Abstract
Background
SARS-CoV-2 nucleocapsid antigen can be detected in plasma, but little is known about its performance as a diagnostic test for acute SARS-CoV-2 infection or infectious viral shedding among nonhospitalized individuals.
Methods
We used data generated from anterior nasal and blood samples collected in a longitudinal household cohort of SARS-CoV-2 cases and contacts. Participants were classified as true positives if polymerase chain reaction (PCR) positive for SARS-CoV-2 and as true negatives if PCR negative and seronegative. Infectious viral shedding was determined by the cytopathic effect from viral culture. Stratified by 7 days after symptom onset, we constructed receiver operating characteristic (ROC) curves to describe optimized accuracy (Youden index), optimized sensitivity, and specificity.
Results
Of 80 participants, 58 (73%) were true positives while 22 (27%) were true negatives. Using the manufacturer's cutoff of 1.25 pg/mL for evaluating infection, sensitivity was higher from 0 to 7 days (77.6% [95% confidence interval {CI}, 64%–88.2%]) than from 8 to 14 days (43.2% [95% CI, 31.1%–54.5%]) after symptom onset; specificity was unchanged at 100% (95% CI, 88.1%–100%). This test had higher sensitivity (100% [95% CI, 88.4%–100%]) and lower specificity (65% [95% CI, 40.8%–84.6%]) for infectious viral shedding as compared with infection, particularly within the first week of symptom onset. Although the presence of N-antigen correlated with infectious viral shedding (r = 0.63; P < .01), sensitivity still declined over time. Additional cutoffs from ROC curves were identified to optimize sensitivity and specificity.
Conclusions
We found that this SARS-CoV-2 N-antigen test was highly sensitive for detecting early but not late infectious viral shedding, making it a viable screening test for community-dwelling individuals to inform isolation practices.
Keywords: blood, infectiousness, infectivity, nucleocapsid antigen, performance
Evaluation of the SARS-CoV-2 nucleocapsid antigen blood test from our longitudinally sampled cohort suggests that it is a highly sensitive test for detecting early infectious viral shedding, making it a viable screening test for community-dwelling individuals to inform isolation practices.
As of April 2022, >847 million severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) tests have been performed in the United States [1], resulting in >80 million infections diagnosed. Screening and diagnostic tests have been developed to detect SARS-CoV-2 RNA, antibodies, or antigens [2–4]. Nucleocapsid protein (N-antigen) was identified as one of the predominantly expressed proteins that could have comparable performance with nucleic acid amplification tests, particularly early in the course of disease [5]. Although antigen testing is generally performed with nasal and saliva biospecimens, emerging evidence suggests that detecting viral proteins in plasma might represent a novel approach to improve the screening or diagnosis of SARS-CoV-2 infection [6–9], depending on its test performance characteristics in various populations.
Although almost all reports of N-antigen in the plasma involve characterization of hospitalized individuals [10, 11], there are few reports among mildly ill or asymptomatic individuals, groups that constitute the majority of individuals infected with SARS-CoV-2. One study comparing the single-molecule array (Simoa) SARS-CoV-2 N-antigen assay (Quanterix, Billerica, Massachusetts) [12] in plasma samples using a cutoff of 1.25 pg/mL with nasal RNA positivity evaluated test performance characteristics among long-term care and hospitalized individuals; the authors found 100% specificity and 97.5% sensitivity [13]. This study did not include individuals living in the community, an important population with potentially different performance characteristics.
There has been a growing evidence base that nasal and saliva antigen testing can provide some indication about infectiousness, but these estimates are imprecise. The challenge with routine molecular testing for SARS-CoV-2 is that it cannot differentiate between the active virus replication (infectious virus) from persistent viral shedding (noninfectious virus). Nasal and saliva antigen testing can provide some indication about infectiousness, but these estimates are imprecise and no single laboratory test has been able to serve as a reliable predictor of infectious viral shedding [14–17]. As there is an urgent need to identify a test to predict infectiousness, we must continue to explore how novel diagnostic approaches may improve our ability to predict infectious viral shedding from blood-based N-antigen tests.
Utilizing a nonhospitalized cohort of individuals infected with SARS-CoV-2 and their household contacts [18], we aimed to evaluate the sensitivity and specificity of the SARS-CoV-2 N-antigen test on plasma (Simoa technology) as compared to infection, stratifying by time since symptom onset. We further investigated the presence and magnitude of SARS-CoV-2 N-antigen in plasma over time and its relationship with the levels and duration of infectious virus measured in nasal samples.
