Abstract
Major species differences in tryptophan (Trp) metabolism and disposition exist with important physiological, functional and toxicity implications. Unlike mammalian and other species in which plasma Trp exists largely bound to albumin, teleosts and other aquatic species possess little or no albumin, such that Trp entry into their tissues is not hampered, neither is that of environmental chemicals and toxins, hence the need for strict measures to safeguard their aquatic environments. In species sensitive to toxicity of excess Trp, hepatic Trp 2,3-dioxygenase (TDO) lacks the free apoenzyme and its glucocorticoid induction mechanism. These species, which are largely herbivorous, however, dispose of Trp more rapidly and their TDO is activated by smaller doses of Trp than Trp-tolerant species. In general, sensitive species may possess a higher indoleamine 2,3-dioxygenase (IDO) activity which equips them to resist immune insults up to a point. Of the enzymes of the kynurenine pathway beyond TDO and IDO, 2-amino-3-carboxymuconic acid-6-semialdehyde decarboxylase (ACMSD) determines the extent of progress of the pathway towards NAD+ synthesis and its activity varies across species, with the domestic cat (Felis catus) being the leading species possessing the highest activity, hence its inability to utilise Trp for NAD+ synthesis. The paucity of current knowledge of Trp metabolism and disposition in wild carnivores, invertebrates and many other animal species described here underscores the need for further studies of the physiology of these species and its interaction with Trp metabolism.
Keywords: Albumin; kynurenine pathway; indoleamine 2,3-dioxygenase; plasma tryptophan; serotonin; tryptophan 2,3-dioxygenase; tryptophan toxicity
Introduction
Many species differences in metabolism and disposition of the essential amino acid L-tryptophan (Trp) exist. Remarkable differences are seen when comparing species such as fish and humans. For example: (1) the rainbow trout (Oncorhynchus mykiss) utilises 60% of dietary Trp for protein synthesis,1 whereas humans hardly use 1%, if at all, as, in a person in nitrogen balance, the Trp released from protein breakdown is reutilised for protein synthesis 2; (2) the plasma Trp-binding protein, albumin, is totally absent from the Antarctic toothfish, the New Zealand eel3,4 and many other aquatic species (see below), whereas, in humans, albumin is the major plasma protein with a normal range of 35 to 50 g/L. Interest in species differences in Trp metabolism probably began with the demonstration during 1949 to 1954 of differences in the conversion of Trp via the kynurenine (Kyn) pathway (KP) to oxidised nicotinamide-adenine dinucleotide (NAD+), with the domestic cat (Felis catus) being unable to utilise Trp instead of nicotinic acid and to possess a high activity of picolinic acid carboxylase (2-amino-3-carboxymuconic acid-6-semialdehyde decarboxylase: ACMSD), a key enzyme whose increased activity can undermine the production and subsequent conversion of quinolinic acid (QA) into NAD+.5 Studies that followed involved assessment of species differences in activity of the major Trp-degrading enzyme, hepatic Trp 2,3-dioxygenase (TDO: formerly Trp pyrrolase; EC.1.13.11.11), probably prompted in part by the observations by Knox6 that adrenalectomized rats cannot handle exogenous Trp efficiently and do not survive repeated injections of the amino acid due to the absence of the glucocorticoid TDO induction mechanism, but can survive after administration of cortisol. A number of other animal species were subsequently shown to suffer from excess Trp and to lack the glucocorticoid induction mechanism, and the concept that these species, which are mainly herbivores, lack the free apoenzyme form of TDO that is inducible by glucocorticoids was proposed.7 A wide array of comparative studies of various aspects of Trp biochemistry and physiology in animal, including aquatic, species have since been reported and the purpose of the present review is to provide an updated wider account of this fascinating area of research that has a wide range of biological, clinical and toxicological implications. The following text is not intended to be a comprehensive one, but of sufficient content to illustrate the general features of, and key advances in research on, Trp metabolism across a range of animal species and to stimulate further Trp research across many species, particularly wild and aquatic ones, to fill in the many gaps in our knowledge of the physiology of these species and its interaction with Trp metabolism. Wherever applicable, data from humans will be the comparative reference.
Overview of Tryptophan Metabolism
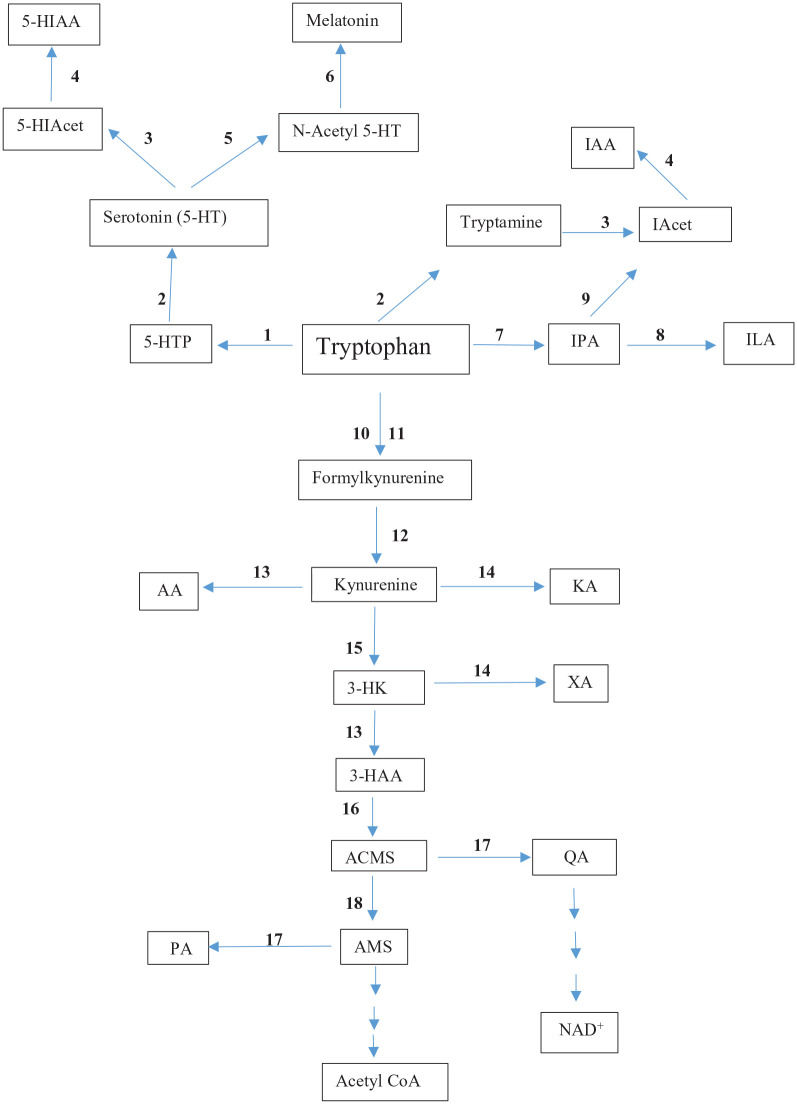
Detailed accounts of the Trp degradative pathways have been published2,8,9 and the following is a brief overview that will be followed later on in this text by detailed species comparisons. Apart from being essential for protein synthesis, Trp is metabolised in mammals by 4 pathways (Figure 1), 3 of which (the serotonin and melatonin, the tryptamine, and the indolepyruvate) are of quantitatively minor importance, though of a great functional one. The fourth, the kynurenine (Kyn) pathway (KP), is quantitatively the most important, accounting for 95% of dietary Trp degradation. The 3 minor pathways exist in both the periphery and brain. The KP exists mainly in the liver, where 90% of dietary Trp is degraded, with the remaining 5% occurring elsewhere. The host microbiota also degrade Trp, mainly to indoles, but some can also synthesise Trp. Microbiota may therefore play an important role in human, and particularly in ruminant, health.
Figure 1.
The tryptophan degradative pathways.
Abbreviations: Acet, acetaldehyde; ACMS, 2-Amino-3-carboxymuconic acid-6-semialdehyde: also known as acroleyl aminofumarate, AMS, 2-Aminomuconic acid -6-semialdehyde; AA, anthranilic acid; 3-HAA, 3-hydroxyanthranilic acid; 5-HIAcet, 5-hydroxyindoleacetaldehyde; 5-HIAA, 3-hydroxyindoleacetic acid; 3-HK, 3-hydroxykynurenine; 5-HT, 5-hydroxytryptamine or serotonin; 5-HTP, 5-hydroxytryptophan; IAcet, indole acetaldehyde; IAA, indol-3-ylacetic acid; ILA, indol-3-yllactic acid; IPA, indol-3-ylpyruvic acid; KA, kynurenic acid; PA, picolinic acid; QA, quinolinic acid; XA, xanthurenic acid.
Bold numbers represent enzymes of the different pathways, as follows: 1 (tryptophan hydroxylase); 2 (aromatic L-amino acid decarboxylase); 3 monoamine oxidase); 4 (aldehyde dehydrogenase); 5 (alkyl amine N-acetyl transferase); 6 (hydroxyindole o-methyl transferase); 7 (tryptophan aminotransferase); 8 (indole lactate dehydrogenase); 9 (indole pyruvate decarboxylase); 10 (tryptophan 2,3-dioxygenase); 11 (indoleamine 2,3-dioxygenase); 12 (N′-formylkynurenine formamidase); 13 (kynureninase); 14 (kynurenine aminotransferase); 15 (kynurenine monooxygenase: also known as kynurenine hydroxylase); 16 (3-hydroxyanthranilic acid 34-dioxygenase); 17 (non-enzymic cyclisation); 18 (2-Amino-3-carboxymuconic acid-6-semialdehyde decarboxylase (ACMSD: also known as picolinate carboxylase).
The serotonin or hydroxylation pathway is controlled by the first enzyme, Trp hydroxylase (TPH), although the second enzyme (aromatic L amino acid decarboxylase: ALAAD) which is pyridoxal 5′-phosphate (PLP)-dependent, could also be limiting under conditions of vitamin B6 deficiency, particularly in humans, wherein its activity in brain is very low,10 or with certain drugs inactivating the PLP cofactor. Brain [Trp] is the major determinant of cerebral serotonin synthesis, as its cerebral levels (5-25 µM) are well below the Km (50 µM) of TPH2.11 In mammals, TPH2 is the brain enzyme isoform, as distinct from the TPH1 isoform in the periphery. TPH2 is therefore at most only 50% saturated with its Trp substrate.12It is notable that both TPH isoforms are expressed in the rainbow trout brain.13 Melatonin is synthesised from 5-HT in both the pineal and periphery and its synthesis is also subject to availability of the serotonin precursor Trp.14 Alkylamine N-acetyl transferase, is the rate-limiting enzyme of melatonin synthesis.
The indolepyruvate (IPA) or transamination pathway and the tryptamine or decarboxylation pathway have in common the end product indol-3-ylacetic acid (IAA), a plant auxin. Not shown in Figure 1 is the conversion of IPA into kynurenic acid (KA), a KP metabolite, via an unstable kynuric intermediate that is produced by the action of reactive oxygen species (ROS) on the enol form of IPA and then cyclisation into KA.
The main products and intermediates of the above 3 pathways exert a range of important functions in both the central nervous system and periphery that impact many physiological processes of particular importance in immune, neurological and psychological disorders.
The (oxidative) KP is controlled mainly by the first enzyme: TDO mainly in liver and indoleamine 2,3-dioxygenase (IDO) elsewhere. The hepatic KP in most mammals contains all the enzymes necessary for the conversion of Trp into NAD+, whereas extrahepatically, not all enzymes are expressed. Accordingly, metabolite formation outside the liver will depend on the enzymes present. Kyn can however be metabolised outside the liver, especially in kidney. NAD+ is synthesised in the main de novo pathway from quinolinic acid (QA), the ‘Preiss-Handler’ pathway from nicotinic acid or the ‘salvage’ pathway from nicotinamide (see Badawy8 for enzymatic details). As shown in Figure 1, the KP produces a range of metabolites. These Kyn metabolites possess various biological activities both affecting, and being influenced by, many physiological processes, including the endocrine, haematopoietic, intermediary metabolism, immune, neuronal and vital body systems, with major clinical implications for a wide range of disorders.8,9,15
Tryptophan Disposition
Dietary tryptophan disposition
Plasma (or serum) is the major source of dietary Trp for utilisation by tissues. In humans and many other animal species, Trp exists largely bound to albumin, with a small fraction (5%-10%) being free. The proportion of free Trp varies according to the analytical procedure, with ultrafiltration of plasma samples being more accurate than equilibrium dialysis.16 Heparinised plasma contains higher free [Trp], because of the lipolytic action of heparin causing a non-esterified fatty acid (NEFA)-mediated Trp displacement from albumin-binding sites. Differences in levels of free Trp occur across many species and are determined by variations in concentrations of the 2 major physiological determinants of Trp binding: the binder albumin and the displacers NEFA. Drugs and other chemicals with a high albumin binding affinity can also cause direct displacement of bound Trp (eg, salicylate) and this is an important issue to consider in Trp metabolic and clinical studies in humans and in exposure of various species to industrial chemicals. Albumin levels can be decreased, leading to release of bound Trp, in liver cirrhosis, late pregnancy and infections, whereas those of NEFA are subject to modulation by nutritional, pharmacological and physiological factors, for example, by fasting, intake of the 3 major dietary classes, the antilipolytic insulin and nicotinic acid and the lipolytic catecholamines and phosphodiesterase inhibitors.16 Thus, as free Trp is a labile parameter subject to modulation by many factors, albumin levels represent the more stable determinant of Trp binding. Trp binding is generally expressed as the percentage free Trp (100 × [free Trp]/[total Trp]). The following are accounts of species differences in albumin and NEFA which can impact Trp binding and hence its availability.
Species differences in plasma albumin
Table 1 illustrates the wide range of plasma albumin concentrations (0-59 g/L) in various animal species. As not all species listed have been examined for Trp binding to albumin, the albumin values given can provide an indication of the likely level of Trp binding in future studies of these species. As stated above, the normal range of plasma albumin in humans is 35 to 50 g/L. Albumin levels of many species fall within this range, including some Trp-tolerant and Trp-sensitive species, all the herbivores and ruminants listed and carnivores and Perissodactyls. By contrast, most Trp-sensitive species have albumin levels of ⩽35 g/L and fish have even-lower levels varying between 0 and 20 g/L. In such species, Trp availability to tissues is likely to be greater than that in those with higher albumin levels and, as will be discussed below, the flux of plasma free Trp through the hepatic KP and other Trp-degradative pathways could be an important factor in their Trp metabolism. That changes in albumin can alter Trp binding has been demonstrated under various conditions, including increased binding by addition of albumin in vitro72 and decreased binding in hepatic cirrhosis73 and in late pregnancy in rats.19 In this latter study, a decrease in serum [albumin] of 19% on day 16 of pregnancy was associated with a significant 33% increase in the % free Trp (from 4.54% to 6.03%). On day 20, the decrease in albumin was little altered (21%), whereas the % free Trp rose by more than expected (by 121%) due to a simultaneous 67% elevation of [NEFA]. A minimum decrease in albumin of ~20% can therefore be expected to increase significantly plasma free Trp within any particular species or experimental setting. Simultaneous elevation of NEFA and depletion of albumin can therefore provide the maximum impact on plasma Trp binding.
Table 1.
Plasma or serum albumin concentration in various animal species.
| [Ref] | [Ref] | ||||
|---|---|---|---|---|---|
| Mammals | Wild animals | ||||
| Trp-tolerant | Carnivores | Hattingh et al54 | |||
| Human (42) | 46.7 | Badawy et al17 | Hyena (Crocuta crocuta) (4) | 28.4 | |
| (114) | 49.6 | Badawy and Dougherty18 | Leopard (Panthera pardus) (6) | 38.5 | |
| Rat (6) | 43.8 | Badawy19 | Lion (Panthera leo) (8) | 29.0 | |
| (6) | 41.4 | Badawy et al20 | Perissodactyls | ||
| Mouse (8) | 33.2 | Kaburagi et al21 | Rhinoceros (Ceratotherium semum) (8) | 24.1 | |
| (25) | 28.0 | Viuff et al22 | Bergkwagga (Equis zebra) (4) | 36.8 | |
| (12) | 22.2 | Bottari et al23 | Zebra (Equus Burchelli) (9) | 32.0 | |
| Pig (9) | 25.8 | Sansom et al24 | Horse | 38.0 | Sanz et al55 |
| (⩾2) | 40.0 | Lardinois and Page25 | 33.5 | Monzón and Villavicencio36 | |
| Chicken (6) | 14.5 | Jiang et al26 | Omnivores | ||
| Turkey (18) | 22.5 | Oso et al27 | Warthog (Phocochoerus aethiopicus) (8) | 25.2 | Hattingh et al54 |
| Pigeon (30) | 20.7 | Orakpoghenor et al28 | Herbivores & Ruminants | ||
| Dog (20) | 28.2 | Bonatto et al29 | Baboon (Papio ursinus) (6) | 45.5 | Hattingh et al54 |
| Trp-sensitive | Blesbuck (Damaliscus Dorcas phillipsi) (5) | 43.5 | |||
| Cat (8) | 28.0 | Sakai et al30 | |||
| (19) | 33.7 | Debosschere et al31 | Buffalo (Syncerus caffer)17 | 42.5 | |
| Cow (65) | 35.7 | Smuts et al32 | Duiker (Sylvicapra grimmia) (3) | 35.7 | |
| (75) | 35.7 | Cattaneo et al33 | Eland (Taurotragus oryx) (11) | 46.5 | |
| Frog (5-7) | 19.3 | MacDonald et al34 | Elephant (Loxodonta africana) (14) | 41.4 | |
| Gerbil (8) | 37.0 | Grando et al35 | Hippopotamus (Hippopotamus amphibius) (11) | 54.9 | |
| (12) | 22.2 | Bottari et al23 | Impala (Aepyceros melampus melampus) (12) | 35.9 | |
| Guinea pig (20) | 30.0 | Monzón and Villavicencio36 | Blue Wildebeest (Connochaetes taurinus) (9) | 49.1 | |
| (30) | 24.6 | Uniyal et al37 | Kudu (Tragelaphus strepsiceros) (7) | 43.4 | |
| Hamster (5) | 32.7 | Jantawong et al38 | Waterbuck (Kobus ellipsiprymnus) (5) | 45.1 | |
| Steer (51) | 30.0 | Herrick et al39 | Rooi hartebeest (Aecelaphus caama) (3) | 48.7 | |
| (60) | 34.5 | Chen et al40 | Roan (Hyppotragus equinus) (9) | 40.1 | |
| Rabbit (15) | 27.0 | Ayyat et al41 | Bontbuck (Damaliscus dorcas dorcas) (8) | 59.2 | |
| Sheep (9) | 32.3 | da S. dos Santos et al42 | Steenbuck (Raphicerus campestris) (8) | 45.2 | |
| Lamb (19) | 38.8 | Sidki and Hirst43 | Fish | ||
| Ewes (46) | 51.3 | Sidki and Hirst43 | Antarctic toothfish (pooled plasma) |
00.0 | Metcalf et al3 |
| Other species | Atlantic salmon (Salmo salar)20 | 17.5 | Wade et al56 | ||
| Goat (5) | 39.9 | Kusumanti and Sugiharto44 | |||
| Marsupials | (10) | 19.5 | Bernhoft et al57 | ||
| Kangaroo (>30) | 31.0 | Wilson and Hoskins45 | Maillou and Nimmo (1993) (10) | 18.0 | Maillou and Nimmo58 |
| Rhesus monkey (8) | 39.3 | Chen et al46 | Block fish (Channa punctatus) | ||
| (24) | 38.7 | Wu et al47 | (5 × 3) | 15.0 | Bharti and Rasool59 |
| Green monkey (33) | 40.0 | Chichester et al48 | Cobia (Rachycentron canadum) | ||
| (8) | 58.1 | Niimi et al49 | (20 × 3) | 9.0 | Huang et al60 |
| Vervet monkey (18) | 37.0 | Fincham et al50 | Common Carp (Cyprinus carpio) | ||
| Mormoset monkey (134) | 43.1 | Davy et al51 | (90) | 10.0 | Hoseini et al61 |
| (48) | 16.0 | Ali et al62 | |||
| Elasmobranchii | Fellows and Hird52 | 00.0 | De Smet et al63 | ||
| Port Jackson shark | 0.0 | Male goldfish (Carassius auratus) | 1.9 | İnanan et al64 | |
| Draughtboard shark | 0.0 | New Zealand eel (pooled plasma) | 00.0 | Metcalf et al4 | |
| Gummy shark | 0.0 | ||||
| Common stingray | 0.0 | Nile tilapia (Oreochromis niloticus) | 39.5 | Abdelghany et al65 | |
| Eagle ray | 0.0 | Pacu (Piaractus mesopotamicus) | 1.97 | Silva and Mercer66 | |
| Melbourne Skate | 0.0 | Rainbow trout ((Oncorhynchus mykiss) | 5.6 | Gültepe67 | |
| Sand tiger shark | 0.0 | Otway53 | 20.0 | Vazirzadeh et al68 | |
| Petromyzone | Fellows and Hird52 | Silver catfish (Rhamdia quelen) | 4.5 | Pianesso et al69 | |
| Lamprey | 0.0 | Striped catfish (Pangasianodon hypophthalmus) | 20.8 | Bera et al70 | |
| Crustaceae | Fellows and Hird52 | Turbot (Scophthalmus maximus) | 17.9 | Jia et al71 | |
| Southern Rock | Long snouted boarfish | 00.0 | Fellows and Hird52 | ||
| Lobster | 0.0 | Globefish | 00.0 | Fellows and Hird52 | |
| Yabbie | 0.0 |
Numbers in parentheses next to species are those of animals tested. Where no numbers are given indicates too many or information not given. Albumin concentration is expressed in g/L.
