Abstract
We have developed a novel system to study monocytic function after human immunodeficiency virus type 1 (HIV-1) infection by infecting a series of human macrophage hybridoma cell lines with HIV-1. Since ethanol has detrimental effects on immune function, we investigated the effect of ethanol and its metabolites acetaldehyde and acetate on monocytic function by utilizing one human macrophage hybridoma cell line, clone 43, as well as primary monocytes. Pretreatment of clone 43 and primary monocytes with ethanol and its metabolites resulted in diminished accessory cell function for mitogen-, anti-CD3-, and antigen-induced T-cell proliferation. The decreased accessory cell function was associated with reduced interleukin 1α (IL-1α), IL-1β, and tumor necrosis factor alpha production with loss of intracellular cytokine and mRNA production and the induction of transforming growth factor β. In ethanol-, acetaldehyde-, and acetate-treated HIV-1-infected clone 43 cells (43HIV), there was a more rapid loss (3 days after infection) of accessory cell function at a lower infecting dose of HIV-1 than that in untreated 43HIV cells. We also observed a more rapid loss of surface class II antigen expression in the ethanol-, acetaldehyde-, and acetate-treated 43HIV cells, but no change in surface expression of CD80 or CD86. Ethanol-induced impairment of monocytic function may compound the immunologic defects of AIDS, making the infected individual more susceptible to the complications of the disease.
It has been suggested that ethanol abuse may play an important role in the transmission and progression of human immunodeficiency virus type 1 (HIV-1) disease as well as increasing the susceptibility to opportunistic infections (2, 13, 16, 28, 32). Ethanol has detrimental effects on immune function, decreasing absolute numbers of T and B cells and altering immunoglobulin (Ig) and cytokine production, especially that of tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-6 (5, 17, 18, 21, 25). It can also affect monocytic function, impairing the capacity to present soluble antigen to major histocompatibility complex (MHC)-matched responder T cells (37). Ethanol has other effects on monocytic function, inducing downregulation of Fcγ receptors and inhibiting tumoricidal activity (4). Furthermore, it can suppress TNF-α production and induce transforming growth factor β (TGF-β) and prostaglandin E2 production, which hinders the generation of normal immune responses (27, 38). Ethanol exposure increases the survival of mycobacteria in macrophages and impairs delayed-type hypersensitivity reactions (7). The microbial killing activity of monocytes and macrophages for other organisms may be hampered by ethanol exposure, including the ability to kill Candida albicans (42).
Monocytes are important cells in HIV-1 infection, serving as a reservoir for virus and the portal of entry of HIV-1 into the central nervous system (22, 24). A number of monocyte defects have been reported both in HIV-1-infected patients and in vitro, including defective accessory cell function and impaired cell surface antigen expression (14, 29). Since ethanol appears to induce monocytic defects that are similar to those produced after HIV-1 infection (impaired accessory cell function and altered cytokine production), exposure either before or after HIV-1 infection may enhance HIV-1 replication or compound immunologic defects, making the infected individual more susceptible to the complications of the disease.
Our laboratory has studied HIV-1–monocyte interactions by utilizing a series of monocyte and macrophage hybridomas obtained by fusing a mutagenized hypoxanthine guanine phosphoribosyltransferase (HGPRT)-deficient promonocytic cell line U937 with either gamma interferon (IFN-γ)-activated monocytes or macrophages obtained by allowing monocytes to mature in Teflon bag cultures (33). These cell lines which can be uniformly infected with HIV-1 possess many normal monocytic functions, including antigen presentation, class II antigen expression, and cytokine production including IL-1α, IL-1β, IL-6, IL-10, and IL-12 (30, 34, 41). Using this system as well as primary HIV-1-infected monocytes, we have demonstrated that monocytic function (antigen presentation and cytokine production) is markedly impaired after HIV-1 infection. In this study, we investigated the effect of ethanol on monocytic function after HIV-1 infection by utilizing a human macrophage hybridoma cell line, clone 43, and primary HIV-1-infected monocytes.
MATERIALS AND METHODS
Monocyte isolation.
Mononuclear cells were separated from buffy coats obtained from normal healthy volunteers by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) density gradient centrifugation by methods previously established in our laboratory (33). The cells were washed three times with sterile phosphate-buffered saline (PBS) and resuspended in RPMI 1640 (GIBCO, Grand Island, N.Y.) supplemented with 10% fetal calf serum (GIBCO), 2 mM l-glutamine, and 1% penicillin–streptomycin (GIBCO), henceforth called complete medium (CM). Freshly isolated peripheral blood mononuclear cells (PBMCs) were incubated in CM at 37°C in culture flasks and allowed to adhere for 45 min. The nonadherent cells were removed, and the adherent cells washed with sterile PBS, harvested with a rubber policeman, and stained with monocyte-specific anti-CD14 monoclonal antibodies to assess the purity of the population. Ninety percent of the cells isolated expressed CD14.
Human macrophage hybridomas.
Human macrophage hybridomas were obtained by fusing macrophages (obtained by allowing monocytes to mature in Teflon bag cultures) with an HGPRT-deficient promonocytic line, U937, as previously described (33). For this study, we utilized one previously characterized clone, clone 43, which is stable in long-term culture (30).
Ethanol, acetaldehyde, and acetate treatment and HIV-1 infection.
The human macrophage hybridomas or primary monocytes were treated with ethanol (25, 75, and 150 mM) for 16 h. We chose this concentration range to cover levels that occur in normal individuals and in chronic alcoholics after ethanol exposure. The 25 and 75 mM doses approximate blood alcohol levels which are achieved after the moderate and excessive intake of ethanol, respectively, in normal individuals. The 150 mM dose approximates the acute ethanol intake in a chronic alcoholic (36). We also pretreated the cells with the same concentrations of the ethanol metabolites acetaldehyde and acetate. We measured the concentrations of ethanol, acetaldehyde, and acetate in the culture supernatants at the end of the 16-h treatment period by previously established methods (9, 20). After the ethanol, acetaldehyde, and acetate treatment, the cells were infected with HIV-1. Monocytes or clone 43 cells were infected with HIV-1BaL or HIV-1IIIB as previously described (30, 34, 41). These reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. Dilutions of HIV-1-containing supernatant standardized to contain equivalent amounts of virus on the basis of reverse transcriptase activity (80,000 cpm/ml) were incubated with the monocytic cells for 90 min followed by three washes with PBS. For cytokine determination, clone 43 and primary monocytes were treated with ethanol, acetaldehyde, and acetate (25, 75, and 150 mM) and stimulated with lipopolysaccharide (LPS) (0.1 to 10 μg/ml) (Sigma Chemical Company, St. Louis, Mo.) for 16 h.
Surface and intracytoplasmic immunofluorescence.
Clone 43 cells were stained by indirect methods as previously described with various monoclonal antibodies (MAbs) (see below) or isotype-matched controls followed by affinity-purified F(ab′)2 fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Ig (Tago, Burlingame, Calif.) and analyzed by flow cytometry gating on the live cells (33). Anti-HLA-DR antibodies were purchased from Becton Dickinson (Mountain View, Calif.); anti-p24, anti-CD80 (B7-1), and anti-CD86 (B7-2) were purchased from Accurate Antibodies (Westbury, N.Y.); and W6/32 (anti-class I framework) was obtained from the American Type Culture Collection (Rockville, Md.). For intracytoplasmic staining, infected and uninfected cells were fixed with 70% ethanol for 30 min at 4°C. The cells were washed three times with PBS and stained with W6/32, anti-p24, anti-HLA-DR antibodies or an isotype control MAb followed by affinity-purified FITC-conjugated F(ab′)2 goat anti-mouse IgG antibody as described above (30, 34).
