ABSTRACT
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first identified in December 2019 and emerged into an ongoing global pandemic. Both the pandemic itself and the associated public restrictive measures of social mobility established with different intensity over different periods in various countries have significantly affected the everyday activities and lifestyles of people all over the world. The impact of lockdown and quarantine measures on hypertension incidence and blood pressure (BP) control is an important topic that requires further investigation. The aim of this review is: a) to present the current evidence regarding the actual effects of public restrictive measures on BP levels and control, originating primarily from studies investigating the impact of public restrictive measures on BP control with the use of various BP phenotypes; b) to summarize the possible pandemic-related effects of factors known to affect BP levels, including both traditional (e.g. dietary habits including alcohol and sodium intake, body weight, smoking and physical activity) and non-traditional (e.g. sleep patterns, air pollution, environmental noise, delayed diagnosis and medication adherence) ones.
Keywords: blood pressure, COVID-19, hypertension, lockdown, quarantine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first identified in China in December 2019 and since then has emerged into an ongoing global pandemic [1]. At 2.5 years after the initial outbreak, COVID-19 has been established as a major source of morbidity and mortality worldwide, infecting >550 million patients, with numbers continuously increasing [2]. The progression and prognosis of COVID-19 is influenced by older age and comorbidities that are common in older individuals, such as hypertension, obesity, diabetes mellitus and pulmonary, cardiovascular and kidney disease [3]. These conditions were associated in more than half of COVID-19 patients, while a third of patients had multiple associated comorbidities [4].
Apart from the direct clinical impact on healthcare systems worldwide, the COVID-19 pandemic has also induced important changes in everyday life, influencing people's everyday activities. To minimize the virus transmission and control the pandemic, many governments enforced public preventive measures including quarantine (i.e. government enforced isolation measures that are applied to individuals presumed to have been exposed to COVID-19, reporting either recent foreign travel history or contact with COVID-19-positive cases) and nationwide lockdowns (i.e. government enforced restrictions aimed at reducing population movement and physical interaction, including stay-at-home orders and social distancing, such as cancellation of public gatherings, closure of schools and public transportation, suspension of markets and non-essential services/activities like gyms) of different intensity (mild versus strict), frequency and duration (e.g. in the USA, each individual state put lockdowns in place of various lengths ranging from 20 to 267 days) over different periods [5].
These measures brought significant changes to people's lives by impacting both on their mental health and lifestyle behaviours [6]. In this context, the COVID-19 pandemic may also have led to adverse changes in the health behaviours of patients with hypertension and other cardiovascular diseases [6, 7]. Hence, in addition to alterations in daily life due to social distancing and economic changes, other traditional (e.g. dietary habits including alcohol and sodium intake, body weight, smoking and physical activity) and non-traditional risk factors (e.g. sleep patterns, air pollution, environmental noise, delayed diagnosis and medication adherence) affecting blood pressure (BP) control might also have exhibited significant changes during the COVID-19 pandemic and lockdown periods [6, 8, 9]. Additionally, the temporary collapse of some healthcare systems and fear of contagion may have limited access to care, especially for chronic conditions such as hypertension. Preliminary studies, mostly involving home BP monitoring, attempted to evaluate the actual effects of lockdown on BP levels and control using various BP phenotypes. Therefore, the aim of this review is to summarize the existing evidence on the impact of public restrictive measures during the COVID-19 pandemic on BP levels per se and factors affecting hypertension incidence and BP control and discuss potential long-term implications.
For the purpose of this review, we conducted literature searches in the PubMed and Scopus databases up to June 2022 using the following keywords (in different combinations using simple Boolean operators): ‘SARS-CoV-2’, ‘COVID’, ‘COVID-19’, ‘COVID19’, ‘lockdown’, ‘quarantine’, ‘confinement’, ‘blood pressure’, ‘hypertension’, ‘blood pressure control’, ‘body weight’, ‘obesity’, ‘alcohol’, ‘ethanol’, ‘sodium’, ‘salt’, ‘sleep’, ‘insomnia’, ‘adherence’, ‘compliance’, ‘physical activity’, ‘exercise’, ‘sedentary behaviour’, ‘smoking’, ‘cigarette’, ‘tobacco’, ‘pollution’, ‘environmental noise’. The literature search for the specific questions of the effects of public restrictive measures on BP levels and control during the COVID-19 pandemic is described in more detail in the relevant section.
COVID-19 and hypertension
The association between COVID-19 and hypertension was identified early in the pandemic. Reports from Europe and the USA suggest that hypertension was the most frequent comorbidity among patients hospitalized for COVID-19 infection [10, 11] as well as among COVID-19 patients admitted to intensive care units [12–14]. Consequently, early after the onset of the pandemic, some authors suggested that a significant association between prevalent hypertension and COVID-19–related mortality may exist [10, 11, 15]. However, seminal studies in the field showed that these associations may be affected by several confounders, including older age and multiple comorbidities, especially diabetes, obesity and cardiovascular, pulmonary and kidney disease [3]. As such, there is little direct evidence to indicate that hypertension per se is an independent risk factor for either COVID-19 infection or severe COVID-19 disease [13, 14].
In addition, early in the pandemic course, some authors without supporting clinical evidence suggested that treatment with renin–angiotensin system (RAS) blockers may be associated with higher risk of severe COVID-19 [16], based on the fact that both SARS-CoV and SARS-CoV-2 bind to their target cells through angiotensin-converting enzyme 2 (ACE2) located in the cell membrane of respiratory epithelial cells [17]. However, these concerns were not confirmed by subsequent observational studies and randomized clinical trials, which demonstrated that the use of RAS blockers is not associated with COVID-19 infection or severe disease and that their discontinuation did not appear to improve outcomes, while their use may also confer a benefit through organ protection [13, 18–21].
Effects of public restrictive measures on BP levels and control during the COVID-19 pandemic
Almost 2.5 years after the start of COVID-19, several studies examining the consequences of the pandemic on BP control in different countries have been published. To explore this field, we conducted a literature search in the PubMed and Scopus databases up to June 2022, using the terms ‘SARS-CoV-2’, ‘COVID’, ‘COVID-19’, ‘COVID19’, ‘lockdown’, ‘quarantine’, ‘confinement’, ‘blood pressure’, ‘hypertension’ and ‘blood pressure control’, described in Supplementary Table 1. The flow diagram of the selection process for studies examining these specific parameters is shown in Supplementary Fig. 1. Most of these studies used office and home BP measurements, but there are also a couple of studies reporting significant changes in 24-h ambulatory BP levels as well as in visits to the emergency department for severe hypertension (Table 1).