Species differences in plasma tryptophan binding to albumin
Table 2 lists the total plasma Trp concentration in a wide range of species, with a smaller number of species (36) in which also free Trp was measured and the % free Trp calculated. As shown, Trp-sensitive species (cat, channel catfish, cow, frog, guinea pig, hamster, rabbit, sheep and steer) possess less total Trp in plasma than Trp-tolerant species. Other species with even-lower plasma total [Trp] include many teleosts, lamprey and crustacea. Binding expressed as the % free Trp varies among most species between 6.29% and 26.39%, but is minimal in aquatic species, with % free Trp values of 81% to 100%.
Table 2.
Plasma tryptophan concentrations and binding in various animal species.
| Species (n) | Tryptophan | |||
|---|---|---|---|---|
| Total | Free | % Free | [Reference] | |
| Vertebrates | ||||
| Human (?) | 65.6 | 8.5 | 12.96 | Fuller and Roush74 |
| (48) | 43.0 | 5.4 | 12.56 | Badawy et al75 |
| (114) | 63.0 | 5.3 | 8.41 | Badawy and Dougherty18 |
| (2628) | 68.0 | Ulvik et al76 | ||
| Rat (?) | 155.7 | 16.8 | 10.79 | Fuller and Roush74 |
| (6) | 78.1 | 8.3 | 10.63 | Lane et al77 |
| (8) | 89.5 | 8.4 | 9.41 | Badawy and Evans7 |
| (5) | 67.0 | 6.6 | 9.80 | Fellows and Hird52 |
| (10) | 198.8 | 21.5 | 10.81 | Allegri et al78 |
| Mouse (?) | 93 | 22.0 | 23.50 | Fuller and Roush74 |
| (4 × 3) | 91.6 | 11.8 | 12.80 | Badawy and Evans7 |
| (10) | 131.5 | 16.8 | 12.78 | Allegri et al78 |
| Pig (?) | 109.7 | 20.7 | 18.87 | Fuller and Roush74 |
| (6) | 85.3 | 12.5 | 14.65 | Badawy and Evans7 |
| (6) | 53.0 | Koopmans et al79 | ||
| Dog (?) | 50.9 | 5.0 | 9.82 | Fuller and Roush74 |
| (6) | 72.0 | Chiang et al80 | ||
| Guinea pig(?) | 30.4 | 6.7 | 22.04 | Fuller and Roush74 |
| (10) | 55.5 | 5.0 | 9.01 | Badawy and Evans7 |
| (8) | 72.8 | 7.7 | 10.58 | Allegri et al78 |
| (6) | 57.6 | Shukla and Chandra81 | ||
| (8) | 72.9 | 7.7 | 10.56 | Allegri et al78 |
| Golden hamster (5) | 36.0 | 9.5 | 26.39 | Badawy and Evans7 |
| Mongolian gerbil (4) | 87.4 | 5.5 | 6.29 | Badawy and Evans7 |
| Cat (?) | 46.0 | 5.3 | 11.52 | Fuller and Roush74 |
| (1) | 28.7 | 5.3 | 18.47 | Badawy and Evans7 |
| (8) | 51.0 | Sakai et al30 | ||
| Rabbit (?) | 64.6 | 6.2 | 9.60 | Fuller and Roush74 |
| (4) | 62.2 | 8.0 | 12.86 | Badawy and Evans7 |
| (5) | 74.8 | 8.2 | 10.96 | Ragazzi et al82 |
| Herbivores | ||||
| Cow(?) | 52.9 | 9.4 | 17.77 | Fuller and Roush74 |
| (4) | 28.0 | Kollmann et al83 | ||
| Heifer (12) | 22.0 | Kollmann et al83 | ||
| Steer (5) | 24.8 | 2.2 | 8.87 | Badawy and Evans7 |
| (4) | 18.0 | Marín and Larraín84 | ||
| Sheep (?) | 71.5 | 8.4 | 11.75 | Fuller and Roush74 |
| (6) | 41.1 | 8.3 | 20.19 | Badawy and Evans7 |
| (9) | 15.0 | Hofford et al85 | ||
| Goat (12) | 9.0 | Ma et al86 | ||
| Horse (?) | 13.50 | Fuller and Roush74 | ||
| Primates | ||||
| Rhesus monkey (?) | 34.3 | 3.7 | 10.79 | Fuller and Roush74 |
| Green monkey (?) | 28.9 | 4.7 | 16.26 | Fuller and Roush74 |
| Mormoset monkey (?) | 43.6 | 7.6 | 17.43 | Fuller and Roush74 |
| Vervet monkey (15) | 42.0 | 7.2 | 17.14 | Chamberlain et al87 |
| Marsupials | ||||
| Kangaroo(3) | 20.0 | 1.2 | 94.00 | Fellows and Hird52 |
| Amphibians | ||||
| Frog (4 × 4) | 17.6 | 14.6 | 82.95 | Badawy and Evans7 |
| (6-7) | 6.4 (October) |
Emelyanova et al88 | ||
| 28.7 (December-January) |
||||
| 9.2 (April) | ||||
| Avians | ||||
| Chicken (?) | 75.4 | 25.8 | 34.22 | Fuller and Roush74 |
| 91.8 | 21.5 | 23.42 | Badawy and Evans7 | |
| Turkey (?) | 28.09 | Fuller and Roush74 | ||
| (4) | 92.5 | 16.8 | 18.16 | Badawy and Evans7 |
| (66) | 112.6 | Middendorf et al89 | ||
| Pigeon (6) | 58.2 | 18.0 | 30.90 | Lane et al77 |
| Teleosts | ||||
| Rainbow trout (4) | 15.0 | 13.0 | 86.70 | Fellows and Hird52 |
| (4-5) | 38.0 | 37.4 | 98.00 | Walton et al1 |
| (15) | 25.0 | Wacyk et al90 | ||
| (5 × 20) | 68.5 | Hajirezaee et al91 | ||
| (8) | 73.0 | Lepage et al92 | ||
| (3 × 2) | 31.0 | Yamamoto et al93 | ||
| Carp (C auratus) (7) | 44.0 | Van Der Boon et al94 | ||
| Channel catfish (3 × 3) | 20.4 | Pohlenz et al95 | ||
| Globefish (4) | 7.4 | 6.0 | 81.00 | Fellows and Hird52 |
| Long-snouted boarfish (4) | 26.0 | 24.0 | 92.31 | Fellows and Hird52 |
| Petromyzones | ||||
| Short-headed lamprey (4) | 7.0 | 7.0 | 100.00 | Fellows and Hird52 |
| Elasmobranchii | Fellows and Hird52 | |||
| Port Jackson shark (5) | 21.0 | 21.0 | 100.00 | Fellows and Hird52 |
| Draughtboard shark (4) | 42.0 | 42.0 | 100.00 | Fellows and Hird52 |
| Gummy shark (2) | 40.0 | 39.0 | 97.00 | Fellows and Hird52 |
| Common stingaree (3) | 81.0 | 75.0 | 93.00 | Fellows and Hird52 |
| Eagle ray (2) | 34.0 | 33.0 | 97.00 | Fellows and Hird52 |
| Melbourne skate (1) | 81.0 | 75.0 | 92.60 | Fellows and Hird52 |
| Round skate (1) | 20.0 | 20.0 | 100.00 | Fellows and Hird52 |
| Crustacea | ||||
| Southern rock lobster (4) | 30.0 | 20.0 | 33.30 | Fellows and Hird52 |
| Yabbie (4) | 19.0 | 19.0 | 100.00 | Fellows and Hird52 |
Numbers of animals (n) are shown in parentheses. Concentrations of free and total Trp are in µM. Trp binding is expressed as the percentage free Trp (100 × [free Trp]/[total Trp]).
Relationship between plasma tryptophan binding and albumin concentration
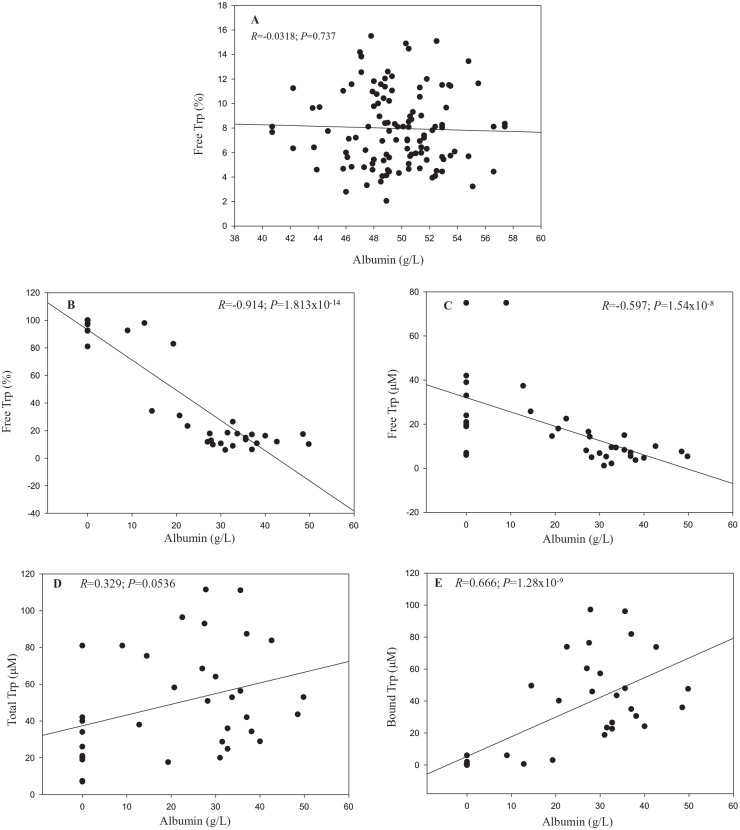
As stated above, a ~ 20% decrease in plasma [albumin] can cause a significant increase in the % free Trp. Figure 2 shows the relationship between these 2 parameters in normal human subjects (Figure 2A) and across various species (Figure 2B–E). In our previous study,18 in fasting human subjects (n = 114), average plasma albumin and % free Trp were 49.6 ± 0.3 g/l and 8.40% ± 0.21%, respectively (means ± SEM). However, there was no significant (Pearson Product Moment) correlation between these 2 parameters (r = −0.0318, P = .737) (Figure 2A) nor between albumin and free Trp, total Trp or bound Trp (r = 0.114-0.143; P > .1) (data not shown), thus reflecting individual variations in a single species for albumin values within the normal range. By contrast, comparison of 35 different species with wider variations in plasma albumin revealed highly significant negative correlations between albumin and either the % free Trp (Figure 2B) or [free Trp] (Figure 2C). A borderline positive correlation was, however, observed between [albumin] and [total Trp] (r = 0.329; P = .0536) (Figure 2D), whereas a highly significant positive correlation was observed between [albumin] and bound Trp (Figure 2E).
Figure 2.
Correlations between plasma albumin and tryptophan concentrations and tryptophan binding in humans and other species.
(A) Healthy human volunteers (n = 114) with mean albumin values of 49.6 g/l (range: 40.7-57.4). (B–E) correlations with 35 different animal species between albumin and the % free Trp (B), free Trp (C), total Trp (D) and bound Trp (E).
Physiological role of albumin across species
A brief account of this role can point to potential species differences in Trp binding as a function of albumin levels and rate of degradation. In vertebrates, albumin is the major plasma protein and is essential for normal body physiology. Its main functions include maintenance of intravascular oncotic pressure, being responsible for ~80% of colloidal osmotic pressure, binding and transport of substances, with a high capacity for binding water, and free radical scavenging through its SH content.96,97 Albumin is synthesised only in liver, at a rate in humans of ~ 0.2 g/kg body weight per day. Its daily metabolism depends on plasma levels and occurs at a fixed ~ 10% rate per day.98 Its degradation is inversely related to its plasma levels, with human albumin having a half-life of about 19 to 21 days.99,100 The albumin T1/2 of horse (19-20 days), cow (14-19 days) and sheep (14-28 days)101 are closer to that of human and are parallelled by the plasma albumin values in Table 1 here. Some species with lower plasma [albumin] than the above species, such as the cat, mouse, rabbit, pig and dog (25.8-28.2 g/l; see Table 1 here) have shorter albumin half-lives of 1.5 to 8.2 days.102,103A plot of averaged plasma [albumin] versus the albumin T1/2 of these 9 species (Figure 3) confirms the above inverse relationship between albumin concentration and its rate of degradation. It is remarkable that a highly significant positive correlation between [albumin] and its T1/2 is observed with a relatively small number (9) of species with moderate differences in their plasma [albumin]. Adding 13 species from Table 1 with zero albumin, such as sharks, skates, rays and eels to the calculations only raised the correlation coefficient slightly to 0.923, but much more significantly to a P value of 9.76 × 10−14. Species differences in albumin degradation can be due to a range of determinants, for example, rate of synthesis, disposition across compartments and expression of the neonatal Fc receptor (FcRn) that binds to albumin and protects it against lysosomal proteolysis.103,104Engineering this receptor prolongs the albumin T1/2105and infusion of albumin into an analbuminaemic human neonate prolongs the T1/2 of the infused albumin to 115 days, as compared with the ~ 20-day duration in normal subjects.106 These latter authors also observed that plasma [Trp] was extremely low in this infant, and suggested that this Trp deficiency might have impaired albumin synthesis. FcRn binding to albumin of various animal species requires systematic investigation, as currently available data are extremely limited.
Figure 3.
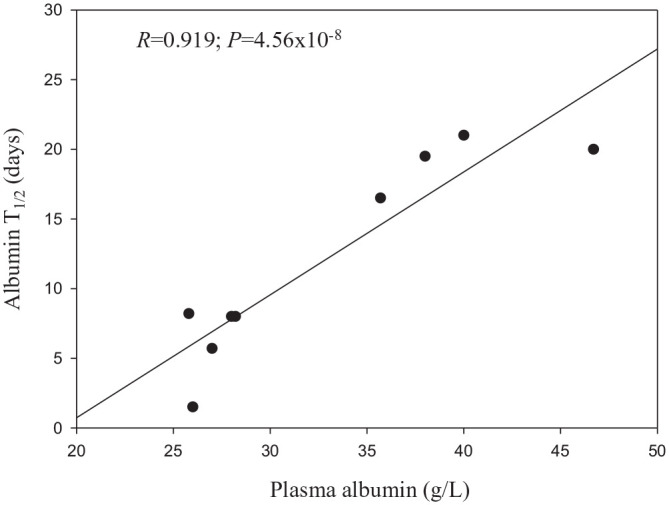
Correlation between plasma albumin concentration and turnover rate.
Pearson moment product correlations were made between plasma [albumin] and albumin T1/2 for the 9 animal species described in the text, the T1/2 of which rose in the following order: mouse < rabbit < cat = dog < pig < cow < horse < man < sheep.
Prolonging the albumin half-life has been used to enhance the transport function of albumin for therapeutic purposes, for example albumin-based therapeutics with tailored FcRn binding properties to study and improve drug pharmacokinetics.22,100Binding of chemicals by albumin can also be used as a biomarker of recent marine life exposure, for example, albumin-benz(α)pyrene diol epoxide adducts.107
In contrast with vertebrates and others, aquatic species show marked differences in plasma albumin. Of the25 aquatic species listed in Table 1, albumin values (in g/L) varied between zero (n = 14), 1.9 to 4.4 (n = 2), 9 to 20.8 (n = 8) and 39.5 (n = 1: Nile tilapia). It is almost certain that the lower albumin in aquatic life is related to osmotic activity, fluid and electrolyte balance and needs within the aquatic macro-environment, as well as the species microenvironment. A discussion of these aspects is outside the scope of the present article, but it is of great interest to note that urea plays a major role as a more suitable substitute for albumin under these conditions. For example, urea levels in shark blood are very high (0.19-6 M), thus enabling them to have an osmotic pressure higher than that in sea water, and teleosts also have raised urea levels, but ~ 100-fold lower than in sharks.108 Whereas the impact of differences in albumin turnover on Trp binding may be difficult to assess, the potential effects of low plasma albumin on Trp binding and its consequences are certainly by-products of the physiological adaptations of aquatic species to their environments.