METHODS
Patient Consent Statement
The study protocol was reviewed by the University of California, San Francisco (UCSF) and Centers for Disease Control and Prevention institutional review boards (IRBs) and was determined to meet criteria for public health surveillance activities and did not require IRB oversight, according to the federal regulations summarized in 45 Code of Federal Regulations 46.102(1)(2). Written informed consent was obtained from all participants.
Study Design, Participants, and Procedures
We used data generated from a subgroup of participants enrolled in our natural history study of acute SARS-CoV-2 infectivity [18]. The parent study was an observational longitudinal cohort based in the San Francisco Bay Area, which has been previously described in detail [19]. In brief, index cases with ≥1 household member were identified from SARS-CoV-2 RNA-positive individuals at UCSF-affiliated health facilities; households were enrolled within 5 days of symptom onset. Interview-administered questionnaires and blood samples were obtained at enrollment, and at days 9, 14, 21, and 28 after the index case's symptom onset. Anterior nasal specimens were self-collected daily through day 14 and on days 17, 19, 21, and 28. For this analysis, we used the plasma and nasal biospecimens collected from consecutive participants enrolled into the parent cohort from 1 September 2020 through 13 April 2021. This included overlap with the time of the introduction of SARS-CoV-2 vaccines and thus some participants were vaccinated prior to enrollment. The nasal samples were processed at UCSF. Blood samples were processed at Labcorp-Monogram Biosciences, Inc, with plasma stored at −80°C after isolation via centrifugation of heparinized blood. On the day of analysis, the plasma was thawed, centrifuged for 5 minutes at 10 000g, and plated in Quanterix well plates. Assays were performed in accordance with the manufacturer’s instructions.
Measurements
Blood-Based
We measured SARS-CoV-2 N-antigen in the plasma using the Simoa technology, Quanterix automated paramagnetic microbead-based immunoassay [13]. This technology offers a 1000-fold greater sensitivity than the traditional immunoassay [20, 21]. Quantitative anti–SARS-CoV-2 spike immunoglobulin G (IgG) from the plasma was assessed using a 3-step paramagnetic microbead-based sandwich enzyme-linked immunosorbent assay that also used the Simoa platform. The results were reported as a signal-to-cutoff value, which was determined to be 0.77 µg/mL [22]. The lower limit of quantification was 0.098 µg/mL, and the upper limit of quantification was 338 µg/mL.
Nasal-Based
We used reverse-transcription polymerase chain reaction (RT-PCR) SARS-CoV-2 RNA and viral culture data from multiple nasal samples over time. Longitudinal RT-PCR RNA and infectious viral shedding data were generated from the self-collected anterior nasal swabs by the Andino laboratory at UCSF. This lab used the KingFisher platform to target nucleocapsid (N) and envelope (E) gene regions; a SARS-CoV-2 RNA-positive result was defined as having any level of RNA positivity in the N and E gene regions. Cytopathic effect (CPE) was assessed with Vero-hACE2-TMPRSS2 cells in a Biosafety Level 3 laboratory. We defined infection as RT-PCR RNA positivity and defined infectious viral shedding as CPE positive.
Questionnaire-Based
We determined symptom status and onset based on data obtained from our interview-administered questionnaires. In addition to symptom data, these questionnaires yielded data on sociodemographic characteristics, medical history, and clinical course of acute coronavirus disease 2019 (COVID-19); the instruments, also used in a related study of COVID-19 recovery, have been described in detail elsewhere [23].
Classification of Infection Status
We defined true positives as participants with evidence of SARS-CoV-2 infection from at least 1 RT-PCR RNA-positive result from the clinical or research laboratories. We did not include serological data in this assessment because of the potential for false-positive results [24]. We defined true negatives as participants with no longitudinal evidence of a RT-PCR RNA-positive result from the clinical or research laboratories. In our assessment of true negatives, participants were required to have negative IgG antibodies throughout the follow-up period. However, the negative status of vaccinated individuals was not dependent on anti-spike IgG antibodies.