Table 1:
Studies examining the effects of public restrictive measures on BP levels and BP control during the COVID-19 pandemic based on different BP measurements.
| Author, year | Location | Study design | Participants | Main results |
|---|---|---|---|---|
| Office BP | ||||
| Laffin et al., 2021 [26] | USA | Longitudinal cohort study | 464 585 individuals participating in an annual employer-sponsored wellness program (2018–2020) | • ↑ monthly mean changes during the pandemic (SBP: from 1.10 to 2.50 mmHg and DBP: from 0.14 to 0.53 mmHg); larger increases in women, older participants (SBP) and younger participants (DBP)• During the pandemic, a larger proportion of participants were up-categorized than down-categorized according to 2017 AHA/ACC BP (26.8% versus 22.0%, P < .0001) |
| HBPM | ||||
| Shah et al., 2021 [27] | USA | Cohort study | 72 706 hypertensive patients | • ↑ mean monthly SBP (131.6 versus 127.5 mmHg, P < .001), DBP (80.2 versus 79.2 mmHg, P < .001) and MAP (97.4 versus 95.3 mmHg, P < .001) during pandemic compared to pre-pandemic period• ↑ participants with uncontrolled hypertension during the pandemic (19% versus 15%) |
| Zhang et al., 2021 [22] | China | Longitudinal cohort study | 7394 hypertensive elderly patients (60–80 years old) [Wuhan (n = 283) compared with other provinces of China (n = 7111)] | Patients in Wuhan versus non-Wuhan areas (five phases: pre-epidemic, incubation, developing, outbreak and plateau):• ↑ average morning SBP during incubation (134.7 versus 131.3 mmHg, P < .001), developing (135.1 versus 131.6 mmHg, P < .001) and outbreak phase (133.9 versus 130.8 mmHg, P < .001) and marginally higher during the plateau phase of the COVID-19 pandemic (131.2 versus 130.1 mmHg, P = .06)• ↑ differences in morning SBP (ΔSBP) from pre-epidemic levels during incubation (2.6 versus 0.1 mmHg, P < .001), developing (3.3 versus 0.4 mmHg, P < .001) and outbreak phase (1.7 versus −0.4 mmHg, P < .001) |
| Girerd et al., 2022 [30] | France | Retrospective cohort study | 2273 hypertensive patients | During lockdown ↓ SBP by 3 mmHg (95% CI −2.4 to −3.9) and DBP by 1.5 mmHg (95% CI −1.4 to −2.2) (all P < .001). In linear mixed effect models, only pre-lockdown BP levels but not age or sex had a significant effect on the SBP change; participants with higher SBP had a greater decrease in SBP during the lockdown |
| Pengo et al., 2022 [29] | Italy | Cohort study | 126 hypertensive patients | ↓ Average home SBP (123.23 versus 125.05 mmHg, P = .008) and DBP (74.45 versus 75.28 mmHg, P = .023) during lockdown compared with pre-lockdown period. Patients with uncontrolled HBP showed the most consistent decrease in SBP (130.00 versus 136.06 mmHg, P = 0.001) and DBP (78.78 versus 81.30 mmHg, P = .018) |
| Zhang et al. 2022 [23] | China | Longitudinal cohort study | 3724 hypertensive elderly patients (60–80 years old) (Wuhan n = 240) compared with other provinces of China (n = 3484) | Patients with anxiety versus patients without anxiety (five phases: pre-epidemic, incubation, developing, outbreak and plateau):• ↑ average morning SBP during incubation (132.7 versus 131.3 mmHg, P = .03), developing (132.8 versus 131.5 mmHg, P = .05), outbreak (132.9 versus 130.7 mmHg, P < .001) and plateau period of the COVID-19 pandemic (132.7 versus 130.0 mmHg, P < .001)• ↑ differences in morning SBP (ΔSBP) from pre-epidemic during outbreak (0.9 versus −0.3 mmHg, P = .01) and plateau phase (0.8 versus −0.9 mmHg, P < .001)• ↑ uncontrolled BP during the incubation (23.3% versus 20.4%, P = .02), developing (21.8% versus 20.8%, P = .02), outbreak (21.8% versus 18.5%, P = .01) and plateau period (29.8% versus 18.5%, P < .001)• ↑ higher risk of incident cardiovascular events during the 1-year follow-up period of the COVID-19 pandemic [HR 2.47 (95% CI 1.10–5.58), P = .03] |
| Office BP and HBPM | ||||
| Kobayashi et al, 2021 [22] | Japan | Retrospective cohort study | 748 patients (who visited regularly clinics for any lifestyle-related or chronic disease) | • ↑ office SBP (138.6 ± 18.6 versus 136.5 ± 17.5 mmHg, P < .001), DBP (79.0 ± 12.2 versus 78.2 ± 12.0 mmHg, P = .03) and MAP (98.9 ± 12.5 versus 97.6 ± 12.0 mmHg, P = .002) during the pandemic compared with the pre-pandemic• ↓ home SBP (126.9 ± 10.2 versus 128.2 ± 10.3 mmHg, P < .001), DBP (75.2 ± 9.0 versus 75.8 ± 8.8 mmHg, P = .01) and MAP (92.5 ± 8.1 versus 93.3 ± 7.9 mmHg, P = .001) during the pandemic compared with the pre-pandemic• ↑ white coat hypertension prevalence (17% versus 13%, P < .001) during the pandemic compared with the pre-pandemic |
| Feitosa et al., 2022 [28] | Brazil | Cohort study | 57 768 individuals (n = 24 227 untreated and n = 27 699 treated) | Main analysis (three periods: pre-pandemic era, early pandemic phase and late pandemic phase)• ↑ prevalence of high OBP and HBPM among untreated participants; ↓ prevalence of high OBP and HBPM among treated participants during the early pandemic period compared with pre-pandemicSubanalysis (495 untreated and 987 treated patients with available repeated measurements, same study periods)• No significant differences in rates of high BP before and during the two phases of the pandemic• No significant differences in BP levels, except for lower OBP and HBPM levels during the early pandemic period among treated participants (office SBP/DBP: 129 ± 18/80 ± 12 versus 134 ± 21/83 ± 12 mmHg, P < .01; home SBP/DBP 124 ± 15/77 ± 10 versus 127 ± 16/78 ± 11 mmHg, P < .05) |
| ABPM | ||||
| Celik et al., 2021 [24] | Turkey | Retrospective study | 142 patients with essential hypertension | • ↑ ambulatory BP during the pandemic (24-h SBP: 130.2 ± 6.4 versus 124.0 ± 5.6 mmHg, p<0.001; daytime SBP: 135.2 ± 6.9 versus 128.5 ± 5.2 mmHg, P < .001; nighttime SBP 120.4 ± 5.8 versus 116.0 ± 5.2 mmHg, P < .001). Similar results for 24-h, day- and nighttime DBP• ↑ ambulatory BP in patients with higher anxiety levels than those with low (24-h SBP: 134.0 ± 5.8 versus 128.0 ± 5.6, P < .001; daytime SBP: 139.0 ± 6.2 versus 132.0 ± 5.5, P < .001; nighttime SBP: 123.0 ± 5.4 versus 118.0 ± 5.4 mmHg, P < .001). Similar results for DBP |
| Emergency department | ||||
| Fosco et al., 2020 [28] | Argentina | Retrospective cohort study | 12 144 patients visited the Emergency Department | ↑ patients presenting to the emergency department with severe hypertension (BP ≥160/100 mmHg) (23.9% versus 15.5%, P < .001) |
HBPM, home BP monitoring; OBP, office BP.