Species differences in plasma non-esterified fatty acids (NEFA)
As the physiological displacers of albumin-bound Trp, NEFA play an important role in determining plasma free [Trp] and hence its flux and availability. Table 3 shows the wide species variations in plasma [NEFA]. The report by Fellows et al,130 is the most comprehensive. Comparing differences among 7 species, these authors reported plasma [NEFA] levels of 0.04-0.53 mEq/L and in the following decreasing order: pigeon > rat > domestic fowl > human = cane toad > Eastern grey kangaroo > axolot. They also compared [NEFA] in aquatic species. Levels in 5 teleosts varied between 0.09 and 0.64 mEq/L, thus resembling those in the above species. By contrast, apart for a [NEFA] of 0.13 mEq/L in white-spotted stingaree, 0.1 in Short headed lamprey, 0.04 in Yabbe and 0.02 in Southern rock lobster, NEFA were not detectable in the common stingaree, eagle ray, southern fiddler, Port Jackson shark, draughtboard shark or Melbourne skate. As described in Table 2 here, binding of Trp to albumin was studied by the same group52 in many of these species.
Table 3.
Species differences in plasma nonesterified fatty acid (NEFA) concentration.
| Species (n) | [NEFA] | [Reference] | Species | [NEFA] | [Reference] |
|---|---|---|---|---|---|
| 1. Trp-tolerant: | Teleostomi | ||||
| Human | Rainbow trout (5) | 0.26 | Fellows et al130 | ||
| Non-fasting | <0.9 | UK NHS Sources109 | Long-nosed flathead (3) | 0.59 | Fellows et al130 |
| Fasting range | 0.5-2.8 | UK NHS Sources109 | Sand flathead (1) | 0.64 | Fellows et al130 |
| Fasting (114) | 0.32 | Dougherty et al110* | Globe fish (5) | 0.15 | Fellows et al130 |
| Rat (6) | 0.31 | Badawy et al111 | Long snouted boarfish (2) | 0.09 | Fellows et al130 |
| Mouse (4-5) | 0.90 | Wei et al112 | Elasmobranchii | ||
| (13-18) | 1.20 | Ohlsson et al113 | White-spotted stingaree (4) | 0.13 | Fellows et al130 |
| Pig (9) | 0.57 | Faris et al114 | Common stingaree (5) | 0 | Fellows et al130 |
| Dog (5) | 0.29 | Bertolucci et al115 | Eagle ray (4) | 0 | Fellows et al130 |
| Horse (12) | 0.10 | Spears et al116 | Southern fiddler (5) | 0 | Fellows et al130 |
| Horse (6: fasting) | 0.46 | Breidenbach et al117 | Port Jackson shark (5) | 0 | Fellows et al130 |
| Pony (6: fasting) | 0.24 | Breidenbach et al117 | Draughtboard shark (I) | 0 | Fellows et al130 |
| Turkey (42) | 0.21 | Bacon et al118 | Melbourne skate (1) | 0 | Fellows et al130 |
| Chicken (6) | 0.11 | Vincent and Brackenbury119 | Petromyzones | ||
| Trout | 0.32 | Librán-Pérez et al120 | Short headed lamprey (6) | 0.10 | Fellows et al130 |
| 2. Trp-sensitive: | Crustacea | ||||
| Guinea pig (10) | 0.12 | Jones121 | Southern rock lobster (5) | 0.02 | Fellows et al130 |
| Golden hamster (17) | 0.20 | Bravo et al122 | Yabbie (6) | 0.04 | Fellows et al130 |
| Mongolian gerbil (16) | 0.55 | Baggia123 | Others | ||
| Obese desert gerbil (30) | 0.64 | Gouaref et al124 | Kangaroo (6) | 0.01 | Fellows et al130 |
| Cat (51: fasting) | 0.71 | Barkai and Allweis125 | Pigeon (6) | 0.53 | Fellows et al130 |
| Cattle (many studies) | <0.2 | Drackley126 | Cane Toad (4) | 3 | Fellows et al130 |
| Sheep (12) | 0.47 | Leat and Ford127 | Axolot (3)** | 0.04 | Fellows et al130 |
| Rabbit (26) | 0.17 | El-Desoky et al128 | |||
| Catfish (African: 6) | 0.44 | Van Heeswijk et al129 |
[NEFA] was determined but not reported in this study. **Blood was taken from cut liver.
Despite the available information on albumin and NEFA levels, it is difficult to dissociate the impact of one or the other on Trp binding, except in species in which albumin is either absent or very low. Here, differences in [NEFA] are unlikely to have a significant impact. It is of interest, however, that where albumin is absent, NEFA are also absent (compare species in Tables 1 and 3). The potential impact of species differences in [NEFA] is, however, difficult to assess, except for changes within a single species in response to stress and dietary manipulations, for which significant evidence exists. As the data in Table 3 show, there is no dichotomy in plasma [NEFA] between Trp-tolerant or – sensitive species or between vertebrates and teleosts. Nutritional, metabolic and other physiological factors, and differences are potential explanations.
Fate of Plasma Tryptophan in Species
Physiological aspects
Plasma Trp levels are determined by dietary protein intake and metabolism in tissues. Trp is essential for protein synthesis, and also for NAD+ synthesis in the absence of adequate intake of niacin (nicotinic acid or nicotinamide: the 2 forms of vitamin B3). With protein synthesis, wide variations in dietary Trp requirements exist among species. Thus, as stated in the Introduction, whereas humans hardly use 1%, if at all, the rainbow trout uses 60%.1 However, many studies of dietary Trp requirements by teleosts are controversial and suggest that requirements are subject to many determinants and confounders other than growth and wellbeing.131 In most mammals, NAD+ synthesis from Trp is quantitatively more important than that from niacin, with only 1 mg of niacin arising from intake of 60 mg of Trp in humans.8,132 Species are known to differ in their nutritional Trp requirements and their Trp:niacin ratios reflect their ability to convert Trp into niacin with any defects being attributable to altered activities of KP enzymes, as will be described further below. Examples of Trp:niacin ratios in species other than humans are: rats: 33:1133 or 40-53:1,134,135 turkeys: 103-119:1, chick 45:1, broiler chicken 47-54:1,136,137Pekin and mule ducks 172-181:1137and both cat5,66,138 and rainbow trout139 lack an efficient conversion of Trp into niacin. The mouse has an even-more efficient conversion rate than the rat.140
The primary determinant of plasma Trp disposition is activity of the major Trp-degrading enzyme, liver TDO. This is best illustrated by the observed 9.3 to 12.7-fold increase in plasma [Trp]141,142 and the 10.6-fold elevation of brain [Trp]143 following deletion of the mouse TDO2 gene. By contrast, no changes in brain [Trp] occur after deletion of the genes encoding the other Trp-degrading enzyme indoleamine 23-dioxygenase (IDO) isoforms 1 and 2.143 Plasma Trp availability is also controlled at the secondary, but more immediate, level by its albumin binding and, additionally for uptake by the brain, by extent of competition from a number of neutral (competing) amino acids (CAA), mainly Val, Leu, Ile, Phe and Tyr, with the ratio of [free Trp]/[CAA] or [total Trp]/[CAA] being a predictor of likely changes in brain [Trp] and hence serotonin synthesis. Many studies involving measurement of this ratio have been performed in humans to assess the role of serotonin in behavioural and other disorders using the acute Trp depletion (ATD) and loading (ATL) tests, by administering amino acid mixtures respectively deficient or supplemented with Trp (see review by144). Originally proposed for use in rats,145,146 the test was adopted for humans147 and the formulations reported by Young et al148 became standard. Other species underwent the ATD and/or ATL test, including vervet monkey to study aggression,87rat to study catecholamines149 and pigeon150 to study injurious behaviour.151These latter authors suggested that ATD may be a useful test to explore in avian species the role of serotonin in abnormal behaviours such as feather picking in laying hens or compulsive feather picking in parrots.
Irrespective of TDO activity, the flux of plasma free Trp down the Kyn and other pathways of Trp degradation can play an important role in production of Trp metabolites. Flux is determined primarily by free Trp levels through changes in albumin and NEFA.72,152 The low levels in, or absence of albumin from, aquatic species is likely to facilitate Trp entry into tissues and thus enhance its utilisation and degradation. Similar changes can also be expected under stressful conditions leading to elevation of NEFA, for example, after exercise in camels.153
Toxicity aspects
It is important at this point to establish the safety or otherwise of Trp, especially at high levels, among various species before discussing in detail Trp metabolism, as the latter plays a key role in Trp disposition and hence its potential toxicity. As stated earlier, the first report of a harmful effect of a large dose of Trp was that of death of adrenalectomized rats, which could be prevented by cortisol administration, suggesting that TDO synthesis is important for ‘detoxifying’ Trp.6 There then followed reports of harmful effects of Trp in other species: death from large or repeated doses of Trp administered to steer,154,155the channel (USA) catfish Ictalurus punctatus,156 the Mongolian gerbil (Meriones unguiculatus)157 and the intact guinea pig.158
Little information is available from toxicological studies on safety of Trp in other species, though a certain degree of speculation is possible. For example, the high Trp intake by carnivorous wild animals is unlikely to be harmful given their Trp-rich protein consumption, though they may suffer potential cardiovascular abnormalities mediated by homocysteine produced from protein-derived methionine. Cardiomyopathy associated with coronary arteriosclerosis has been reported in the Eurasian lynx.159While death of wild animals has many causes, it would be of interest to assess the potential involvement of homocysteine and frequency of cardiovascular abnormalities in these species. Plasma homocysteine in horse is within the normal range for humans (<15 µM)160and Trp is not toxic to horse, at least at doses of up to 120 mg/kg body weight (producing blood Trp levels of 350 µM).161 Trp exerts no effect on reaction time to startle or clinical pathology parameters in horse. The above authors observed no calmative effect of Trp (ie, for the horse to travel at a lower speed). While Trp may exert a calmative effect in excitable horses, evidence for this is controversial.162 Several studies have established the role of dietary Trp in stimulating growth and immunity and decreasing anxiety in fish species.163-166 Trp administration is well tolerated by Rhesus monkey (Macaca mulatta) and inhibits self-injurious behaviour in males with a history of such behaviour.167 Clearly the safety of Trp in many other species requires assessment.
From available evidence so far, Trp toxicity is associated with the absence of the TDO apoenzyme and its glucocorticoid induction mechanism. Table 4 lists species possessing and lacking apo-TDO and/or its glucocorticoid induction mechanism in relation to Trp toxicity. A clear distinction between the above 2 parameters and toxicity is obvious, except for the rainbow trout, in which Trp is safe (see above) despite the absence of the TDO apoenzyme.168 It is also unknown if the trout TDO is inducible by cortisol. Indirect evidence for induction in another teleost, Meagre (Argyrosomus regius) is suggested by the finding169 that stress-induced elevation of plasma cortisol is associated with increased production of Kyn and quinolinic acid (QA) in liver in dietary Trp-supplemented fish. As will be discussed below, stress in meagre receiving the basal diet increases the flux of Trp through TDO, increasing both liver Trp and Kyn simultaneously. Whereas turkey liver TDO is inducible by cortisol,170 indirect evidence suggests that chicken and pig liver TDO may also be inducible by glucocorticoids. Thus, Bordetella avium infection of poults increases serum corticosterone concentration and enhances TDO activity,171and treatment of weaned piglets with Diquat enhances TDO mRNA expression,172 most likely by elevating circulating cortisol.173 Cortisol does not induce TDO activity in Ictalurus punctatus.156 These latter authors also reported the failure of Trp to activate the enzyme at ⩾4 hours, though this could be explained by a potential activation by the dose given having taken place at earlier time intervals, as species lacking apo-TDO appear to respond rapidly to activation by Trp.7 Much work is clearly required to assess glucocorticoid induction of TDO in other species in relation to Trp toxicity. Potential mechanisms of Trp toxicity in Trp-sensitive species are discussed below under IDO.
Table 4.
Summary of species differences in plasma tryptophan, liver apo-TDO and its induction by glucocorticoids, and sensitivity to tryptophan.
| Species | Low plasma [Trp] | NO Apo-TDO | Glucocorticoid induction | Trp toxicity |
|---|---|---|---|---|
| A- Trp-tolerant species | ||||
| Human | + | |||
| Rat | + | |||
| Mouse | + | |||
| Turkey | + | |||
| Rainbow trout | + | ? | ||
| Chicken | (+) | |||
| Pig | (+) | |||
| B- Trp-sensitive species | ||||
| Cat | + | + | + | |
| Frog | + | + | + | |
| Gerbil | + | + | ||
| Guinea pig | + | + | + | |
| Hamster | + | + | + | |
| Ox | + | + | ? | + |
| Sheep | + | + | ? | + |
| Rabbit | + | + | + | |
| Catfish | + | + | + | |
Symbols: +, present; ?, unknown; (+), indirect evidence; blank, absent.
Although fish species lack or possess very little albumin to bind Trp and generally have low Trp levels, these limitations could benefit fish in at least 2 ways. (1) Because of their low TDO activity, the low plasma Trp does not impose an immediate (strong) burden on TDO. (2) The absence of albumin enables Trp to have a much easier access to liver and other tissues, with the Trp flux playing a particularly important role in production of kynurenine metabolites. For brain, Trp availability can enhance serotonin synthesis, whereas for liver, synthesis of KP metabolites, niacin and NAD+ will depend on the KP enzyme complement. Lack of albumin in most aquatic species, however, also facilitates tissue uptake of industrial chemicals and other pollutants and consequent organ damage and death, thus further emphasising the need to protect these vulnerable species by strengthening measures to control the disposal of pollutants.
Metabolic aspects
Given that TDO activity and its induction by glucocorticoids are likely to be involved in Trp toxicity, it is important to assess the role of TDO and other enzymes of Trp metabolism along the KP in some detail, especially as this is the most studied Trp-metabolic pathway in animal species. This will be followed by a discussion of the role of Kyn and other pathway metabolites in immune modulation and other physiological processes.
Species Differences in Enzymes of the KP
Most studies of species differences in enzymes of the KP have been limited to relatively small numbers of species and have focused on the 2 rate-limiting enzymes of the pathway, TDO and IDO, with very few comparative studies of subsequent pathway enzymes. The most detailed of these studies are those involving TDO enzymatic determination,7 development of a TDO assay procedure in liver cytosol from multiple species174 and a comparison of TDO and IDO in metazoans.175 However, as will be discussed below, studies of urinary Trp metabolite excretion can also provide indirect indicators of enzyme activities. The following are accounts of data available for individual enzymes of the KP
Tryptophan 2,3-dioxygenase (TDO)
Species comparisons
In addition to assays of TDO activity in various species discussed above, other measures of enzyme activity have been published and involved determination of TDO activity in vivo by measuring the evolution of 14CO2 from administered 2-14C Trp and formation of niacin or NAD+ from exogenous Trp. For example, Green et al182 reported that TDO activity in vivo in gerbils is much lower than that in rats and is not inducible by cortisol. These authors also reported that, whereas TDO induction by cortisol in rats decreases brain Trp and serotonin synthesis and turnover, no changes in brain indoles are observed in gerbils. By contrast, α-methyl tryptophan, a potent activator of TDO, is effective in activating liver TDO and decreasing serotonin in both species. As the comparisons in Table 5 show, the studies listed reported variations in the relative TDO activities among species, possibly because of experimental and other study differences. For example, whereas in one study174only the holoenzyme was assayed (ie, in the absence of added cofactor) in pooled liver cytosols obtained from commercial suppliers, the total (holoenzyme + apoenzyme) activity was measured in the presence of added haematin in the other 4 studies. The rat TDO activity in these latter 4 studies was the highest or second highest among species, whereas that in the other study174 was the second lowest. The rat TDO holoenzyme activity is by no means the highest among the various species studied.7This latter study showed a clear distinction in TDO activity between species that tolerate, and those that are sensitive to, excess Trp, with the latter lacking the inactive haem-free apoenzyme and the former possessing both forms.
Table 5.
Comparison of enzymes of the kynurenine pathway of tryptophan metabolism across animal species.
| Enzyme | Species comparisons | Source | [Reference] |
|---|---|---|---|
| TDO: | rat > guinea pig = mouse > rabbit | liver homogenate | Allegri et al78 |
| IDO: | rat > rabbit > guinea pig > mouse | ||
| KMO: | mouse > rat > guinea pig > rabbit | ||
| KAT: | rat > mouse > rabbit > guinea pig | ||
| KYNU: | guinea pig > rat > mouse > rabbit | ||
| 3-HAAO: | guinea pig > rabbit > rat> mouse | ||
| ACMSD: | guinea pig > rat > mouse > rabbit | ||
| TDO: | pig > rat > turkey > chicken > mouse > frog > hamster > guinea pig > rabbit = gerbil > ox = sheep | liver homogenate | Badawy and Evans7 |
| TDO: | rat > rabbit > mouse > gerbil | liver homogenate | Fujigaki et al176 |
| TDO: | mouse > dog > monkey > rat > human | Liver cytosol (based on Vmax) | Wang et al174 |
| TDO: | rat > rabbit > mouse > gerbil | liver | Murakami and Saito177 |
| IDO: | rabbit > gerbil > mouse > rat | lung | |
| gerbil > rabbit > mouse > rat | brain | ||
| KMO: | rat > gerbil > mouse > rabbit | liver | |
| Mouse > rat = gerbil > rabbit | lung | ||
| KYNU: | mouse > gerbil > rabbit > rat | liver | |
| IDO1: | platypus > opossum > mouse | transfected cell lysates | Yuasa et al178 |
| IDO2: | platypus > mouse > opossum | ||
| TDO: | rat > cat | liver | DeCastro et al179 |
| KAT: | rat > cat | kidney | |
| KMO: | cat > rat | liver, kidney | |
| KYNU: | rat ⩾ cat | liver | |
| cat ⩾ rat | kidney | ||
| KYNU | Suncus (shrew) > rat > human | liver | Ishikawa et al180 |
| 3-HAAO: | sunfish > bass > terrapin > chicken > | liver | Lan and Gholson181 |
| frog > crayfish (zero) | |||
| ACMSD: | bass > sunfish > terrapin > frog > chicken | liver | |
| AMSD: | frog > terrapin > chicken > sunfish | liver | |
| 3-HAAO: | rat > cat > beef | liver | |
| ACMSD: | cat > rat > beef | liver | |
| AMSD: | cat > rat = beef | kidney | |
| KYNU: | rat ⩾ cat | liver | Ikeda et al5 |
| 3-HAAO: | rat > cat | liver | |
| ACMSD: | cat > lizard > frog > cattle > pig > pigeon > rabbit > mouse > guinea pig > chicken > human > hamster > toad > rat | liver |
Comparisons of TDO among strains
Strain differences in TDO activity have been studied largely in mice, but also in rat strains developed for alcohol preference. In mice, TDO activity differs among strains as follows: C57BL > AKR > C3H = DBA.183 TDO of the C57BL is double that of the CBA strain.184,185 TDO activity in albino NCL mice is 52% higher than in Swiss albino mice.186 The C57BL strain is notable for its preference over other strains for alcohol intake in a free choice drinking situation.185 In rats, TDO activity is comparable among the Wistar, heterozygous Gunn and Sprague-Dawley, and higher than in the Long-Evans strain.187 However, TDO activity of Sprague-Dawley rats was reported to be moderately (31%) higher than that of the Wistar strain.188 Rat strains have also been developed to prefer alcohol and have been shown to possess a higher TDO activity over their corresponding controls, for example, the Indiana preferring (P) versus non-preferring (nP) and the Sardinian preferring (SP) versus non-preferring (SnP) strains.189 The higher TDO activity may be due to the higher [corticosterone] in both preferring strains and, additionally enhanced gene expression of the enzyme, as demonstrated with the SP strain and also with the C57BL versus CBA.189 TDO also exists in flies. The enzyme in Drosophila melanogaster has been assayed without added haematin and its activity appears to be equally distributed between head, thorax and abdomen.190 Expressed per weight, TDO is more active in flies than in rats. Activity of the enzyme in flies is also strain-dependent in the following descending order: BW > Sevelon > Oregon-R, with minimal activities in several other strains.190
TDO in other species
In addition to mammals, TDO exists in many non-mammalian species, including teleosts [rainbow trout,168 Senegal soles (Solea senegalensis),166 Atlantic salmon (Salmo salar), Japanese rice fish (Oryzias latipes) and zebra fish (Danio rerio).131 It also exists in poults,171 honey bees,191 mosquitoes192 and across metazoans.175 The TDO of the mosquito Culex pipiens appears to be fully saturated with haem,193 thus possibly existing in the holoenzyme form, and, its kynurenine pathway is limited to production of xanthurenic acid. Wild carnivores are almost certain to express TDO, otherwise they would be unable to handle their high Trp intake.