Statistical Analyses
We assessed the test performance characteristics (sensitivity and specificity) of the SARS-CoV-2 plasma N-antigen against infection (gold standard) and against infectious viral shedding. Evaluation of sensitivity and sensitivity was restricted to the first 14 days of symptom onset, stratified by 0–7 days and 8–14 days. We constructed ROC curves (sensitivity plotted against 1-specificity to varying concentrations of nucleocapsid protein) for each outcome (RNA viral shedding, infectious viral shedding). To demonstrate the range of sensitivity and specificity estimates available from the test, we used the ROC curves to describe values of the N-antigen test that included the manufacturer's cutoff of 1.25 pg/mL, Youden index (sensitivity + specificity – 1) [25], optimized sensitivity, and optimized specificity.We used specimens from the full 28-day observational period to assess the correlation between N-antigen in plasma samples and infectious viral shedding in nasal samples. For analyzing the within-subject correlation between these 2 variables, we used the “analysis of variance” with CPE (infectious viral shedding) as the outcome variable and N-antigen as the predictor variable. The direction of correlation was assessed using a linear regression model. For assessment of between-subject correlation, we used weighted correlation coefficient, using number of observations as weight [26]. All statistical calculations were performed using Stata version 16.1 software (StataCorp LLC, College Station, Texas).
RESULTS
Clinical Description of Study Participants
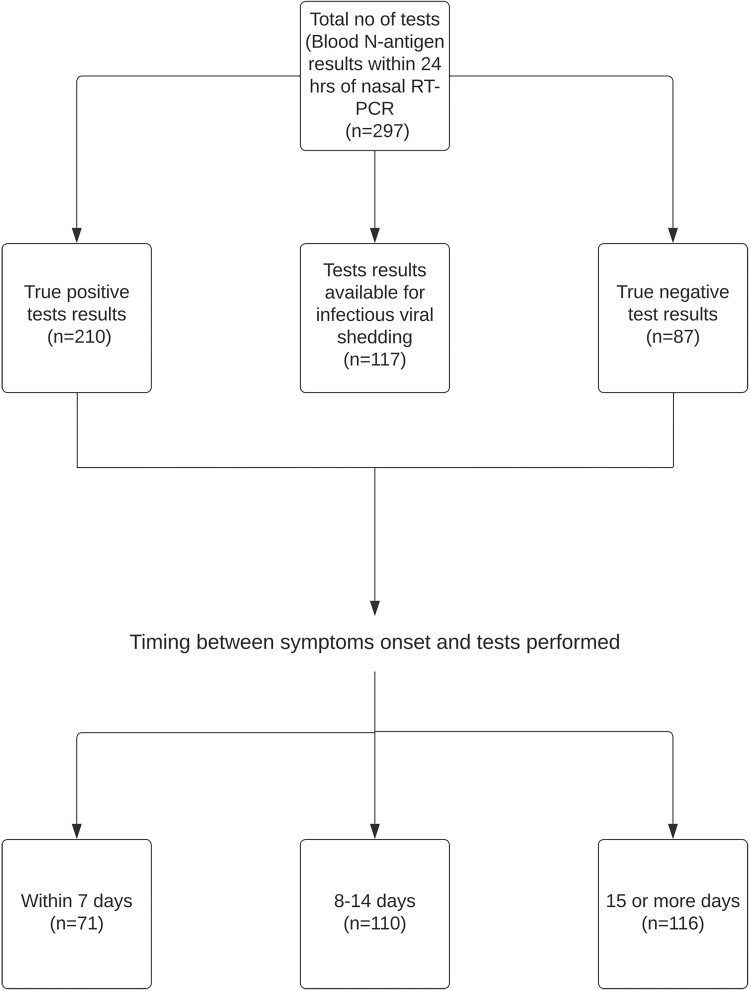
The analysis cohort contained 80 individuals (Table 1); 44 (55%) were women. Fifty-eight (73%) individuals were true positives, and 22 (27%) individuals were true negatives. Fifty-three of 58 (92%) SARS-CoV-2–infected individuals were symptomatic, and 5 (8%) had asymptomatic infection. Four of 22 (18.2%) individuals without SARS-CoV-2 infection reported some symptoms during the quarantine period even though they did not test positive or seroconvert. A total of 297 specimens had nasal RT-PCR and corresponding N-antigen results within 24 hours. Of these, 71 (24%) were within 7 days of symptom onset, 110 (37%) were between 8 and 14 days, and the remaining 116 (39%) were ≥15 days (Figure 1).
Table 1.