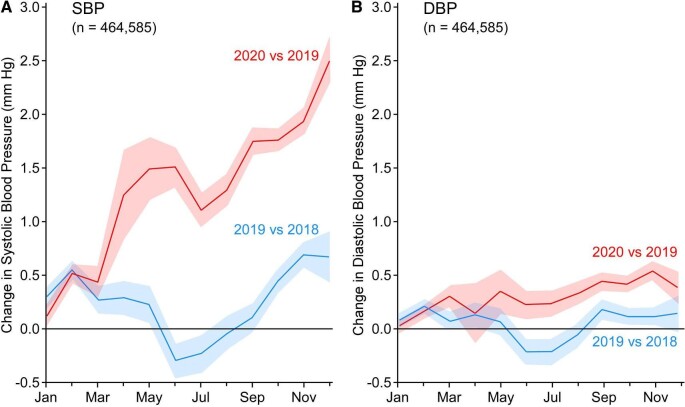
Most of the existing evidence supports a worse BP control pattern during the initial phase of lockdown. In a study from China, Zhang et al. [22] used longitudinal data of home BP monitored via a smartphone application in 7394 elderly hypertensive patients living in Wuhan versus other cities of China in order to examine the impact of the COVID-19 outbreak on BP levels. A short-term increase in morning home systolic BP (SBP) was evident during the early phases of the pandemic (from December 2019 to February 2020) in Wuhan compared with non-Wuhan patients and then returned to normal at the plateau phase. Patients in Wuhan also had an increased regimen change in antihypertensive drugs during the outbreak compared with non-Wuhan patients. Expectedly, Wuhan patients were more likely to check their BP via the application, while doctors were less likely to monitor the app for BP control during the pandemic [22]. In a subsequent study from the same research group aimed at evaluating the association of anxiety status with BP fluctuations and cardiovascular events during the COVID-19 outbreak in China, patients with anxiety had a higher average morning home SBP during the pandemic and an increased risk of incident cardiovascular events during the 1-year follow-up {hazard ratio [HR] 2.47 [95% confidence interval CI) 1.10–5.58], P = .03} [23]. Moreover, the rates of uncontrolled BP in patients with anxiety were higher than in those without anxiety [23]. This negative effect of pandemic-associated anxiety on BP control was also noted by another, albeit small, study including 142 hypertensive patients from Turkey [24]. In this study, Celik et al. [24] showed that 24-h daytime and nighttime SBP and diastolic BP (DBP) levels were significantly higher during the pandemic, with individuals with higher anxiety scores presenting higher ambulatory SBP and DBP. Moreover, a Japanese study from Kobayashi et al. [25] including 748 participants with valid office BP readings showed an increase in office BP during the pandemic compared with pre-pandemic levels, as well as an increase in the prevalence of white coat hypertension (from 13% to 17%; P < .001). Another US study including 464 585 participants with valid annual office BP measurements (2018–2020) showed increased BP levels during the pandemic period compared with the previous year (mean change in SBP from +1.10 to +2.50 mmHg and in DBP from +0.14 to +0.53 mmHg) (Fig. 1) [26]. A recent population-based analysis of home BP data from 72 706 participants from the USA showed that during the first months of the pandemic and national lockdown periods (April–August 2020), home SBP/DBP levels were significantly increased compared with pre-COVID-19 levels (131.6/80.2 versus 127.5/79.2 mmHg; P < .001 for both). Similarly, the proportion of participants with uncontrolled hypertension also rose during the same period (19% versus 15%) [27]. Lastly, in a cohort study from Argentina comparing the number of patients visiting the emergency department with severe hypertension (SBP ≥160 mmHg and/or DBP ≥100 mmHg) reported an increase in severe hypertension cases in post- versus pre-lockdown (23.9% versus 15.5% of the total visits in the emergency department; P < .001) and post-lockdown versus interannual reference (23.9% versus 17.6%; P < .001) [28].
Figure 1:
BP changes during the COVID-19 pandemic in the USA. Mean changes (with 95% CIs) in (A) SBP and (B) DBP from the preceding year. From Laffin et al. [26].
In contrast to the above, there are also some studies reporting better BP control during the initial phase of the COVID-19 lockdown period. In the aforementioned study from Kobayashi et al. [25] there was a significant decrease in home BP levels (SBP/DBP: 128.2 ± 10.3/75.8 ± 8.8 to 126.9 ± 10.2/75.2 ± 9.0 mmHg; P < .001/P = .01, respectively). Moreover, in a cohort study including 126 treated hypertensive patients from Italy, Pengo et al. [29] reported that home BP was significantly decreased during lockdown (SBP/DBP: 123.23/74.45 versus 125.05/75.28 mmHg; P = .008 and P = .023 respectively), with more profound effects in patients with uncontrolled BP. The same results were found in a French study (2273 participants), in which there was a decrease in home SBP/DBP by 3.0/1.5 mmHg during lockdown compared to pre-lockdown periods; these decreases were more significant in patients with higher BP [30]. Finally, in the only study to date examining the medium and long-term effects of COVID-19 lockdown on BP (n = 57 768 individuals from Brazil), Feitosa et al. [31] reported a trend toward a higher prevalence of high office and home BP among untreated participants but a lower prevalence of high office and home BP among treated participants during the early pandemic period compared with the corresponding period in 2019. In a subanalysis including 495 untreated and 987 treated patients with available repeated office and home BP measurements during the same periods, there were no significant differences in prevalence rates of both office and home high BP before and during the pandemic; similarly, both office and home BP levels were similar before and during the pandemic.