Antigen, mitogen, anti-CD3, and TT-induced proliferation.
Ethanol-, acetaldehyde-, and acetate-treated and untreated HIV-1-infected or uninfected monocytes or 43 and 43HIV were used as accessory cells in antigen-, mitogen-, anti-CD3-, and tetanus toxoid (TT)-induced T-cell proliferation. For these experiments, T cells were obtained from MHC-matched PBMCs from normal blood donors and were monocyte depleted with a nylon wool column (41). PBMCs were incubated on the column for 45 min and eluted with warm CM. T cells isolated in this manner failed to respond to TT (Wyeth Pharmaceuticals, West Point, Pa.) (4 to 40 μg/ml), phytohemagglutinin (PHA) (0.01 to 1.0 μg/ml) (Sigma), concanavalin A (ConA) (0.01 to 1.0 μg/ml) (Sigma), pokeweed mitogen (PWM) (0.1 to 1.0 μg/ml) (Sigma), or the anti-CD3 MAb 446 (5 μg/ml) as previously described (41). Monocyte-depleted T cells (105) were cocultured either with various concentrations of irradiated (6,000 rads, cesium source) ethanol-, acetaldehyde-, and acetate-treated 43 and 43HIV cells or with autologous HIV-1-infected and uninfected monocytes, cells (103 to 105), and PHA (0.01 to 1.0 μg/ml) (Sigma), (ConA (0.01 to 1.0 μg/ml) (Sigma), PWM (Sigma) (0.01 to 1.0 μg/ml), and MAb 446 (5 μg/ml) in 0.2 ml of CM in triplicate round-bottom microtiter plates (Linbro, Oxnard, Calif.) at 37°C in a 5% CO2 incubator for 3 days. TT (4.0 to 40 μg/ml [Wyeth])-stimulated cells were maintained in culture for 5 days. The 446 MAb has been previously characterized. It is an antibody directed against the γ-chain of the CD3 complex. This antibody can stimulate T cells by using the FcR expressed on macrophages to cross-link the antigen receptor (41). The TT-stimulated T cells were maintained in culture for 5 days at 37°C in a 5% CO2 incubator. In some experiments, various concentrations of IL-1α and IL-1β (0.1 to 10 U/ml) (Boehringer Mannheim, Indianapolis, Ind.) and anti-TGFβ (10 μg/ml) (R & D, Minneapolis, Minn.) were added to the cultures.
Determination of a toxic effect.
The viability of the 43 and 43HIV cells and HIV-1-infected and uninfected monocytes treated with ethanol, acetaldehyde, and acetate was assessed by propidium iodide staining followed by flow cytometric analysis (34). The viability of T cells cocultured with ethanol-, acetaldehyde-, and acetate-treated monocytic cells was also determined. T cells (105) were cocultured with various concentrations (103 to 105) of 43HIV or 43 cells or HIV-1-infected or uninfected monocytes. The cells were dual stained with FITC-labeled anti-CD3 MAb (Leu 4; Becton Dickinson) and propidium iodide and analyzed by flow cytometry as previously described to determine the viability of the T cells (34).
Detection of DNA strand breaks associated with apoptosis.
The 43 and 43HIV cells, the HIV-1-infected and uninfected monocytes, and the cocultured T cells were assessed for apoptosis after the ethanol, acetaldehyde, and acetate treatments. The cells were washed three times and suspended in cacodylate buffer consisting of 0.2 M potassium, 25 mM Tris-HCl (pH 6.6), 2.5 mM cobalt chloride (CoCl2), 0.25 μg of BSA per ml, 100 U of terminal transferase, and 0.5 M biotin 6-UTP for 30 min at 37°C. The cells were then fixed in 1% formaldehyde for 25 min at 4°C, washed in PBS, and resuspended in 100 μl of sodium citrate buffer consisting of 2.5 μg of fluoresceinated avidin per ml, 0.1% Triton X-100, and 5% (wt/vol) nonfat dry milk for 30 min at 25°C in the dark. The cells were rinsed in propidium iodide buffer (5 μg of propidium iodide per ml, 0.1% RNAse A) and analyzed by flow cytometry for DNA strand breaks (fluorescein) and changes in cell cycle (propidium iodide).
Cytokine assay.
IL-1α, IL-1β, TNF-α, and TGF-β levels in the culture supernatants and cell lysates (see below) from ethanol-, acetaldehyde-, and acetate-treated and untreated HIV-1-infected and uninfected primary monocytes as well as 43 and 43HIV cells were measured with commercially available kits (R & D).
Generation of 43 cell and monocyte lysates.
Lysates of ethanol-, acetaldehyde-, and acetate-treated LPS and phorbol myristate acetate (PMA)-stimulated 43 cells and monocytes were generated by freezing (−40°C) and thawing the cells twice. The cells were sonicated and spun at high speed (12,000 rpm) for 10 min. Supernatant from the lysed cells was collected for IL-1α, IL-1β, TNF-α, and TGF-β determination as described above.
Detection of IL-1α, IL-1β, TNF-α, and TGF-β mRNA by slot analysis.
RNA was extracted from 2 × 106 cells with RNAzol (Linnai Technologies, Dallas, Tex.). RNA probes were prepared from different cDNA clones for slot blot analysis of the cytokines. The M31A clone was used for TNF-α, the pHTGF-b clone was used for TGF-β, the pmIL1AcDNA clone was used for IL-1α, and the psm201 clone was used for IL-1β. 32P-labeled antisense RNA transcripts were generated with T7 polymerase after linearization. Equivalent amounts of mRNA from the ethanol-, acetaldehyde-, and acetate-treated LPS-stimulated monocytes and 43 cells were applied to nitrocellulose filters and then probed for IL-1α, IL-1β, TNF-α, and TGF-β for 16 h at 65°C. The filters were washed first in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% sodium dodecyl sulfate (SDS) three times and then with 0.5× SSC plus 1% SDS at 42°C, followed by exposure to X-ray film overnight. The filters were stripped and then probed for glucose 3-phosphate dehydrogenase (G3PDH) mRNA, which served as the internal control.
RESULTS
Immunosuppressive effect of ethanol, acetaldehyde, and acetate on the 43 hybridoma cells.
Previous studies have suggested that ethanol may have immunosuppressive effects on monocytic function (4, 7, 27, 38, 42). Peripheral blood monocytes represent an extremely heterogeneous population of cells. A model system utilizing a clonal system representing subpopulations of human macrophages may eliminate variability in analysis of monocytic function after infection with pathogens. This may be particularly important when analyzing monocytic function after HIV-1 infection, where highly variable results may relate to incomplete monocyte infection. Therefore, we determined if ethanol and its metabolites acetaldehyde and acetate had any effect on accessory cell function in the uninfected 43 cells. We pretreated the 43 cells with various concentrations of ethanol, acetaldehyde, and acetate (25, 75, and 150 mM) for 16 h as previously described (38) and then cocultured the cells with monocyte-depleted MHC-matched responder T cells pulsed with PHA, ConA, PWM, MAb 446, and TT. Other time points were used for the ethanol, acetaldehyde, and acetate treatments, but the results were not as consistent as those at the 16-h time point (data not shown). There was also no significant evaporation of ethanol, acetaldehyde, and acetate from the cultures. Although pretreatment with different concentrations of ethanol, acetaldehyde, and acetate had no effect on the viability of the 43 cells and cocultured T cells and there was no induction of apoptosis, there was a dose-dependent decrease in the T-cell proliferative responses to PHA, ConA, PWM, MAb 446, and TT (Table 1). Since we observed decreased T-cell proliferation when the ethanol-, acetaldehyde-, and acetate-treated 43 cells were used as accessory cells, we wanted to validate these findings in primary monocytes. Consonant with the data from the 43 cells, there was no change in the viability or induction of apoptosis in the treated monocytes or cocultured T cells. Pretreatment of the primary monocytes with ethanol, acetaldehyde, and acetate (25, 75, and 150 mM) also resulted in a dose-dependent impairment of T-cell proliferation induced by PHA, ConA, PWM, MAb 446, and TT (Table 2).