Characteristics of Study Participants
| Characteristic | SARS-CoV-2 Infection Status | Total (N = 80) | |
|---|---|---|---|
| Infection Present (TP) (n = 58) |
Infection Absent (TN) (n = 22) |
||
| Classification | |||
| Index case | 34 (58.6) | 0 (0) | 34 (42.5) |
| Household contact | 24 (41.4) | 22 (100) | 46 (57.5) |
| Age, y | |||
| Mean (range) | 39.92 (16–68) | 33.68 (15–56) | 37.98 (15–68) |
| <25 y | 6 (10.3) | 6 (27.3) | 12 (15.0) |
| 25–55 y | 42 (72.4) | 13 (59.1) | 55 (68.7) |
| 56–65 y | 6 (10.3) | 3 (13.6) | 9 (11.3) |
| >65 y | 4 (7.0) | 0 (0) | 4 (5.0) |
| BMI, kg/m2, mean (range) | 28.7 (18.4–56.7) | 25.24 (18.5–48) | 27.8 (18.42–56.7) |
| ≤24.9 | 18 (31.0) | 13 (59.1) | 31 (38.8) |
| 25–29.9 | 19 (32.7) | 6 (27.3) | 25 (31.2) |
| ≥30.0 | 17 (29.3) | 1 (4.5) | 18 (22.5) |
| Not reported/unknown | 4 (7.0) | 2 (9.1) | 6 (7.5) |
| Biological sex | |||
| Female | 32 (55.2) | 12 (54.5) | 44 (55.0) |
| Male | 26 (44.8) | 10 (45.5) | 36 (45.0) |
| Race/ethnicity | |||
| Hispanic/Latino | 19 (32.8) | 7 (31.8) | 26 (32.5) |
| Non-Hispanic/Latino | 39 (67.2) | 13 (59.1) | 52 (65) |
| Prefer not to answer | 0 (0) | 2 (9.1) | 2 (2.5) |
| Self-reported comorbiditiesa | 18 (31.1) | 5 (22.7) | 23 (28.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TN, true negative; TP, true positive.
Participants self-reported the following comorbidities: autoimmune disease, cancer, diabetes, heart disease, lung disease, and kidney disease.
Figure 1.
Description of study cohort. Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Levels and Trends of Plasma N-Antigen
Among true positives, the level of N-antigen in plasma was higher for symptomatic individuals compared to asymptomatic individuals (mean, 37.5 vs 10.8 pg/mL). The mean peak level of N-antigen was 29.6 pg/mL (standard deviation, 108.6), with a subsequent exponential decay over time (Supplementary Figure 1). The median duration of N-antigen positivity in the plasma was 14 days from symptom onset (interquartile range, 8–21 days).
Test Characteristics of Plasma N-Antigen Against SARS-CoV-2 Infection
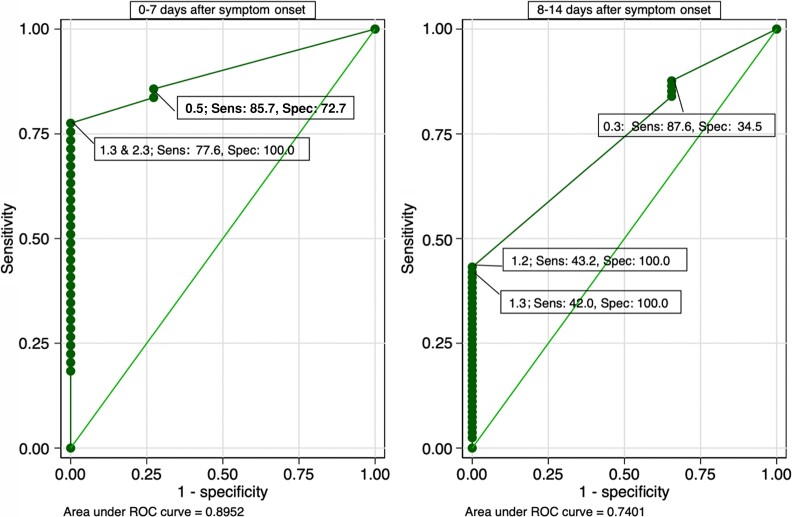
In the evaluation of the test for infection, sensitivity was higher from 0 to 7 days than 8 to 14 days after symptom onset (Supplementary Figure 2), whereas specificity remained stable over the 14-day observation period. Using at a cutoff of 1.25 pg/mL from day 0 to day 7, sensitivity was 77.6% (95% confidence interval {CI}, 64%–88.2%), and specificity was 100% (95% CI, 84.6%–100%). From days 0–7 to days 8–14, sensitivity decreased from 77.6% to 43.2% (95% CI, 31.1%–54.5%) whereas specificity was unchanged at 100% (95% CI, 88.1%–100).
While there were points along the ROC curve that optimized both sensitivity and specificity, we found that the following pattern was consistent for further optimizing sensitivity or specificity: decreasing the cutoff increased sensitivity while increasing the cutoff increased specificity (Figure 2). By decreasing the cutoff to 0.48 pg/mL on the ROC curve evaluating infection from day 0 to day 7, for example, sensitivity increased to 86%. We observed a similar pattern from day 8 to day 14: By decreasing the cutoff to 0.26 pg/mL, sensitivity was optimized up to 87.6%. In situations where the cutoff was decreased to optimize or increase sensitivity, there was a concurrent decline in specificity and vice versa.
