Abstract
Background
Social determinants of health (SDOH) have been associated with coronavirus disease 2019 (COVID-19) outcomes. We examined patterns in COVID-19–related mortality by SDOH and compared these patterns to those for non–COVID-19 mortality.
Methods
Residents of Ontario, Canada, aged ≥20 years were followed from 1 March 2020 to 2 March 2021. COVID-19–related death was defined as death within 30 days following or 7 days prior to a positive COVID-19 test. Area-level SDOH from the 2016 census included median household income; proportion with diploma or higher educational attainment; proportion essential workers, racially minoritized groups, recent immigrants, apartment buildings, and high-density housing; and average household size. We examined associations between SDOH and COVID-19–related mortality, and non-COVID-19 mortality using cause-specific hazard models.
Results
Of 11 810 255 individuals, we observed 3880 COVID-19–related deaths and 88 107 non–COVID-19 deaths. After accounting for demographics, baseline health, and other area-level SDOH, the following were associated with increased hazards of COVID-19–related death (hazard ratio [95% confidence interval]: lower income (1.30 [1.04–1.62]), lower educational attainment (1.27 [1.07–1.52]), higher proportions essential workers (1.28 [1.05–1.57]), racially minoritized groups (1.42 [1.08–1.87]), apartment buildings (1.25 [1.07–1.46]), and large vs medium household size (1.30 [1.12–1.50]). Areas with higher proportion racially minoritized groups were associated with a lower hazard of non–COVID-19 mortality (0.88 [0.84–0.92]).
Conclusions
Area-level SDOH are associated with COVID-19–related mortality after accounting for demographic and clinical factors. COVID-19 has reversed patterns of lower non–COVID-19 mortality among racially minoritized groups. Pandemic responses should include strategies to address disproportionate risks and inequitable coverage of preventive interventions associated with SDOH.
Keywords: social determinants of health, COVID-19, mortality, inequality, race/ethnicity
Area-level social determinants of health (SDOH) are associated with coronavirus disease 2019 (COVID-19)–related mortality, after accounting for individual demographic and clinical factors. COVID-19 has reversed patterns of lower non–COVID-19 mortality among racially minoritized groups.
Increasing evidence has confirmed the central role of social determinants of health (SDOH) in shaping variations in coronavirus disease 2019 (COVID-19) disease burden and severity [1–6]. Across high-income countries, rates of COVID-19 diagnoses and deaths have been consistently correlated with socioeconomic status (SES) [5, 7] and disproportionately affecting racially minoritized groups [3, 8–10].
In the context of infectious disease, social and structural inequalities may shape differential health outcomes through differences in susceptibility, contact patterns and networks [11, 12] and reach/uptake of prevention interventions (eg, access to testing [12, 13], effective isolation and quarantine [14], ability to reduce nonhousehold contacts [15], access to vaccines [16]), and quality of treatment [17, 18].
To date, most studies have focused on SDOH such as SES as a composite index [5, 6, 13] and race/ethnicity as proxies for structural racism (biological differences [19], if any, are not the sole explanation for observed disparities by race/ethnicity) [3, 8, 10]. Few studies have examined other SDOH such as educational attainment and occupation and housing conditions, and even fewer have examined several SDOH in conjunction [1, 2]. Moreover, studies on the relationship between SDOH and COVID-19 deaths were often conducted among diagnosed cases or hospitalized populations [7]. Although outcomes such as case fatality among diagnosed cases and mortality while hospitalized provided important information regarding disease severity by SDOH, these analyses are prone to collider biases [20]. For example, SDOH and severe COVID-19 outcomes both affect likelihoods of being diagnosed/hospitalized; restricting analyses among samples of diagnosed/hospitalized cases could distort the relationship between SDOH and COVID-19 outcomes [3, 5, 7].
In Canada, provisional Vital Statistics Deaths data have demonstrated higher age-standardized COVID-19–related mortality among urban residents (vs rural), lower-income areas, higher ethnocultural concentration areas, and residents of apartment buildings (vs detached homes) [21]. However, existing studies were not able to account for potential confounders such as comorbidities. Moreover, to date, no studies have estimated COVID-19–related mortality while at the same time accounting for mortality unrelated to COVID-19, which is a competing risk for COVID-19–related mortality [22]. Such an inquiry also provides opportunities to understand whether the same patterns of inequities drive both COVID-19 and non–COVID-19–related mortality.
Using population-based data among 11.8 million adults in Ontario, Canada, we examined differential patterns in COVID-19–related mortality across a set of area-level SDOH including SES (median household income, proportion with diploma or higher educational attainment, proportion essential workers), ethnic diversity (proportion racially minoritized groups, proportion recent immigrations), and housing conditions (proportion apartment buildings, proportion high-density housing, average household size). We assessed whether patterns in COVID-19–related mortality by SDOH can be explained by demographics, baseline health, and other area-level SDOH. We also compared patterns by SDOH in COVID-19–related mortality vs those in non–COVID-19 mortality and in COVID-19 case fatality.
METHODS
Study Design and Residents
We conducted a population-based, retrospective, cohort study of community-dwelling adults in Ontario, Canada, a setting with universal healthcare [23]. Individuals aged ≥20 years residing in Ontario as of 1 March 2020 and having a valid health card were identified using Ontario's Registered Persons Database (RPDB) and followed through 2 March 2021. We excluded residents in long-term care homes because they are not included in Canadian census data from which SDOH variables were determined [24, 25]. Data use was authorized under Section 45 of Ontario's Personal Health Information Protection Act, which does not require ethics review.
Outcomes
Our primary outcome was COVID-19–related death, defined as death within 30 days following or 7 days prior to a positive COVID-19 test. Test result and date were determined based on records in the Ontario Laboratories Information System and the Public Health Case and Contact Management Solution (CCM). Date of death was determined using CCM and RPDB. We estimated that use of both CCM and RPDB capture 99.3% of COVID-19–related deaths (Supplementary Table 1). The secondary outcome was non–COVID-19 death, defined as death without any history of a positive COVID-19 test. COVID-19–related mortality and non–COVID-19 mortality were estimated using the full cohort as the denominator. COVID-19 case fatality was estimated using the subset of the cohort that was diagnosed with COVID-19 as the denominator.
We restricted our analyses to COVID-19–related deaths observed up to 2 March 2021 and cases diagnosed prior to 31 January 2021. Therefore, our analyses capture the first and second waves of regional pandemic representing the original strain of the virus (>95%) or the Alpha variant [26, 27].