Of note, in February 2021, the European Society of Hypertension (ESH) COVID-19 Task Force initiated the ESH ABPM COVID-19 Study (NCT05167240) [32]. This is an ongoing, multicentre (involving 34 hypertension centres in Europe and Israel) study with the main goal to determine the impact of COVID-19 lockdown on BP levels and BP variability [through the comparison of ambulatory BP monitoring (ABPM) results obtained before and during lockdown) in already-treated hypertensive patients.
Impact of public restrictive measures on traditional and non-traditional factors affecting BP levels during the COVID-19 pandemic
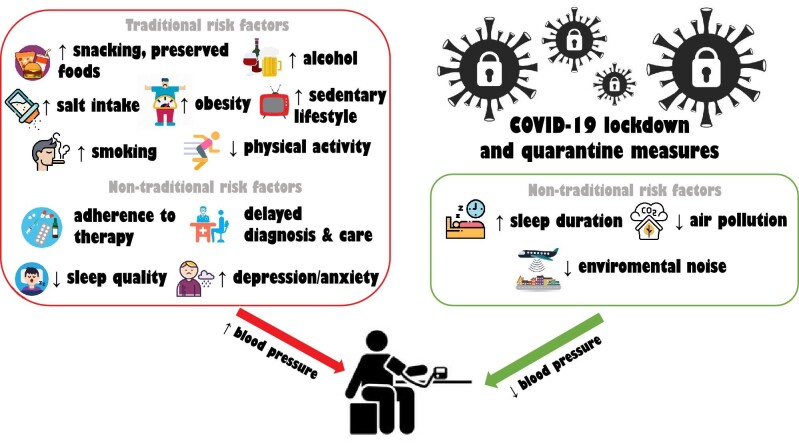
Lockdown and quarantine measures may have had both negative and positive effects on traditional and non-traditional factors affecting BP levels, including alcohol consumption, body weight and dietary habits, smoking, physical activity, dietary sodium intake, sleep quality and duration and sleep disorders, adherence to therapy, delayed diagnosis and care of BP, air pollution and environmental noise (Fig. 2).
Figure 2:
Adverse and beneficial effects of public restrictive measures on BP levels during the COVID-19 pandemic.
Possible negative effects
Body weight and dietary habits
Obesity is associated with increased hypertension incidence, and reducing weight towards an ideal body weight has beneficial effects on BP levels [33]. Apart from the strong association with adverse outcomes in hypertension, obesity is also one of the strongest independent predictors of COVID-19 severity [34]. With regards to patients with end-stage kidney disease, in a recent subanalysis of the European Renal Association COVID-19 Database (ERACODA) in 3160 patients on kidney function replacement therapy, obesity was independently associated with an increased risk of mortality at 3 months [adjusted HR 1.71 (95% CI 1.27–2.30) in patients with a body mass index (BMI) ≥35 kg/m2 compared with a BMI of 18.5–24.9] [35].
Body weight is another factor that might have been influenced negatively during COVID-19 restrictive measures (Table 2) [36–41]. In a longitudinal study, young adults gained ≈3.5 pounds (1.6 kg) on average during COVID-19 quarantine measures in the USA compared with baseline values from the previous 2 years [42]. Further, a large (2060 adults) cross-sectional study showed that 30% of the participants reported an increase in BMI during the COVID-19 lockdown period [43]. In a cross-sectional study from France including 536 individuals >50 years of age, Berard et al. [44] showed that one in four participants had gained weight during this period. Finally, a recent meta-analysis in 3339 individuals with pre-existing eating disorders and obesity reported a pooled prevalence rate of weight gain of 52%; the respective prevalence rate of deterioration in eating disorders was 65% [37]. Depression and anxiety were considered to be major determinants for this deterioration [37].
Table 2:
Systematic reviews and meta-analyses examining the impact of public restrictive measures on body weight levels and obesity during the COVID-19 pandemic.
| Author, year | Included studies | Participants | Results |
|---|---|---|---|
| Bakaloudi et al., 2021 [36] | 38 studies (6 in meta-analysis) | 59 711 adults and adolescents >16 years of age | ↑ body weight [WMD 1.57 (95% CI 1.01–2.14)], ↑ BMI [WMD 0.31 (95% CI 0.17–0.45)] |
| Sideli et al., 2021 [37] | 26 studies | 3399 participants with eating disorders and obesity | ↑ body weight pooled prevalence 52% (95% CI 25–78, k = 4). Pooled prevalence of symptomatic deterioration in eating disorders 65% (95% CI 48–81, k = 10). No change in BMI [WMD 0.11 (95% CI −0.20–0.42) |
| Khan et al., 2022 [38] | 41 studies | 469 362 participants | No quantitative synthesis of the data. 7.2–72.4% of participants with ↑ body weight (mean gain from 0.6 ± 1.3 to 3.0 ± 2.4 kg) and 11.1–32.0% of participants ↓ body weight (mean loss from 2.0 ± 1.4 to 2.9 ± 1.5 kg). Determinants of body weight gain: obesity, female sex, age <25 years and >45 years, ↓ sleep time/quality, pre-existing poor dietary quality, stress, ↓ physical activity, comorbidities |
| La Fauci et al., 2022 [39] | 20 studies | 818 743 children and adolescents | No quantitative synthesis of the data. Most of the included studies reported ↑ body weight and ↑ BMI |
| Chang et al. 2021 [40] | 12 studies | 4710 children and adolescents | ↑ body weight [WMD 2.67 (95% CI 2.12–3.23)], ↑ BMI [WMD 0.77 (95% CI 0.33–1.20)], ↑ in obesity rates [OR 1.23 (95% CI 1.10–1.37)] and ↑ overweight [OR 1.17 (95% CI 1.06–1.29)] |
| Daniels et al. 2022 [41] | 14 studies | 37 674 (most of the participants from the general population) | No quantitative synthesis of the data. A qualitative synthesis indicated a trend of weight gain during the pandemic, with 12 of 14 studies reporting ↑ body weight and ↑ BMI |
WMD, weighted mean difference.