Species TDO response to glucocorticoids and tryptophan
As stated above, species sensitive to Trp toxicity lack the TDO apoenzyme and are also unresponsive to its induction by glucocorticoids. As shown by Knox6 glucocorticoid induction of apo-TDO synthesis protects adrenalectomized rats against Trp toxicity. Glucocorticoid induction of TDO can therefore be regarded as a Trp detoxification mechanism. Species lacking the haem-free apoenzyme, however, process Trp somewhat differently from those possessing the apoenzyme in an apparent attempt to dispose of it rapidly. For example, their TDO is activated earlier and by smaller doses of Trp than the rat TDO. Thus, at 30 minutes after Trp administration, the TDO of golden hamster > guinea pig > frog is activated by a 50 mg/kg dose and maximally by a 150 mg/kg dose, whereas the rat TDO is unresponsive to any Trp dosage up to 500 mg/kg at this time-interval.7 Whereas haem (haematin) and its precursor 5-aminolaevulinate and agents that displace Trp from albumin-binding sites, such as ethanol and salicylate, activate the rat TDO by cofactor or substrate- mediated mechanisms, they fail to do so in guinea pigs or golden hamsters.7,194 Different responses are also observed in mouse strains. Thus, TDO of the C57BL strain exhibits a greater response to induction by cortisol or dexamethasone than other strains.183,189 By contrast to TDO, Tyr aminotransferase induction by dexamethasone or cortisol is comparable between C57BL and CBA.189 In rabbits, where the glucocorticoid induction mechanism is absent, repeated triamcinolone acetonide administration, which induces TDO in rats,195 does not decrease plasma [Trp], but decreases plasma [Tyr],196 suggesting induction of Tyr aminotransferase, but not TDO. Response of TDO to Trp, however, is less variable among mouse strains, with C57BL = DBA = AKR > C3H.183
Trp, however, has been reported not to activate TDO at 4 to 16 hours after administration to the channel catfish (Ictalurus punctatus).156 These latter authors suggested seasonal variations as an explanation, though activation at an earlier time-interval is also possible. If this fish TDO does not respond to Trp, it could be especially vulnerable to its toxicity, as suggested by the rapid death of half the treated fish within 12 to 16 hours,156 relative to steers dying from Trp over 1 to 7 days.154
Regulation of TDO
TDO regulation has been extensively studied following the pioneering work of W. Eugene Knox, Olga Greengard, Phillip Feigelson and others in the ~ 1950s with the discovery of its induction by glucocorticoids and activation by Trp. TDO has since been at the centre of the concept of enzyme regulation. Many reviews of TDO regulation have been published. Briefly, the enzyme is regulated by at least 4 major mechanisms: hormonal induction by glucocorticoids, substrate activation and stabilisation by Trp, cofactor activation by haem and feedback or end-product inhibition by NAD(P)H. While this is a general outline of these mechanisms, more complex aspects are involved, such as the roles of insulin, glucagon and other hormones and of the haem cofactor in the glucocorticoid mechanism, and the ability of some kynurenine metabolites (KA and 3-HAA) to activate TDO.8
Functions of TDO
TDO performs a range of important functions.8,15 These include detoxification of excess Trp, control of Trp availability to other tissues, including the brain for serotonin synthesis, regulation of hepatic haem biosynthesis by its preferential utilisation of the small regulatory-haem pool in the hepatic cytosol, control of production of kynurenine metabolites and of NAD+ synthesis. These functions and the resulting physiological changes extend across many body systems with important implications for a range of health issues.
Indoleamine 2,3-dioxygenase (IDO)
Species comparisons and expressions of IDO
As well as the comparative studies of IDO activity in the mammalian species outlined in Table 5, and that comparing IDO activity in mice, opossum (Monodelphis domestica) and the semiaquatic mammal the platypus (Ornithorhynchus anatinus),178 most other studies of IDO were performed in aquatic species. In the Yuasa et al178 study, IDO1 activity in descending order was: platypus > opossum > mouse, and IDO2 was platypus > mouse > opossum. IDO is expressed in many fish species, including the rainbow trout (Oncorhynchus mykiss),197 the grass carp (Ctenopharyngodon idella)198 and many other teleosts.199 The IDO of the grass carp can be activated by the haem precursor 5-aminolaevulinate, thus suggesting that it is not fully haem-saturated. As well as in the mammalian species studied above, IDO is also expressed in invertebrates in at least 15 species within 6 phyla.175
IDO isoforms and variants and their functional differences
All vertebrae express both IDO1 and IDO2. IDO1 is expressed in human, gorilla, macaque, goat, chiru, yak, cattle, bat, domestic cat, rat, giant panda, dog, hamster, Tasmanian devil and all other mammalian species.199 IDO2 is also expressed in the above mammals and in amphibia, avians, reptiles (lizards and alligators) and certain teleosts.199 Seven teleost and 2 turtle species express both isoforms, whereas many other teleosts express only IDO2.199 As well as IDO1 and IDO2, an IDO3 variant is expressed in a mollusc (M. yessoensis) and variants across many mammalian and other species with altered catalytic activity have also been identified.175
Kinetic parameters differ among IDO isoforms. With IDO1, substrate (L-Trp) affinity (expressed as the Km in µM) is generally relatively high (14-22 µM for human < mouse < platypus), compared with Japanese Medaka (351 µM).199 In rat and mouse tissues, predominantly placenta, lung and intestine, the IDO1 Km varies between 20 and 50 µM.8 IDO1 thus exhibits a greater affinity for its Trp substrate, compared with TDO2, whose Km is 190 µM. The greater capacity of TDO2 towards Trp enables it to handle large increases in dietary [Trp], whereas, under these conditions, IDO1 is at a disadvantage, as its activity is inhibited by Trp200 with a Ki of 50 µM for the human enzyme.201 IDO1 inhibition by Trp involves reversed sequence of binding of O2 and Trp: with O2 binding preceding that of Trp at low [Trp] and the opposite sequence occurring at high [Trp].202 IDO2 has a much reduced affinity for its Trp substrate, with Km values in the high mM range, except that (81 µM) of the lizard (Anolis carolinensis).199 While IDO2 of many species exhibits a very low affinity for its Trp substrate, occasionally wild-type species IDO2 shows comparable affinity to that of IDO1, for example, the sponge Amphimedon queenslandica, with Km values of 4.7 and 7.7 µM respectively.175 Variants with amino acid residue modifications can alter the affinity of IDOs considerably in both directions.175
IDO and immune activity
IDO’s major role is that of interaction with the immune system.203 Immune activation induces IDO1 and results in accelerated Trp degradation along the KP, leading to depletion of Trp and simultaneous production of its immuno-reactive and other metabolites. In particular, 3-HK, 3-HAA and QA cause immunosuppression of allogeneic T-cell proliferation additively204 and by an apoptotic mechanism.205 At 10 µM, induction of apoptosis is significant with 3-HAA > QA > 3-HK, with Kyn or anthranilic acid (AA) being inactive.205 The Kyn transamination product kynurenic acid (KA) possesses both pro- and anti-inflammatory properties206,207 and is the KP metabolite with the greatest affinity for the aryl hydrocarbon receptor (AhR), followed by xanthurenic acid (XA), with Kyn itself being the weakest.208 While Kyn has generally been assumed to be an active KP ligand of the AhR, its role in vivo is likely to be that of the KA precursor, in view of the importance of substrate availability for kynurenine aminotransferase, given the high Km of the enzyme for Kyn (0.96-4.7 mM).8 The importance of KA in AhR activation is further suggested by the discovery of a new metabolic immune check-point: IL-4-induced gene 1 (IL4I1 or L-phenylalanine oxidase), which activates the AhR via KA and also the Trp transamination metabolite IPA.209
The AhR, a ligand activated transcription factor, can elicit both destructive and protective effects through binding respectively to exogenous or endogenous ligands. Exogenous ligands are activated to toxic metabolites through induction of cytochrome P-450 enzymes and this presents danger to aquatic and other wild life when exposed to industrial chemicals. Exogenous AhR ligands include the chemical 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and other halogenated aromatic hydrocarbons, for example, dibenzofurans and biphenyls, polycyclic aromatic hydrocarbons, such as 3-methylcholanthrene, benzo-[a]-pyrene, benzanthracenes and benzoflavones, indirubin and 6-formylindolo(3,2-b) carbazole (FICZ). Their harmful effects depend on the rate of their metabolic clearance, with effects of the high affinity AhR ligand TCDD lasting for days, because of its poor metabolism due to its 4 Cl atoms impeding access by metabolising enzymes.210
Endogenous ligands derived from Trp metabolism via the KP are KA, XA and Kyn, whereas many other ligands are derived from other Trp-metabolic pathways and include indole, indol-3-ylacetic acid, indol-3-yllactic acid, indol-3-ylpyruvic acid, indol-3-ylaldehyde and indoxyl sulphate.211-215 AhR activation by endogenous ligands is generally associated with positive immune changes, but excessive activation can induce negative effects. The AhR controls IDO1 expression through an autocrine loop of AhR-IL-6-STAT3 signalling216,217 and whereas KA induces IL-6, IL-6 generation by inflammation can induce IDO1 to produce enough KA to activate the AhR.208
Another important function of the AhR is control of expression of poly (ADP-ribose) polymerases (PARPs).209,218 PARPs are NAD+ consumers and their excessive activation can deplete this vital cellular effector and also ATP, resulting in cell death.
Because of the wider exposure of aquatic species to environmental pollutants and other chemicals, the role of the AhR in their susceptibility to chemical toxicity has received a great deal of research interest, a detailed discussion of which is outside the scope of this review. However, a number of aspects of AhR biology in aquatic species are noteworthy, for example, the lower affinity of the AhRs of the frog, salamander and other extant amphibian orders to TCDD, compared with vertebrates and teleosts,219 the greater sensitivity to AhR induction by β-naphthoflavone of brown trout > rainbow trout > brook trout > European eel > white sturgeon > Atlantic salmon over several other fish species,220 the wide variations in AhR activation potencies of a broad range of poly aromatic hydrocarbon and heterocyclic compounds in fish, avian and mammalian systems,221 the wide variations in susceptibility of fish species to early life mortality induced by TCDD, with trout showing the least susceptibility and zebra fish the greatest222 and the tissue specificity of AhR expression in rainbow trout as a function of the CYP isoforms.223
Whereas the role of the major cytokine and IDO1 inducer interferon-γ (IFN-γ) in vertebrate physiopathology is well known, less information is available on its role and that of its variants in teleosts224 and potentially other aquatic species. Despite the large literature on the AhR and the likely IDO1 induction by IFN-γ in aquatic species, Trp metabolism along the KP has received little attention in these species. Persistent exposure of the common minke whale (Balaenoptera acutorostrata) to organochlorines downregulates liver kynureninase,225 suggesting a potential elevation of kynurenine and KA levels and a consequent AhR activation. As will be discussed below, inhibition of N′-formylkynurenine formamidase by the organophosphate insecticide diazinon paradoxically raises levels of Kyn and KA in mice and a potentially similar effect in aquatic species could occur. Clearly more studies of the KP in aquatic species are desirable.
Regulation of IDO
Unlike TDO, IDO is not inducible by glucocorticoids and its activation by Trp is limited, partly because of its high affinity for Trp (Km = 20-50 µM) and its inhibition by [Trp] ⩾50 µM (see above). Interferons (IFNs) are the major IDO effectors, with IFN-γ being the strongest inducer.226 Glucocorticoids, however, exert differential effects on IDO induction by IFNs, namely inhibition and potentiation. Thus, IDO induction by IFN-α is inhibited by dexamethasone, whereas that by IFN-γ is potentiated226 and this may explain in part the efficacy of dexamethasone in therapy of COVID-19 infection, wherein IFN-α appears to play a major role.227 Other proinflammatory cytokines such as interleukin IL-1β, IL-6 and tumour necrosis factor-α (TNF-α) also induce IDO or potentiate its induction by IFN-γ, whereas anti-inflammatory cytokines such as IL-4, IL-10 and TGF-β inhibit IDO induction by IFN-γ.228 Thus, the balance between proinflammatory and anti-inflammatory cytokines determines the IDO status. NO is also a negative IDO effector.
Although IDO is a haemoprotein, many enzymatic studies using tissue preparations do not include added cofactor, thus suggesting that the enzyme exists fully saturated with haem. An exception to this notion is that of haem activation of the grass carp IDO described above. However, in instances where newly synthesised apo-IDO is studied, addition of haem activates the enzyme, whereas haem deprivation inhibits it.203 Regulation of IDO is a complex and multi-faceted process extending beyond these aspects, as detailed in the excellent review by Yeung et al.203
As TDO and IDO are both hemoproteins, the question of whether their activities could be influenced by changes in body iron levels and whether species differences in these levels could be involved has not been studied in detail. Species differences in body Fe have been compared in a limited number of studies. Thus, whole blood [Fe] is the highest in dog > horse > rat > human > rabbit > hamster > mouse.229 Wide variations in the total body Fe content in 3 Clarias (walking catfish) species have been reported.230 Of 8 fish species in India, C. fasciata shows the largest Fe content.231 As far as we could ascertain, no comparative studies of any potential effect of differences in iron content on the above 2 hemoproteins have been reported. However, differences in Fe content could, at least theoretically, influence the 2 enzymes, especially if Fe levels are extremely large or small. The wide variation in body Fe in above 3 Clarias could render these species a suitable model for such a study. In experimental Fe deficiency in rats, liver TDO activity is doubled and the response of the enzyme to induction by glucocorticoids or activation by Trp is not impaired.232 The increased TDO activity in this study232 however appears to be glucocorticoid-mediated, rather than Fe-related, as Fe-deficiency induced weight loss, akin to that caused by starvation, which is associated with a corticosterone-mediated TDO induction.233 In this model of iron deficiency anaemia, which induces significant decreases in levels of blood haemoglobin, serum Fe and liver non-heme iron, levels the major hepatic hemoprotein, cytochrome P-450, which utilises ~ 66% of hepatic heme,234 are not impaired.235 As far as we could ascertain, the potential effects of iron overload on TDO activity are unknown. By contrast, the effects of Fe on IDO1 activity appear to be cell- and/or tissue-dependent. Thus, Fe activates IDO1 in brain, and cultured microglia and the purified human recombinant enzyme,236 but inhibits the IFN-γ induction of the enzyme in Hep-2 cells.237 Whereas iron deficiency anaemia can exert profound effects on the immune system including cytokine abnormalities,238,239 sequestration of Fe blocks its IDO activation.236 Clearly more systematic studies in this area are warranted.
Does IDO play a role in tryptophan toxicity?
The mechanism of the toxic effects of Trp in sensitive species is not fully delineated. Knox6 suggested that the absence of glucocorticoid induction of TDO by adrenalectomy in rats diverts Trp metabolism to other pathways, the products of which may be toxic. 5-Hydroxytryptamine was implicated in death of rats treated with large doses of Trp, based on increased death by co-administration of the monoamine oxidase inhibitor pargyline and protection by the TPH inhibitor p-chlorophenylalanine.240 In cattle which are prone to emphysema, ruminant bacteria may enhance the conversion of excess Trp to indole metabolites.155 In fact, Trp metabolism in steer occurs mainly (78%) in indole-forming pathways, with the kynurenine pathway contributing only ~10%. Many indoles are AhR agonists, including indole itself, 5-hydroxyindole, 3-methylindole, indol-3-ylacetic acid and tryptamine. 3-Methylindole causes pulmonary oedema and emphysema in cattle and goats.241 Moffett and Namboodiri242 suggested that Trp toxicity in Trp-sensitive species may involve immune activation with consequent IDO induction and production of immunosuppressive kynurenine metabolites. However, this implies an initial development of an inflammatory state to elicit toxicity. In this regard, it would be of interest to know if Trp-sensitive species are more prone to infections and if the greater induction of IDO by LPS reported in gerbils243 (see above) could be demonstrated in other Trp-sensitive species.
Serotonin is unlikely to be involved in Trp toxicity, as excess Trp exerts a substrate inhibition of TPH,244 hence the failure of brain 5-HT to rise further beyond levels observed at brain [Trp] of ⩾ 79 µM by doses of Trp of ⩾ 25 mg/kg body weight in rats.245 Although p-chlorophenylalanine irreversibly inhibits TPH thereby decreasing 5-HT synthesis, it also inhibits the production of IFN-γ in human blood cells at a relatively low concentration (5 µM),246 an effect likely to weaken or block IDO1 induction by this cytokine. Whereas microbiota in ruminants play a significant role in Trp metabolism, the indole metabolites produced can collectively induce a significant activation of the AhR and a consequent induction of IDO. However, in non-ruminant Trp-sensitive species, the role of gut microbiota and/or IDO in Trp toxicity is less clear and thus requires investigation. A rank score of IDO1 activity in the limited comparative studies summarised in Table 5 suggests that the 3 Trp-sensitive species (gerbil = rabbit > guinea pig) possess higher IDO activity than the 2 Trp-tolerant species (rat > mouse). Heyes et al243 showed that basal IDO activity in gerbil lung is 13.2-fold greater than that in rats and that IDO induction by lipopolysaccharide (LPS: endotoxin) is 153-fold greater in gerbil. From this limited information, it can be suggested that IDO may play a role in Trp toxicity in Trp-sensitive species, but more comparative studies in both sensitive and tolerant species are required before this possibility can be established with certainty.