Covariates
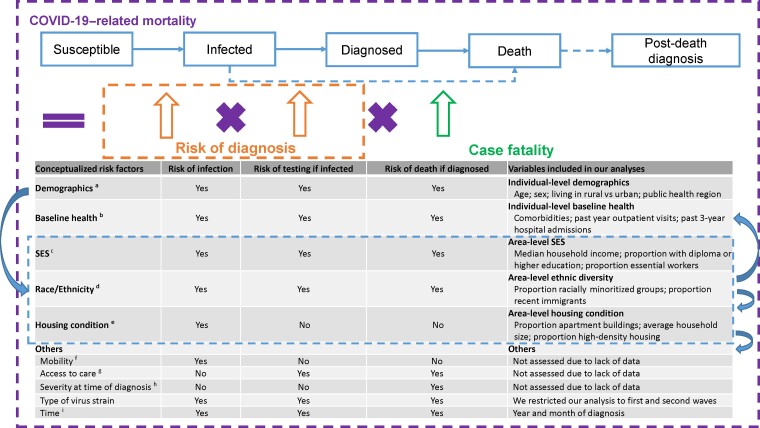
Based on available data and existing literature [4, 7, 8, 10, 12, 28], we developed a conceptual framework to select SDOH variables and potential confounders for the relationship between SDOH and outcomes, as hypothesized along the risk pathway of COVID-19–related mortality, including risk of infection, risk of testing if infected, and risk of death if diagnosed. Rationales of variable selection are detailed in Figure 1.
Figure 1.
Conceptualization of risk factors for COVID-19–related mortality. Based on the conceptualized factors, we sourced data, where available, at the individual level, otherwise at the area level. aAreas where an individual resides might reflect contact rates in communities and healthcare system capacity and quality and therefore associated with risk of infection, testing, and death [1, 2, 12]. bIndividual's baseline health (eg, comorbidities) has been correlated with susceptibility to COVID-19 infection and severity of infection and therefore associated with risk of infection, testing, and death [4]. cOccupation (eg, essential workers) might reflect contact rates at work and therefore be associated with risk of infection and testing [12, 29]. Income and education might affect exposure to the virus through working or living conditions, while also reflecting access to healthcare services, and therefore be associated with risk of infection, testing, and death [12, 30]. dRacially minoritized groups might be subject to systemic racism and socioeconomic inequalities, affecting the risk pathway of COVID-19–related mortality [3, 8]. eHousing conditions might reflect contact rates within household and be associated with risk of infection [12, 28, 31]. fWe assume mobility is a mediator for the relationship between social determinants of health (SDOH) and risk of infection. gWe assume access to care is a mediator for the relationship between SDOH and risk of testing and death. hWe assume severity at time of diagnosis is a mediator for the relationship between SDOH and risk of death. iA change occurred in August 2020 regarding clinical practice with respect to the use of steroids to treat COVID-19. Abbreviations: COVID-19, coronavirus disease 2019; SES, socioeconomic status; SDOH, Social determinants of health.
Our primary covariates included area-level SDOH, derived from the 2016 census at dissemination area (DA) level, the smallest geographic unit (representing 400–700 residents) for which census data are reported [24]. Area-level SDOH included factors that reflect SES (median household income, proportion with diploma or higher educational attainment, proportion essential workers), ethnic diversity (proportion racially minoritized groups, proportion recent immigrants), and housing conditions (proportion apartment buildings, proportion high-density housing, average household size). Proportion essential workers was defined as the proportion of working people in the DA who self-identify as working in sales, trades, manufacturing, or agriculture. Proportion racially minoritized groups was defined as the proportion of people who self-identify as non-White and non-Indigenous. Proportion apartment buildings was defined as the proportion of buildings that are apartments. For each SDOH variable, we ranked DAs at the city (for income) or provincial level (for other SDOH) and then categorized them into quintiles. For example, a DA being in income quintile 1 means it is among the highest 20% of DAs in its city by median household income. Detailed definitions of these variables are shown in Table 1 footnotes.
Table 1.
Characteristics of Overall Community-Dwelling Adults in Ontario, Canada, and Those Who Died Related to Coronavirus Disease 2019 and Other Causes
| Characteristic | Number of Individuals Residing in Ontario as of 1 March 2020 | Number of COVID-19–related Deathsa Between 1 March 2020 and 2 March 2021 | Number of Non–COVID-19 Deathsb Between 1 March 2020 and 2 March 2021 |
|---|---|---|---|
| Total | 11 810 255 | 3880 | 88 107 |
| Age (median, interquartile range), yc | 48 (34–62) | 81 (72–88) | 77 (65–86) |
| Age category, yc | |||
| ȃ20–34 | 3 143 764 (26.6%) | 23 (0.6%) | 2289 (2.6%) |
| ȃ35–49 | 3 009 493 (25.5%) | 84 (2.2%) | 4149 (4.7%) |
| ȃ50–64 | 3 099 010 (26.2%) | 399 (10.3%) | 14 334 (16.3%) |
| ȃ65–74 | 1 487 522 (12.6%) | 710 (18.3%) | 17 897 (20.3%) |
| ȃ75–84 | 769 255 (6.5%) | 1140 (29.4%) | 22 900 (26.0%) |
| ȃ85+ | 301 211 (2.6%) | 1524 (39.3%) | 26 538 (30.1%) |
| Male | 5 777 603 (48.9%) | 2249 (58.0%) | 48 501 (55.0%) |
| Residing in a rural aread | 1 192 569 (10.1%) | 138 (3.6%) | 11 614 (13.2%) |
| Comorbiditye | |||
| ȃAsthma | 1 750 679 (14.8%) | 752 (19.4%) | 14 671 (16.7%) |
| ȃChronic obstructive pulmonary disease | 290 131 (2.5%) | 643 (16.6%) | 17 064 (19.4%) |