Alterations in dietary habits and nutritional patterns contributed significantly to the body weight increase during the COVID-19 lockdown. Studies from Europe reported lower nutritional quality in eating patterns during the lockdown compared with the pre-COVID era [45]. Along the same line, several studies conducted in Europe and Latin America showed an increase in the consumption of unhealthy snacks (i.e. food items rich in salt and calories and poor in nutrients) during the same period of public restrictive measures [45, 46]. Remarkably, among various population groups affected by the COVID-19 lockdown, overweight and obese people were more prone to impair their dietary patterns and lifestyles; in particular, they reported eating and snacking more during home confinement and had a lower frequency of consumption of fruits and vegetables [47].
Alcohol consumption
The association between alcohol consumption and hypertension is a long-established one. Previous clinical trials and meta-analyses suggest that a reduction of alcohol consumption has a beneficial effect on BP levels and cardiovascular health [48, 49]. Conversely, binge drinking has a strong pressor effect on BP [33].
During the COVID-19 pandemic, changes in alcohol consumption patterns and resultant effects were evident [46, 50]. Most data about changes in alcohol consumption during the COVID-19 lockdown derive from web-based surveys and the results are not uniform. While in some studies alcohol consumption remained unchanged [51–53], many studies report an increase in binge drinking and solitary drinking during lockdown compared with pre-lockdown years [47, 54–56]. Moreover, in a cohort study from the UK, an increased relative risk for binge drinking and alcohol consumption frequency during lockdown was reported [risk ratio (RR) 1.48 (95% CI 1.27–1.73) and RR 1.38 (95% CI 1.26–1.51)] [57]. Consistent with the above findings, market research in Europe, Australia and the USA during the first lockdown period showed an increase in alcohol sales [58–60]. Although some studies observed an increase in drinking among women in relation to men [55, 61], others did not observe sex differences in alcohol use patterns [62]. Overall, there is a growing body of evidence that during the COVID-19 pandemic, there has been a tendency towards increased alcohol consumption and alterations of alcohol consumption patterns. Medium- and long-term consequences in cardiovascular health should be investigated in depth.
Smoking
Acutely, smoking exerts a catecholamine-mediated hypertensive and tachycardic effect [63]. The chronic effects of smoking on BP have also been established. Studies using ABPM have shown that smokers exhibit higher daily BP levels than non-smokers [64]. In addition, smoking has been linked with masked hypertension [65]. Renovascular hypertension is also more common in smokers [63]. Overall, smoking increases CVD risk for hypertensive patients and smoking cessation represents one of the most effective strategies to diminish this risk [63].
Data on smoking behaviour alterations throughout the COVID-19 lockdown derive mainly from web-based surveys, the majority of which included participants from the general population and a few only included smokers. Their results are again not homogeneous. In the general population, Sun et al. [66] showed that the overall percentage of smokers increased marginally during the pandemic (from 12.8% to 13.6%), but other studies revealed either similar [67] or decreased rates of smoking prevalence [68, 69]. Among smokers, the changes in smoking habits were variable. A few studies showed that during the lockdown tobacco consumption remained stable in the majority of smokers (studies from the USA [70], the Netherlands [71] and France [72]). In other studies, the majority increased smoking frequency and the total number of cigarettes smoked per day during the quarantine (studies from Poland [73], Israel [74], Australia [75] and Belgium [76]), whereas there is also an Italian study that revealed a decrease in smoking during lockdown (P < .001) [68]. With regards to heavy smoking (>10 cigarettes/day), a study from the USA showed an increased prevalence of this habit among smokers, from 5.8% before to 7.9% during the pandemic [67].
In the aforementioned studies, several factors have been identified to be associated with changes in smoking habits. Among them, younger age [67, 72, 76] and higher depression, stress or anxiety levels [72, 75] have been associated with increased smoking. As for education and current living status, the findings are not consistent. Guignard et al. [72] linked higher education level and living in overcrowded housing with higher odds of increased smoking, whereas Vanderbruggen et al. [76] showed that lower education and living alone increased the odds.
Although not uniform, these imply a significant worsening in smoking behaviour following the pandemic's outbreak.
Physical activity
The antihypertensive role of physical activity is firmly established. Despite a transient increase in SBP during acute exercise, BP falls below baseline levels after completing training, a phenomenon called ‘post-exercise hypotension’ that persists for hours [77]. In a recent meta-analysis of randomized controlled trials (RCTs) assessing the effects of exercise on ambulatory BP in hypertensive patients, significant decreases in 24-h SBP/DBP (−5.4 mmHg/−3.0 mmHg) daytime and nighttime SBP/DBP levels were confirmed [78]. As such, the latest European Society of Cardiology/ESH hypertension guidelines acknowledge the importance of physical activity in the management of hypertensive patients, recommending regular physical activity as a first-line lifestyle modification (class of recommendation, I; level of evidence, A) [33, 79].
Pandemic-related public restrictive measures reduced unprecedentedly the amount of physical activity through confining people to their homes and cultivating sedentary behaviours. A preliminary large cross-sectional study of 3800 individuals showed a decline in time spent on vigorous physical activity and walking [16.8% (P < .001) and 58.2% (P < .001), respectively] and an increase of 23.8% (P < .001) in sedentary time, with these effects being more markedly manifested in men and previously more active individuals [80]. Similar results were reported by other studies [75, 81, 82]. Subsequent meta-analyses confirmed the above findings by showing a significant decline in physical activity and a marked increment in sedentary lifestyle in all age groups [83–87] (Table 3). This shift in physical activity profile exerted a negative psychological impact, as a relationship between psychological distress (i.e. stress, anxiety, depression) and physical activity levels during the pandemic was demonstrated [88]. The same profile of lockdown-attributed physical inactivity was also affirmed in the elderly [87] and patients with chronic comorbidities, although in the latter case most of relevant studies were based on self-reported questionnaires and not on objective physical activity measurements [86]. Of note, only one study so far has investigated the effects of lockdown on physical activity in hypertensive patients [89]. The main findings of this study revealed an increase in sedentary behaviour with prolonged continuous sitting and shorter breaks, a decreased number of steps per day and a shorter duration of light and moderate/vigorous physical activity, with these deleterious changes being more prominent on weekends [89]. Notably, a specific behaviour that negatively affected physical activity during the pandemic was an increase in television viewing time. In a longitudinal study with 631 participants, the hours spent watching television were significantly increased from 0.9 ± 0.8 to 1.7 ± 1.4 h/day [82], while other studies reported an increase in the percentage of participants watching television >2 h/day (10.4% before versus 24.3% during the pandemic) [90].