Figure 2.
Receiver operating characteristic curve for N-antigen concentration for detection of severe acute respiratory syndrome coronavirus 2 infection. A total of 71 tests for 0–7 days and 110 tests for 8–14 days after symptom onset were included in the analysis. Abbreviations: ROC, receiver operating characteristic; Sens, sensitivity; Spec, specificity.
Test Characteristics of Plasma N-Antigen Against Infectious Viral Shedding
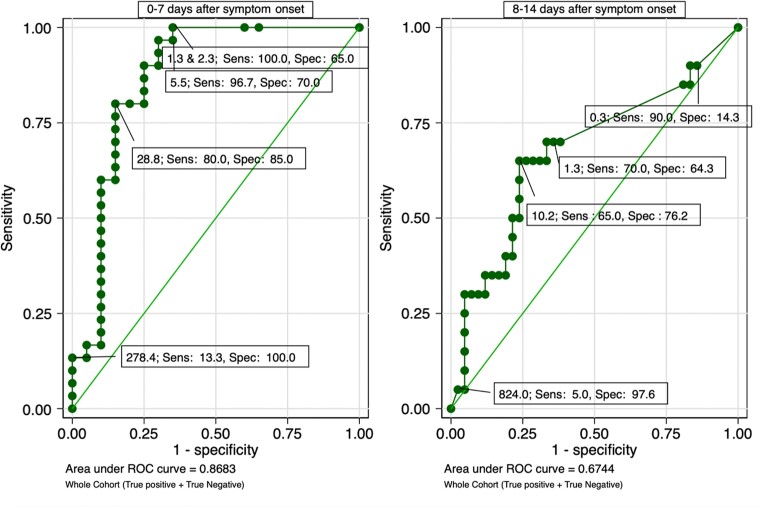
In the evaluation of the test for infectious viral shedding, the test had higher sensitivity and lower specificity for infectious viral shedding as compared with infection. Using a cutoff of 1.25 pg/mL to evaluate for infectious viral shedding within 7 days of symptom onset, we found a sensitivity of 100% (95% CI, 88.4%–100%) and specificity of 65% (95% CI, 40.8%–84.6%). By following the ROC curve nearly to its end, we were able to optimize specificity to 100%, but the cutoff increased to 278 pg/mL and sensitivity was very low at 13.3%. Given any cutoff, sensitivity decreased over the 14-day period, which also occurred in the evaluation for infection. Even in the case of infectious viral shedding when we used Youden index, sensitivity still decreased from 96.7% in the first 7 days (0–7 days) to 65% (95% CI, 45.7%–88.1%) in the second 7 days (8–14 days). Returning to the cutoff of 1.25 pg/mL to compare specificity over the 2 periods (0–7 days vs 8–14 days), the estimate was similar at 64%–65% (Figure 3). The test performance for detection of SARS-CoV-2 infectious viral shedding was similar among truly infected individuals rather and the entire study population (Supplementary Figure 3). Over time, we observed that the presence of N-antigen correlated with infectious viral shedding (r = 0.63; P < .01). More details on cutoffs that optimize sensitivity and specificity can be found in Table 2.
Figure 3.
Receiver operating characteristic curve for N-antigen concentration for detection of severe acute respiratory syndrome coronavirus 2 infectious viral shedding. A total of 71 tests for 0–7 days and 110 tests for 8–14 days after symptom onset were included in the analysis. Abbreviations: ROC, receiver operating characteristic; Sens, sensitivity; Spec, specificity.
Table 2.