TABLE 1.
Effects of ethanol, acetaldehyde, and acetate on accessory cell function in clone 43 cellsa
| Treatment (mM) | Fold increase after stimulation with b:
|
||||
|---|---|---|---|---|---|
| PHA | ConA | PWM | MAb 446 | TT | |
| None | 135 ± 64 | 70 ± 34 | 28 ± 14 | 20 ± 9 | 6 ± 3 |
| Ethanol | |||||
| 25 | 103 ± 34 | 43 ± 21 | 18 ± 9 | 15 ± 7 | 4 ± 2 |
| 75 | 40 ± 17 | 14 ± 7 | 13 ± 4 | 11 ± 3 | 3 ± 1.5 |
| 150 | 14 ± 8 | 9 ± 5 | 7 ± 3 | 5 ± 3 | 1 ± 0.5 |
| Acetaldehyde | |||||
| 25 | 99 ± 37 | 45 ± 33 | 17 ± 1 | 14 ± 7 | 4.5 ± 1.7 |
| 75 | 39 ± 16 | 15 ± 8 | 14 ± 5 | 13 ± 7 | 3.1 ± 1.6 |
| 150 | 13 ± 7 | 8 ± 4 | 10 ± 5 | 10 ± 4 | 1.8 ± 0.9 |
| Acetate | |||||
| 25 | 101 ± 38 | 45 ± 23 | 20 ± 9 | 16 ± 8 | 4.5 ± 3.1 |
| 75 | 42 ± 20 | 15 ± 8 | 12 ± 5 | 10 ± 4 | 3.1 ± 1.5 |
| 150 | 15 ± 7 | 10 ± 6 | 7.5 ± 4 | 6 ± 2 | 1.1 ± 0.9 |
Some clone 43 cells (104) were maintained in culture, while others were treated with various concentrations (25, 75, and 150 mM) of ethanol, acetaldehyde, and acetate for 16 h, washed with PBS, cocultured with 105 monocyte-depleted PBMCs, and stimulated with PHA (1 μg/ml), ConA (1 μg/ml), PWM (1 μg/ml), and MAb 446 (anti-CD3 [5 μg/ml]) for 72 h. TT (4 μg/ml)-stimulated cultures were harvested after 5 days. [3H]thymidine (1 μCi) was added for the last 16 h, the cells were harvested, and the incorporated counts per minute were analyzed.
Data are the means ± standard errors from the pooled data of three separate experiments and are expressed as fold increases (stimulation index) in accessory cell function over levels in unstimulated cultures.
TABLE 2.
Effects of ethanol, acetaldehyde, and acetate on accessory cell function in monocytesa
| Treatment (mM) | Fold increase after stimulation withb:
|
||||
|---|---|---|---|---|---|
| PHA | ConA | PWM | MAb 446 | TT | |
| None | 265 ± 70 | 110 ± 30 | 57 ± 18 | 27 ± 9 | 7 ± 3 |
| Ethanol | |||||
| 25 | 151 ± 50 | 67 ± 20 | 35 ± 10 | 19 ± 8 | 4.5 ± 1.8 |
| 75 | 81 ± 24 | 53 ± 18 | 21 ± 7 | 12 ± 5 | 2.5 ± 1 |
| 150 | 27 ± 9 | 19 ± 9 | 10 ± 4 | 7 ± 2 | 1 ± 0.9 |
| Acetaldehyde | |||||
| 25 | 45 ± 51 | 71 ± 22 | 38 ± 14 | 21 ± 10 | 5 ± 2.5 |
| 75 | 78 ± 20 | 52 ± 23 | 19 ± 7 | 13 ± 8 | 3 ± 1 |
| 150 | 30 ± 11 | 21 ± 7 | 11 ± 5 | 8 ± 3 | 1 ± 0.7 |
| Acetate | |||||
| 25 | 148 ± 45 | 71 ± 21 | 31 ± 13 | 23 ± 10 | 5 ± 2 |
| 75 | 79 ± 20 | 57 ± 19 | 2 ± 10 | 13 ± 4 | 3 ± 1.1 |
| 150 | 29 ± 10 | 17 ± 9 | 12 ± 5 | 8 ± 3 | 1.5 ± 0.7 |
Some cells (104) were maintained in culture, while others were treated with various concentrations of ethanol, acetaldehyde, and acetate as described for Table 1.
Data are the means ± standard errors from the pooled data of three separate experiments and are represented as fold increases (stimulation index) in accessory cell function over levels in unstimulated cultures.
Effect of ethanol, acetaldehyde, and acetate treatment on cytokine production.
Alteration in cytokine production by the ethanol-, acetaldehyde-, and acetate-treated 43 cells and monocytes may account for the decreased T-cell proliferation observed in response to stimulation with the mitogens, MAb 446, and TT. Since ethanol has previously been reported to affect production of IL-1, TNF-α, and TGF-β (8, 38), we first measured constitutive and induced (LPS [0.1 to 10.0 μg/ml]) production in the 43 cells and primary monocytes treated with ethanol, acetaldehyde, and acetate at concentrations (25, 75, and 150 mM) at which we noted decreased T-cell proliferation in response to stimulation by the mitogens, MAb 446, and TT. Production of IL-1α, IL-1β, and TNF-α was markedly reduced after ethanol, acetaldehyde, and acetate treatment, but there was induction of TGF-β production (Table 3). To validate the results in the 43 cells, we again performed similar experiments with primary monocytes treated with the same concentrations (25, 75, and 150 mM) of ethanol, acetaldehyde, and acetate. Significant reductions in IL-1α, IL-1β, and TNF-α production from primary monocytes were observed when comparing ethanol-, acetaldehyde-, and acetate-treated to untreated monocytes (Table 4). The induction of TGF-β by ethanol, acetaldehyde, and acetate treatment of the primary monocytes was also significant (Table 4). Since changes in cytokine production in the monocytes and 43 cells may be impairing T-cell proliferation in response to mitogens, anti-CD-3, and TT, we attempted to restore proliferation by adding IL-1α and IL-1β (0.1 to 10 U/ml) to the cultures. Exogenous IL-1α and IL-1β failed to restore T-cell proliferation at any concentration tested. However, when we added anti-TGF-β antibody (10 μg/ml) along with either IL-1α (5 U/ml) or IL-1β (5 U/ml), there was recovery of T-cell proliferation in response to stimulation with mitogens, MAb 446, and TT (data not shown).
TABLE 3.