Table 3:
Systematic reviews and meta-analyses examining the impact of public restrictive measures on physical activity during the COVID-19 pandemic.
| Author, Year | Included studies (physical activity definition) | Participants | Results |
|---|---|---|---|
| Pérez-Gisbert et al., 2021 [142] | 5 studies (2 studies accelerometers, 3 questionnaires) | 667 patients with chronic diseases | ↓ physical activity levels [SMD −0.29 (95% CI −0.40 to −0.18), P < .00001, I2 = 13%] |
| Ng et al., 2022 [86] | 36 studies (5 studies accelerometer/ pedometer, 8 questionnaires, 9 no clear definition) | 800 256 participants with or without chronic diseases | ↓ step count [SMD −2.789 (95% CI −3.667 to −1.912), P < .01, I2 = 100%], ↓ METS minutes per week [SMD −0.164 (95% CI −0.303 to −0.025), P = .02, I2 = 77%], ↓ physical activity duration [SMD = −0.068 (95% CI = −0.097 to −0.039), P < .01, I2 = 0%], ↑ sedentary time [SMD = 0.09 (95% CI 0.006–0.180), P = .04, I2 = 84%] |
| Wunsch et al., 2022 [84] | 57 studies (17 accelerometers/pedometers, 40 questionnaires) | 119 094 participants | ↓ physical activity [z = −0.18 (95% CI −0.30 to −0.06), P < .001] |
| Stockwell et al., 2021 [83] | 66 studies (5 studies accelerometer/pedometer, 61 questionnaires) | 86 981 participants (healthy adults and children, patients with medical conditions) | ↓ in physical activity and ↑ in sedentary behaviours during lockdown |
| Oliveira et al., 2022 [143] | 25 studies (4 studies accelerometer/pedometer, 21 questionnaires) | 15 964 elderly participants | ↓ in physical activity caused by ↑ in sitting time, ↓ in METs, ↓ in the number of steps, ↓ in exercise frequency and duration |
METs, metabolic equivalent tasks; SMD, standardized mean difference.
Overall, most of these studies were either retrospective or cross-sectional and were based on self-reported online questionnaires and did not objectively assess pre-lockdown physical activity; therefore, the results may have been affected by recall bias and mirror a ‘perceived’ instead of an attested physical activity reduction [91]. Furthermore, it would be interesting to examine by properly designed studies whether physical activity returns to usual levels during the periods when the restrictive measures are relaxed, since regaining the levels of physical activity after time intervals of deconditioning, weight gain, etc. could be a complex issue.
Dietary sodium intake
Salt intake has long been proposed as a pivotal factor of the pathogenesis of essential hypertension, based on numerous observational studies and RCTs revealing an inverse relationship between sodium intake and BP [92].
The consumption of high-sodium processed and preserved foods (such as snacks, sauces and frozen, canned and instant foods) increased during the pandemic [93]. A study investigating the diet changes of 938 French individuals found that salt intake increased from 2.9 g/day before to 3.2 g/day during lockdown (P < .001) [94], whereas in the Pandemic-against-LifeStyle (PaLS) study, more than one-third of the responders stated they added salt to meals [95]. As mentioned before, the consumption of snacks, which represent high-sodium products, was significantly increased [46, 96] and the percentage of participants experiencing such a dietary change exceeded 50% in some surveys [73, 97]. The American Frozen Food Institute confirmed this tendency by describing spikes in frozen food sales [98]. The hypertensive effects of these changes have not yet been investigated by properly designed studies.
Sleep quality and sleep disorders
Mounting evidence from experimental and observational studies suggests that poor sleep quality is associated with hypertension. A meta-analysis of 29 studies with 45 041 patients demonstrated that poor sleep quality increased significantly the risk for hypertension [odds ratio 1.48 (95% CI 1.13–1.95)], whereas poor sleepers had a higher average SBP [mean difference 4.37 mmHg (95% CI −0.69–9.42)] and DBP [mean difference 1.25 mmHg (95% CI −1.20–3.70)] than normal sleepers [99]. There is also a relationship between sleep disturbances, including insomnia and sleep disruption due to shift work, and hypertension [100].
National lockdowns forcing people to stay home and altering rapidly their daily routine prompted serious alterations in sleep quality. According to a meta-analysis including 493 475 individuals from 49 countries, sleep disorders, including poor sleep quality and insomnia, irrespective of any covariate, had an estimated prevalence of 40.49% (95% CI 37.56–43.48) [101]. Relevant studies revealed that sleep quality decreased substantially during the pandemic [102–104], sleeping medication use increased [103, 105] and poorer sleep quality was associated with higher levels of depression, stress and anxiety [75, 104]. In support of the above, insomnia became significantly more common [105–107], both in terms of new-onset or worsened symptoms [105] and with women being more affected [105–107]. A recent meta-analysis showed insomnia to have a pooled prevalence of 23.87% (95% CI 15.74–34.48) in populations affected by COVID-19, a percentage significantly higher than in the pre-pandemic period [108].
A special group that displayed severe sleep disorders during the COVID-19 pandemic are healthcare workers. The experience of intense psychological burden and harsh shift-work schedules render the sleeping status of healthcare workers a fragile issue; sleep disorders were given a pooled prevalence of 44% among them in the latest meta-analysis of 70 related studies [103]. In a cross-sectional study from the USA, the majority of healthcare workers reported a reduction in sleep duration and experienced both daytime sleepiness and insomnia [109]. Furthermore, in another study, shift work was linked with deteriorating sleep quality whereas non-shift work was linked with improved sleep quality [110]. Finally, Cénat et al. [108] showed that healthcare workers were more affected by insomnia than other professionals (z = 2.69, P < .05).
All this evidence may justify the creation of the novel terms ‘COVID-somnia’ or ‘coronasomnia’ that reflect the increased rates of insomnia in the context of the COVID-19 pandemic [111].
Adherence to therapy
Medication non-adherence is a well-described determinant of poor BP control that exposes the patients to an increased risk of cardiovascular morbidity [112, 113]. A recent meta-analysis suggested a pooled prevalence of 31.2% (95% CI 3.3–86.1) among patients with apparent treatment-resistant hypertension [114].
