these animals, and potentially humans, are exposed to infected ticks at a high frequency in nature.
Human granulocytic ehrlichiosis (HGE), first described in the upper midwest United States (3), is a newly emerging, tick-borne disease found with increasing frequency in at least eleven U.S. states and in several European countries (23). In the eastern United States, nymphal-stage Ixodes scapularis ticks are known to be vectors for transmission of the HGE agent (19, 21). Transmission from nymphal-stage Ixodes ticks occurs predominantly during the summer months of May through July, a period which coincides with the seasonal distribution of the majority of cases of HGE (4). If infected adult I. scapularis ticks feed on large mammals, such as deer, these mammals may serve as sentinels for regions where there is a high risk for transmission (5).
Deer participate in the maintenance of the tick life cycle as hosts for adult stages, but their role as reservoirs is controversial. White-tailed deer (Odocoileus virginianus) are often found to be infected with Ehrlichia species and are a proven reservoir for Ehrlichia chaffeensis (16). Recently, Dawson et al. described the presence of novel Ehrlichia species 16S rRNA gene sequences in the blood of white-tailed deer with E. chaffeensis antibodies and interpreted the findings as evidence of infection with a new uncultured species (6). The presence of a high rate of natural infection in deer by such Ehrlichia species is problematic when indirect immunofluorescent antibody (IFA) tests are used, owing to serologic cross-reactivity among tick-transmitted Ehrlichia species. Therefore, the use of immunoblots that employ specific HGE agent or E. chaffeensis antigens can be useful in identifying the infecting species (7, 24).
In order to assess whether deer may become naturally infected by the HGE agent or E. chaffeensis and act as markers of natural transmission or as reservoirs of infection, we performed IFA tests and immunoblots on white-tailed deer from northwest Wisconsin and Maryland.
MATERIALS AND METHODS
Sample collection.
Blood was obtained from the peritoneal cavities of 43 deer shot during the 1994 fall hunting season and from 294 deer during the 1995 fall hunting season in northwestern Wisconsin. The 1994 hunt season deer sera were collected at one checkpoint site in Washburn County, and the 1995 hunt season deer sera were collected in six counties of northwestern Wisconsin, including Barron, Bayfield, Burnett, Douglas, Sawyer, and Washburn Counties, that have a high population density of I. scapularis ticks and reported cases of HGE. The sera were separated from clotted blood and stored frozen at −20°C until used. Sera from 12 southwestern Maryland deer, collected in Charles County in 1992 to 1993, were provided courtesy of Abdu F. Azad, University of Maryland School of Medicine.
IFA testing.
Serum samples from white-tailed deer were tested for either Ehrlichia equi or HGE agent antibodies and for E. chaffeensis antibodies with the IFA test (7). E. equi MRK or the HGE agent Webster strain cultivated in HL60 cells (11) and E. chaffeensis (Arkansas strain; courtesy of J. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.) cultivated in DH82 cells were used as antigens. Briefly, HL60 and DH82 cells that were approximately 90 to 100% infected with either E. equi or the HGE agent and E. chaffeensis, respectively, were centrifuged at low speed and reconstituted in 0.1 M phosphate-buffered saline (PBS) with 2% fetal bovine serum and 0.05% sodium azide solution. The optimal cell concentration was determined empirically, and cells were applied to 12-well Teflon-coated slides, air dried, fixed in acetone, and stored at −70°C until used. The sera were diluted 1:80 in PBS with 0.5% non-fat dry milk (PBSM) and incubated with the antigen for 1 h at room temperature in a humidified chamber. After being washed with PBS, rinsed in deionized water, and air dried, the secondary antibody, fluorescein isothiocyanate-labeled rabbit anti-deer immunoglobulin G (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) diluted 1:50 in PBSM, was incubated with each antigen well for 1 h at room temperature in a humidified chamber. The slides were then incubated in PBS with 0.005% Evans blue for 5 min, washed, and air dried as described above. Slides were then covered with PBS-glycerol mounting medium and examined by epifluorescence microscopy for the presence of fluorescent intracellular aggregates with the morphology of ehrlichial morulae. A previously identified positive and a negative control serum were used with each run. All serum samples that were positive at a 1:80 dilution were titrated until typical fluorescent ehrlichial morula morphology was no longer detected. Sera still reactive in dilutions of 1:2,560 were not further titrated.