Effects of ethanol, acetaldehyde, and acetate on IL-1α, IL-1β, TNF-α, and TGF-β production in clone 43 cellsa
| Treatment | Production (pg/ml) of cytokineb:
|
|||
|---|---|---|---|---|
| IL-1α | IL-1β | TNF-α | TGF-β | |
| None | 0 | 0 | 0 | 0 |
| LPS (1 μg/ml) | 302 ± 100 | 799 ± 147 | 535 ± 135 | 0 |
| Ethanol | ||||
| 25 mM | 175 ± 43 | 165 ± 37 | 179 ± 81 | 75 ± 31 |
| 75 mM | 101 ± 31 | 85 ± 37 | 50 ± 71 | 25 ± 43 |
| 150 mM | 37 ± 10 | 57 ± 14 | 78 ± 34 | 250 ± 84 |
| Acetaldehyde | ||||
| 25 mM | 168 ± 37 | 159 ± 50 | 180 ± 90 | 62 ± 20 |
| 75 mM | 99 ± 22 | 130 ± 50 | 157 ± 68 | 101 ± 30 |
| 150 mM | 40 ± 13 | 52 ± 20 | 73 ± 29 | 239 ± 70 |
| Acetate | ||||
| 25 mM | 164 ± 40 | 153 ± 37 | 173 ± 70 | 39 ± 10 |
| 75 mM | 99 ± 24 | 79 ± 30 | 134 ± 80 | 110 ± 30 |
| 150 mM | 39 ± 13 | 59 ± 20 | 85 ± 30 | 210 ± 73 |
Some clone 43 cells were maintained in culture, while others were treated with various concentrations (25, 75, and 150 mM) of ethanol, acetaldehyde, and acetate for 16 h, washed in PBS, and stimulated with LPS (1 μg/ml) for 16 h. Cytokine determinations were performed with the culture supernatant by a direct-binding enzyme-linked immunosorbent assay.
Data represent the means ± standard errors from three separate experiments.
TABLE 4.
Effects of ethanol, acetaldehyde, and acetate on IL-1α, IL-1β, TNF-α, and TGF-β production in primary monocytesa
| Treatment | Production (pg/ml) of cytokineb:
|
|||
|---|---|---|---|---|
| IL-1α | IL-1β | TNF-α | TGF-β | |
| None | 0 | 0 | 0 | 0 |
| LPS (1 μg/ml) | 376 ± 123 | 877 ± 233 | 543 ± 156 | 433 ± 203 |
| Ethanol | ||||
| 25 mM | 250 ± 84 | 595 ± 170 | 375 ± 97 | 180 ± 57 |
| 75 mM | 165 ± 54 | 275 ± 84 | 235 ± 84 | 275 ± 135 |
| 150 mM | 95 ± 34 | 101 ± 30 | 125 ± 70 | 375 ± 70 |
| Acetaldehyde | ||||
| 25 mM | 247 ± 101 | 734 ± 211 | 395 ± 100 | 175 ± 57 |
| 75 mM | 175 ± 87 | 347 ± 121 | 247 ± 87 | 271 ± 121 |
| 150 mM | 97 ± 31 | 121 ± 41 | 130 ± 87 | 325 ± 121 |
| Acetate | ||||
| 25 mM | 270 ± 101 | 627 ± 121 | 427 ± 121 | 148 ± 57 |
| 75 mM | 151 ± 29 | 285 ± 95 | 297 ± 98 | 256 ± 98 |
| 150 mM | 87 ± 27 | 141 ± 42 | 150 ± 72 | 340 ± 120 |
Significant reductions in IL-1α, IL-1β, and TNF-α production were observed when ethanol-, acetaldehyde-, and acetate-treated LPS-stimulated monocytes were compared with untreated monocytes.
These data represent values of four separate experiments and are expressed as the means ± standard errors.
Effect of ethanol, acetaldehyde, and acetate on intracellular cytokine production.
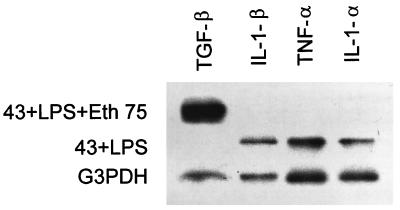
Two possible mechanisms may explain the effect of ethanol, acetaldehyde, and acetate treatment in reducing IL-1α, IL-1β, and TNF-α synthesis: there may be either impaired protein production or impaired release of cytokine from the cells. To clarify the effects of ethanol, acetaldehyde, and acetate, we simultaneously measured the production of IL-1α, IL-1β, and TNF-α in culture supernatant and cell lysates from ethanol-, acetaldehyde-, and acetate-treated LPS-stimulated monocytes and 43 cells. Consistent with the secretion data, intracellular levels of IL-1-α, IL-1-β, and TNF-α detected in the ethanol-, acetaldehyde-, and acetate-treated lysates from the 43 cells were reduced, indicating that there was decreased production of all three cytokines and not just inhibition of release where stable or increased levels of IL-1α, IL-1β, and TNF-α would be expected (Table 5). Similar results were observed with the primary monocytes (data not shown). To further delineate the site of the inhibitory effect of ethanol, acetaldehyde, and acetate on IL-1α, IL-1β, and TNF-α, we next assessed the level of IL-1α, IL-1β, and TNF-α mRNA in the presence or absence of ethanol, acetaldehyde, and acetate (75 mM) by slot blot analysis. After ethanol treatment, there was loss of mRNA for IL-1α, IL-1β, and TNF-α in the 43 cells and monocytes and induction of TGF-β mRNA (Fig. 1). Similar results were observed with 43 cells treated with acetaldehyde and acetate in the monocytes (data not shown).
TABLE 5.
Effects of ethanol, acetaldehyde, and acetate on intracellular production of IL-1α, IL-1β, TNF-α, and TGF-β in clone 43 cellsa
| Treatment | Production (pg/ml) of cytokineb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IL-1α
|
IL-1β
|
TNF-α
|
TGF-β
|
|||||
| Supernatant | Lysate | Supernatant | Lysate | Supernatant | Lysate | Supernatant | Lysate | |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LPS (1 μg/ml) | 398 ± 150 | 295 ± 173 | 925 ± 257 | 853 ± 212 | 675 ± 200 | 723 ± 275 | 498 ± 190 | 412 ± 212 |
| Ethanol | ||||||||
| 25 mM | 298 ± 125 | 275 ± 155 | 735 ± 189 | 631 ± 210 | 521 ± 134 | 575 ± 121 | 93 ± 41 | 108 ± 53 |
| 75 mM | 175 ± 74 | 198 ± 101 | 361 ± 91 | 315 ± 150 | 295 ± 151 | 287 ± 111 | 153 ± 73 | 178 ± 98 |
| 150 mM | 85 ± 21 | 103 ± 53 | 138 ± 73 | 178 ± 73 | 173 ± 81 | 113 ± 53 | 311 ± 98 | 275 ± 178 |
| Acetaldehyde | ||||||||
| 25 mM | 308 ± 123 | 190 ± 103 | 801 ± 210 | 601 ± 110 | 595 ± 121 | 624 ± 138 | 98 ± 48 | 58 ± 21 |
| 75 mM | 137 ± 81 | 153 ± 84 | 312 ± 112 | 258 ± 98 | 307 ± 131 | 298 ± 151 | 126 ± 51 | 101 ± 63 |
| 150 mM | 75 ± 19 | 83 ± 24 | 101 ± 37 | 97 ± 21 | 121 ± 67 | 151 ± 63 | 321 ± 101 | 298 ± 98 |
| Acetate | ||||||||
| 25 mM | 281 ± 121 | 273 ± 119 | 834 ± 177 | 703 ± 198 | 627 ± 100 | 598 ± 103 | 87 ± 35 | 77 ± 32 |
| 75 mM | 130 ± 62 | 128 ± 66 | 780 ± 130 | 299 ± 101 | 433 ± 190 | 397 ± 113 | 157 ± 70 | 144 ± 95 |
| 150 mM | 74 ± 21 | 63 ± 18 | 250 ± 110 | 101 ± 60 | 111 ± 39 | 98 ± 17 | 357 ± 180 | 401 ± 155 |
The clone 43 cells were treated with various concentrations (25, 75, and 150 mM) of ethanol, acetaldehyde, and acetate for 16 h, washed in PBS, and stimulated with LPS (1 μg/ml) for 16 h. Cytokine determinations were performed simultaneously with the culture supernatants and cell lysates by direct-binding enzyme-linked immunosorbent assay.