| ȃHypertension | 3 085 359 (26.1%) | 3205 (82.6%) | 63 356 (71.9%) |
| ȃDiabetes | 1 471 040 (12.5%) | 1847 (47.6%) | 32 328 (36.7%) |
| ȃCongestive heart failure | 264 194 (2.2%) | 988 (25.5%) | 22 696 (25.8%) |
| ȃDementia or frailty score >15f | 164 518 (1.4%) | 1215 (31.3%) | 18 742 (21.3%) |
| ȃCancerg | 242 667 (2.1%) | 235 (6.1%) | 15 663 (17.8%) |
| ȃChronic kidney diseaseh | |||
| ȃȃWith no recent dialysis | 277 564 (2.4%) | 937 (24.1%) | 16 286 (18.5%) |
| ȃȃWith recent (last 3 mo) dialysis | 11 131 (0.1%) | 95 (2.4%) | 1723 (2.0%) |
| ȃImmunocompromisedi | 89 318 (0.8%) | 130 (3.4%) | 3997 (4.5%) |
| ȃAdvanced liver diseasej | 86 612 (0.7%) | 103 (2.7%) | 4337 (4.9%) |
| ȃCardiac ischemic diseasek | 359 120 (3.0%) | 707 (18.2%) | 15 166 (17.2%) |
| ȃIschemic stroke or transient ischemic attackl | 112 634 (1.0%) | 370 (9.5%) | 6994 (7.9%) |
| Hospital admissions, past 3 y | |||
| ȃ0 | 10 278 277 (87.0%) | 1934 (49.8%) | 40 188 (45.6%) |
| ȃ1 | 1 112 902 (9.4%) | 856 (22.1%) | 20 623 (23.4%) |
| ȃ2 | 265 192 (2.2%) | 503 (13.0%) | 11 539 (13.1%) |
| ȃ3 or more | 153 884 (1.3%) | 587 (15.1%) | 15 757 (17.9%) |
| Outpatient physician visits, past y | |||
| ȃ0–1 | 4 054 472 (34.3%) | 313 (8.1%) | 10 673 (12.1%) |
| ȃ2–4 | 3 111 063 (26.3%) | 608 (15.7%) | 13 598 (15.4%) |
| ȃ5–8 | 2 320 703 (19.6%) | 882 (22.7%) | 16 897 (19.2%) |
| ȃ9–14 | 1 429 868 (12.1%) | 926 (23.9%) | 18 545 (21.0%) |
| ȃ15 or more | 894 149 (7.6%) | 1151 (29.7%) | 28 394 (32.2%) |
| Income quintile (1 = highest)m,n | |||
| ȃ1 | 2 351 451 (19.9%) | 479 (12.3%) | 14 152 (16.1%) |
| ȃ2 | 2 343 768 (19.8%) | 552 (14.2%) | 14 613 (16.6%) |
| ȃ3 | 2 364 379 (20.0%) | 776 (20.0%) | 17 011 (19.3%) |
| ȃ4 | 2 337 045 (19.8%) | 933 (24.0%) | 19 418 (22.0%) |
| ȃ5 | 2 301 617 (19.5%) | 1120 (28.9%) | 22 469 (25.5%) |
| ȃMissing | 111 995 (0.9%) | 20 (0.5%) | 444 (0.5%) |
| Educational attainment quintile (1 = highest)m,o | |||
| ȃ1 | 2 490 287 (21.1%) | 638 (16.4%) | 14 904 (16.9%) |
| ȃ2 | 2 513 154 (21.3%) | 781 (20.1%) | 17 337 (19.7%) |
| ȃ3 | 2 443 398 (20.7%) | 729 (18.8%) | 17 755 (20.2%) |
| ȃ4 | 2 260 406 (19.1%) | 846 (21.8%) | 19 110 (21.7%) |
| ȃ5 | 1 970 234 (16.7%) | 852 (22.0%) | 18 328 (20.8%) |
| ȃMissing | 132 776 (1.1%) | 34 (0.9%) | 673 (0.8%) |
| Proportion essential workers quintile (1 = lowest)m,p | |||
| ȃ1 | 2 533 697 (21.5%) | 705 (18.2%) | 14 830 (16.8%) |
| ȃ2 | 2 592 332 (21.9%) | 780 (20.1%) | 17 367 (19.7%) |
| ȃ3 | 2 315 922 (19.6%) | 760 (19.6%) | 18 453 (20.9%) |
| ȃ4 | 2 217 021 (18.8%) | 794 (20.5%) | 18 163 (20.6%) |
| ȃ5 | 2 018 450 (17.1%) | 807 (20.8%) | 18 620 (21.1%) |
| ȃMissing | 132 833 (1.1%) | 34 (0.9%) | 674 (0.8%) |
| Proportion racially minoritized groups quintile(1 = lowest)m,q | |||
| ȃ1 | 1 826 634 (15.5%) | 260 (6.7%) | 18 046 (20.5%) |
| ȃ2 | 1 954 891 (16.6%) | 454 (11.7%) | 18 424 (20.9%) |
| ȃ3 | 2 105 986 (17.8%) | 666 (17.2%) | 17 568 (19.9%) |
| ȃ4 | 2 564 575 (21.7%) | 964 (24.8%) | 16 729 (19.0%) |
| ȃ5 | 3 225 565 (27.3%) | 1502 (38.7%) | 16 672 (18.9%) |
| ȃMissing | 132 604 (1.1%) | 34 (0.9%) | 668 (0.8%) |
| Proportion recent immigrants (1 = lowest)m,r | |||
| ȃ1 | 5 983 539 (50.7%) | 1499 (38.6%) | 52 336 (59.4%) |
| ȃ2 | 2 412 998 (20.4%) | 880 (22.7%) | 16 208 (18.4%) |
| ȃ3 | 3 236 805 (27.4%) | 1464 (37.7%) | 18 402 (20.9%) |
| ȃMissing | 176 913 (1.5%) | 37 (1.0%) | 1161 (1.3%) |
| Proportion apartment buildings (1 = lowest)m,s | |||
| ȃ1 | 6 605 697 (55.9%) | 1613 (41.6%) | 42 666 (48.4%) |
| ȃ2 | 2 120 840 (18.0%) | 687 (17.7%) | 18 576 (21.1%) |
| ȃ3 | 2 944 390 (24.9%) | 1545 (39.8%) | 26 093 (29.6%) |
| ȃMissing | 139 328 (1.2%) | 35 (0.9%) | 772 (0.9%) |
| Average household size quintile (1 = lowest)m,t | |||
| ȃ1 | 2 325 763 (19.7%) | 1028 (26.5%) | 25 171 (28.6%) |
| ȃ2 | 2 064 823 (17.5%) | 571 (14.7%) | 19 138 (21.7%) |
| ȃ3 | 1 582 415 (13.4%) | 405 (10.4%) | 12 471 (14.2%) |
| ȃ4 | 2 722 878 (23.1%) | 861 (22.2%) | 17 930 (20.4%) |
| ȃ5 | 2 975 277 (25.2%) | 980 (25.3%) | 12 625 (14.3%) |
| ȃMissing | 139 099 (1.2%) | 35 (0.9%) | 772 (0.9%) |
| Proportion high-density housing (1 = lowest)m,u | |||
| ȃ1 | 3 983 354 (33.7%) | 1018 (26.2%) | 31 975 (36.3%) |
| ȃ2 | 2 559 526 (21.7%) | 675 (17.4%) | 20 016 (22.7%) |
| ȃ3 | 2 289 131 (19.4%) | 722 (18.6%) | 15 862 (18.0%) |
| ȃ4 | 2 679 342 (22.7%) | 1370 (35.3%) | 17 732 (20.1%) |
| ȃMissing | 298 902 (2.5%) | 95 (2.4%) | 2522 (2.9%) |
Databases used for creation of individual-level characteristics included the following: Discharge Abstract Database, National Ambulatory Care Reporting System, Ontario Health Insurance Plan provider billings, Ontario Drug Benefits Plan, Continuing Care Reporting System, Canadian Organ Replacement Registry, and Ontario Cancer Registry.