Most relevant studies suggest that during the COVID-19 lockdown, patients’ compliance with antihypertensive treatment was reduced. The underlying causes could be economical, psychological or related to social distancing and to problematic follow-up by physicians. A recent US study revealed that hypertensive patients reporting cost-related medication non-adherence were less likely to receive their prescribed treatment and efficiently control their BP during the lockdown period [115]. In addition, an Ethiopian study in 409 patients showed that the prevalence of poor adherence according to the Morisky Medication Adherence Scale was up to 72% [116]. Beyond that, social restrictions hindered the patients’ regular follow-up visits to their physicians and the renewal or alteration of any prescriptions, thus worsening further their medication compliance [117].
It must be noted however that not all studies reported decreased adherence to prescribed medication. Indirect evidence from a German study comparing the number of patients receiving cardiovascular medications from pharmacies between the first trimester in 2019 and 2020 showed an increased number of patients receiving all studied medications from pharmacies during the lockdown period (i.e. the first trimester of 2020), with the largest increase in the age group of 18–40 years, suggesting that an improved adherence pattern might be present in some populations [118]. Given that this segment of the population is frequently studying or working, the reduced time requirements for these activities, together with pandemic-derived health concerns, may have allowed them to focus on compliance. In addition, in the above-mentioned Ethiopian study, patients with lower income had better adherence to medication during the COVID-19 lockdown, probably because citizens under the poverty line were exempt from insurance premiums or tended to follow medical advice more correctly [116].
Delayed diagnosis and care on BP control
During lockdown, drastic confinement measures impeded or delayed diagnosis and regular care for hypertensive patients. Relevant data can be drawn from two recent electronic surveys originating from the Centres of Excellence of the ESH. The first study [117], providing data from 52 centres from 20 European and 3 non‑European countries, showed that during the pandemic the number of patients treated per week decreased by 90% compared with the pre-lockdown era, with 60% of patients reporting limited access to medical consultations. The vast majority of Centres of Excellence (85%) experienced a shutdown lasting a median of 9 weeks, whereas more than half of them could not offer 24-h ABPM to their patients [117]. The second study [119] included data from 54 Centres of Excellence from 18 European and 3 non-European countries during the consecutive years 2019–2020 and showed dramatic decreases in hypertension-related diagnostic and therapeutic procedures during the first pandemic year, with the largest reductions being manifested during the first lockdown. In this period, the median reduction in ABPM use was 50.7%, ultrasound of renal arteries 47.1%, computed tomography/magnetic resonance imaging of renal arteries 50%, percutaneous angioplasties of renal arteries 57.1% and laboratory tests for catecholamines 46.9% and for renin/aldosterone 41% [119]. Taken together, these results confirmed a pandemic-related compromise in routine care and diagnostic investigation of hypertensive patients, hindering BP diagnosis and control.
Socio-economic difficulties
Several socio-economic factors (e.g. social isolation, low socio-economic status, financial instability or economic recession) have been previously associated with hypertension, as they are considered to generate excessive chronic psychological stress and anxiety, both responsible for sympathetic overactivation [120]. In a recent meta-analysis, psychosocial stress was associated with an increased risk of hypertension [OR 2.40 (95% CI 1.65–3.49)] [121]. In the case of the COVID-19 pandemic, several studies suggest that public restrictive measures through increasing unemployment rates, financial strain and insecurity resulted in considerable increases of psychological distress [122–124]. These phenomena could have probably negatively impacted BP levels and hypertension control; however, the actual impacts of these effects on hypertension incidence and BP control remain to be further investigated by studies focusing on these particular questions.
Possible positive effects
Sleep duration
Accumulated evidence from experimental and observational studies suggests that sleep deprivation acts as a risk factor for hypertension through impeding nocturnal dipping and increasing morning BP [125]. Of note, these hypertensive effects of short sleep are more pronounced in female than male individuals [126] and in those <65 years of age [127].
Despite the aforementioned negative effects of the lockdown on sleep quality, accumulated evidence suggests that public restrictive measures have a beneficial impact on sleep duration. Since the beginning of lockdown, several studies have reported slight increases in total sleep duration [102, 128, 129], delayed sleep chronotypes produced by a later shift of sleep timing [103, 104, 128–130] and decreases in social jetlag (i.e. the difference in mid-sleep between work days and free days), indicating that sleep schedules became more consistent throughout the week [102, 129, 130]. These results revealed that in spite of its poorer quality, the overall time of sleeping was increased.
Environmental noise
The exposure to excess levels of environmental and transportation-associated noise, particularly aircraft noise, is increasingly recognized as a significant risk factor for hypertension, coronary artery disease, heart failure and stroke [131]. Traffic noise during nighttime disrupts sleep architecture and quality, while during daytime it activates the sympathetic nervous system and hypothalamic–pituitary–adrenal axis. In combination, these changes lead to autonomic imbalance, metabolic abnormalities, oxidative stress and inflammation, resulting in endothelial and vascular dysfunction [131, 132]. Arterial stiffness and hypertension are the ultimate epiphenomena. A recent meta-analysis of 11 cohort studies found that the pooled RR of hypertension for every 10-dB increment of noise was 1.13 (95% CI 0.99–1.28) [133].
Following the beginning of the pandemic, traffic noise was diminished due to wide suspension of public transport and flights, a fact that could exert beneficial effects on BP levels. Wojciechowska et al. [134] have published the only study so far shedding light on this subject. In a previous case–control analysis comparing participants exposed to aircraft noise with a day-evening-night level >60 dB and unexposed individuals, the authors showed that long-lasting aircraft noise was associated with a higher office DBP, nighttime DBP and pulse wave velocity (PWV) [135]. In the current study they showed that short-term noise reduction during lockdown was linked with a significant decrease in 24-h SBP (117.9 versus 121.2 mmHg; P = .034), 24-h DBP (72.0 versus 75.1 mmHg; P = .003) and PWV (8.8 versus 10.2 m/s; P = .001) in the exposed group. In addition, the difference in PWV reduction between the exposed and unexposed subjects was also significant in adjusted analysis accounting for covariates (−1.49 versus −0.35 m/s; P = .017) [134].