Data represent means ± standard errors from three separate experiments.
FIG. 1.
mRNA expression in ethanol-treated clone 43 cells. mRNA was extracted from the clone 43 cells and probed for IL-1α, IL-1β, TNF-α, and TGF-β expression after ethanol (Eth) treatment (75 mM for 16 h). G3PDH served as the internal control. The figure shows results from a representative experiment repeated three times.
Class II, antigen, CD-80, and CD-86 expression.
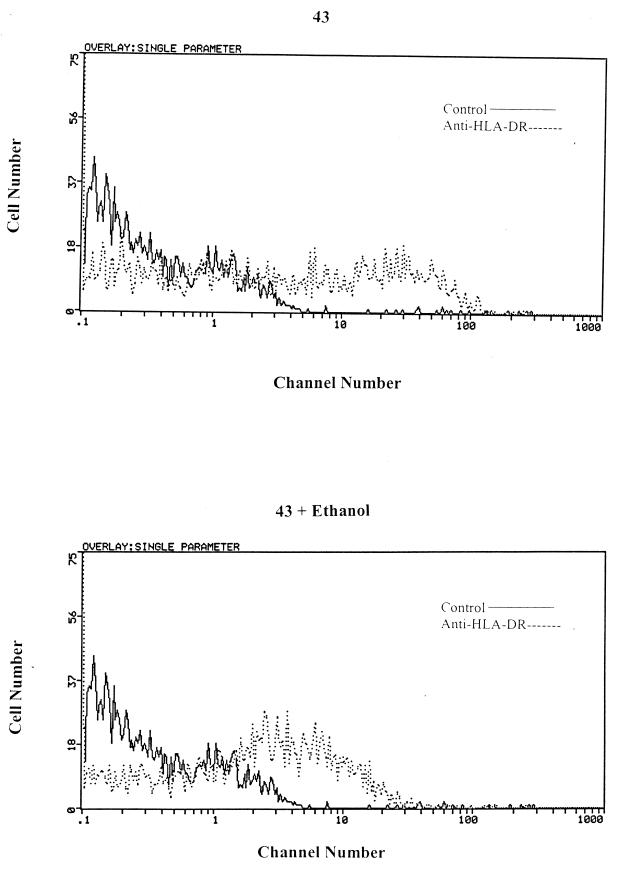
The loss of IL-1α and IL-1β and induction of TGF-β would explain in part the inability of the 43 cells and primary monocytes to induce T-cell proliferation in response to stimulation with mitogens, MAb 446, and TT. However, ethanol, acetaldehyde, and acetate may have additional deleterious effects on monocytic function, which may further contribute to the impairment of accessory cell function. To determine the effect of ethanol, acetaldehyde, and acetate on the expression of class II antigen and the costimulatory molecules CD80 and CD86, immunofluorescence was performed with untreated and treated 43 cells (IFN-γ at 100 U for 48 h) and primary monocytes. There was a modest reduction in the percentage of HLA-DR+ cells (untreated versus treated) after ethanol (65% versus 35% and 51% versus 41%) (Fig. 2), acetaldehyde, or acetate treatment, but no change in either class I, CD80, or CD86 expression in the 43 cells and primary monocytes (data not shown). The last possibility is that ethanol-, acetaldehyde-, and acetate-treated monocytes or 43 cells may be directly toxic to or may induce apoptosis in the cocultured T cells. The viability of the mitogen-, MAb 446-, and TT-stimulated and bystander T cells was unchanged after coculture with the ethanol-, acetaldehyde-, and acetate-treated cells, and there was no induction of apoptosis (data not shown). Studies have suggested that there may be an increased incidence of HIV-1 infection among alcoholics, suggesting that there is an association between ethanol intake and HIV-1 infection (2, 13, 16, 28, 32). Since some of the defects that occurred after ethanol, acetaldehyde, and acetate treatment are similar to those noted following HIV-1 infection of monocytes, we wanted to determine if the presence of ethanol, acetaldehyde, and acetate would either accelerate or enhance the defects seen after HIV-1 infection.
FIG. 2.
HLA-DR expression after ethanol treatment. Clone 43 was treated with 75 mM ethanol for 16 h. Surface immunofluorescence was performed with FITC-labeled anti-HLA-DR MAbs gated on live cells. The figure shows results from a representative of an experiment repeated three times.
Effect of ethanol on the kinetics of loss of accessory cell function after HIV-1 infection.
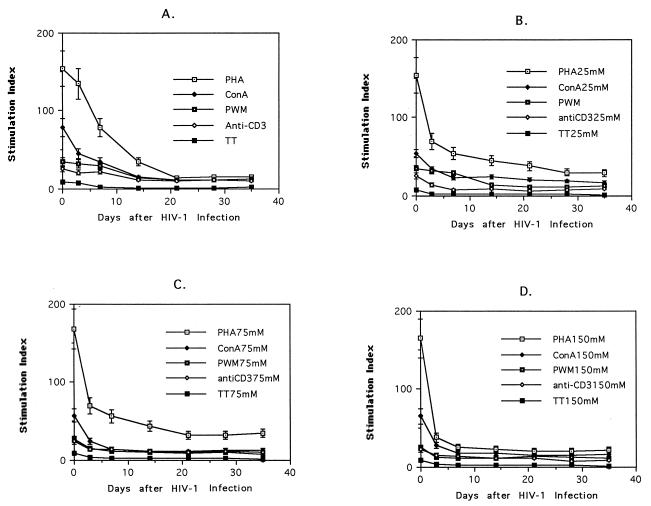
We pretreated 43 cells and primary monocytes with various concentrations (25, 75, and 150 mM) of ethanol, acetaldehyde, and acetate and then infected the cells with HIV-1. The amount of HIV-1 required (HIV-1IIIB or HIV-1BaL, with supernatant standardized to 80,000 cpm by reverse transcriptase activity) for infection as determined by intracytoplasmic staining with an anti-p24 MAb determined 48 h after infection was less (40,000 cpm) in the ethanol-, acetaldehyde-, and acetate-treated cells than in the control cells (data not shown). After ethanol treatment (25, 75, and 150 mM), the HIV-1-infected 43 cells (43HIV) lost accessory cell function for PHA, ConA, PWM, MAb 446, and TT 3 days after HIV-1 infection compared to 7 days in the non-ethanol-treated 43HIV cells (Fig. 3). Acetaldehyde and acetate had a similar effect on the kinetics of loss of accessory cell function (data not shown). Because we have previously demonstrated that HIV-1 infection induced multiple accessory cell defects in the 43HIV cells, we wanted to determine if the ethanol, acetaldehyde, or acetate pretreatment accelerated the development of these defects.
FIG. 3.