Western blotting.
All of the IFA-positive deer sera (titer ≥80) were assayed by immunoblotting with Renografin density gradient-purified HGE agent (Webster strain) and E. chaffeensis (Arkansas strain) as the antigens (2). Uninfected HL60 and DH82 cell lysates were used as negative control antigens. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot preparation and staining were performed as previously described (2). Alkaline phosphatase-labeled rabbit anti-deer immunoglobulin G, (Kirkegaard and Perry Laboratories) diluted 1:100 in 1% PBSM and 1% normal rabbit serum, was used as a secondary antibody.
Minimal criteria for interpreting antibodies as belonging to the Ehrlichia phagocytophila group and E. chaffeensis by immunoblotting were bands at 44 kDa for the HGE agent Webster strain antigen and at 28 to 29 kDa for the E. chaffeensis Arkansas strain antigen, respectively. The precise localization of these bands was confirmed by comparing the 44-kDa antigen detected with a monoclonal antibody specific for the E. phagocytophila group 44-kDa antigen (unpublished data) and with monoclonal antibody 1A9 (courtesy of Didier Raoult, Marseille, France) and monoclonal antibody 3C7 (courtesy of Dave Walker, Galveston, Tex.), which react with 22-, 28-, and 29-kDa proteins of E. chaffeensis.
RESULTS
Antibody detection: 1994 hunt season, Wisconsin.
Of 43 white-tailed deer sera collected in Washburn County, Wis., from the 1994 hunt season, 20 (47%) were found to contain antibodies against E. equi by IFA testing and 9 (21%) contained antibodies reactive with E. chaffeensis antigen (Table 1). Of the 20 E. equi-positive sera, seven (35%) were found to have antibodies against E. chaffeensis; thus, seven of the nine (78%) E. chaffeensis antibody-positive sera also had antibodies reactive with E. equi (Table 1). Of these seven dually positive sera, three had a fourfold or higher difference in titer for E. equi antibodies compared to E. chaffeensis antibodies, and the remaining four had only a twofold difference in titer. Titers of the 20 E. equi-positive sera ranged from 80 to 1,280 with 80% of the titers being greater than or equal to 160 (geometric mean titer [GMT], 279), and titers of the nine E. chaffeensis-positive sera ranged from 80 to 320 with 78% of the titers being greater than or equal to 160 (GMT, 160).
TABLE 1.
IFA test results for deer sera from Wisconsin and Maryland
| Yr and location of sample (no. tested) | No. (%) positive for:
|
||
|---|---|---|---|
| E. equi-HGE agent | E. chaffeensis | Both | |
| 1994, Wisconsin (43) | 20 (47) | 9 (21) | 7 (16) |
| 1995, Wisconsin (294) | 176 (60) | 6 (2) | 6 (2) |
| 1992–1993, Maryland (12) | 3 (25) | 10 (83) | 3 (25) |
1995 hunt season, Wisconsin.
Of 294 white-tailed deer sera collected from the 1995 hunt season in northwest Wisconsin, 176 (60%) contained antibodies against HGE agent antigen and 6 (2%) were reactive with E. chaffeensis antigen (Table 1). All six of the E. chaffeensis-positive sera also had antibodies reactive with the HGE agent (Table 1). Of these six dually positive sera, one had a sixfold-higher titer for HGE agent antibodies than E. chaffeensis antibodies, and the remaining five had either the same titer or only a twofold difference in titer for HGE agent and E. chaffeensis antibodies. The range of titers for these HGE agent IFA-positive sera was from 80 to 2,560 with 168 of the 176 (95%) positive sera having titers greater than or equal to 160 (GMT, 314), and the range of titers for the six E. chaffeensis-positive sera was from 160 to 320 (GMT, 320).
1992 to 1993, Maryland.