N′-Formylkynurenine formamidase
Formamidase exists mainly in liver, kidney and brain, with a Km of 50 to 180 µM,8 and is widely distributed across species, including flies,190 mosquitos192 cattle and rainbow trout.247 In mice, formamidase activity is high in liver, almost double that in kidney and deletion of its gene almost completely abolishes its activity in liver and reduces it by 87% in kidney.248 Deletion, however, does not inactivate some formamidase variants or enzymes with formamidase activity, as suggested by their presence in Drosophila melanogaster,249 pig liver250 and mouse brain.251 Deletion of the formamidase gene does not influence plasma [Trp].248 Inhibition studied of the liver enzyme from rainbow trout and cattle247 show that Trp is a poor inhibitor, whereas Kyn and 3-HK cause significant inhibition at 50-150 µM. By contrast, certain metal cations are powerful inhibitors at 1 mM. In rainbow trout, HgCl2 and ZnCl2 cause 84% and 95% inhibition, whereas in cattle, ZnCl2, BaCl2 and CaCl2 cause 50%, 81% and 86% inhibition respectively. Studies with purified formamidase from adult chicken liver show that Ag+ is the strongest inhibitor, causing 89% inhibition at 10 µM.252 It is currently unknown if formamidase inhibition by metal cations can paradoxically lead to elevation of Kyn and KA levels, as is the case with the organophosphate insecticide diazinon, which causes 5-fold elevation of plasma [Kyn] and greatly increased urinary [KA] and [XA] and to a lesser extent urinary [QA],253 suggesting increased transamination of Kyn to KA and of 3-HK to XA and that, in view of the relatively greater affinity of KMO for Kyn and of kynureninase for 3-HK (14-25 and 77 µM, respectively), greater conversion of kynurenine to the immunosuppressive metabolites 3-HK, 3-HAA and QA can be expected with formamidase inhibition.
Other enzymes of the Kynurenine pathway
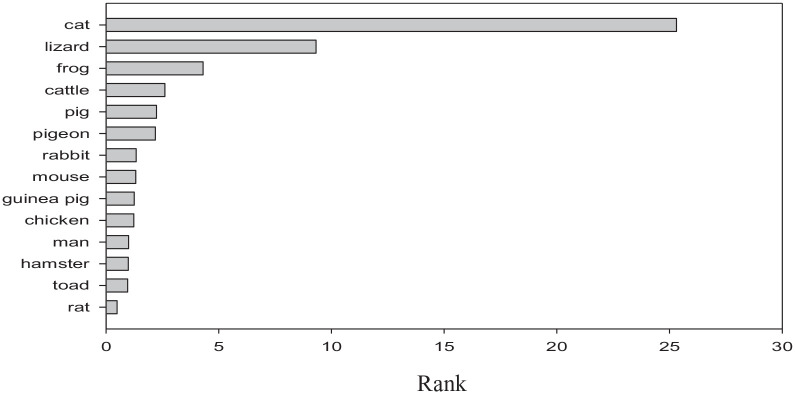
The 2 studies in Table 5 comparing liver KMO activity across 5 species suggest the following decreasing order: gerbil > rat = guinea pig > mouse > rabbit. In liver, KMO activity is also higher in rats > cats. Other tissues contain lower enzyme activity and, in lung, the enzyme is highest in mouse > rat = gerbil > rabbit. Thus, there is no distinctive pattern of differences in KMO between Trp-sensitive and –tolerant species. KMO is the gateway for production of 3-HK, 3-HAA and QA and also, by inference, NAD+. Its activity is therefore vital for immune, neuronal and other cellular activities. Also, as shown in Table 5, little information is available to establish a distinction between Trp-sensitive or – tolerant species in activities of kynurenine aminotransferase, kynureninase or 3-hydroxyanthranilic acid 3,4-dioxygenase, perhaps because of the different experimental conditions between studies. The only exception is the lower activities of TDO, KAT and KMO in cats, relative to those in rats.179 Only activity of hepatic 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) (picolinate carboxylase) has been compared across 14 species in a single study by Ikeda et al5 Assigning a score of 1 for the human ACMSD activity in Figure 4, of the species studied by Ikeda et al5 the rat enzyme exhibited only ~50%, whereas that of toad and hamster was comparable to that, of the human enzyme. All other 10 species listed exhibited greater enzyme activities than humans. In particular, frog < lizard < cat showed the highest activities. ACMSD is an important KP enzyme, as its activity controls the progress of the pathway towards QA and hence NAD+ synthesis or towards picolinic acid (PA) and eventual formation of acetyl CoA. Inhibition of ACMSD in humans by phthalates increases QA production and its urinary excretion.254 The particularly higher ACMSD activity in the above 3 species renders them unable to effectively utilise Trp as a source of NAD+. In the cat, this is further compromised by the lower activity of 3-HAAO compared to the rat, though the 2 species do not differ in quinolinate phosphoribosyltransferase (QPRT) or AMS dehydrogenase.5
Figure 4.
Ranking of ACMSD activity among species.
Ranking was based on levels of enzyme activity similarly expressed in a single study by Ikeda et al.5
Urinary metabolite excretion patterns can provide indirect pointers to enzyme activities. In rat strains, urinary excretion of Trp metabolites of the KP is similar among the Wistar, heterozygous Gunn and Sprague-Dawley strains and higher than in Long-Evans, consistent with the lower TDO activity in the latter strain.187 Data on urinary excretion of kynurenine and other KP metabolites were compared in 4 species.255 Urinary kynurenine levels were highest in dog > man > cat > rat (23.6, 14.3, 1.4, and 0.5 µmol/day respectively: n = 5, 9, 2 and 5, respectively). After Trp loading, kynurenine metabolite excretion as a % of the administered dose was highest in dog = rat > man > cat, thus further confirming the low TDO activity in cats.
Species differences in efficiency of tryptophan conversion to niacin and NAD+
In mammals, Trp is a more efficient precursor of NAD+ than the 2 forms of niacin, nicotinamide and nicotinic acid.256,257 The elegant work of the group of D. A. Bender on kinetic and other activities of enzymes involved in NAD+ synthesis from its various precursors has established the mechanisms behind this concept (see Badawy8 for discussion and references). As discussed above, ACMSD activity is a major determinant of the progress of the KP towards QA and NAD+ synthesis in the de novo pathway or diversion towards PA and eventual formation of acetyl CoA. As the cat possesses the greatest ACMSD activity among species, its ability to form NAD+ from Trp is severely impaired, with its production of nicotinic acid ribonucleotide from 3-HK being only 11% of that of the rat,5 hence its reliance of exogenous niacin in the Preiss-Handler pathway from nicotinic acid and/or the salvage pathway from nicotinamide. Trp is also a poor precursor of NAD+ synthesis in the channel catfish Ictalurus punctatus,258 the rainbow and brook trout and many other salmonid species, due to their high ACMSD activity139,259,260 Other than frog, (see above), very little is known about ACMSD activity or efficiency of Trp conversion to NAD+ in other Trp-sensitive species.
Species Differences in the Serotonin Pathway
As far as we could ascertain, little work has been published on species differences in Trp metabolism by pathways other than the KP. Only the serotonin pathway has received some attention and available comparative data are discussed below. No attempt will therefore be made here to consider the tryptamine or indolepyruvate pathways, nor microbiota Trp metabolism.
While serotonin is synthesised from Trp in a whole range of species and performs important functions in many physiological processes, including mood in humans,148 aggression in vervet monkey,87 injurious behaviour in pigeon, hens and parrots150 or catecholamines metabolism in rats,149 surprisingly little comparative studies of serotonin pathway activity across species or strains thereof have been reported and these are mainly concerned with response of the pathway to manipulations, such as stress.261 alterations in dietary Trp,262 monoamine transporters in humans, rats and mice,263 behavioural variability in Medaka fish strains264 and mouse strain differences in serotonin-specific reuptake inhibitor sensitivity.265
Table 6 summarises data published on species content of brain Trp and its 5-hydroxyindole metabolites 5-HT and 5-HIAA and a few species or strain comparisons. It is clear that much information is required to fill in the gaps in our knowledge of serotonin metabolism in various species. The brain Trp concentrations in rats and gerbils reported by Green et al182 appear excessive, at least in rats, compared to other data in the Table. Whereas no clear distinction exists between Trp-sensitive and – tolerant species in 5-HT metabolism, the data in Table 6 suggest provisionally that teleosts form less 5-HT than vertebrates and this appears consistent with their lower plasma [Trp]. In the absence of more detailed information, no other conclusions can be drawn from the data in Table 6, particularly given that brain 5-HT levels are controlled by several mechanisms, including brain [Trp] and activities of TPH2, ALAAD and MAO. TPH2 is widely distributed across species, even if its activity varies with species, for example, the rat brain TPH2 is more active than that of human or chimpanzee.272 TPH2 activity is, however, limited mainly by brain [Trp] because of the partial saturation of the enzyme with its substrate. ALAAD activity also varies across species. Thus, in a detailed study by Robins et al10 the brain enzyme activity in decreasing order was as follows: guinea pig > mouse > hamster > rat > rabbit > chicken > squirrel monkey > cat > turtle > carp > Macaca monkey > Cynomolgus monkey, with the human brain enzyme activity being lower than the last of these species. By contrast with the brain, the liver ALAAD is most active in rat > mouse > guinea pig > hamster > rabbit,10 with the human enzyme activity averaging just below that of turtle. With MAOA, the subtype that prefers 5-HT as substrate, few studies of species differences have been published, for example, gene functional variability in macaque species in relation to aggressive behaviour273 and oxidative capacity of the enzyme in human, rabbit, rat, mouse, bovine and subhuman primate liver.274
Table 6.
Concentrations of tryptophan and its 5-hydroxyindole metabolites in brains of various species.
| Species | Trp | 5-HTP | 5-HT | 5-HIAA | Brain tissue | [Reference] |
|---|---|---|---|---|---|---|
| Rat (10) | 33.3 | 2.95 | 1.88 | whole brain | Green et al182 | |
| Gerbil (6-9) | 29.7 | 3.22 | 2.30 | |||
| Rat* (6) | 18.02 | 0.22 | 2.64 | 1.86 | 4 brain structures | Lane et al77 |
| Pigeon* (6) | 25.25 | 0.64 | 3.00 | 0.86 | ||
| Rat (26) | 12.53 | 2.72 | 1.73 | whole brain | Curzon and Knott266 | |
| (42) | 12.49 | 2.89 | 2.14 | whole brain | Badawy and Evans184 | |
| Mouse | ||||||
| (CBA/Ca) (6) | 9.01 | 5.33 | 2.25 | whole brain | Badawy et al185 | |
| (C57BL (6) | 7.00 | 4.09 | 2.04 | whole brain | ||
| Weaned pig (8) | 4.30 | hypothalamus | Lv et al172 | |||
| Guinea pig | 11.46 | 2.60 | 2.72 | whole brain | Shukla and Chandra81 | |
| Golden hamster | 12.60 | 4.29 | hypothalamus | Payne et al267 | ||
| 10.55 | 5.49 | mid-brain | ||||
| Rhesus monkey | 0.21 | CSF | Weld et al167 | |||
| Meagre* (7-10) | 7.35 | 0.57 | whole brain | Herrera et al169 | ||
| Senegalese soles (10) | 39.17 | 1.70 | whole brain | Salamanca et al166 | ||
| Medaka fish (Oryzias Sp): | Audira et al264 | |||||
| O. dancena | 1.03 | |||||
| O. latipes | 0.74 | |||||
| O. woworae | 1.15 | |||||
| O. sinesis | 0.77 | |||||
| Common carp | 1.59 | whole brain | Jia et al268 | |||
| Rainbow trout | 10.00 | 1.4 | 2.55 | whole brain | Johnston et al269 | |
| Rainbow trout: | 12.24 | 8.39 | 1.86 | telencephalon | Lepage et al270 | |
| 9.79 | 4.24 | 0.32 | hypothalamus | |||
| 2.45 | 1.25 | 0.39 | brain stem | |||
| 1.22 | 1.50 | 0.35 | optic tectum | |||
| South American | 7.45 | 1.93 | averaged fore-and hind-brain | Morandini et al271 | ||
| Cichlid (6) | ||||||
All values are in µM. When expressed differently by the authors (eg, in µg/g of tissue or per mg of protein) they were converted into µM by the present authors. *Values were erroneously presented in mg/g tissue, but should have been in µg/g, otherwise they would be 1000-fold higher than presented above.
Conclusions and Comments
Whereas the present review has provided an updated comparison of tryptophan metabolism and disposition across a range of animal species, it has exposed the many gaps to be addressed in our knowledge in this area in many species. It is hoped that this account will provide a platform and a stimulus for further studies of species differences in Trp metabolism. A number of aspects are noteworthy in this regard. The wide species variations in plasma albumin can predict likely levels of Trp binding and availability to tissues of many species. In wild carnivores, much of plasma Trp can be expected to be albumin-bound. In aquatic species, the low levels or absence of albumin suggests a largely unimpeded entry into tissues of environmental pollutants: a vulnerability that reinforces the need for stringent safeguards against industrial pollution. A limited number of species have been shown to be sensitive to toxicity of excess Trp due to their lack the free apoenzyme form of Trp 2,3-dioxygenase and its glucocorticoid induction mechanism. While wild carnivores are almost certain to tolerate excess Trp by virtue of their Trp-rich prey diet, many aquatic species could be vulnerable to Trp. Because aquatic species are particularly vulnerable to the harmful effects of chemical pollutants, the AhR has received much attention in immune studies in these species. However, by contrast, little is known about changes in IDO1 elicited by the immune insults induced by AhR activation by chemicals. The potential role of IDO in Trp toxicity requires assessment in comparative studies. The importance of the kynurenine pathway in species physiology further extends to synthesis of the vital cellular effector NAD+ with ACMSD activity playing a decisive role in this process. Nutritional studies in species should explore the role of this enzyme.85
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AA-BB received no financial support for the research, authorship, and/or publication of this article. GJG is supported by the National Health and Medical Research Council (NHMRC) and Macquarie University.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AA-BB wrote the first draft. AA-BB and GJG revised it and approved the final version.
AA, anthranilic acid; ACMSD, 2-amino-3-carboxymuconic acid-6-semialdehyde decarboxylase; ALAAD, aromatic L-amino acid decarboxylase; 3-HAA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine or serotonin; IDO, indoleamine 2,3-dioxygenase; IAA, indol-3-ylacetic acid; IPA, indol-3-ylpyruvic acid; KA, kynurenic acid; Kyn, kynurenine; NAD+, oxidised nicotinamide-adenine dinucleotide; PA, picolinic acid; PLP, pyridoxal 5′-phosphate; QA, quinolinic acid; ROS, reactive oxygen species; Trp, L-tryptophan; TDO, Trp 2,3-dioxygenase; TPH, Trp hydroxylase; XA, xanthurenic acid.