Kinetics of loss of accessory cell function for alcohol-treated and untreated 43HIV cells. 43HIV cells were pretreated with ethanol at 0 mM (A), 25 mM (B), 75 mM (C), and 150 mM (D) for 16 h and then pulsed with various concentrations of PHA (1 to 0.01 μg/ml), ConA (1 to 0.01 μg/ml), PWM (1 to 0.01 μg/ml), and MAb 446 (1 μg/ml) after 72 h TT (4 μg/ml)-induced T-cell proliferation was measured after 5 days. T-cell proliferation after mitogen, antigen, and anti-CD3 stimulation was determined by thymidine incorporation.
Kinetics of loss of cytokine production and class II expression.
To determine if the kinetics of loss of IL-1α, IL-1β, and TNF-α production were altered by ethanol, acetaldehyde, or acetate treatment, we measured constitutive and induced (LPS [0.1 to 10 μg/ml]) levels of IL-1α, IL-1β, and TNF-α in the culture supernatants of untreated and treated (25, 75, and 150 mM) 43HIV cells (Table 6). LPS-induced IL-1α, IL-1β, and TNF-α production by the 43HIV cells was decreased after ethanol treatment, but this reduction occurred more rapidly 7 days after infection instead of the 2 weeks that was observed in the untreated 43HIV cells. The 43HIV cells did not produce TGF-β at any time point tested after infection, but following treatment with ethanol, there was induction of TGF-β (Table 6). Acetaldehyde and acetate had a similar effect on LPS-induced IL-1α, IL-1β, TNF-α, and TGF-β production in the 43HIV cells (Table 6).
TABLE 6.
Effects of ethanol, acetaldehyde, and acetate on IL-1α, IL-1β, TNF-α, and TGF-β production in 43HIV cellsa
| Treatment (mM) | Production (pg/ml) of cytokineb:
|
|||
|---|---|---|---|---|
| IL-1α | IL-1β | TNF-α | TGF-β | |
| None | 135 ± 40 | 385 ± 95 | 265 ± 81 | 0 |
| Ethanol | ||||
| 25 | 75 ± 21 | 180 ± 65 | 111 ± 30 | 175 ± 30 |
| 75 | 37 ± 8 | 95 ± 30 | 37 ± 8 | 125 ± 70 |
| 150 | 15 ± 5 | 53 ± 10 | 25 ± 5 | 195 ± 85 |
| Acetaldehyde | ||||
| 25 | 80 ± 25 | 173 ± 45 | 105 ± 27 | 64 ± 14 |
| 75 | 51 ± 11 | 84 ± 24 | 39 ± 10 | 110 ± 39 |
| 150 | 19 ± 4 | 54 ± 10 | 27 ± 5 | 175 ± 50 |
| Acetate | ||||
| 25 | 74 ± 19 | 157 ± 50 | 97 ± 14 | 54 ± 20 |
| 75 | 50 ± 19 | 71 ± 17 | 42 ± 14 | 130 ± 40 |
| 150 | 25 ± 10 | 45 ± 9 | 25 ± 6 | 210 ± 80 |
43HIV cells 7 days after infection were treated with 25, 75, and 150 mM ethanol, acetaldehyde, and acetate for 16 h and then stimulated with LPS (1 μg/ml). Immunoreactive cytokines in the culture supernatants were measured by direct-binding enzyme-linked immunosorbent assay.
Data represent the means ± standard errors from three separate experiments.
Class II antigen expression.
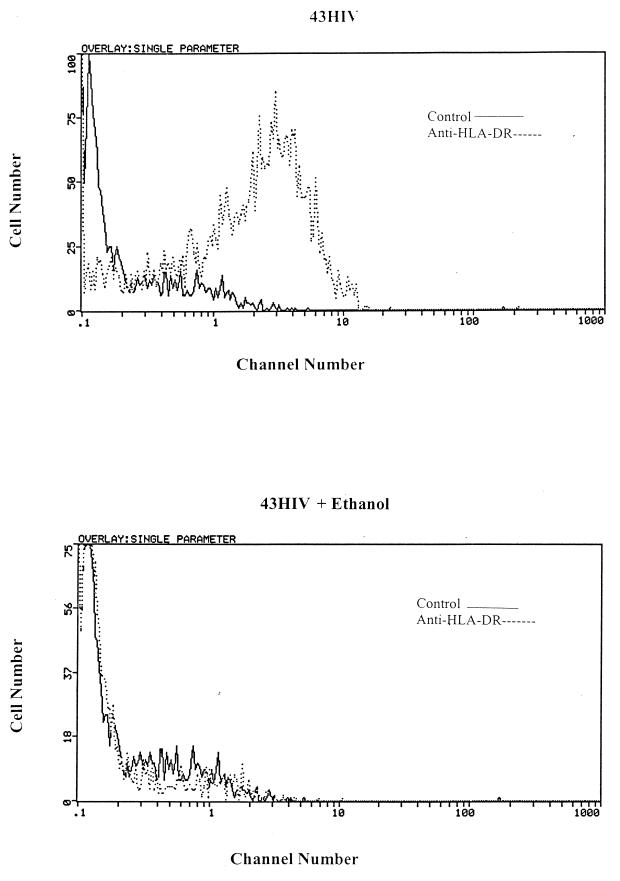
One of the most pronounced effects of HIV-1 infection in the 43HIV cells and other human macrophage hybridoma cell lines was the ablation of class II antigen expression. Since other monocytic defects appeared to occur more rapidly in the ethanol-, acetaldehyde-, and acetate-treated cells, we assessed whether there was more rapid loss of HLA-DR expression in the ethanol-treated HIV-1-infected 43 cells. Serial staining of the ethanol-treated 43HIV cells with anti-class II antibodies revealed the loss of surface HLA-DR 7 days after HIV-1 infection instead of 14 days in the non-ethanol-treated HIV-1-infected cells (Fig. 4). Consistent with the ethanol experiments, acetaldehyde and acetate caused comparable reductions in surface HLA-DR expression 7 days after HIV-1 infection (data not shown).
FIG. 4.
HLA-DR expression in ethanol-treated and untreated 43HIV cells. 43HIV cells were maintained in culture, while others were treated with 75 mM ethanol for 16 h. Surface immunofluorescence was performed with FITC-labeled anti-HLA-DR MAbs gated on live cells. The figure shows results from a representative experiment repeated three times.
DISCUSSION
We have previously described aberrant monocyte function after HIV-1 infection, as determined with our human macrophage hybridoma system and primary HIV-1BaL-infected monocytes. These included defects in class II antigen expression, cytokine production, and accessory cell function for antigen-, mitogen-, and anti-CD-3-induced T-cell proliferation. We have extended these studies to investigate the effect of ethanol and its metabolites acetaldehyde and acetate on monocytic function after HIV-1 infection. The data presented here have demonstrated that ethanol may cause defects in monocytic function which could contribute to the progression and/or manifestations of HIV-1 infection. In both the human macrophage hybridoma cell line, clone 43, and primary monocytes, there was progressive impairment of accessory cell function for mitogen-, anti-CD-3-, and antigen-induced T-cell proliferation after pretreatment of the cells with increasing concentrations of ethanol, acetaldehyde, and acetate (Tables 1 and 2). There also was no difference between the effects of ethanol, acetaldehyde, and acetate on immune function, which is consistent with the results of previous studies (31). The concentrations of acetaldehyde and acetate used in our experiments are 100-fold more than those observed in vivo (15). We utilized these concentrations since they had been previously published in other studies to evaluate the effect of ethanol, acetaldehyde, and acetate on T-cell proliferation (38, 39). The purpose of using equivalent amounts of acetaldehyde and acetate was to demonstrate a similar effect of the ethanol metabolites and not to mimic in vivo concentrations.