Of the 12 white-tailed deer sera collected from southern Maryland, three (25%) were reactive with E. equi antigen and 10 (83%) reacted with E. chaffeensis antigen (Table 1). All three of the E. equi-positive sera were also reactive with E. chaffeensis (Table 1). Of these three dually positive sera, one had a fourfold-higher titer for E. chaffeensis antibodies than E. equi antibodies, and the remaining two had either the same titer or only a twofold difference in titer for E. chaffeensis and E. equi antibodies. Titers of the E. equi-reactive deer sera were 80, 160, and 1,280 (GMT, 254), and the titers of the E. chaffeensis-reactive sera ranged from 160 to 1,280 (GMT, 343).
Western blotting.
Immunoblot results for Wisconsin and Maryland deer sera are shown in Table 2. All 20 E. equi IFA-positive 1994 hunt season white-tailed deer sera and all three E. equi IFA-positive Maryland deer sera reacted with a 44-kDa HGE agent protein by immunoblotting. A total of 23 randomly selected HGE agent IFA-positive 1995 hunt season white-tailed deer sera with a wide range of titers were tested by immunoblotting, and all sera were found to react with the 44-kDa HGE agent protein. Three of the nine E. chaffeensis IFA-positive 1994 Wisconsin deer sera and all six of the E. chaffeensis IFA-positive 1995 Wisconsin deer sera reacted with the 28- to 29-kDa E. chaffeensis proteins; all sera with reactions to the E. chaffeensis 28- to 29-kDa antigen also reacted with the 44-kDa HGE agent antigen. Overall, 3 of 43 (7%) and 6 of 294 (2%) Wisconsin deer sera obtained in 1994 and 1995, respectively, contained antibodies reactive with the 28- to 29-kDa E. chaffeensis antigens.
TABLE 2.
Immunoblot results on deer sera from Wisconsin and Maryland
| Yr and location of sample | No. positive/no. tested (%)
|
||
|---|---|---|---|
| HGE agent (44-kDa band) | E. chaffeensis (28–29-kDa band) | Both | |
| 1994, Wisconsin | 20/20 (100) | 3/9 (33) | 3/7 (43) |
| 1995, Wisconsin | 23/23 (100) | 6/6 (100) | 6/6 (100) |
| 1992–1993, Maryland | 3/3 (100) | 8/10 (80) | 3/3 (100) |
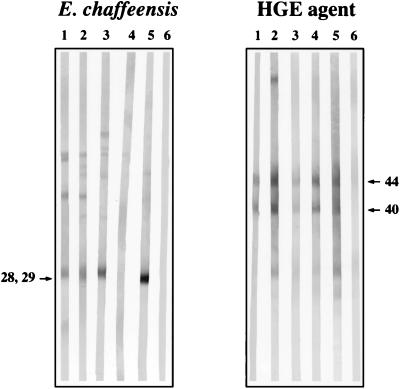
Of 10 E. chaffeensis IFA-reactive Maryland deer sera, eight reacted with the 28- to 29-kDa E. chaffeensis proteins. All three of the Maryland deer sera that were IFA positive for both E. equi-HGE agent and E. chaffeensis reacted with both the 44-kDa protein of the HGE agent and the 28- to 29-kDa proteins of E. chaffeensis. None of the IFA-positive deer sera reacted with HL60 or DH82 antigen. Representative results for deer sera reactive with HGE agent and E. chaffeensis antigens by immunoblotting are shown in Fig. 1.
FIG. 1.
Immunoblotting to differentiate E. chaffeensis and HGE agent antibodies in deer sera. (Left panel) Lane 1, Maryland sample 8 (E. chaffeensis IFA positive only); lane 2, Maryland sample 10 (E. chaffeensis and HGE agent IFA positive); lane 3, Wisconsin sample C8 (E. chaffeensis and HGE agent IFA positive); lane 4, Wisconsin sample SP10 (E. chaffeensis and HGE agent IFA-negative control); lane 5, mouse anti-E. chaffeensis monoclonal antibody 1A9; lane 6, normal mouse serum. (Right panel) Lane 1, Wisconsin sample C9 (HGE agent IFA positive only); lane 2, Wisconsin sample C43 (HGE agent IFA positive only); lane 3, Wisconsin sample C8 (E. chaffeensis and HGE agent IFA positive); lane 4, Maryland sample 10 (E. chaffeensis and HGE agent IFA positive); lane 5, Wisconsin sample SP15 (HGE agent IFA-positive control); lane 6, Wisconsin sample SP10 (E. chaffeensis and HGE agent IFA-negative control). Numbers beside the gels are molecular sizes of the diagnostically significant 28- to 29-kDa antigen of E. chaffeensis and 44-kDa antigen of the HGE agent.