References
- 1. Walton MJ, Coloso RM, Cowey CB, Adron JW, Knox D. The effects of dietary tryptophan levels on growth and metabolism of rainbow trout (Salmo gairdneri). Br J Nutr. 1984;51:279-287. [DOI] [PubMed] [Google Scholar]
- 2. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:101-197. [DOI] [PubMed] [Google Scholar]
- 3. Metcalf VJ, Brennan SO, George PM. The Antarctic toothfish (Dissostichus mawsoni) lacks plasma albumin and utilises high density lipoprotein as its major palmitate binding protein. Comp Biochem Physiol B. 1999;124:147-155. [DOI] [PubMed] [Google Scholar]
- 4. Metcalf VJ, Brennan SO, Chambers G, George PM. High density lipoprotein (HDL), and not albumin, is the major palmitate binding protein in New Zealand long-finned (Anguilla dieffenbachii) and short-finned eel (Anguilla australis schmidtii) plasma. Biochim Biophys Acta. 1999;1429:467-475. [DOI] [PubMed] [Google Scholar]
- 5. Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide II. A role of picolinic acid carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem. 1965;240:1395-1401. [PubMed] [Google Scholar]
- 6. Knox WE. The regulation of tryptophan pyrrolase activity by tryptophan. Adv Enzyme Regul. 1966;4:287-297. [DOI] [PubMed] [Google Scholar]
- 7. Badawy AA, Evans M. Animal liver tryptophan pyrrolases: absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976;158:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938-1178646917692020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badawy AA. Tryptophan metabolism: a versatile area providing multiple targets for pharmacological intervention. Egypt J Basic Clin Pharmacol. 2019;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robins E, Robins JM, Croninger AB, Moses SG, Spencer SJ, Hudgens RW. The low level of 5-hydroxytryptophan decarboxylase in human brain. Biochem Med. 1967;1:240-251. [Google Scholar]
- 11. Gál EM. Tryptophan-5-hydroxylase: function and control. Adv Biochem Psychopharmacol. 1974;11:1-11. [PubMed] [Google Scholar]
- 12. Carlsson A, Lindqvist M. Dependence of 5-HT and catecholamine synthesis on concentrations of precursor amino-acids in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1978;303:157-164. [DOI] [PubMed] [Google Scholar]
- 13. Chivite M, Leal E, Míguez JM, Cerdá-Reverter JM. Distribution of two isoforms of tryptophan hydroxylase in the brain of rainbow trout (Oncorhynchus mykiss). An in situ hybridization study. Brain Struct Funct. 2021;226:2265-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sánchez S, Sánchez CL, Paredes SD, Rodriguez AB, Barriga C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J Appl Biomed. 2008;6:177-186. [Google Scholar]
- 15. Badawy AA. Kynurenine pathway and human systems. Exp Gerontol. 2020;129:110770. [DOI] [PubMed] [Google Scholar]
- 16. Badawy AA. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809-815. [DOI] [PubMed] [Google Scholar]
- 17. Badawy AA-B, Morgan CJ, Llewelyn MB, Albuquerque SRJ, Farmer A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with the chronic fatigue syndrome. J Psychopharmacol. 2005;19:385-391. [DOI] [PubMed] [Google Scholar]
- 18. Badawy AA, Dougherty DM. Assessment of the human kynurenine pathway: comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int J Tryptophan Res. 2016;9:31-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badawy AA. Effects of pregnancy on tryptophan metabolism and disposition in the rat. Biochem J. 1988;255:369-372. [PMC free article] [PubMed] [Google Scholar]
- 20. Badawy AA, Morgan CJ, Dacey A, Stoppard T. The effects of lofepramine and desmethylimipramine on tryptophan metabolism and disposition in the rat. Biochem Pharmacol. 1991;42:921-929. [DOI] [PubMed] [Google Scholar]
- 21. Kaburagi T, Yamano T, Fukushima Y, Yoshino H, Mito N, Sato K. Effect of Lactobacillus johnsonii La1 on immune function and serum albumin in aged and malnourished aged mice. Nutrition. 2007;23:342-350. [DOI] [PubMed] [Google Scholar]
- 22. Viuff D, Antunes F, Evans L, et al. Generation of a double transgenic humanized neonatal fc receptor (FcRn)/albumin mouse to study the pharmacokinetics of albumin-linked drugs. J Control Release. 2016;223:22-30. [DOI] [PubMed] [Google Scholar]
- 23. Bottari NB, Tonin AA, Fighera R, et al. Neospora caninum and Toxoplasma gondii: relationship between hepatic lesions, cytological and biochemical analysis of the cavitary liquid during the acute phase of the diseases in experimental models. Exp Parasitol. 2014;136:68-73. [DOI] [PubMed] [Google Scholar]
- 24. Sansom BF, Beer RJ, Kitchenham BA. Changes in the concentrations of serum urea nitrogen, albumin, globulin, sodium and inorganic phosphorus in weaner pigs infected with Trichuris suis. J Comp Pathol. 1974;84:409-415. [DOI] [PubMed] [Google Scholar]
- 25. Lardinois R, Page LA. Serum albumin, prealbumin, and postalbumin in perinatal pigs. Dev Biol. 1969;19:261-280. [DOI] [PubMed] [Google Scholar]
- 26. Jiang J, Liu S, Jamal T, et al. Effects of dietary sweeteners supplementation on growth performance, serum biochemicals, and jejunal physiological functions of broiler chickens. Poult Sci. 2020;99:3948-3958. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oso AO, Williams GA, Oluwatosin OO, et al. Effect of dietary supplementation with arginine on haematological indices, serum chemistry, carcass yield, gut microflora, and lymphoid organs of growing turkeys. Livestock Sci. 2017;198:58-64. [Google Scholar]
- 28. Orakpoghenor O, Markus TP, Ogbuagu NE, et al. Age-dependent variations in haematological and serum biochemical parameters of domestic pigeons (Columba livia domestica). Heliyon. 2021;7:e07486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonatto NCM, de Oliveira PL, Mancebo AM, et al. Postprandial lipemia causes oxidative stress in dogs. Res Vet Sci. 2021;136:277-286. [DOI] [PubMed] [Google Scholar]
- 30. Sakai K, Maeda S, Yonezawa T, Matsuki N. Decreased plasma amino acid concentrations in cats with chronic gastrointestinal diseases and their possible contribution in the inflammatory response. Vet Immunol Immunopathol. 2018;195:1-6. [DOI] [PubMed] [Google Scholar]
- 31. Debosschere Y, Depuydt E, Pauwelyn G, et al. Safety and immunomodulatory properties of equine peripheral blood-derived mesenchymal stem cells in healthy cats. Vet Immunol Immunopathol. 2020;227:110083. [DOI] [PubMed] [Google Scholar]
- 32. Smuts MP, de Bruyn S, Thompson PN, Holm DE. Serum albumin concentration of donor cows as an indicator of developmental competence of oocytes. Theriogenology. 2019;125:184-192. [DOI] [PubMed] [Google Scholar]
- 33. Cattaneo L, Lopreiato V, Piccioli-Cappelli F, Trevisi E, Minuti A. Plasma albumin-to-globulin ratio before dry-off as a possible index of inflammatory status and performance in the subsequent lactation in dairy cows. J Dairy Sci. 2021;104:8228-8242. [DOI] [PubMed] [Google Scholar]
- 34. MacDonald JA, Degenhardt T, Baynes JW, Storey KB. Glycation of wood frog (Rana sylvatica) hemoglobin and blood proteins: in vivo and in vitro studies. Cryobiology. 2009;59:223-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grando TH, Baldissera MD, Gressler LT, et al. Melaleuca alternifolia anthelmintic activity in gerbils experimentally infected by Haemonchus contortus. Exp Parasitol. 2016;170:177-183. [DOI] [PubMed] [Google Scholar]
- 36. Monzón CM, Villavicencio VI. Serum proteins in Guinea-pigs and horses infected with Trypanosoma evansi (Steel, 1885). Vet Parasitol. 1990;36:295-301. [DOI] [PubMed] [Google Scholar]
- 37. Uniyal S, Garg AK, Jadhav SE, Chaturvedi VK, Mohanta RK. Comparative efficacy of zinc supplementation from different sources on nutrient digestibility, hemato-biochemistry and anti-oxidant activity in guinea pigs. Livestock Sci. 2017;204:59-64. [Google Scholar]
- 38. Jantawong C, Priprem A, Intuyod K, et al. Curcumin-loaded nanocomplexes: acute and chronic toxicity studies in mice and hamsters. Toxicol Rep. 2021;8:1346-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herrick RT, Jones TP, Sperber JL, Richeson JT, Brown TR, Lawrence TE. Assessment of changes in complete blood count and serum chemistry in fed Holstein steers with or without liver abscesses. Appl Animal Sci. 2020;36:256-264. [Google Scholar]
- 40. Chen GJ, Zhang R, Wu JH, et al. Effects of soybean lecithin supplementation on growth performance, serum metabolites, ruminal fermentation and microbial flora of beef steers. Livestock Sci. 2020;240:104121. [Google Scholar]
- 41. Ayyat MS, Abd El-Latif KM, Helal AA, Al-Sagheer AA. New Zealand white rabbits tolerance to chronic thermal stress at different dietary energy/protein levels. Anim Feed Sci Technol. 2021;278:114992. [Google Scholar]
- 42. da S, dos Santos D, Klauck V, Campigotto G, et al. Benefits of the inclusion of açai oil in the diet of dairy sheep in heat stress on health and milk production and quality. J Therm Biol. 2019;84:250-258. [DOI] [PubMed] [Google Scholar]
- 43. Sidki A, Hirst DH. Establishing albumin levels in sheep serum by a specific fluoroimmunoassay. Vet J. 1998;156:67-72. [DOI] [PubMed] [Google Scholar]
- 44. Kusumanti E, Sugiharto S. Effect of dietary supplementation of binahong leaf meal, betel nut meal or their combination on serum albumin and globulin, fecal endoparasites and bacterial counts in milk of Saanen goats suffering from subclinical mastitis. Agric Nat Resour. 2017;51:415-419. [Google Scholar]
- 45. Wilson GR, Hoskins L. Haematology and blood chemistry of the red kangaroo Megaleia rufa in captivity. Aust Vet J. 1975;51:146-149. [DOI] [PubMed] [Google Scholar]
- 46. Chen Z-L, Zeng W, Cheng AC, et al. Six-month repeated dose toxicity of orally administered metacavir in rhesus monkeys. Exp Toxicol Pathol. 2011;63:379-385. [DOI] [PubMed] [Google Scholar]
- 47. Wu ML, Gong L, Qian C, Liang ZG, Zeng W. Characteristics of blood chemistry, hematology, and lymphocyte subsets in pregnant rhesus monkeys. Chin J Nat Med. 2015;13:409-414. [DOI] [PubMed] [Google Scholar]
- 48. Chichester L, Gee MK, Jorgensen MJ, Kaplan JR. Hematology and clinical chemistry measures during and after pregnancy and age- and sex-specific reference intervals in African green monkeys (Chlorocebus aethiops sabaeus). J Am Assoc Lab Anim Sci. 2015;54:359-367. [PMC free article] [PubMed] [Google Scholar]
- 49. Niimi K, Morishita H, Usui M, et al. Measurement of the α1-proteinase inhibitor (α1-antitrypsin) of common marmoset and intestinal protein loss in wasting syndrome. Biosci Rep. 2019;39:1-14. doi: 10.1042/BSR20190562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fincham JE, Faber M, Weight MJ, et al. Diets realistic for westernised people significantly effect lipoproteins, calcium, zinc, vitamins C, E, B6 and haematology in Vervet monkeys. Atherosclerosis. 1987;66:191-203. [DOI] [PubMed] [Google Scholar]
- 51. Davy CW, Jackson MR, Walker J. Reference intervals for some clinical chemical parameters in the marmoset (Callithrix jacchus): effect of age and sex. Lab Anim. 1984;18:135-142. [DOI] [PubMed] [Google Scholar]
- 52. Fellows FC, Hird FJ. A comparative study of the binding of L-tryptophan and bilirubin by plasma proteins. Arch Biochem Biophys. 1982;216:93-100. [DOI] [PubMed] [Google Scholar]
- 53. Otway NM. Serum biochemical reference intervals for free-living sand Tiger sharks (Carcharias taurus) from east Australian waters. Vet Clin Pathol. 2015;44:262-274. [DOI] [PubMed] [Google Scholar]
- 54. Hattingh J, Bomzon L, Marcus E, et al. Concentration and composition of plasma proteins in wild mammals. Comp Biochem Physiol. 1983;75:441-445. [DOI] [PubMed] [Google Scholar]
- 55. Sanz MG, Schnider DR, Mealey KA. Relative deficiency in albumin methionine content is associated with decreased antioxidant capacity of equine plasma. J Equine Vet Sci. 2021;96:103277. [DOI] [PubMed] [Google Scholar]
- 56. Wade NM, Clark TD, Maynard BT, et al. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J Therm Biol. 2019;80:64-74. [DOI] [PubMed] [Google Scholar]
- 57. Bernhoft A, Høgåsen HR, Rosenlund G, et al. Effects of dietary deoxynivalenol or ochratoxin A on performance and selected health indices in Atlantic salmon (Salmo salar). Food Chem Toxicol. 2018;121:374-386. [DOI] [PubMed] [Google Scholar]
- 58. Maillou J, Nimmo IA. Identification and some properties of an albumin-like protein in the serum of pre-spawning Atlantic salmon (Salmo salar). Comp Biochem Physiol. 1993;104:401-405. [Google Scholar]
- 59. Bharti S, Rasool F. Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol Rep. 2021;8:443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang JS, Guo ZX, Zhang JD, et al. Effects of hypoxia-reoxygenation conditions on serum chemistry indicators and gill and liver tissues of cobia (Rachycentron canadum). Aquac Rep. 2021;20:100692. [Google Scholar]
- 61. Hoseini SM, Hedayati A, Taheri Mirghaed A, Ghelichpour M. Toxic effects of copper sulfate and copper nanoparticles on minerals, enzymes, thyroid hormones and protein fractions of plasma and histopathology in common carp Cyprinus carpio. Exp Toxicol Pathol. 2016;68:493-503. [DOI] [PubMed] [Google Scholar]
- 62. Ali Z, Yousafzai AM, Sher N, et al. Toxicity and bioaccumulation of manganese and chromium in different organs of common carp (Cyprinus carpio) fish. Toxicol Rep. 2021;8:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Smet H, Blust R, Moens L. Absence of albumin in the plasma of the common carp Cyprinus carpio: binding of fatty acids to high density lipoprotein. Fish Physiol Biochem. 1998;19:71-81. [Google Scholar]
- 64. İnanan BE, Acar Ü, İnanan T. Effects of dietary Ferula elaeochytris root powder concentrations on haematology, serum biochemical parameters, spermatozoa parameters, and oxidative status in tissues of males goldfish (Carassius auratus). Aquaculture. 2021;544:737087. [Google Scholar]
- 65. Abdelghany MF, El-Sawy HB, Abd El-Hameed SAA, Khames MK, Abdel-Latif HMR, Naiel MAE. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020;107:277-288. [DOI] [PubMed] [Google Scholar]
- 66. Silva SV, Mercer JR. Effect of protein intake on amino acid catabolism and gluconeogenesis by isolated hepatocytes from the cat (Felis domestica). Comp Biochem Physiol. 1985;80:603-607. [DOI] [PubMed] [Google Scholar]
- 67. Gültepe N. Protective effect of D-limonene derived from orange peel essential oil against Yersinia ruckeri in rainbow trout. Aquac Rep. 2020;18:100417. [Google Scholar]
- 68. Vazirzadeh A, Roosta H, Masoumi H, Farhadi A, Jeffs A. Long-term effects of three probiotics, singular or combined, on serum innate immune parameters and expressions of cytokine genes in rainbow trout during grow-out. Fish Shellfish Immunol. 2020;98:748-757. [DOI] [PubMed] [Google Scholar]
- 69. Pianesso D, Radünz Neto J, da Silva LP, et al. Determination of tryptophan requirements for juvenile silver catfish (Rhamdia quelen) and its effects on growth performance, plasma and hepatic metabolites and digestive enzymes activity . Animal Feed Science and Technology. 2015;210:172-183. [Google Scholar]
- 70. Bera KK, Kumar S, Paul T, Prasad KP, Shukla SP, Kumar K. Triclosan induces immunosuppression and reduces survivability of striped catfish Pangasianodon hypophthalmus during the challenge to a fish pathogenic bacterium Edwardsiella tarda. Environ Res. 2020;186:109575. [DOI] [PubMed] [Google Scholar]
- 71. Jia Y, Gao Y, Chen X, Huang B. Determination of optimal fasting time before blood sampling to get baseline data on serum biochemical characteristics in juvenile turbot ( Scophthalmus maximus). Aquaculture. 2018;487:83-88. [Google Scholar]
- 72. Smith SA, Pogson CI. The metabolism of L-tryptophan by isolated rat liver cells. Effect of albumin binding and amino acid competition on oxidatin of tryptophan by tryptophan 2,3-dioxygenase. Biochem J. 1980;186:977-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hijikata Y, Hara K, Shiozaki Y, Murata K, Sameshima Y. Determination of free tryptophan in plasma and its clinical applications. J Clin Chem Clin Biochem. 1984;22:291-299. [DOI] [PubMed] [Google Scholar]
- 74. Fuller RW, Roush BW. Binding of tryptophan to plasma proteins in several species. Comp. Biochem. Physiol. 1973;46:273-276. [DOI] [PubMed] [Google Scholar]
- 75. Badawy AA, Lake SL, Dougherty DM. Mechanisms of the pellagragenic effect of leucine: stimulation of hepatic tryptophan oxidation by administration of branched-chain amino acids to healthy human volunteers and the role of plasma free tryptophan and total kynurenines. Int J Tryptophan Res. 2014;7:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ulvik A, Theofylaktopoulou D, Midttun Ø, Nygård O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2016;98:934-940. [DOI] [PubMed] [Google Scholar]
- 77. Lane JD, Smith JE, Aprison MH. Comparison of free tryptophan, bound tryptophan and tyrosine in plasma, and tryptophan, 5-hydroxytryptophan, serotonin, 5-hydroxyindoleacetic acid, tyrosine, dopamine and norepinephrine in brain parts of rat and pigeon. Comp. Biochem Physiol. 1976;53:469-472. [DOI] [PubMed] [Google Scholar]
- 78. Allegri G, Costa CV, Bertazzo A, Biasiolo M, Ragazzi E. Enzyme activities of tryptophan metabolism along the kynurenine pathway in various species of animals. Il Farmaco. 2003;58:829-836. [DOI] [PubMed] [Google Scholar]
- 79. Koopmans SJ, Ruis M, Dekker R, van Diepen H, Korte M, Mroz Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol Behav. 2005;85:469-478. [DOI] [PubMed] [Google Scholar]
- 80. Chiang C-F, Larsen JA, Sahtout M, Horoschak RE, Yu ZS, Fascetti AJ. Impact of storage temperature, storage duration, and deproteinization on plasma amino acid concentrations in dogs. Res Vet Sci. 2021;136:416-421. [DOI] [PubMed] [Google Scholar]
- 81. Shukla GS, Chandra SV. Effect of nickel on aromatic amino acids and biogenic amines in brain of guinea pigs. Toxicol Lett. 1979;3:369-372. [Google Scholar]
- 82. Ragazzi E, Costa CV, Caparrotta L, Biasiolo M, Bertazzo A, Allegri G. Enzyme activities along the tryptophan-nicotinic acid pathway in alloxan diabetic rabbits. Biochim Biophys Acta. 2002;1571:9-17. [DOI] [PubMed] [Google Scholar]
- 83. Kollmann MT, Locher M, Hirche F, Eder K, Meyer HH, Bruckmaier RM. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest Anim Endocrinol. 2006;34:14-24. [DOI] [PubMed] [Google Scholar]
- 84. Marín GA, Larraín RE. Changes in behavior and plasma metabolites after tryptophan supplementation in steers. J Vet Behav. 2019;32:24-29. [Google Scholar]
- 85. Hofford JM, Milakofsky L, Pell S, Vogel W. A profile of amino acid and catecholamine levels during endotoxin-induced acute lung injury in sheep: searching for potential markers of the acute respiratory distress syndrome. J Lab Clin Med. 1996;128:545-551. [DOI] [PubMed] [Google Scholar]