Ethanol has been reported to have many immunosuppressive effects, such as decreasing absolute numbers of T and B cells and circulating levels of IgG, increasing IgA and IgM levels, and impairing T-cell migration (5, 17, 18, 21, 25). In animal model systems, the immunosuppressive effect of ethanol has been further demonstrated. In a rat model, ethanol consumption resulted in decreased thymic weight, low T-cell counts, and impaired macrophage function compared to those of the control animals (26). In murine systems, it has been demonstrated that ethanol consumption decreased total numbers of lymphocytes in the spleen, caused impaired mitogen proliferation and responses to T-cell-dependent antigens, as well as defective phagocytosis by monocytes, and decreased NK cell activity and antibody-dependent antibody cytotoxicity (17).
In our system, ethanol, acetaldehyde, and acetate affected cytokine production. In the 43 cells and primary monocytes treated with ethanol, acetaldehyde, and acetate, there was loss of production of IL-1α, IL-1β, and TNF-α along with the induction of TGF-β (Tables 3 and 4). This may be particularly important, since the decreased production of these cytokines could impair normal immune responses to HIV-1 as well as to other pathogens (8, 38), accelerating the course of the disease. However, the defective T-cell proliferation after ethanol, acetaldehyde, and acetate in response to mitogens, MAb 446, and TT could be restored with the addition of exogenous IL-1α or IL-1β and anti-TGF-β antibodies. Ethanol, acetaldehyde, and acetate inhibited the cellular production of IL-1α, IL-1β, and TNF-α, since there were reduced intracellular levels of cytokine and inhibition of transcription of mRNA after treatment (Fig. 1 and Table 5). This is similar to our previous studies demonstrating that HIV-1 infection of the human macrophage hybridomas was associated with loss of mRNA for IL-1α, IL-1β, and TNF-α (34). There was not a general inhibition of cytokine mRNA production, since TGF-β was induced after treatment of the cells. Different concentrations of ethanol, acetaldehyde, and acetate may have different effects on TGF-β production in monocytic cells. Although there was more TGF-β production from the untreated monocytes after LPS stimulation, lower concentrations of ethanol, acetaldehyde, and acetate appeared to suppress TGF-β production. There was no detectable TGF-β from the monocytes in the absence of LPS stimulation. Although TGF-β is present in many tissues, spontaneous production by monocytes has been variably observed (35). It is conceivable that ethanol, acetaldehyde, and acetate may be having an effect on the regulation of DNA binding proteins necessary for IL-1α, IL-1β, and TNF-α or inducing a suppressor protein. Studies are presently under way comparing DNA binding proteins from ethanol-, acetaldehyde-, and acetate-treated and untreated 43 cells and primary monocytes. Cytokine synthesis was also affected in the chronically HIV-1-infected 43 cells (43HIV). There was reduced production of IL-1α, IL-1β, and TNF-α and induction of TGF-β 7 days after infection when the 43HIV cells were treated with ethanol, acetaldehyde, and acetate compared to the levels at 14 days in the untreated cells (Table 6). We also observed decreased surface expression of HLA-DR after ethanol treatment in both the uninfected 43 and 43HIV cells (Fig. 2 and 4). Taken together with the early loss of accessory cell function (Fig. 3), these results suggest that ethanol, acetaldehyde, and acetate have an additive detrimental effect on monocytic function after HIV-1 infection.
The effect of ethanol and its metabolites on cytokine production may play a particularly important role in the progression of HIV-1 disease. Macrophage-derived cytokines, especially IL-10 and IL-12, may be important in the regulation of responses against HIV-1 and other pathogens (11). The level of IL-10 mRNA is increased in patients with more advanced HIV-1 disease compared to that in patients with asymptomatic disease and has been implicated in apoptosis and in shifting T-cell helper responses from a Th1 to a Th2 pattern (1, 12). Such a switch is associated with progression to AIDS (11). IL-12 enhances cytolytic activity and is important in promoting Th1 responses (19). Decreased IL-12 production may play an important role in HIV-1 immunopathogenesis, since IL-12 has been reported to correct mitogen and antigen responses of HIV-1-infected individuals in vitro (10). In an animal model system, ethanol has been reported to switch cytokine production to a Th2 pattern after a retroviral infection. C57/BL6 mice who were exposed to ethanol 10 weeks prior to infection with LP-MB5, a murine leukemia virus that causes murine AIDS, had decreased IL-2 and IFN-γ production and increased IL-5, IL-6, IL-10, and TNF-α production and induced hypergammaglobulinemia (40). In these animals, there was also decreased mitogen proliferation.
Ethanol, acetaldehyde, and acetate in our system appeared to facilitate HIV-1 infection of the 43 cells. These findings are in line with recent evidence suggesting that ethanol may enhance HIV-1 replication in PBMCs infected with HIV-1. When PBMCs from volunteers who were infused with ethanol were compared to PBMCs from volunteers infused with saline, there was a significant increase in p24 antigen production after HIV-1 infection from the ethanol-treated individuals (3). In another study, when PBMCs were collected before and after ethanol exposure, HIV-1 replication in vitro as determined by p24 levels and syncytium formation was increased after infection (6). Our findings along with those of the studies cited above further suggest that ethanol and its metabolites may accelerate the course of HIV-1 infection.
ACKNOWLEDGMENTS
K.S. was supported by National Institutes of Health grant CA R-29-256990 and the Irma T. Hirschl Career Development Trust.