DISCUSSION
HGE occurs frequently in Wisconsin and the upper Midwest (4), and cases have also been reported from the northeastern United States and California (1, 10, 22). In contrast, human monocytic ehrlichiosis (HME) occurs predominantly in the south central, southeastern, and mid-Atlantic regions of the United States. The northern states have an abundance of I. scapularis ticks that transmit not only the HGE agent but also the causative agents of Lyme disease and babesiosis (8, 20), while the southern regions have an abundance of Amblyomma americanum ticks that transmit E. chaffeensis. White-tailed deer play a role in the emergence of Lyme disease and HME, in part by hosting the adult reproductive stages of I. scapularis ticks and all life stages of A. americanum. Areas in the northeast United States with high population densities of deer are correlated with the frequent occurrence of Lyme disease (8), and clear associations between E. chaffeensis-infected white-tailed deer and the presence of A. americanum ticks have been recognized in the southern United States (17). From these observations, it can be speculated that white-tailed deer may be useful sentinels that reflect the frequency of natural transmission of these tick-borne diseases. The high seropositivity rate detected for HGE agent antibodies in deer from Wisconsin (58%) and Maryland (25%) and the high seropositivity rate detected for E. chaffeensis antibodies in deer from Maryland (83%) suggest that a high degree of human risk for acquiring these tick-borne illnesses exists in these same regions.
Serologic studies used to determine infection rate can be confounded, since the HGE agent and E. chaffeensis both infect white-tailed deer and each species may induce serologic cross-reactions (4, 23, 24). In addition, white-tailed deer are frequently found to be infected by the newly described white-tailed deer ehrlichia that may also contribute to serologic cross-reactivity (6, 15). A more definitive identification of the infecting agent might be achieved by the use of specific immunoblots that would circumvent problems with IFA serologic cross-reactivity (2, 7, 12, 24). Here, we show that all of the deer with antibodies to the HGE agent detected by IFA testing and also tested by immunoblotting have antibodies reactive with an E. phagocytophila genogroup-specific antigen. These results confirm a high rate of infection with the HGE agent among wild white-tailed deer in Wisconsin as well as in Maryland.
Of deer sera from Maryland and Wisconsin that were found to have antibodies reactive with E. chaffeensis by IFA testing, 80 and 60%, respectively, were also positive by immunoblotting. These results confirm a high E. chaffeensis infection rate for white-tailed deer in Maryland and suggest that a low level of E. chaffeensis transmission occurs in Wisconsin. A higher proportion of sera obtained in 1994 contained E. chaffeensis antibodies according to IFA testing than the cohort obtained in 1995. The percentage of those with antibodies to the 28- to 29-kDa E. chaffeensis antigens was low overall in both years but significantly different (7 versus 2%; P < 0.003, χ2 test). The reasons for this difference are not known but may relate to environmental factors, differences in geographic location, and differences in the local tick populations. It is interesting that all of the 1994 deer were captured in Washburn County, a relatively rural region where HGE is highly endemic but A. americanum ticks have not been found. Because both the IFA and immunoblot tests were reactive with these sera, it is unlikely to represent a technical abnormality.