Abbreviation: COVID-19, coronavirus disease 2019.
Death within 30 days following or 7 days prior to a laboratory-confirmed positive COVID-19 test.
Death without a laboratory-confirmed positive COVID-19 test. We did not include those who died more than 7 days prior or 30 days after a positive COVID-19 test in our definition of non–COVID-19 death, as we aimed to determine patterns of mortality by area-level social determinants of health without COVID-19 in our secondary outcome, limiting the assessment of the potential longer-term impact of COVID-19 on the outcome.
Age as of 1 March 2020.
We defined rural as being located outside the commuting zone of a city with a population >10 000 [32].
The look-back window for comorbidities was since 1991, unless otherwise specified.
Frailty score >15 in the last 5 years.
Treatment in last 6 months or diagnosis in last year.
Diagnosis in the last 5 years.
Immunocompromised defined as diagnosed with human immunodeficiency virus (regardless of CD4 count) between 1991 and present, or had an organ or bone marrow transplant, or had another immunodeficient condition in the last 20 years.
Advanced liver disease defined as diagnosis of cirrhosis or decompensated cirrhosis.
Diagnosis in last 5 years or had a procedure in last 20 years.
Inpatient diagnosis in the last 20 years.
Area-level variables at the level of the census dissemination area.
Income quintile has variable cutoff values in each city or census area in order to take cost of living into account. A census dissemination area being in quintile 1 means it is among the highest 20% of dissemination areas in its city by median household income.
First quintile represents areas with 0%–4.1% of people aged 25–64 years without a diploma; second quintile, 4.1%–7.5%; third quintile, 7.5%–11.4%; fourth quintile, 11.4%–17.1%; and fifth quintile, 17.1%–94.3%.
First quintile represents 0%–32.5% of working people in the area who self-identified as working in an essential job, including sales, trades, manufacturing, and agriculture; second quintile, 32.5%–42.3%; third quintile, 42.3%–49.8%; fourth quintile, 50.0%–57.5%; and fifth quintile, 57.5%–114.3%.
First quintile represents 0%–2.2% of people in the area who self-identified as racially minoritized groups; second quintile, 2.2%–7.5%; third quintile: 7.5%–18.7%; fourth quintile, 18.7%–43.5%; and fifth quintile, 43.5%–100%.
First category represents 0%–2.1% of people in the area being recent immigrants who came to Canada within the last 5 years; second category, 2.1%–4.7%; and third category, 4.7%–41.2%. The high frequency of zeros permitted the creation of only 3 categories (ie, the lower 3 quintiles combined and the fourth and fifth quintiles).
First category, 0%–7.3% of buildings in the area are apartment buildings; second category, 7.4%–37.7%; and third category, 37.7%–100%. The high frequency of zeros permitted the creation of only 3 categories (ie, the lower 3 quintiles combined and the fourth and fifth quintiles).
First quintile represents 0–2.1 people/dwelling; second quintile, 2.2–2.4; third quintile, 2.5–2.6; fourth quintile, 2.7–3; and fifth quintile, 3.1–5.7.
First category represents 0%–2.6% of households are considered high-density housing; second category, 2.7%–5.2%; third category, 5.3%–8.7%; fourth category, >8.7%. The high frequency of zeros permitted the creation of only 4 categories (the lower 2 quintiles combined); “housing density/housing suitability” refers to whether a private household is “living in suitable accommodations” according to the National Occupancy Standard, that is, whether the dwelling has enough bedrooms for the size and composition of the household. A household is deemed to be living in suitable accommodations (non–high-density housing) if its dwelling has enough bedrooms, as calculated using the National Occupancy Standard.
All covariates other than SDOH were measured at the individual level, including age, sex (male vs female), other demographics (living in rural [32] vs urban area, public health region), and baseline health (a set of comorbidity variables, Table 1; past 3-year hospital admission; past year outpatient physician visits).
All datasets were linked using unique encoded identifiers [33] and analyzed at ICES.
Statistical Analyses
We examined and compared the demographics, baseline health, and SDOH of the full cohort, individuals who died related to COVID-19, and individuals who died without COVID-19 using descriptive statistics.
To examine the relationship between SDOH and COVID-19–related mortality, we used cause-specific hazard models [22, 34], where deaths without a positive COVID-19 test were treated as competing risk events (Supplementary Figure 1). We fitted unadjusted model and a set of adjusted models with a priori defined serial adjustment to assess the impact of different confounders. The models were fitted using the PHREG procedure of SAS [35]. Proportional hazard assumptions were assessed using scaled Schoenfeld residuals testing [36] (Supplementary Table 2).
To compare patterns by SDOH in non–COVID-19 mortality to those in COVID-19–related mortality, we repeated analyses using cause-specific hazard models to examine the relationship between SDOH and non–COVID-19 mortality, treating COVID-19 diagnosis as a competing risk.
To compare patterns by SDOH in COVID-19–related mortality with those in COVID-19 case fatality, we used multivariable logistic regression models to examine the associations between SDOH and COVID-19–related death among those who tested positive for COVID-19.
To quantify the absolute differences by area-level SDOH in COVID-19–related mortality, we used Fine and Gray subdistribution hazard models [22, 37]. Based on the fitted models adjusted for individual-level demographics and baseline health, we estimated the adjusted marginal cumulative incidence functions [38] and calculated the difference in the 1-year cumulative probability of COVID-19–related death between the most (SDOH level with the worst outcome; eg, lowest income quintile) and the least (SDOH level with the best outcome; eg, highest income quintile) at-risk group for each SDOH variable.
All analyses were conducted using SAS 9.4 [35]. R 4.1.2 was used to generate figures [39]. The confidence intervals (CIs) were derived from a robust sandwich covariance matrix to account for clustering by DA [40].
RESULTS
Of 11 810 255 community-dwelling adults (median age, 48 years) included, 206 671 (1.75%) tested positive for COVID-19, 3880 (0.03%) died related to COVID-19, and 88 107 (0.75%) died without a COVID-19 diagnosis. Individuals with missing data (N = 111 955, 0.9%) on area-level SDOH were excluded from the multivariable regression analyses (Supplementary Figure 2).
Deaths related to COVID-19 were disproportionately concentrated among older adults, males, and individuals living in urban areas (Table 1). COVID-19–related deaths were also disproportionately concentrated among individuals living with a comorbidity and those with more prior healthcare use (Table 1). Compared with the full cohort, COVID-19–related deaths were overrepresented in areas with less social advantage (eg, 28.9% vs 19.5% lived in the lowest-income areas) and in areas with a higher proportion of racially minoritized groups (38.7% vs 27.3%) and recent immigrants (37.7% vs 27.4%; Table 1).