REFERENCES
- 1.Akridge R E, Oyafuso L K M, Reed S G. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–5789. [PubMed] [Google Scholar]
- 2.Avins A, Woods W, Lindow C, Hudes E, Clark W, Hulley S. HIV infection and risk factors among heterosexuals in alcohol treatment programs. JAMA. 1994;271:515–518. [PubMed] [Google Scholar]
- 3.Bagasra O, Bachman S, Jew L, Tawodros R, Carter J, Boden G, Ryan I, Pomerantz R. Increased human immunodeficiency virus type I replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathologic mechanisms. J Infect Dis. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- 4.Bagasra O, Howeedy A, Kajacsy-Balla A. Macrophage function in chronic experimental alcoholism. I. Modulation of surface receptors and phagocytosis. Immunology. 1988;65:405–409. [PMC free article] [PubMed] [Google Scholar]
- 5.Bagasra O, Howeedy R, Dorio R, Kajdacy-Balla A. Functional analysis of T-cell subsets in chronic experimental alcoholism. Immunology. 1987;61:63–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Balla A, Lischner H, Pomerantz R, Bagasra O. Human studies on alcohol and susceptibility to HIV infection. Alcohol. 1994;11:99–103. doi: 10.1016/0741-8329(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez L, Young L. Ethanol augments intracellular survival of mycobacterium avium complex and impairs macrophage responses to cytokines. J Infect Dis. 1991;163:1286–1292. doi: 10.1093/infdis/163.6.1286. [DOI] [PubMed] [Google Scholar]
- 8.Bermudez L, et al. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–420. [PubMed] [Google Scholar]
- 9.Carpenter S P, Lasker J M, Raucy J L. Expression, induction, and catalytic activity of the ethanol inducible cytokine p450 (CYP2E1) in human fetal liver and hepatocytes. Mol Pharmacol. 1996;49:260–268. [PubMed] [Google Scholar]
- 10.Clerici M, Lucey D, Berzofsky J, Pinto L, Wynn T, Blatt S, Dolen M, Hendrix C, Wolf S, Shearer G. Restoration of HIV-1 specific cell mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 11.Clerici M, Shearer G M. The TH1-TH2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Wynn T A, Berzofsky J, Blatt S, Hendrick C, Scher A, Coffman R, Shearer G. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Investig. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crum R, Galai N, Cohn S, Celetano D, Vlahow D. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: a prospective analysis. Alcohol Clin Exp Res. 1996;20:364–370. doi: 10.1111/j.1530-0277.1996.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 14.Ennen J, Seipp I, Norley S G, Kurth R. Decreased accessory cell function of macrophages after infection with human immunodeficiency virus type 1 in vitro. Eur J Immunol. 1990;20:2451–2456. doi: 10.1002/eji.1830201114. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman S, Dvorak G, MacGregor R. Host defense during prolonged alcohol consumption in a controlled environment. Arch Intern Med. 1977;137:1539–1543. [PubMed] [Google Scholar]
- 16.Jacobson J, Warner T, Sacks H, Lieber C. Human immunodeficiency virus and hepatitis B infections in a New York City alcoholic population. J Stud Alcohol. 1992;53:76–79. doi: 10.15288/jsa.1992.53.76. [DOI] [PubMed] [Google Scholar]
- 17.Jerrells T, Marietta C, Bone G, Weight F, Eckardt M. Ethanol-associated immunosuppression. Psychological, neuropsychiatric, and substance abuse aspects of AIDS. AIDS. 1988;4:177–188. [Google Scholar]
- 18.Kaelin R. Influence of ethanol on human T-lymphocyte migration. J Clin Lab Med. 1984;104:752. [PubMed] [Google Scholar]
- 19.Kobayaski M, Fitz L, Ryan M, Hevick R, Clark S, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF): a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–839. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraner J L, Lasker J M, Corcoran G B, Roy S B, Raucy J L. Induction of P4502 by acetone in isolated rabbit hepatocytes. Role of increased protein and mRNA synthesis. Biochem Pharmacol. 1993;49:260–268. doi: 10.1016/0006-2952(93)90049-3. [DOI] [PubMed] [Google Scholar]
- 21.MacGregor R. Alcohol and immune defense. JAMA. 1986;256:1474–1479. [PubMed] [Google Scholar]
- 22.Mann D, Gartner S, LeSane F, Buchnow H, Popovic M. HIV-1 transmission and function of virus-infected monocytes/macrophages. J Immunol. 1990;144:2152–2158. [PubMed] [Google Scholar]
- 23.Mann D L, Gartner S, Lesane F, Blattner W A, Popovic M. Cell surface antigens and function of monocytes and a monocyte-like cell line before and after infection with HIV. Clin Immunol Immunopathol. 1990;54:174–183. doi: 10.1016/0090-1229(90)90079-6. [DOI] [PubMed] [Google Scholar]
- 24.Meyaard L, Schuitemaker H, Miedema F. T-cell dysfunction in HIV infection: energy due to defective antigen-presenting cell function. Immunol Today. 1993;14:123. doi: 10.1016/0167-5699(93)90279-T. [DOI] [PubMed] [Google Scholar]
- 25.Mili F, Flanders D, Boring J, Annest J, DeStefano F. The association of alcohol drinking and drinking cessation to measures of the immune system in middle aged men. Alcohol Clin Exp Res. 1992;16:688–694. doi: 10.1111/j.1530-0277.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 26.Mufti S, Prabhala R, Mariiguchi S, Sipes I, Watson R. Functional and numerical alterations induced by ethanol in the cellular immune system. Immunopharmacology. 1988;15:84–93. doi: 10.1016/0162-3109(88)90055-0. [DOI] [PubMed] [Google Scholar]
- 27.Nelson S, Bagby G, Bainton B, Summer W. The effects of acute and chronic alcoholism on tumor necrosis factor and inflammatory responses. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- 28.Penkower L, Dew M, Kingsley L, Becker J, Satz P, Schaerf F, Sheridan K. Behavioral, health and psychosocial factors and risk for HIV infection among sexually active men: the multicenter AIDS cohort study. Am J Public Health. 1991;81:194–196. doi: 10.2105/ajph.81.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petit A J C, Termette M, Terpstra R E, de Goede R Y E, van Lier R A W, Miedema F. Decreased accessory cell function by human monocytic cells after infection with HIV. J Immunol. 1988;140:1485–1489. [PubMed] [Google Scholar]
- 30.Polyak S, Chen H, Hirsch D, George I, Herschberg R, Sperber K. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J Immunol. 1997;159:2177–2188. [PubMed] [Google Scholar]
- 31.Roselle G A, Mendenhall C C. Alteration of in vitro human function by ethanol, acetaldehyde and acetate. J Clin Lab Immunol. 1982;9:33–37. [PubMed] [Google Scholar]
- 32.Scheidt D, Windle M. The alcoholics in treatment HIV risk (ATRISK) study: gender, ethnic and geographic group comparisons. J Stud Alcohol. 1995;56:300–308. doi: 10.15288/jsa.1995.56.300. [DOI] [PubMed] [Google Scholar]
- 33.Sperber K, Bauer J, Pizzimenti A, Najfeld V, Mayer L. Identification of subpopulations of human macrophages through the generation of human macrophage hybridomas. J Immunol Methods. 1990;129:31–40. doi: 10.1016/0022-1759(90)90417-t. [DOI] [PubMed] [Google Scholar]
- 34.Sperber K, Hamrung G, Louie M J, Kalb T, Banerjee R, Choi H S, Parnetto F, Mayer L. Progressive impairment of monocytic function in HIV-1 infected human macrophage hybridomas. AIDS Res Hum Retroviruses. 1993;9:657–667. doi: 10.1089/aid.1993.9.657. [DOI] [PubMed] [Google Scholar]
- 35.Sporn M B, Roberts R B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;199:1017–1034. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo G, Poppolo M, Verma B, Catalano D. Regulatory potential of ethanol and retonoic acid on human monocyte function. Alcohol Clin Exp Res. 1994;18:548–554. doi: 10.1111/j.1530-0277.1994.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 37.Szabo G, Verma B, Catalano D. Selective inhibition of antigen-specific T lymphocyte proliferation by acute ethanol exposure: the role of impaired antigen presentation capacity and mediator production. J Leukocyte Biol. 1993;54:534–544. doi: 10.1002/jlb.54.6.534. [DOI] [PubMed] [Google Scholar]
- 38.Szabo G, Verma B K, Fogarasi M, Catalano D. Induction of TGF-β and prostaglandin E2 by ethanol in human monocytes. J Leukocyte Biol. 1992;52:602–610. doi: 10.1002/jlb.52.6.602. [DOI] [PubMed] [Google Scholar]
- 39.Tisman G, Herbert V. In vitro myelosuppression and immunosuppression by ethanol. J Clin Investig. 1973;52:1410–1414. doi: 10.1172/JCI107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Watson R. Chronic ethanol consumption before retrovirus infection is a cofactor in the development of immune dysfunction during murine AIDS. Alcohol Clin Exp Res. 1994;18:976–981. doi: 10.1111/j.1530-0277.1994.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoo J, Chen H, Kraus T, Hirsch D, Poylak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 42.Zuiable A, Wiener E, Wickramasinghe S N. In vitro effects of ethanol on the phagocytic and microbial killing activities of normal monocytes and monocyte-derived macrophages. Clin Lab Haemotol. 1992;14:137–147. doi: 10.1111/j.1365-2257.1992.tb01071.x. [DOI] [PubMed] [Google Scholar]