Interestingly, 9 of 337 (2.7%) deer from Wisconsin and 3 of 12 (25%) deer from Maryland were found by immunoblotting to have specific antibodies that confirm prior infection with both the HGE agent and E. chaffeensis. This finding could also result from infection by either an unidentified Ehrlichia species or the white-tailed deer ehrlichia, since this species has not been cultivated and its antigens have not been investigated. The presence of antibodies that react with both E. chaffeensis and the HGE agent in deer could be anticipated in regions where both Ehrlichia species and appropriate tick vectors exist, since deer are often hosts to numerous ticks. Thus, immunoblots that can identify species-specific antibodies are potentially useful tools for differentiating among infecting Ehrlichia species, but additional study is still required to assess specificity in well-defined populations with known infections.
Adult I. scapularis ticks are believed to feed primarily on larger mammals, such as the white-tailed deer, and less frequently on medium-sized and smaller mammals (9). The feeding cycle for adult-stage I. scapularis ticks begins in mid-October and continues until April (8). Serologic evidence of infection with the HGE agent in white-tailed deer during the fall of 1994 and 1995 supports the hypothesis that a high degree of transmission from adult-stage Ixodes ticks is occurring and is in agreement with the work of Belongia et al. and Dawson et al. (5, 6), who have found deer naturally infected with the HGE agent. However, the percentage of seropositive deer in the study of Belongia et al. is much lower than the percentage that we report here (5). This discrepancy may be due to differences in technique or sensitivity and specificity of antigen used or may be the result of differences in areas where samples were collected. All of our samples were collected in six counties in northwestern Wisconsin that are known to have a high population density of I. scapularis ticks and are also areas with a high incidence of HGE in humans (4), whereas samples in the study of Belongia et al. were collected from 22 different counties within Wisconsin.
Although white-tailed deer are a known reservoir for E. chaffeensis, transmission and occurrence of HME is also determined by the geographical distribution of A. americanum ticks (17). The confirmation of a high percentage of E. chaffeensis-infected deer in Maryland, where A. americanum ticks are abundant, supports this premise. Additionally, a high percentage of HGE agent-infected deer in areas of Wisconsin with high population densities of I. scapularis ticks and a lower percentage of HGE agent-infected deer in a region of Maryland where I. scapularis ticks are not as abundant support this finding. The discovery of a low percentage of E. chaffeensis-infected deer in Wisconsin, where A. americanum ticks are not found, is somewhat surprising; however, the presence of Dermacentor variabilis ticks that may be competent for transmission of E. chaffeensis could explain this finding.
The potential contribution of white-tailed deer as reservoirs for the HGE agent is controversial, and it has been suggested that the major reservoir for the HGE agent and Borrelia burgdorferi in the eastern United States is the white-footed mouse, Peromyscus leucopus (14, 21). This hypothesis would predict that natural maintenance of the HGE agent would be similar to that of B. burgdorferi and would rely solely upon a small-mammal–tick cycle. Levin and Fish have shown that the difference in the percentage of HGE agent infection between nymphal- and adult-stage I. scapularis ticks is significantly more pronounced than for B. burgdorferi, suggesting the contribution of an alternate reservoir host on which nymphal-stage I. scapularis ticks would feed and acquire the infectious agent (13). Ruminants become persistently but subclinically infected by E. phagocytophila organisms, and it is believed that wild deer may be an important reservoir in Europe (18). Thus, definitive evidence of white-tailed deer as reservoirs for the HGE agent will depend upon experimental transmission studies and the demonstration that immature stages of I. scapularis ticks effectively obtain blood meals from deer with reasonable frequency in nature.
Serologic evidence of naturally infected white-tailed deer in areas where Ixodes species or A. americanum ticks that transmit the HGE agent and E. chaffeensis, respectively, are endemic suggests that deer may be used as markers of natural transmission. Therefore, a high incidence of naturally infected deer in areas with a dense population of ticks would put humans living in these regions at a high risk of acquiring HGE or HME. By examining deer, wild rodent, and tick populations, it may be possible to predict which geographical areas present the greatest threat to humans. A clearer understanding of the complexities of perpetuation and transmission of the agent of HGE would allow for improved strategies for control and prevention of HGE in humans who reside in or venture into tick-infested areas.
ACKNOWLEDGMENTS
This work was supported in part by grant 050-9938-7203 from the Duluth Clinic Foundation and by National Institutes of Health grant R01 AI41213-01.