Area-level SDOH and COVID-19–related Mortality
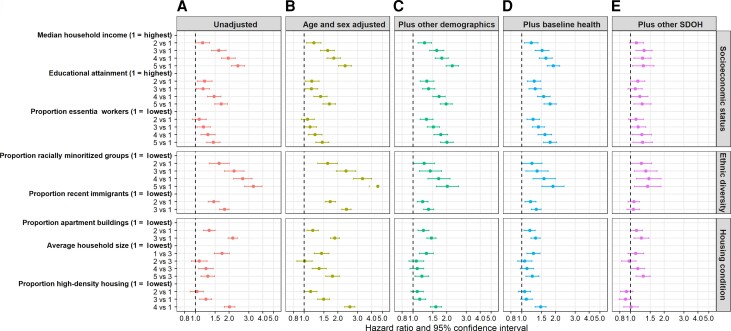
In the unadjusted models, areas with lower SES, higher ethnic diversity, higher proportion of apartment buildings and high-density housing, and lowest or highest household size (vs medium) were associated with increased hazard of COVID-19–related death (Figure 2A , Supplementary Table 3). We observed a dose–response relationship between all area-level SDOH variables and COVID-19–related mortality, except for household size (medium household size was associated with the lowest COVID-19–related mortality and was treated as the reference group; Figure 2).
Figure 2.
Associations between area-level SDOH and coronavirus disease 2019 (COVID-19)–related mortality among community-dwelling adult populations aged ≥20 years in Ontario, Canada, between 1 March 2020 and 2 March 2021. Results from unadjusted model (A) and models with serial adjustment of potential confounders (B–E). Cause-specific hazard models were used for COVID-19–related mortality analyses. COVID-19–related death defined as death within 30 days following or 7 days prior to a positive COVID-19 test. Other demographic variables included whether individuals reside in rural vs urban area and the public health region where individuals reside. Baseline health variables included comorbidities (listed in Table 1), number of hospital admissions in the past 3 years, and outpatient physician visits in the past year. Other SDOH variables are shown in the figure per the y-axis. Detailed definitions of SDOH variables are shown in Table 1 footnotes. Abbreviation: SDOH, social determinants of health.
Adjustment for individual-level demographics either attenuated or amplified the associations between COVID-19–related mortality and area-level SES (Figure 2A–2C ). Further adjustment for baseline health slightly reduced the associations between COVID-19–related mortality and SES (Figure 2C, 2D ). After further adjustment for other area-level SDOH, SES remained an independent determinant of COVID-19–related mortality, although the magnitude of association was greatly reduced (Figure 2D, 2E ). Fully adjusted hazard ratios (aHRs; 95% CIs) were 1.30 (1.04–1.62) for lowest vs highest income, 1.27 (1.07–1.52) for lowest vs highest proportion with diploma or higher educational attainment, and 1.28 (1.05–1.57) for highest vs lowest proportion essential workers (Figure 2E, Supplementary Table 3).
Adjustment for age and sex increased the magnitude of associations between area-level ethnic diversity and COVID-19–related mortality (Figure 2A, 2B ). Additional adjustment for other individual-level demographics largely reduced the magnitude of associations (Figure 2B, 2C ). Further adjustment for baseline health had a minimal influence on the associations (Figure 2C, 2D ). Additional adjustment of other area-level SDOH reduced the magnitude of associations between COVID-19–related mortality and proportion racially minoritized groups and nullified the association between COVID-19–related mortality and proportion recent immigrants (Figure 2D, 2E ). The fully aHR (95% CI) was 1.42 (1.08–1.87) for highest vs lowest proportion racially minoritized groups (Figure 2E, Supplementary Table 3).
After adjustment for individual-level demographics, baseline health, and other area-level SDOH, proportion apartment buildings was independently associated with increased hazard of COVID-19–related death (aHR, 1.25; 95% CI, 1.07–1.46), while proportion high-density housing was not (Figure 2E , Supplementary Table 3). The nonmonotonic relationship between COVID-19–related mortality and area-level household size persisted after full adjustment. The fully aHR (95% CI) was 1.30 (1.12–1.50) for highest vs medium area-level household size (Figure 2E, Supplementary Table 3).
Area-level SDOH and Non–COVID-19 Mortality and COVID-19 Case Fatality
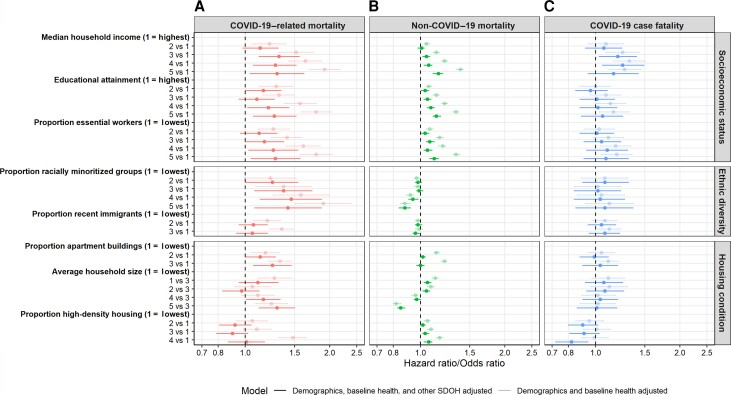
In contrast to the pattern with COVID-19–related mortality, areas with higher proportion racially minoritized groups (highest vs lowest: aHR, 0.88; 95% CI, .84–.92) and large household size (highest vs medium: aHR, 0.85; 95% CI, .83–.88) were independently associated with decreased hazard of non–COVID-19 death (Figure 3A, 3B; Supplementary Table 4). Only lower area-level income was independently associated with increased COVID-19 case fatality (Figure 3C, Supplementary Table 4).
Figure 3.
Comparison of patterns by area-level SDOH in COVID-19–related mortality (A), non–COVID-19 mortality (B), and COVID-19 case fatality (C) among community-dwelling adult populations aged ≥20 years in Ontario, Canada, 1 March 2020–2 March 2021. Multivariable cause-specific hazard models and a logistic regression model were used to estimate cause-specific mortalities and case fatality, respectively. Death within 30 days following or 7 days prior to a positive COVID-19 test was considered in calculations of COVID-19 case fatality and COVID-19–related mortality. Death without a positive COVID-19 test was considered non–COVID-19 mortality. Demographic variables included age, sex, whether individuals reside in rural vs urban area, and the public health region where individuals reside. Baseline health variables included comorbidities (listed in Table 1), number of hospital admissions in the past 3 years, and outpatient physician visits in the past year. Other SDOH variables are shown per the y-axis. Detailed definitions of SDOH variables are shown in Table 1 footnotes. The case fatality model additionally adjusted for month of COVID-19 test. Abbreviations: COVID-19, coronavirus disease 2019; SDOH, social determinants of health.