Summary of N-Antigen Test Performance From Receiver Operating Characteristic Curves
| Cutoff | Diagnosis of Infection (Within 7 d After Symptom Onset) | Diagnosis of Infection (8–14 d After Symptom Onset) | Diagnosis of Infectious Viral Shedding (Within 7 d After Symptom Onset) | Diagnosis of Infectious Viral Shedding (8–14 d After Symptom Onset) |
|---|---|---|---|---|
| Manufacturer's cutoff | 1.3a | 1.3 | 1.3 | 1.3 |
| Sensitivity | 77.6 | 42.0 | 100.0 | 70.0 |
| Specificity | 100.0 | 100.0 | 65.0 | 64.3 |
| Cutoff for highest Youden index | 2.3 | 1.2 | 5.5 | 10.2 |
| Sensitivity | 77.6 | 43.2 | 96.7 | 65.0 |
| Specificity | 100.0 | 100.0 | 70.0 | 76.2 |
| Cutoff optimizing sensitivity | 0.5 | 0.3 | 2.3 | 0.3 |
| Sensitivity | 85.7 | 87.6 | 100.0 | 90.0 |
| Specificity | 72.7 | 34.5 | 65.0 | 14.3 |
| Cutoff optimizing specificity | 2.3 | 1.2 | 278.4 | 824.0 |
| Sensitivity | 77.6 | 43.2 | 13.3 | 5.0 |
| Specificity | 100.0 | 100.0 | 100 .0 | 97.6 |
Cutoff for N-antigen concentration is shown as picograms per milliliter, and sensitivity and specificity as percentage. Youden index was calculated as (sensitivity + specificity – 1). Sensitivity was calculated as the number of true positives / (number of true positives + number of false negatives). Specificity was calculated as the number of true negatives / (number of true negatives + number of false positives).
The manufacturer's cutoff of 1.25 pg/mL is rounded off to 1.3.
DISCUSSION
In this longitudinally sampled cohort, our findings provide novel evidence in support of the evaluation of N-antigen plasma testing for infectious virus shedding from the nasopharynx. We found that this SARS-CoV-2 N-antigen plasma test was highly sensitive for detecting early infectious viral shedding. The correlation between plasma N-antigen and infectious viral shedding was strong, which was consistent with previous studies describing the first week of acute illness [27–29]. In contrast to RT-PCR testing, antigen testing has the potential to differentiate between the infectious (ie, replication competent) and noninfectious virus [30] [31, 32]. Given the relatively poor specificity to identify infectiousness, this test would not be appropriate for use to test out of quarantine or isolation at the manufacturer's cutoff, but as a highly sensitive test, it may be a viable screening test for community-dwelling individuals to inform their isolation practices.
Compared with other studies evaluating infection, N-antigen plasma testing in our study performed with similar sensitivity and specificity for infection; other studies included outpatients as well as hospitalized individuals and reported sensitivity ranging from 62% to 81.4% and specificity of 93% to 100% [4–10, 33–36]. Several studies assessing performance of nasal N-antigen tests reported higher sensitivity ranging from 80% to 98.3% and specificity from 96.8% to 100% [37–40]. In concordance with our findings (specificity of 100% for infection), the outstanding specificity of the N-antigen plasma tests may be ideal in some settings as confirmatory testing, for example point-of-care finger stick testing, pediatric or add-on laboratory testing, and blood banking.
In the evaluation of infection and infectious viral shedding, the ROC curve could be used to identify cutoffs that improve the test performance and optimize it more for screening or confirmatory testing. Depending on the objectives and setting, practitioners can elect how they use the N-antigen plasma test. For example, this test was less sensitive in identifying infected individuals in our nonhospitalized cohort than previously reported with this test in a hospitalized and long-term-care cohort [13]; if a cutoff below current manufacturer recommendations were to be used, however, sensitivity of infection would increase to more acceptable levels in which it could be used as a screening test. Although the specificity of our N-antigen plasma test for infection was outstanding, the specificity for detection of early infectious viral shedding was more moderate, so increasing the cutoff to high levels is a potential solution to optimizing specificity. If specificity were to be outstanding (100%), then this test could be used in hospital-based environments, for example, as an approach to testing out of isolation, instead of alternative imperfect approaches such as cycle threshold values.
This study has several limitations. We included a small number of outpatients, and our findings should be verified in a larger cohort. The study population is not representative of all individuals with SARS-CoV-2, as we studied few asymptomatic individuals as there were few included, and screening asymptomatic individuals would be presumably a useful purpose for this test (eg, blood banks). We did not study variant-specific effects, and the circulating variants during the study period preceded the Delta and Omicron surges in the United States. We also included relatively few vaccinated individuals, and no individuals receiving antiviral therapy; these factors might affect test performance and are becoming more widespread in the current era of SARS-CoV-2.
In conclusion, we demonstrated several ways that the N-antigen plasma test can be used to detect infection and infectious viral shedding. Our evaluation of the test for infection was comparable to other rapid nasal and plasma N-antigen tests available for commercial use. Finally, this study draws on a rigorous sampled longitudinal cohort tested for infectious viral shedding, and the evaluation of N-antigen plasma testing in this cohort provided novel data. Our findings support this test as a suitable screening test for identifying early SARS-CoV-2 infectiousness. Given that the feasibility of this test may make its use preferrable in some settings, this test could be of use in many clinical practice and research settings if our results are confirmed in larger and more diverse cohorts.