REFERENCES
- 1.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Asanovich K M, Bakken J S, Madigan J E, Aguero-Rosenfeld M, Wormser G P, Dumler J S. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J Infect Dis. 1997;176:1029–1034. doi: 10.1086/516529. [DOI] [PubMed] [Google Scholar]
- 3.Bakken J S, Dumler J S, Chen S-M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 4.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 5.Belongia E A, Reed K D, Mitchell P D, Kolbert C P, Persing D H, Gill J S, Kazmierczak J J. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–1468. doi: 10.1128/jcm.35.6.1465-1468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson J, Warner C, Baker V, Ewing S, Stallknecht D, Davidson W, Kocan A, Lockhart J, Olson J. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus) J Parasitol. 1996;82:52–58. [PubMed] [Google Scholar]
- 7.Dumler J S, Asanovich K M, Bakken J S, Richter P, Kimsey R, Madigan J E. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic ehrlichia. J Clin Microbiol. 1995;33:1098–1103. doi: 10.1128/jcm.33.5.1098-1103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish, D. 1995. Environmental risk and prevention of Lyme disease. Am. J. Med. 8(Suppl. 4A):2S–9S. [DOI] [PubMed]
- 9.Fish D, Dowler R C. Host associations of ticks (Acari: Ixodidae) parasitizing medium-sized mammals in a Lyme Disease endemic area of southern New York. J Med Entomol. 1989;26:200–209. doi: 10.1093/jmedent/26.3.200. [DOI] [PubMed] [Google Scholar]
- 10.Gerwirtz A S, Cornbleet P J, Vugia D J, Traver C, Niederhuber J, Kolbert C P, Persing D H. Human granulocytic ehrlichiosis: report of a case in northern California. Clin Infect Dis. 1996;23:653–654. doi: 10.1093/clinids/23.3.653. [DOI] [PubMed] [Google Scholar]
- 11.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 12.IJdo J W, Zhang Y, Hodzic E, Magnarelli L A, Wilson M L, Telford III S R, Barthold S W, Fikrig E. The early humoral response in human granulocytic ehrlichiosis. J Infect Dis. 1997;176:687–692. doi: 10.1086/514091. [DOI] [PubMed] [Google Scholar]
- 13.Levin M L, Fish D. Programs and abstracts of the Thirteenth Sesqui-Annual Meeting of the American Society for Rickettsiology. 1997. Incongruity in the natural cycles of the agents of HGE and Lyme disease, abstr. 101. [Google Scholar]
- 14.Levine J F, Wilson M L, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 15.Little S E, Dawson J E, Lockhart J M, Stallknecht D E, Warner C K, Davidson W R. Development and use of specific polymerase chain reaction for the detection of an organism resembling Ehrlichia sp. in white-tailed deer. J Wildl Dis. 1997;33:246–253. doi: 10.7589/0090-3558-33.2.246. [DOI] [PubMed] [Google Scholar]
- 16.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E. Site-specific geographic association between Amblyomma americanum (Acari: Ixodidae) infestations and Ehrlichia chaffeensis-reactive (Rickettsiales: Ehrlichieae) antibodies in white-tailed deer. J Med Entomol. 1996;33:153–158. doi: 10.1093/jmedent/33.1.153. [DOI] [PubMed] [Google Scholar]
- 18.McDiarmid A. Modern trends in animal health and husbandry. Some infectious diseases of free-living wildlife. Br Vet J. 1965;121:245–257. [Google Scholar]
- 19.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 20.Persing D H, Conrad P A. Babesiosis: new insights from phylogenetic analysis. Infect Agents Dis. 1995;4:182–195. [PubMed] [Google Scholar]
- 21.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telford S R, III, Lepore T H, Snow P, Dawson J E. Human granulocytic ehrlichiosis in Massachusetts. Ann Intern Med. 1995;123:277–279. doi: 10.7326/0003-4819-123-4-199508150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong S J, Brady G S, Dumler J S. Serological responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York State. J Clin Microbiol. 1997;35:2198–2205. doi: 10.1128/jcm.35.9.2198-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]