- 86. Ma H, Yue C, Zhang W, Bu D, Jia Z. Ruminal disappearance, intestinal digestibility, and plasma tryptophan response of rumen-protected tryptophan in Cashmere goats. Small Rumin Res. 2012;107:22-27. [Google Scholar]
- 87. Chamberlain B, Ervin FR, Pihl RO, Young SN. The effect of raising or lowering tryptophan levels on aggression in vervet monkeys. Pharmacol Biochem Behav. 1987;28:503-510. [DOI] [PubMed] [Google Scholar]
- 88. Emelyanova LV, Koroleva EM, Savina MV. Glucose and free amino acids in the blood of lampreys (Lampetra fluviatilis L.) and frogs (Rana temporaria L.) under prolonged starvation. Comp Biochem Physiol. 2004;138:527-532. [DOI] [PubMed] [Google Scholar]
- 89. Middendorf L, Radko D, Düngelhoef K, et al. Amino acid pattern in the liver and blood of fattening turkeys suffering from hepatic lipidosis. Poult Sci. 2019;98:3950-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wacyk J, Powell M, Rodnick K, Overturf K, Hill RA, Hardy R. Dietary protein source significantly alters growth performance, plasma variables and hepatic gene expression in rainbow trout (Oncorhynchus mykiss) fed amino acid balanced diets. Aquaculture. 2012;356-357:223-234. [Google Scholar]
- 91. Hajirezaee S, Rafieepour A, Shafiei S. A NMR-based metabonomic study on the ameliorating effects of Ginkgo biloba extract in rainbow trout, Oncorhynchus mykiss exposed to organophosphate pesticide, diazinon. Aquaculture. 2019;513:734450. [DOI] [PubMed] [Google Scholar]
- 92. Lepage O, Larson ET, Mayer I, Winberg S. Serotonin, but not melatonin, plays a role in shaping dominant–subordinate relationships and aggression in rainbow trout. Horm Behav. 2005;48:233-242. [DOI] [PubMed] [Google Scholar]
- 93. Yamamoto T, Unuma T, Akiyama T. The influence of dietary protein and fat levels on tissue free amino acid levels of fingerling rainbow trout Oncorhynchus mykiss. Aquaculture. 2000;182:353-372. [Google Scholar]
- 94. Van Der Boon J, Verhagen MAW, Van Den Thillart GEE, Addink ADF. Free amino acids in whole blood and plasma of two cyprinids, Cyprinus carpio and Carassius auratus (Cyprinidae: Teleostei). Comp Biochem Physiol. 1991;99:391-399. [Google Scholar]
- 95. Pohlenz C, Buentello A, Bakke AM, Gatlin DM. Free dietary glutamine improves intestinal morphology and increases enterocyte migration rates, but has limited effects on plasma amino acid profile and growth performance of channel catfish Ictalurus punctatus. Aquaculture. 2012;370-371:32-39. [Google Scholar]
- 96. Hankins J. The role of albumin in fluid and electrolyte balance. J Infus Nurs. 2006;29:260-265. [DOI] [PubMed] [Google Scholar]
- 97. Boldt J. RETRACTED: use of albumin: an update. Br J Anaesth. 2010;104:276-284. [DOI] [PubMed] [Google Scholar]
- 98. Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53:789-803. [DOI] [PubMed] [Google Scholar]
- 99. Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nilsen J, Sandlie I, Roopenian DC, Andersen JT. Animal models for evaluation of albumin-based therapeutics. Curr Opin Chem Eng. 2018;19:68-76. [Google Scholar]
- 101. Dich J, Nielsen K. Metabolism and distribution of 131I-labeled albumin in the pig. Can J Comp Med Vet Sci. 1963;27:269-273. [PMC free article] [PubMed] [Google Scholar]
- 102. Dixon FJ, Maurer PH, Deichmiller MP. Half-lives of homologous serum albumins in several species. Proc Soc Exp Biol Med. 1953;83:287-288. [DOI] [PubMed] [Google Scholar]
- 103. Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex-related fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Latvala S, Jacobsen B, Otteneder MB, Herrmann A, Kronenberg S. Distribution of FcRn across species and tissues. J Histochem Cytochem. 2017;65:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andersen JT, Dalhus B, Viuff D, et al. Extending serum half-life of albumin by engineering neonatal fc receptor (FcRn) binding. J Biol Chem. 2014;289:13492-13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cormode EJ, Lyster DM, Israels S. Analbuminemia in a neonate. J Pediatr. 1975;86:862-867. [DOI] [PubMed] [Google Scholar]
- 107. Padrós J, Pelletier. In vivo formation of (+)-anti-benzo[a]pyrene diol-epoxide–plasma albumin adducts in fish. Mar Environ Res. 2000;50:347-351. [DOI] [PubMed] [Google Scholar]
- 108. Andreeva AM. Structure of fish serum albumins. J Evol Biochem Physiol. 2010;46:135-144. (Original Russian Text © A. M. Andreeva, 2010, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2010, Vol. 46, No. 2, pp. 111-118). [PubMed] [Google Scholar]
- 109. UK NHS South Tees Hospitals: Non-Esterified Fatty Acids (NEFA). [Google Scholar]
- 110. Dougherty DM, Marsh-Richard DM, Mathias CW, et al. Comparison of 50- and 100-g l-tryptophan depletion and loading formulations for altering 5-HT synthesis: pharmacokinetics, side effects, and mood states. Psychopharmacology. 2008;198:431-441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Badawy AA-B, Morgan CJ, Davis NR, Dacey A. High-fat diets increase tryptophan availability to the brain: importance of choice of the control diet. Biochem J. 1984;217:863-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab. 2014;306:E779-E790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ohlsson C, Hammarstedt A, Vandenput L, et al. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am J Physiol Endocrinol Metab. 2017;313:E450-E462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Faris RJ, Boddicker RL, Walker-Daniels J, Li J, Jones DE, Spurlock ME. Inflammation in response to n3 fatty acids in a porcine obesity model. Comp Med. 2012;62:495-503. [PMC free article] [PubMed] [Google Scholar]
- 115. Bertolucci C, Fazio F, Piccione G. Daily rhythms of serum lipids in dogs: influences of lighting and fasting cycles. Comp Med. 2008;58:485-489. [PMC free article] [PubMed] [Google Scholar]
- 116. Spears JW, Lloyd KE, Siciliano P, et al. Chromium propionate increases insulin sensitivity in horses following oral and intravenous carbohydrate administration. J Animal Sci. 2020;98:skaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Breidenbach A, Fuhrmann H, Deegen E, Lindholm A, Sallmann H-P. Studies on Equine lipid Metabolism – 2. lipolytic activities of plasma and tissue lipases in large horses and ponies. J Veter Med Ser A. 1999;46:39-48. [DOI] [PubMed] [Google Scholar]
- 118. Bacon WL, Musser MA, Brown KI. Plasma free fatty acid and neutral lipid concentrations in immature, laying and broody turkey hens. Poult Sci. 1974;53:1154-1160. [DOI] [PubMed] [Google Scholar]
- 119. Vincent R, Brackenbury JH. Plasma free fatty acid profile in male and female domestic fowl at rest and after exercise. Poult Sci. 1987;66:368-372. [DOI] [PubMed] [Google Scholar]
- 120. Librán-Pérez M, Velasco C, López-Patiño MA, Míguez JM, Soengas JL. Counter-regulatory response to a fall in circulating fatty acid levels in rainbow trout. Possible involvement of the hypothalamus-pituitary-interrenal axis. PLoS One. 2014;9:e113291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jones CT. Lipid metabolism and mobilization in the guinea pig during pregnancy. Biochem J. 1976;156:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bravo E, Cantafora A, Calcabrini A, Ortu G. Why prefer the golden Syrian hamster (Mesocricetus auratus) to the Wistar rat in experimental studies on plasma lipoprotein metabolism? Comp Biochem Physiol. 1994;107:347-355. [Google Scholar]
- 123. Baggia S. The role of the adrenal cortex in fat metabolism of Meriones unguiculatus, the Mongolian gerbil. Master’s Theses, University of Richmond; 1983. [Google Scholar]
- 124. Gouaref I, Detaille D, Wiernsperger N, Khan NA, Leverve X, Koceir E-A. The desert gerbil Psammomys obesus as a model for metformin-sensitive nutritional type 2 diabetes to protect hepatocellular metabolic damage: impact of mitochondrial redox state. PLoS One. 2017;12:e0172053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Barkai A, Allweis C. Effect of electrical stimulation of the hypothalamus on plasma free fatty acid concentration in cats. J Lipid Res. 1972;13:725-732. [PubMed] [Google Scholar]
- 126. Drackley JK. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci. 1999;82:2259-2273. [DOI] [PubMed] [Google Scholar]
- 127. Leat WM, Ford EJ. Utilization of free fatty acids by starved and pregnant sheep. Biochem J. 1966;101:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. El-Desoky NI, Hashem NM, Elkomy AG, Abo-Elezz ZR. Improving rabbit doe metabolism and whole reproductive cycle outcomes via fatty acid-rich moringa oleifera leaf extract supplementation in free and nano-encapsulated forms. Animals. 2022;12:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Van Heeswijk JC, Van Pelt J, Van den Thillart GE. Free fatty acid metabolism in the air-breathing African catfish (Clarias gariepinus) during asphyxia. Comp Biochem Physiol. 2005;141:15-21. [DOI] [PubMed] [Google Scholar]
- 130. Fellows FCI, Hird FJ, McLean RM, Walker TI. A survey of the non-esterified fatty acids and binding proteins in the plasmas of selected animals. Comp Biochem Physiol. 1980;67:593-597. [Google Scholar]
- 131. Hoseini SM, Pérez-Jiménez A, Costas B, Azeredo R, Gesto M. Physiological roles of tryptophan in teleosts: current knowledge and perspectives for future studies. Rev Aquacult. 2019;11:3-24. [Google Scholar]
- 132. Horwitt MK, Harvey CC, Rothwell WS, Cutler JL, Haffron D. Tryptophan:niacin relationships in man. J Nutr. 1956;60:1-43.13385712 [Google Scholar]
- 133. Hankes LV, Henderson LM, Brickson WL, Elvehjem CA. Effect of amino acids on the growth of rats on niacin-tryptophan-deficient rations. J Biol Chem. 1948;174:873-881. 1948. [PubMed] [Google Scholar]
- 134. Shibata K, Kondo T, Marugami M, Umezawa C. Increased conversion ratio of tryptophan to niacin by the administration of clofibrate, a hypolipidemic drug, to rats. Biosci Biotechnol Biochem. 1996;60:1455-1459. [DOI] [PubMed] [Google Scholar]
- 135. Shibata K, Toda S. Effects of sex hormones on the metabolism of tryptophan to niacin and to serotonin in male rats. Biosci Biotechnol Biochem. 1997;61:1200-1202. [DOI] [PubMed] [Google Scholar]
- 136. Ruiz N, Harms RH. Conversion of tryptophan into niacin in the turkey (Meleagris gallipavos). Poult Sci. 1990;69:446-450. [DOI] [PubMed] [Google Scholar]
- 137. Chen BJ, Shen TF, Austic RE. Efficiency of tryptophan-niacin conversion in chickens and ducks. Nutr Res. 1996;16:91-104. [Google Scholar]
- 138. da Silva AC, Guerios MF, Monsao SR. The domestic cat as a laboratory animal for experimental nutrition studies: VI. Choline deficiency. J Nutr. 1959;67:537-547. [DOI] [PubMed] [Google Scholar]
- 139. Serrano Ae, Jr, Nagayama F. Liver 3-hydroxyanthranilic acid oxygenase activity in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B. 1991;99:275-280. [DOI] [PubMed] [Google Scholar]
- 140. Thorn SL, Young GS, Kirkland JB. The guinea-pig is a poor animal model for studies of niacin deficiency and presents challenges in any study using purified diets. Br J Nutr. 2007;98:78-85. [DOI] [PubMed] [Google Scholar]
- 141. Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Terakata M, Fukuwatari T, Kadota E, et al. The niacin required for optimum growth can be synthesized from L-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J Nutr. 2013;143:1046-1051. [DOI] [PubMed] [Google Scholar]
- 143. Too LK, Li KM, Suarna C, et al. Deletion of TDO2, IDO-1 and IDO-2 differentially affects mouse behavior and cognitive function. Behav Brain Res. 2016;312:102-117. [DOI] [PubMed] [Google Scholar]
- 144. Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Biggio G, Fadda F, Fanni P, Tagliamonte A, Gessa GL. Rapid depletion of serum tryptophan, brain tryptophan, serotonin and 5-hydroxyindoleacetic acid by a tryptophan-free diet. Life Sci. 1974;14:1321-1329. [DOI] [PubMed] [Google Scholar]
- 146. Gessa GL, Biggio G, Fadda F, Corsini GU, Tagliamonte A. Effect of the oral administration of tryptophan-free amino acid mixtures on serum tryptophan, brain tryptophan and serotonin metabolism. J Neurochem. 1974;22:869-870. [DOI] [PubMed] [Google Scholar]
- 147. Moja EA, Antinoro E, Cesa-Bianchi M, Gessa GL. Increase in stage 4 sleep after ingestion of a tryptophan-free diet in humans. Pharmacol Res Commun. 1984;16:909-914. [DOI] [PubMed] [Google Scholar]
- 148. Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87:173-177. [DOI] [PubMed] [Google Scholar]
- 149. Ardis TC, Cahir M, Elliott JJ, Bell R, Reynolds GP, Cooper SJ. Effect of acute tryptophan depletion on noradrenaline and dopamine in the rat brain. J Psychopharmacol. 2009;23:51-55. [DOI] [PubMed] [Google Scholar]
- 150. Birkl P, Kjaer JB, Szkotnicki W, Forsythe P, Harlander-Matauschek A. Acute tryptophan depletion: the first method validation in an avian species (Gallus gallus domesticus). Poult Sci. 2017;96:3021-3025. [DOI] [PubMed] [Google Scholar]
- 151. Birkl P, Franke L, Bas Rodenburg T, Ellen E, Harlander-Matauschek A. A role for plasma aromatic amino acids in injurious pecking behavior in laying hens. Physiol Behav. 2017;175:88-96. [DOI] [PubMed] [Google Scholar]
- 152. Badawy AA, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-Dioxygenase: time for appraisal. Int J Tryptophan Res. 2019;12:1178646919868978-1178646919869010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kirat D, Hamada M, Moustafa A, Miyasho T. Irisin/FNDC5: A participant in camel metabolism. Saudi J Biol Sci. 2021;28:693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Johnson RJ, Dyer IA. Effect of orally administered tryptophan on tryptophan pyrrolase activity in ovine and bovine. Life Sci. 1966;5:1121-1124. [DOI] [PubMed] [Google Scholar]
- 155. Yang JN, Carlson JR. Effects of high tryptophan doses and two experimental rations on the excretion of urinary tryptophan metabolites in cattle. J Nutr. 1972;102:1655-1665. [DOI] [PubMed] [Google Scholar]
- 156. Brown JN, Dodgen CL. Fish liver tryptophan pyrrolase: the apparent absence of both hormonal regulation and substrate induction. Biochim Biophys Acta. 1968;165:463-469. [DOI] [PubMed] [Google Scholar]
- 157. Baughman KL, Franz JM. Control of tryptophan oxygenase and formamidase activity in the gerbil. Int J Biochem. 1971;2:201-211. [Google Scholar]
- 158. Hvitefelt J, Santti RS. Tryptophan pyrrolase in the liver of guinea pig: the absence of hydrocortisone induction. Biochim Biophys Acta. 1972;258:358-365. [DOI] [PubMed] [Google Scholar]
- 159. Ryser-Degiorgis M-P, Robert N, Meier RK, et al. Cardiomyopathy associated with coronary arteriosclerosis in free-ranging Eurasian lynx (Lynx lynx carpathicus). Front Vet Sci. 2020;7:594952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mitchell KJ, De Clercq D, Stirn M, van Loon G, Schwarzwald CC. Plasma homocysteine concentrations in healthy horses and horses with atrial fibrillation. J Vet Cardiol. 2018;20:276-284. [DOI] [PubMed] [Google Scholar]
- 161. Noble GK, Li X, Zhang D, Sillence MN. Randomised clinical trial on the effect of a single oral administration of L-tryptophan, at three dose rates, on reaction speed, plasma concentration and haemolysis in horses. Vet J. 2016;213:84-86. [DOI] [PubMed] [Google Scholar]
- 162. Grimmett A, Sillence MN. Calmatives for the excitable horse: A review of L-tryptophan. Vet J. 2005;170:24-32. [DOI] [PubMed] [Google Scholar]
- 163. Calheiros AC, Reis RP, Castelar B, Cavalcanti DN, Teixeira VL. Corrigendum to “Ulva spp. as a natural source of phenylalanine and tryptophan to be used as anxiolytics in fish farming” [Aquaculture 509 (2019) 171–177]. Aquaculture. 2020;518:171-177. [Google Scholar]
- 164. Cerqueira M, Schrama D, Silva TS, et al. How tryptophan levels in plant-based aquafeeds affect fish physiology, metabolism and proteome. J Proteomics. 2020;221:103782. [DOI] [PubMed] [Google Scholar]
- 165. Hoseini SM, Taheri Mirghaed A, Ghelichpour M, Pagheh E, Iri Y, Kor A. Effects of dietary tryptophan supplementation and stocking density on growth performance and stress responses in rainbow trout (Oncorhynchus mykiss). Aquaculture. 2020;519:734908. [Google Scholar]
- 166. Salamanca N, Morales E, Ruiz-Azcona P, Herrera M. Endocrine and metabolic effects of trp-enriched diets for attenuation of chronic stress in the Senegal soles (Solea senegalensis). Aquaculture. 2020;523:735173. [Google Scholar]
- 167. Weld KP, Mench JA, Woodward RA, Bolesta MS, Suomi SJ, Higley JD. Effect of tryptophan treatment on self-biting and central nervous system serotonin metabolism in rhesus monkeys (Macaca mulatta). Neuropsychopharmacology. 1998;19:314-321. [DOI] [PubMed] [Google Scholar]
- 168. Serrano AE, Nagayama F. Activity and partial purification of liver tryptophan pyrrolase in rainbow trout. Nippon Suisan Gakkaishi. 1992;58:2367-2371. [Google Scholar]
- 169. Herrera M, Fernández-Alacid L, Sanahuja I, et al. Physiological and metabolic effects of a tryptophan-enriched diet to face up chronic stress in meagre (Argyrosomus regius). Aquaculture. 2020;522:735102. [Google Scholar]
- 170. Yersin AG, Edens FW, Simmons DG. Tryptophan 2,3-dioxygenase activity in turkey poults infected with Bordetella avium. Comp Biochem Physiol. 1990;97:755-759. [DOI] [PubMed] [Google Scholar]
- 171. Edens FW, Yersin AG, Simmons DG. Tryptophan methylester modulation of poult responses to Bordetella avium. Poult Sci. 1999;78:327-335. 1999. [DOI] [PubMed] [Google Scholar]
- 172. Lv M, Yu B, Mao XB, Zheng P, He J, Chen DW. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal. 2012;6:928-934. [DOI] [PubMed] [Google Scholar]
- 173. Doan N, Liu Y, Xiong X, et al. Organic selenium supplement partially alleviated diquat-induced oxidative insults and hepatic metabolic stress in nursery pigs. Br J Nutr. 2020;124:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wang J, Takahashi RH, DeMent K, et al. Development of a mass spectrometry-based tryptophan 2, 3-dioxygenase assay using liver cytosol from multiple species. Anal Biochem. 2018;556:85-90. [DOI] [PubMed] [Google Scholar]
- 175. Yuasa HJ. A comprehensive comparison of the metazoan tryptophan degrading enzymes. BBA Protein Proteomics. 2020;1868:140247. [DOI] [PubMed] [Google Scholar]
- 176. Fujigaki S, Saito K, Takemura M, et al. Species differences in L-tryptophan-kynurenine pathway metabolism: quantification of anthranilic acid and its related enzymes. Arch Biochem Biophys. 1998;358:329-335. [DOI] [PubMed] [Google Scholar]
- 177. Murakami Y, Saito K. Species and cell types difference in tryptophan metabolism. Int J Tryptophan Res. 2013;6:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yuasa HJ, Ball HJ, Ho YF, et al. Characterization and evolution of vertebrate indoleamine 2,3- dioxygenases IDOs from monotremes and marsupials. Comp Biochem Physiol. 2009;153B:137-144. [PubMed] [Google Scholar]