Supplementary Material
Contributor Information
Sujata Mathur, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Michelle C Davidson, Francis I. Proctor Foundation, University of California, San Francisco, California, USA; School of Medicine, University of California, San Francisco, California, USA.
Khamal Anglin, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Scott Lu, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Sarah A Goldberg, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Miguel Garcia-Knight, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Michel Tassetto, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Amethyst Zhang, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Mariela Romero, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Jesus Pineda-Ramirez, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Ruth Diaz-Sanchez, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Paulina Rugart, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Jessica Y Chen, Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA.
Kevin Donohue, School of Medicine, University of California, San Francisco, California, USA.
Joshua R Shak, San Francisco Veterans Affairs Medical Center, San Francisco, California, USA.
Ahmed Chenna, Labcorp-Monogram Biosciences, South San Francisco, California, USA.
John W Winslow, Labcorp-Monogram Biosciences, South San Francisco, California, USA.
Christos J Petropoulos, Labcorp-Monogram Biosciences, South San Francisco, California, USA.
Brandon C Yee, Labcorp-Monogram Biosciences, South San Francisco, California, USA.
Jeremy Lambert, Quanterix Laboratories, Billerica, Massachusetts, USA.
David V Glidden, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
George W Rutherford, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Steven G Deeks, Division of HIV, Infectious Disease, and Global Medicine, Department of Medicine, University of California, San Francisco, California, USA.
Michael J Peluso, Division of HIV, Infectious Disease, and Global Medicine, Department of Medicine, University of California, San Francisco, California, USA.
Raul Andino, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Jeffrey N Martin, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
J Daniel Kelly, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA; Francis I. Proctor Foundation, University of California, San Francisco, California, USA; San Francisco Veterans Affairs Medical Center, San Francisco, California, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participants for making this study possible while acutely infected with SARS-CoV-2. We appreciate the input and support of Claire M. Midgley, Sharon Saydah, Melissa Briggs-Hagen, Glen R. Abedi and other team members who contributed from the US Centers for Disease Control and Prevention. Vero TMPRSS2 hAce2 cells were a kind gift from the NIH.
Author contributions. K. A., M. R., J. P.-R., R. D. S., and P. R. recruited participants, coordinated research visits, administered study questionnaires, and collected clinical data. S. L., S. M., S. A. G., J. Y. C., and K. D. performed data entry and quality control and addressed, or adjudicated issues related to data integrity. S. L. maintained the database and oversaw data management. M. G.-K., M. T., and A. Z. performed the RT-PCR test and viral culture in the laboratory. A. C., J. W. W., C. J. P., B. C. Y., and J. L. performed and oversaw the N-antigen assay at Monogram Biosciences and Quanterix laboratory. S. M. and M. C. D. accessed and verified all data, did the data analysis, and drafted the first and final version of the manuscript. J. R. S., D. V. G., G. W. R., S. G. D., M. J. P., R. A. P., J. N. M., and J. D. K. provided in-depth critical review for manuscript revision. All authors reviewed, edited, and approved the manuscript.
Disclaimer. The funding sources had no role in the writing of the manuscript or the decision for publication.
Financial support . This work was supported by a Centers for Disease Control and Prevention Broad Agency Announcement (75D30120C08009). J. D. K. was supported by the National Institute of Allergy and Infectious Diseases, NIH (award number K23 AI146268).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mathieu E, Ritchie H, Rodés-Guirao L, et al. . Coronavirus pandemic (COVID-19), Our World in Data. 2020. Available at: https://ourworldindata.org/coronavirus-testing. Accessed 19 July 2021.