Adjusted Cumulative Probability of COVID-19–related Death
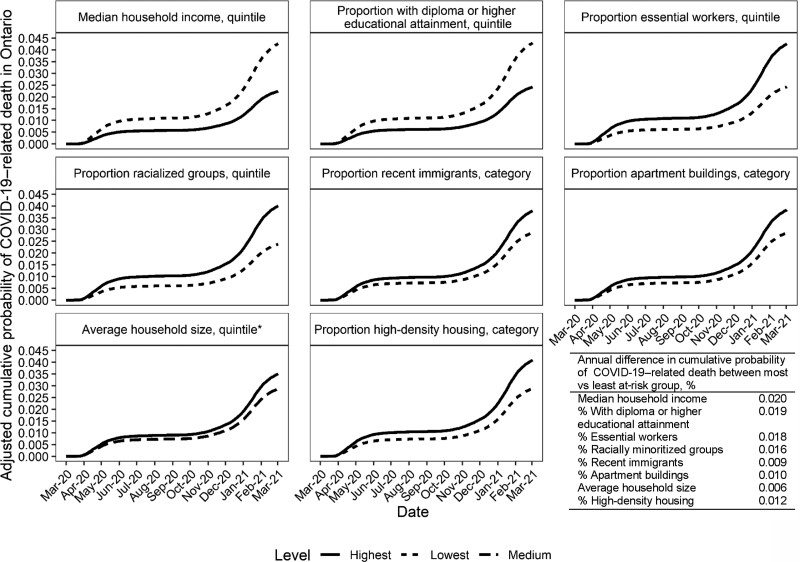
After accounting for individual-level demographics and baseline health, the estimated absolute difference in the cumulative probability of COVID-19–related death over a 1-year period ranged from 0.006% to 0.020%, comparing the most and least at risk SDOH group (Figure 4).
Figure 4.
Adjusted cumulative incidence function of COVID-19–related mortality by area-level social determinants of health (SDOH) among community-dwelling adult populations aged ≥20 years in Ontario, Canada, 1 March 2020–2 March 2021. Death within 30 days following or 7 days prior to a positive COVID-19 test was considered COVID-19–related. Estimates were obtained from the fitted Fine and Gray subdistribution hazard models. The models adjusted for demographics (age, sex, whether individuals reside in rural vs urban area, the public health region where individuals reside), and baseline health (comorbidities; listed in Table 1), number of hospital admissions in the past 3 years, and outpatient physician visits in the past year. Most at risk groups were defined as the SDOH level with the worst outcome, eg, lowest income quintile; least at-risk groups were defined as the SDOH level with the best outcome, eg, highest income quintile.*Areas with medium-level (quintile 3) average household size had the lowest COVID-19–related mortality and was defined as the least at risk group. Abbreviation: COVID-19, coronavirus disease 2019.
DISCUSSION
In a population-based cohort of 11.8 million adults in Ontario, Canada, we found that areas characterized by lower SES, greater ethnic diversity, more apartment buildings, and large vs medium household size were associated with increased hazards of COVID-19–related mortality, after accounting for individual-level demographics, baseline health, and other area-level SDOH. In contrast, areas with higher proportion racially minoritized groups and larger household size were associated with reduced hazard of non–COVID-19 mortality. With the exception of income, the area-level SDOH examined in this study were not independently associated with COVID-19 case fatality.
Our findings mirror those from studies in other countries, including the United Kingdom [4], Switzerland [5], Chile [13], and the United States [6], that have shown that areas with lower SES, measured by a composite index, were associated with increased risk and mortality of COVID-19. Our study demonstrated that specific elements of area-level SES, including income, educational attainment, and essential workers, were each independently associated with elevated hazard of COVID-19–related mortality. For example, individuals working in front-facing essential services who were not amenable to remote work had limited ability to shelter in place during periods of broad-scale restrictions on mobility and were less likely to receive benefits such as paid sick leave [41, 42], leading to heightened exposure risk and barriers to effective quarantine or isolation [12, 14]. The relationship between area-level income and case fatality might reflect delayed diagnosis or access to and quality of clinical care for persons living in lower-income neighborhoods [17, 43, 44]. Emerging evidence suggests that in-hospital mortality with COVID-19 was amplified during periods of higher patient load. Such inpatient surges were most likely to occur in hospitals serving lower-income areas experiencing the highest rates of cases [17, 43–45].
Our finding that areas with a higher proportion racially minoritized groups experienced increased hazard of COVID-19–related mortality but not higher case fatality confirmed findings in other settings [3, 10]. A systematic review of 52 US studies found that African-American/Black and non-White Hispanic populations experienced a disproportionate burden of infections, hospitalization, and COVID-19–related mortality, but not higher in-hospital case fatality, compared with similarly aged White non-Hispanic populations [10]. Studies in the United Kingdom found that minority ethnic groups experienced elevated risk of COVID-19–related mortality [3], higher prevalence of COVID-19 antibodies [46], but similar infection fatality ratio [46] compared with White counterparts. Taken together, the findings suggest that inequalities in COVID-19–related mortality by racially minoritized groups are more likely to stem from disproportionate exposure risks leading to disproportionate risks of acquisition/transmission and barriers to reach/access preventive interventions, as opposed to differences post-diagnosis [3, 10, 12].
In Canada, racially minoritized groups are more likely to work in essential services and more likely to live in larger and higher-density households [30], all of which have been identified as mechanistic risk factors for heightened exposure risk [12, 14]. Prior to COVID-19 and similar to our findings regarding non–COVID-19 mortality during the COVID-19 pandemic, mortality rates in Canada were lower in racially minoritized groups [47]. Similar to findings from the United Kingdom and Sweden [3, 48], COVID-19 has reversed the dose–response pattern of lower non–COVID-19 mortality among racially minoritized groups vs their counterparts.
The nonmonotonic relationship between area-level household size and COVID-19–related mortality might be partially explained by the positive correlation between income and household size (data not shown) and by different contact patterns (eg, individuals who live by themselves might have increased contacts outside the household). Our findings suggest that large household size, regardless of the housing density, might be an independent risk factor for household transmission. In epidemic theory, contact rates are conceptualized as density-dependent or frequency-dependent. Transmissions outside households may be influenced by population density (density-dependent transmission) [49]. Within the same household, contact rates may be better reflected by the frequency-dependent transmission (thus, household size; ie, assuming close interactions among all household members, regardless of the household density) [49].
Strengths of our study include limiting collider bias [20] and leveraging high-quality linked health administrative, surveillance, and health registries data to examine the influence of various confounders, including comorbidities, on the relationship between COVID-19–related mortality and area-level SDOH. Another strength is the competing risk survival analysis approach that allowed us to correctly estimate the marginal probability of COVID-19–related death in the presence of competing events. Our estimates of marginal probability of COVID-19–related death by area-level SDOH provided important insights into the health of each subgroup and permitted the quantification of inequalities on an absolute scale with adjustment of covariates [3, 5, 50], which are meaningful for public health decision-making, including informing strategies such as geographically focused vaccination [51–53].