- 179. de Castro FT, Brown RR, Price JM. The intermediary metabolism of tryptophan by cat and rat tissue preparations. J Biol Chem. 1957;228:777-784. [PubMed] [Google Scholar]
- 180. Ishikawa T, Okuno E, Kawai J, Kido R. Organ distribution, purification and characterization of kynureninase in suncus murinus (insectivore) and anthranilic acid level in the serum. Comp Biochem Physiol. 1989;93B:107-111. [DOI] [PubMed] [Google Scholar]
- 181. Lan SJ, Gholson RK. A comparative study of tryptophan catabolism. J Biol Chem. 1965;240:3934-3937. [PubMed] [Google Scholar]
- 182. Green AR, Sourkes TL, Young SN. Liver and brain tryptophan metabolism following hydrocortisone administration to rats and gerbils. Br J Pharmacol. 1975;53:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Monroe CB. Induction of tryptophan oxygenase and tyrosine aminotransferase in mice. Am J Physiol. 1968;214:1410-1414. [DOI] [PubMed] [Google Scholar]
- 184. Badawy AA, Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976;160:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Badawy AA, Morgan CJ, Lane J, Dhaliwal K, Bradley DM. Liver tryptophan pyrrolase. A major determinant of the lower brain 5-hydroxytryptamine concentration in alcohol-preferring C57BL mice. Biochem J. 1989;264:597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Costa C, De Antoni A, Baccichetti F, Biasiolo M, Allegri G. Metabolites and enzyme activities involved in tryptophan metabolism in two different strains of mouse. Ital J Biochem. 1984;33:319-324. [PubMed] [Google Scholar]
- 187. Costa C, De Antoni A, Baccichetti F, Vanzan S, Appodia M, Allegri G. Strain differences in the tryptophan metabolite excretion and enzyme activities along the kynurenine pathway in rats. Ital J Biochem. 1982;31:412-418. [PubMed] [Google Scholar]
- 188. Shibata K, Hayakawa T, Iwai K. Comparison of the enzyme activities in the tryptophan-NAD pathway between the Wistar and Sprague Dawley strains of rats. Agric Biol Chem. 1986;50:1643-1644. [Google Scholar]
- 189. Bano S. Tryptophan metabolism in relation to mental illness. PhD Thesis, Cardiff University; 1997. [Google Scholar]
- 190. Kaufman S. Studies on tryptophan pyrrolase in drosophila melanogaster. Genetics. 1962;47:807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Dustmann JH. Quantitative untersuchungen zur tryptophan → ommochrom-reaktionskette bei wildtyp und mutanten der honigbiene Apis mellifera. Insect Biochem. 1975;5:429-445. [Google Scholar]
- 192. Han Q, Beerntsen BT, Li J. The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J Insect Physiol. 2007;53:254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Prasad C, French WL. Tryptophan pyrrolase activity during development in mosquito (Culex pipiens). Comp Biochem Physiol. 1971;38:627-629. [Google Scholar]
- 194. Badawy AA, Evans M. Guinea-pig liver tryptophan pyrrolase. Absence of detectable apoenzyme activity and of hormonal induction by cortisol and possible regulation by tryptophan. Biochem J. 1974;138:445-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Peters RF, Richardson MC, Small M, White AM. Some biochemical effects of triamcinolone acetonide on rat liver and muscle. Biochem J. 1970;116:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shoji S. Effects of triamcinolone acetonide on plasma amino acids and urinary urea output in rabbits. Int J Biochem. 1991;23:361-363. [DOI] [PubMed] [Google Scholar]
- 197. Cortés J, Alvarez C, Santana P, Torres E, Mercado L. Indoleamine 2,3-dioxygenase: first evidence of expression in rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol. 2016;65:73-78. [DOI] [PubMed] [Google Scholar]
- 198. Cui Z-W, Zhang X-Y, Zhang X-J, et al. Molecular and functional characterization of the indoleamine 2,3-dioxygenase in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019;89:301-308. [DOI] [PubMed] [Google Scholar]
- 199. Yuasa HJ, Mizuno K, Ball HJ. Low efficiency IDO2 enzymes are conserved in lower vertebrates, whereas higher efficiency IDO1 enzymes are dispensable. FEBS J. 2015;282:2735-2745. [DOI] [PubMed] [Google Scholar]
- 200. Ozaki Y, Reinhard JF, Jr, Nichol CA. Cofactor activity of dihydroflavin mononucleotide and tetrahydrobiopterin for murine epididymal indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 1986;137:1106-1111. [DOI] [PubMed] [Google Scholar]
- 201. Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46:2155-2163. [DOI] [PubMed] [Google Scholar]
- 202. Efimov I, Basran J, Sun X, et al. The mechanism of substrate inhibition in human indoleamine 2,3-dioxygenase. J Am Chem Soc. 2012;134:3034-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci. 2015;129:601-672. [DOI] [PubMed] [Google Scholar]
- 204. Terness P, Bauer TM, Röse L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069-1077. [DOI] [PubMed] [Google Scholar]
- 206. Wirthgen E, Hoeflich A, Rebl A, Günther J. Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. 2018;8:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Badawy AA. Hypothesis kynurenic and quinolinic acids: the main players of the kynurenine pathway and opponents in inflammatory disease. Med Hypotheses. 2018;118:129-138. [DOI] [PubMed] [Google Scholar]
- 208. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Sadik A, Somarribas Patterson LF, Öztürk S, et al. IL4I1 is a metabolic immune checkpoint that activates the AhR and promotes tumor progression. Cell. 2020;182:1252-1270.e34. [DOI] [PubMed] [Google Scholar]
- 210. Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hubbard TD, Murray IA, Bisson WH, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cheng Y, Jin U-H, Allred CD, Jayaraman A, Chapkin RS, Safe S. Aryl hydrocarbon receptor activity of tryptophan metabolites in young adult mouse colonocytes. Drug Metab Dispos. 2015;43:1536-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. 2017;357:806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Puccetti M, Paolicelli G, Oikonomou V, et al. Towards targeting the aryl hydrocarbon receptor in cystic fibrosis. Mediators Inflamm. 2018;2018:1601486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kumar P, Lee J-H, Lee J. Diverse roles of microbial indole compounds in eukaryotic systems. Biol Rev. 2021;96:2522-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Litzenburger UM, Opitz CA, Sahm F, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Dere E, Boverhof DR, Burgoon LD, Zacharewski TR. In vivo-in vitro toxicogenomic comparison of TCDD-elicited gene expression in hepa1c1c7 mouse hepatoma cells and C57BL/6 hepatic tissue. BMC Genomics. 2006;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kazzaz SA, Giani Tagliabue S, Franks DG, et al. An aryl hydrocarbon receptor from the caecilian Gymnopis multiplicata suggests low dioxin affinity in the ancestor of all three amphibian orders. J Comp Endocrinol. 2020;299:113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Doering JA, Wiseman S, Beitel SC, Tendler BJ, Giesy JP, Hecker M. Tissue specificity of aryl hydrocarbon receptor (AhR) mediated responses and relative sensitivity of white sturgeon (Acipenser transmontanus) to an AhR agonist. Aquat Toxicol. 2012;114-115:125-133. [DOI] [PubMed] [Google Scholar]
- 221. Barron MG, Heintz R, Rice SD. Relative potency of pahs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95-100. [DOI] [PubMed] [Google Scholar]
- 222. King-Heiden TC, Mehta V, Xiong KM, et al. Reproductive and developmental toxicity of dioxin in fish. Mol Cell Endocrinol. 2012;354:121-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Burkina V, Zamaratskaia G, Sakalli S, Giang PT, Zlabek V, Rasmussen MK. Tissue-specific expression and activity of cytochrome P450 1A and 3A in rainbow trout (Oncorhynchus mykiss). Toxicol Lett. 2021;341:1-10. [DOI] [PubMed] [Google Scholar]
- 224. Pereiro P, Figueras A, Novoa B. Insights into teleost interferon-gamma biology: an update. Fish Shellfish Immunity. 2019;90:150-164. [DOI] [PubMed] [Google Scholar]
- 225. Niimi S, Imoto M, Kunisue T, et al. Effects of persistent organochlorine exposure on the liver transcriptome of the common minke whale (Balaenoptera acutorostrata) from the North Pacific. Ecotoxicol Environ Saf. 2014;108:95-105. [DOI] [PubMed] [Google Scholar]
- 226. Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents on induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144:1147-1153. [DOI] [PubMed] [Google Scholar]
- 227. Badawy AA. Immunotherapy of COVID-19 with poly (ADP-ribose) polymerase inhibitors: starting with nicotinamide. Biosci Rep. 2020;40:1-18. doi: 10.1042/BSR20202856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Badawy AA. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013;27:878-893. [DOI] [PubMed] [Google Scholar]
- 229. Bahovschi V, Zamboni CB, Silva LFFL, Metairon S, Medeiros IMMA. Differences in iron concentration in whole blood of animal models using NAA. J Phys Conf Ser. 2015;630:630. [Google Scholar]
- 230. Panwar S, Jain S, Varma M, Mohini A. A comparative biochemical study on iron content in various forms of Clarias species. Int J Fisheries and Aquacult Sci. 2015;5:21-25. [Google Scholar]
- 231. Debnath C, Sahoo L, Singha A, Yadav GS, Datta M, Ngachani SV. Protein and mineral compositions of some local fishes of Tripura, India. Indian J Hill Farming. 2014;27:210-218. [Google Scholar]
- 232. Badawy AB, Bailey-Wood R, Evans M, Jacobs A. Proceedings: rat liver tryptophan pyrrolase activity in iron deficiency anaemia. Br J Pharmacol. 1975;54:227-228. [PMC free article] [PubMed] [Google Scholar]
- 233. Badawy AA. Possible involvement of the enhanced tryptophan pyrrolase activity in the corticosterone- and starvation-induced increases in concentrations of nicotinamide-adenine dinucleotides (phosphates) in rat liver. Biochem J. 1981;196:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Badawy AA. Central role of tryptophan pyrrolase in haem metabolism. Biochem Soc Trans. 1979;7:575-583. [DOI] [PubMed] [Google Scholar]
- 235. Bailey-Wood R, Blayney LM, Muir JR, Jacobs A. The effects of iron deficiency on rat liver enzymes. Br J Exp Pathol. 1975;56:193-198. [PMC free article] [PubMed] [Google Scholar]
- 236. Donley DW, Realing M, Gigley JP, Fox JH. Iron activates microglia and directly stimulates indoleamine-2,3-dioxygenase activity in the N171-82Q mouse model of Huntington’s disease. PLoS One. 2021;16:e0250606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Krausse-Opatz B, Wittkop U, Gutzki FM, et al. Free iron ions decrease indoleamine 2,3-dioxygenase expression and reduce IFNgamma-induced inhibition of chlamydia trachomatis infection. Microb Pathog. 2009;46:289-297. [DOI] [PubMed] [Google Scholar]
- 238. Jason J, Archibald LK, Nwanyanwu OC, et al. The effects of iron deficiency on lymphocyte cytokine production and activation: preservation of hepatic iron but not at all cost. Clin Exp Immunol. 2002;126:466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Bergman M, Bessler H, Salman H, Siomin D, Straussberg R, Djaldetti M. In vitro cytokine production in patients with iron deficiency anemia. Clin Immunol. 2004;113:340-344. [DOI] [PubMed] [Google Scholar]
- 240. Hortia A, Carino MA. Modification of the toxic actions of l-tryptophan by pargyline and p-chlorophenylalanine. Biochem Pharmacol. 1970;19:1521-1524. [DOI] [PubMed] [Google Scholar]
- 241. Carlson JR, Yokoyama MT, Dickinson EO. Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science. 1972;176:298-299. [DOI] [PubMed] [Google Scholar]
- 242. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247-265. [DOI] [PubMed] [Google Scholar]
- 243. Heyes MP, Saito K, Chen CY, et al. Species heterogeneity between gerbils and rats: quinolinate production by microglia and astrocytes and accumulations in response to ischemic brain injury and systemic immune activation. J Neurochem. 1997;69:1519-1529. [DOI] [PubMed] [Google Scholar]
- 244. Friedman PA, Kappelman AH, Kaufman S. Partial purification and characterization of tryptophan hydroxylase from rabbit hindbrain. J Biol Chem. 1972;247:4165-4173. [PubMed] [Google Scholar]
- 245. Gál EM, Young RB, Sherman AD. Tryptophan loading: consequent effects on the synthesis of kynurenine and 5-hydroxyindoles in rat brain. J Neurochem. 1972;31:237-244. [DOI] [PubMed] [Google Scholar]
- 246. Kubera M, Kenis G, Bosmans E, Scharpé S, Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-gamma and interleukin-10. Neuropsychopharmacol. 2000;23:89-98. [DOI] [PubMed] [Google Scholar]
- 247. Serrano Ae, Jr, Nagayama F. Inhibition studies on liver arylformamidases of rainbow trout and cattle. Comp Biochem Physiol. 1991;99:281-285. [DOI] [PubMed] [Google Scholar]
- 248. Dobrovolsky VN, Bowyer JF, Pabarcus MK, et al. Effect of arylformamidase (kynurenine formamidase) gene inactivation in mice on enzymatic activity, kynurenine pathway metabolites and phenotype. Biochim Biophys Acta. 2005;1724:163-172. [DOI] [PubMed] [Google Scholar]
- 249. Moore GP, Sullivan DT. The characterization of multiple forms of kynurenine formidase in Drosophila melanogaster. Biochim Biophys Acta. 1975;397:468-477. [DOI] [PubMed] [Google Scholar]
- 250. Shinohara R, Ishiguro I. New formamidase having substrate specificity for o-formylaminoacetophenone in pig liver. Biochim Biophys Acta. 1977;483:409-415. [DOI] [PubMed] [Google Scholar]
- 251. Cumming RB, Walton MF, Fuscoe JC, Taylor BA, Womack JE, Gaertner FH. Genetics of formamidase-5 (brain formamidase) in the mouse: localization of the structural gene on chromosome 14. Biochem Genet. 1979;17:415-431. [DOI] [PubMed] [Google Scholar]
- 252. Bailey CG, Wagner C. Kynurenine formamidase: purification and characterization of the adult chicken liver enzyme and immunochemical analyses of the enzyme of developing chicks. J Biol Chem. 1974;249:4439-4444. [PubMed] [Google Scholar]
- 253. Seifert J, Pewnim T. Alteration of mice L-tryptophan metabolism by the organophosphorous acid triester diazinon. Biochem Pharmacol. 1992;44:2243-2250. [DOI] [PubMed] [Google Scholar]
- 254. Nassan FL, Gunn JA, Hill MM, Coull BA, Hauser R. High phthalate exposure increased urinary concentrations of quinolinic acid, implicated in the pathogenesis of neurological disorders: is this a potential missing link? Environ Res. 2019;172:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Brown RR, Price JM. Quantitative studies on metabolites of tryptophan in the urine of the dog, cat, rat and man. J Biol Chem. 1956;219:985-997. [PubMed] [Google Scholar]
- 256. Williams JN, Feigelson P, Elvehjem CA. Relation of tryptophan and niacin to pyridine nucleotides of tissue. J Biol Chem. 1950;187:597-604. [PubMed] [Google Scholar]
- 257. Bender DA, Magboul BI, Wynick D. Probable mechanisms of regulation of the utilization of dietary tryptophan, nicotinamide and nicotinic acid as precursors of nicotinamide nucleotides in the rat. Br J Nutr. 1982;48:119-127. [DOI] [PubMed] [Google Scholar]
- 258. Ng WK, Serrini G, Zhang Z, Wilson RP. Niacin requirement and inability of tryptophan to act as a precursor of NAD+ in channel catfish, Ictalurus punctatus. Aquaculture. 1997;152:273-285. [Google Scholar]
- 259. Poston HA, DiLorenzo RN, Scott ML. Tryptophan conversion to niacin in the brook trout (Salvelinus fontinalis). Proc Soc Exp Biol Med. 1973;144:110-112. 1973. [DOI] [PubMed] [Google Scholar]
- 260. Poston HA, Combs GF., Jr. Nutritional implications of tryptophan catabolizing enzymes in several species of trout and salmon. Proc Soc Exp Biol Med. 1980;163:452-454. [DOI] [PubMed] [Google Scholar]
- 261. Browne CA, Hanke J, Rose C, et al. Effect of acute swim stress on plasma corticosterone and brain monoamine levels in bidirectionally selected DxH recombinant inbred mouse strains differing in fear recall and extinction. Stress. 2014;17:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Zhang WQ, Smolik CM, Barba-Escobedo PA, et al. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacol. 2015;90:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Shirasaka Y, Lee N, Duan H, Ho H, Pak J, Wang J. Interspecies comparison of the functional characteristics of plasma membrane monoamine transporter (PMAT) between human, rat and mouse. J Chem Neuroanat. 2017;83-84:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Audira G, Siregar P, Chen KH-C, et al. Interspecies behavioral variability of Medaka fish assessed by comparative phenomics. Int J Mol Sci. 2021;22:5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Jin ZL, Chen X-F, Ran YH, et al. Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci Rep. 2017;7:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Curzon G, Knott PJ. Effects on plasma and brain tryptophan in the rat of drugs and hormones that influence the concentration of unesterified fatty acid in the plasma. Br J Pharmacol. 1974;50:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Payne AP, Andrews MJ, Wilson CA. The effects of isolation, grouping and aggressive interactions on indole- and catecholamine levels and apparent turnover in the hypothalamus and midbrain of the male golden hamster. Physiol Behav. 1985;34:911-916. [DOI] [PubMed] [Google Scholar]
- 268. Jia R, Du J, Cao L, et al. Application of transcriptome analysis to understand the adverse effects of hydrogen peroxide exposure on brain function in common carp (Cyprinus carpio). Environ Pollut. 2021;286:117240. [DOI] [PubMed] [Google Scholar]
- 269. Johnston WL, Atkinson JL, Theresa Glanville N. Effect of PCPA or tryptophan on brain serotonin and on consumption of a high protein or high carbohydrate diet by rainbow trout, Oncorhynchus mykiss. J Nutr Biochem. 1992;3:421-428. [Google Scholar]
- 270. Lepage O, Tottmar O, Winberg S. Elevated dietary intake of L-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss). J Exp Biol. 2002;205:3679-3687. [DOI] [PubMed] [Google Scholar]
- 271. Morandini L, Ramallo MR, Moreira RG, et al. Serotonergic outcome, stress and sexual steroid hormones, and growth in a South American cichlid fish fed with an L-tryptophan enriched diet. Gen Comp Endocrinol. 2015;223:27-37. [DOI] [PubMed] [Google Scholar]
- 272. Hong K-W, Sugawara Y, Hasegawa H, et al. A new gain-of-function allele in chimpanzee tryptophan hydroxylase 2 and the comparison of its enzyme activity with that in humans and rats. Neurosci Lett. 2007;412:195-200. [DOI] [PubMed] [Google Scholar]
- 273. Wendland JR, Lesch K-P, Newman TK, et al. Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 2006;36:163-172. [DOI] [PubMed] [Google Scholar]
- 274. Inoue H, Castagnoli K, Van Der Schyf C, Mabic S, Igarashi K, Castagnoli N., Jr. Species-dependent differences in monoamine oxidase A and B-catalyzed oxidation of various C4 substituted 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridinyl derivatives. J Pharmacol Exp Ther. 1999;291:856-864. [PubMed] [Google Scholar]