- 2. Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konrad R, Eberle U, Dangel A, et al. . Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveill 2020; 25:2000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li T, Wang L, Wang H, et al. . Serum SARS-COV-2 nucleocapsid protein: a sensitivity and specificity early diagnostic marker for SARS-COV-2 infection. Front Cell Infect Microbiol 2020; 10:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Che XY, Hao W, Wang Y, et al. . Nucleocapsid protein as early diagnostic marker for SARS. Emerg Infect Dis 2004; 10:1947–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hingrat QL, Visseaux B, Laouenan C, et al. . Detection of SARS-CoV-2 N-antigen in blood during acute COVID-19 provides a sensitive new marker and new testing alternatives. Clin Microbiol Infect 2021; 27:789.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahava MJ, Kurkela S, Kuivanen S, Lappalainen M, Jarva H, Jääskeläinen AJ. Detection of SARS-CoV-2 nucleocapsid antigen from serum can aid in timing of COVID-19 infection. J Virol Methods 2022; 302:114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lebedin YS, Lyang OV, Galstyan AG, Panteleeva AV, Belousov VV, Rebrikov DV. Serum SARS-CoV-2 nucleocapsid antigen detection is essential for primary diagnostics of SARS-CoV-2–associated pneumonia. medRxiv [Preprint]. Posted online 25 September 2020. 10.1101/2020.09.24.20200303. [DOI] [Google Scholar]
- 9. Yokoyama R, Kurano M, Nakano Y, et al. . Association of the serum levels of the nucleocapsid antigen of SARS-CoV-2 with the diagnosis, disease severity, and antibody titers in patients with COVID-19: a retrospective cross-sectional study. Front Microbiol 2021; 12:791489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perna F, Bruzzaniti S, Piemonte E, et al. . Serum levels of SARS-CoV-2 nucleocapsid antigen associate with inflammatory status and disease severity in COVID-19 patients. Clin Immunol 2021; 226:108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogata AF, Maley AM, Wu C, et al. . Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin Chem 2020; 66:1562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quanterix. SARS-CoV-2 N protein antigen test (EUA). Available at: https://www.quanterix.com/simoa-assay-kits/sars-cov-2-n-protein-antigen-test-eua/. Accessed 12 August 2021.
- 13. Shan D, Johnson JM, Fernandes SC, et al. . N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat Commun 2021; 12:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prince-Guerra JL, Almendares O, Nolen LD, et al. . Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pray IW, Ford L, Cole Devlin, et al. . Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singanayagam A, Patel M, Charlett A, et al. . Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhoads D, Peaper DR, She RC, et al. . College of American Pathologists (CAP) Microbiology Committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021; 72:e685–6. [DOI] [PubMed] [Google Scholar]
- 18. Kelly JD, Lu S, Anglin K, et al. Magnitude and determinants of SARS-CoV-2 household transmission: a longitudinal study. Clin Infect Dis 2022; 75:S193–204. [DOI] [PMC free article] [PubMed]
- 19. University of California, San Francisco. Information about LIINC, a COVID-19 (novel coronavirus) study being conducted by UCSF researchers. Available at: https://www.findcovid19.org/en/study-information. Accessed 20 February 2022.
- 20. Rissin DM, Kan CW, Campbell TGD, et al. . Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010; 28:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson DH, Rissin DM, Kan CW, et al. . The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 2016; 21:533–47. [DOI] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration. Simoa semi-quantitative SARS-CoV-2 IgG antibody test: instructions for use. Available at: https://www.fda.gov/media/144764/download. Accessed 22 October 2022.
- 23. Peluso MJ, Kelly JD, Lu S, et al. . Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-CoV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis 2022; 9:ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Latiano A, Tavano F, Panza A, et al. . False-positive results of SARS-CoV-2 IgM/IgG antibody tests in sera stored before the 2020 pandemic in Italy. Int J Infect Dis 2021; 104:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 26. Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ 1995: 310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Knight M, Anglin K, Tassetto M, et al. . Infectious viral shedding of SARS-CoV-2 Delta following vaccination: a longitudinal cohort study. PLoS Pathog 2022; 18:e1010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arons MM, Hatfield KM, Reddy SC, et al. . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465.–. [DOI] [PubMed] [Google Scholar]
- 30. Rhee C, Kanjilal S, Baker M, Klompas M. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis 2021; 72:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widders A, Broom A, Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Health 2020; 25:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Binnicker MJ. Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol 2021; 59:e0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng Q, Ye G, Pan Y, et al. . High performance of SARS-CoV-2N protein antigen chemiluminescence immunoassay as frontline testing for acute phase COVID-19 diagnosis: a retrospective cohort study. Front Med 2021; 8:676560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thudium RF, Stoico MP, Høgdall E, et al. . Early laboratory diagnosis of COVID-19 by antigen detection in blood samples of the SARS-CoV-2 nucleocapsid protein. J Clin Microbiol 2021; 59:e0100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Yu, Ong CM, Yun C, et al. . Diagnostic value of nucleocapsid protein in blood for SARS-CoV-2 infection. Clin Chem 2021; 68:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Che X-Y, Qiu L-W, Pan Y-X, et al. . Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol 2004; 42:2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porte L, Legarraga P, Vollrath V, et al. . Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis 2020; 99:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaimayo C, Kaewnaphan B, Tanlieng N, et al. . Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J 2020; 17:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boum Y, Fai KN, Nikolay B, et al. . Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis 2021; 21:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dinnes J, Deeks JJ, Berhane S, et al. . Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2021; 3:CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.