Limitations include the potential for misclassification due to lack of data on the cause of death. Based on Ontario COVID-19 surveillance data, 92% of recorded all-cause deaths among individuals diagnosed with COVID-19 occurred within 30 days following or 7 days prior to a positive test (Supplementary Figure 3). Other settings have adopted similar definitions of COVID-19–related death to capture the immediate impact of COVID-19 on death [54]. Our estimates of COVID-19–related mortality might be underestimated if missed diagnosis occurs due to lack of testing or false-negative antigen tests [55]. Individuals who do not have provincial health insurance were not captured. If they were more likely to be socially and structurally vulnerable, our estimates might have underestimated the inequalities. We were restricted to area-level SDOH measures in the absence of individual-level measures, which might result in an underestimation of the SDOH–mortality associations [56]. Almost all areas with the highest quintile proportion racially minoritized groups were urban areas. However, stratified analysis by rural/urban revealed that inequalities in COVID-19–related mortality by racially minoritized groups were present in both settings (Supplementary Table 5). We lacked data on the severity of comorbidities and COVID-19 infection, and individuals’ exposures related to contact patterns and physical networks (eg, mobility, physical distancing) and masking, information that could help further explain the relationship between SDOH and COVID-19–related mortality. We did not determine if the associations between SDOH and COVID-19–related mortality differed across age groups or regions or if they changed over time (eg, between pandemic waves or in the context of vaccination) [3, 13], which will be an important next step of research. Indeed, examination of proportional hazard assumptions suggests a time-varying relationship between proportion racially minoritized group and hazard of COVID-19–related mortality (Supplementary Table 2).
Our study demonstrated that area-level social and structural inequalities are associated with COVID-19–related mortality after accounting for age, sex, and clinical factors. The majority of inequalities stem from proximal exposures and reach of, and access to, prevention interventions. COVID-19 has reversed existing patterns of mortality by race/ethnicity, with higher COVID-19–related mortality for racially minoritized groups. Tailored strategies that specifically address and are designed around the risk pathways related to SES, racism, and housing contexts include, but are not limited to, paid sick leave and improved workplace health and safety protocols; outbreak management; and community-led and community-tailored outreach for testing, effective isolation and quarantine, and vaccine programs. Moving forward, the goal of pandemic responses should include improving overall population health by addressing disproportionate acquisition and transmission risks and inequitable coverage of prevention interventions associated with SDOH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. W., J. C. K., and S. M. conceptualized the study. A. C. conducted the data cleaning and statistical analyses. L. W. drafted the manuscript. A. C., S. B., J. S., A. K. C., B. S., P. C. A., J. C. K., and S. M. provided critical input into the results interpretation and preparation of the manuscript.
Acknowledgments. The authors thank IQVIA Solutions Canada for use of their Drug Information File. The authors are grateful to the 14.7 million Ontario residents, without whom this research would be impossible.
Data sharing. The dataset from this study is held securely in coded form at ICES. While legal data-sharing agreements between ICES and data providers (eg, healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer. Some of the material presented here is based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and Ontario Health (OH) (previously known as Cancer Care Ontario). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of the funding sources. No endorsement by ICES, the Ontario Ministry of Health (MOH), Ministry of Long-Term Care (MLTC), CIHI, or OH is intended or should be inferred. The study sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant VR5–172683) and the St. Michael’s Hospital Foundation. This study was also supported by ICES, which is funded by an annual grant from the MOH and the MLTC. S. M. is supported by a Tier 2 Canada Research Chair in Mathematical Modelling and Program Science. J. K. is supported by a Clinician–Scientist Award from the University of Toronto Department of Family and Community Medicine.
Supplementary Material
Contributor Information
Linwei Wang, MAP-Centre for Urban Health Solutions, St. Michael's Hospital, Unity Health Toronto, Toronto, Ontario, Canada.
Andrew Calzavara, ICES, Toronto, Ontario, Canada.
Stefan Baral, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Janet Smylie, MAP-Centre for Urban Health Solutions, St. Michael's Hospital, Unity Health Toronto, Toronto, Ontario, Canada; Well Living House, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Adrienne K Chan, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Department of Medicine, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada; Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Beate Sander, ICES, Toronto, Ontario, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada; Toronto Health Economics and Technology Assessment Collaborative, University Health Network, Toronto, Ontario, Canada; Public Health Ontario, Toronto, Ontario, Canada.
Peter C Austin, ICES, Toronto, Ontario, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada; Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Jeffrey C Kwong, ICES, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Public Health Ontario, Toronto, Ontario, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada; University Health Network, Toronto, Ontario, Canada.
Sharmistha Mishra, MAP-Centre for Urban Health Solutions, St. Michael's Hospital, Unity Health Toronto, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Department of Medicine, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada; Institute of Medical Sciences, University of Toronto, Toronto, Ontario, Canada.
References
- 1. Upshaw TL, Brown C, Smith R, Perri M, Ziegler C, Pinto AD. Social determinants of COVID-19 incidence and outcomes: a rapid review. PLoS One 2021; 16:e0248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green H, Fernandez R, MacPhail C. The social determinants of health and health outcomes among adults during the COVID-19 pandemic: a systematic review. Public Health Nurs 2021; 38:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet 2021; 397:1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riou J, Panczak R, Althaus CL, et al. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: a population-based analysis. Lancet Public Health 2021; 6:e683–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open 2021; 4:e2036462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wachtleri B, Michalsk N, Nowossadeck E, et al. Socioeconomic inequalities and COVID-19—a review of the current international literature. J Health Monit 2020; 5(Suppl 7):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine 2020; 23:100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol 2020; 47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med 2021; 174:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckee C, Noor A, Sattenspiel L. Thinking clearly about social aspects of infectious disease transmission. Nature 2021; 595:205–13. [DOI] [PubMed] [Google Scholar]
- 12. Sundaram ME, Calzavara A, Mishra S, et al. Individual and social determinants of SARS-CoV-2 testing and positivity in Ontario, Canada: a population-wide study. CMAJ 2021; 193:E723–E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mena GE, Martinez PP, Mahmud AS, Marquet PA, Buckee CO, Santillana M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021; 372:eabg5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cevik M, Baral SD, Crozier A, Cassell JA. Support for self-isolation is critical in COVID-19 response. BMJ 2021; 372:n224. [DOI] [PubMed] [Google Scholar]
- 15. Gozzi N, Tizzoni M, Chinazzi M, Ferres L, Vespignani A, Perra N. Estimating the effect of social inequalities on the mitigation of COVID-19 across communities in Santiago de Chile. Nat Commun 2021; 12:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021; 398:1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stephen R, Idriss O. Did hospital capacity affect mortality during the pandemic’s first wave. 7 November 2020. Available at: https://www.health.org.uk/news-and-comment/charts-and-infographics/did-hospital-capacity-affect-mortality-during-the-pandemic. Accessed 15 November 2022.
- 18. Garcia MA, Homan PA, Garcia C, Brown TH. The color of COVID-19: structural racism and the disproportionate impact of the pandemic on older black and Latinx adults. J Gerontol B Psychol Sci Soc Sci 2021; 76:e75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue W, White A. COVID-19 and the rebiologisation of racial difference. Lancet 2021; 398:1479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 2020; 11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Public Health Agency of Canada . Social inequalities in COVID-19 mortality by area- and individual-level characteristics in Canada, January to July/August 2020. Ottawa, ON; 2021.
- 22. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez-Escamilla R, Cunningham K, Moran VH. COVID-19 and maternal and child food and nutrition insecurity: a complex syndemic. Maternal & Child Nutrition 2020; 16:e13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dictionary, Census of Population, 2016: dissemination area (DA) Ottawa: Statistics Canada 2016, modified 2019 January 3. Available at: www12.statcan.gc.ca/census-recensement/2016/ref/dict/geo021-eng.cfm. Accessed 15 November 2022.
- 25. Public Health Ontario . COVID-19 in Ontario—a focus on diversity: January 15, 2020 to May 14, 2020. Ottawa, ON; 2020.
- 26. Public Health Ontario . Enhanced epidemiological summary. Trends of COVID-19 Incidence in Ontario. Ottawa, ON; 2021.
- 27. Ontario COVID-19 Science Advisory Table. Ontario dashboard: percentage of cases caused by different variants in Ontario, 2021.. Available at: https://covid19-sciencetable.ca/ontario-dashboard/#percentcausedbyvariants. Accessed 15 November 2022.
- 28. van Ingen T, Brown KA, Buchan SA, et al. Neighbourhood-level socio-demographic characteristics and risk of COVID-19 incidence and mortality in Ontario, Canada: a population-based study. PLoS One 2022; 17:e0276507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rao A, Ma H, Moloney G, et al. A disproportionate epidemic: COVID-19 cases and deaths among essential workers in Toronto, Canada. Ann Epidemiol 2021; 63:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra S, Ma H, Moloney G, et al. Increasing concentration of COVID-19 by socioeconomic determinants and geography in Toronto, Canada: an observational study. Ann Epidemiol 2022; 65:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmad K, Erqou S, Shah N, et al. Association of poor housing conditions with COVID-19 incidence and mortality across US counties. PLoS One 2020; 15:e0241327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. du Plessis V, Beshiri R, Bollman RD, Clemenson H. Agriculture and Rural Working Paper Series. Working Paper No. 61: Definitions of “Rural”. Statistics Canada, 2002. Available at: https://www150.statcan.gc.ca/n1/en/pub/21-006-x/21-006-x2001003-eng.pdf?st=GoG5Ty5A. Accessed 15 November 2022.
- 33. ICES . Working with ICES data. Toronto: ICES; 2022.Available at: https://www.ices.on.ca/Data-and-Privacy/ICES-data/Working-with-ICES-Data. Accessed 15 November 2022.
- 34. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. SAS Institute, Inc . SAS/ACCESS® 9.4 Interface to ADABAS. Cary, NC: SAS Institute, Inc, 2013. [Google Scholar]
- 36. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982; 69:239–41. [Google Scholar]
- 37. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 38. Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol 2010; 63:46–55. [DOI] [PubMed] [Google Scholar]
- 39.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available at: https://www.R-project.org/. Accessed 15 November 2022.
- 40. Lin DY, Wei L-J. The robust inference for the Cox proportional hazards model. J Am Statist Assoc 1989; 84:1074–8. [Google Scholar]
- 41. Ontario Nonprofit Network . Paid sick days advocacy,2021. Available at: https://theonn.ca/our-work/our-people/decent-work/paid-sick-days/. Accessed 15 November 2022.
- 42. Thompson A, Stall N, Born K, et al. Benefits of paid sick leave during the COVID-19 pandemic. Science Briefs of the Ontario COVID-19 Science Advisory Table 2021; 2:1–3. [Google Scholar]
- 43. Xia Y, Ma H, Buckeridge DL, et al. Mortality trends and lengths of stay among hospitalized COVID-19 patients in Ontario and Québec (Canada): a population-based cohort study of the first three epidemic waves. Int J Infect Dis 2022; 121:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rossman H, Meir T, Somer J, et al. Hospital load and increased COVID-19 related mortality in Israel. Nat Commun 2021; 12:1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gray WK, Navaratnam AV, Day J, et al. Variability in COVID-19 in-hospital mortality rates between national health service trusts and regions in England: a national observational study for the Getting It Right First Time Programme. EClinicalMedicine 2021; 35:100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun 2021; 12:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khan M, Kobayashi K, Lee S, Vang Z. (In) Visible minorities in Canadian health data and research. Population change and lifecourse strategic knowledge cluster discussion paper series/Un Réseau Stratégique de Connaissances Changements de Population et Parcours de Vie Document de Travail2015; 3:5. [Google Scholar]
- 48. Drefahl S, Wallace M, Mussino E, et al. A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun 2020; 11:5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parasite Ecology . Parasite Ecology [Internet] 2013. Available at: https://parasiteecology.wordpress.com/2013/10/17/density-dependent-vs-frequency-dependent-disease-transmission/. Accessed 15 November 2022.
- 50. Asada Y. On the choice of absolute or relative inequality measures. Milbank Q 2010; 88:616–22; discussion 23-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown K, Stall N, Joh E. A strategy for the mass distribution of COVID-19 vaccines in Ontario based on age and neighbourhood. Science Briefs of the Ontario COVID-19 Science Advisory Table2021; 2. [Google Scholar]
- 52. Mishra S, Stall N, Ma H. A vaccination strategy for Ontario COVID-19 hotspots and essential workers. Science Briefs of the Ontario COVID-19 Science Advisory Table2021; 2. [Google Scholar]
- 53. Wrigley-Field E, Kiang MV, Riley AR, et al. Geographically targeted COVID-19 vaccination is more equitable and averts more deaths than age-based thresholds alone. Sci Adv 2021; 7:eabj2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Centre for Evidence-Based Medicine . Public Health England has changed its definition of deaths: here's what it means, 2020. Available at: https://www.cebm.net/covid-19/public-health-england-death-data-revised. Accessed 15 November 2022.
- 55. Centers for Disease Control and Prevention, Infectious Diseases Society of America . 2022COVID-19 Real-time Learning Network: rapid testing. Available at: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/rapid-testing/. Accessed 15 November 2022.
- 56. Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr 2021; 19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.