Air pollution
Key components of air pollution include nitrogen dioxide (NO2) and fine particulate matter (≤2.5 μm; PM2.5) [136]. Inhalation of PM2.5, derived from the combustion of fossil fuels, is an important risk factor for cardiovascular and kidney disease, including increased BP [137–139]. Economic downturns in the past have been associated with decreased mortality rates, and decreased air pollution may be one of the contributing factors [140]. The COVID-19 lockdowns were associated with a dramatic improvement in air quality in many countries that has been estimated to have prevented thousands of air pollution-related deaths [141]. This may have potentially resulted in improved control of BP. However, we did not find studies addressing this hypothesis.
CONCLUSIONS
The COVID-19 pandemic altered dramatically the daily routines of people all over the world through lockdown and quarantine measures. These changes had a severe impact on a plethora of parameters affecting BP levels and control. Despite the fact that some effects (i.e. increased sleep duration, decreased environmental noise and air pollution) may be associated with BP decreases and efficient control, most other changes in individual behaviours (increased alcohol consumption, increased smoking, reduced physical activity, insomnia, high sodium intake, medication non-adherence etc.) may have contributed to increased BP levels and impaired BP control (Fig. 2). However, current evidence regarding the actual consequences of these risk factor changes on BP levels and control lacks homogeneity and is not conclusive. Even if the issues of delayed diagnosis and treatment of cardiovascular and renal diseases, including hypertension, could be partially restored when the activity of healthcare systems returns to ‘normal’, the changes in everyday activities due to the COVID-19 pandemic and related and self-isolation policies may be long lasting, thus future longitudinal studies aimed at investigating the long-term pandemic-related implications on hypertension incidence and BP control are highly encouraged.
Supplementary Material
Contributor Information
Artemios G Karagiannidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Marieta P Theodorakopoulou, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Charles J Ferro, Department of Renal Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Maria Jose Soler, Nephrology Department, Vall d'Hebron University Hospital, Universitat Autònoma de Barcelona, Nephrology and Kidney Transplant Research Group, Vall d'Hebron Research Institute, Barcelona, Spain.
Jean-Michel Halimi, Service de Néphrologie-Hypertension, Dialyses, Transplantation Rénale, CHRU Tours, Tours, France; INSERM SPHERE U1246, Université Tours, Université de Nantes, Tours, France.
Andrzej Januszewicz, Department of Hypertension, National Institute of Cardiology, Warsaw, Poland.
Alexandre Persu, Pole of Cardiovascular Research, Institut de Recherche Expérimentale et Clinique and Division of Cardiology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium.
Reinhold Kreutz, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin; Berlin Institute of Health, Institut für Klinische Pharmakologie und Toxikologie, Berlin, Germany.
Pantelis Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
FUNDING
This article was not supported by any source and represents an original effort of the authors. A.O. and M.J.S. are supported by the Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) funded by the European Union – NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR).
DATA AVAILABILITY STATEMENT
This is a systematic review paper with no new data generated in support of it.
CONFLICT OF INTEREST STATEMENT
A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GlaxoSmithKline, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra AstraZeneca-UAM of chronic kidney disease and electrolytes. He is the previous Editor-in-Chief of CKJ. M.J.S. is the current Editor-in-Chief of CKJ. The other authors have no financial or other relationships to disclose that might lead to a conflict of interest regarding this article. The results presented in this work have not been previously published in whole or part, except in abstract format.
REFERENCES
- 1. Zhu N, Zhang D, Wang Wet al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (10 July 2022, date last accessed). [Google Scholar]
- 3. Iaccarino G, Grassi G, Borghi Cet al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension 2020;76:366–72. [DOI] [PubMed] [Google Scholar]
- 4. Thakur B, Dubey P, Benitez Jet al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep 2021;11:8562. 10.1038/s41598-021-88130-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oraby T, Tyshenko MG, Maldonado JCet al. Modeling the effect of lockdown timing as a COVID-19 control measure in countries with differing social contacts. Sci Rep 2021;11:3354. 10.1038/s41598-021-82873-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreutz R, Dobrowolski P, Prejbisz Aet al. Lifestyle, psychological, socioeconomic and environmental factors and their impact on hypertension during the coronavirus disease 2019 pandemic. J Hypertens 2021;39:1077–89. 10.1097/HJH.0000000000002770 [DOI] [PubMed] [Google Scholar]
- 7. Gori T, Lelieveld J, Münzel T.. Perspective: cardiovascular disease and the Covid-19 pandemic. Basic Res Cardiol 2020;115:32. 10.1007/s00395-020-0792-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freiberg A, Schubert M, Romero Starke Ket al. A rapid review on the influence of COVID-19 lockdown and quarantine measures on modifiable cardiovascular risk factors in the general population. Int J Environ Res Public Health 2021;18:8567. 10.3390/ijerph18168567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Januszewicz A, Wojciechowska W, Prejbisz Aet al. Impact of the COVID‑19 pandemic on blood pressure control and cardiovascular risk profile in patients with hypertension. Pol Arch Intern Med 2021;131:16129. https://www.ncbi.nlm.nih.gov/pubmed/34704702 [DOI] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du Ret al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson S, Hirsch JS, Narasimhan Met al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grasselli G, Zangrillo A, Zanella Aet al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kreutz R, Algharably EAE-H, Azizi Met al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res 2020;116:1688–99. 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savoia C, Volpe M, Kreutz R.. Hypertension, a moving target in COVID-19: current views and perspectives. Circ Res 2021;128:1062–79. 10.1161/CIRCRESAHA.121.318054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu PP, Blet A, Smyth Det al. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;142:68–78. 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 16. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan Y, Shang J, Graham Ret al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020;94:e00127–20. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancia G, Rea F, Ludergnani Met al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–40. 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauer A, Schreinlechner M, Sappler Net al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med 2021;9:863–72. 10.1016/S2213-2600(21)00214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soler MJ, Noordzij M, Abramowicz Det al. Renin-angiotensin system blockers and the risk of COVID-19-related mortality in patients with kidney failure. Clin J Am Soc Nephrol 2021;16:1061–72. 10.2215/CJN.18961220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theodorakopoulou MP, Alexandrou M-E, Boutou AKet al. Renin-angiotensin system blockers during the COVID-19 pandemic: an update for patients with hypertension and chronic kidney disease. Clin Kidney J 2022;15:397–406. 10.1093/ckj/sfab272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang S, Zhou X, Chen Yet al. Changes in home blood pressure monitored among elderly patients with hypertension during the COVID-19 outbreak: a longitudinal study in China leveraging a smartphone-based application. Circ Cardiovasc Qual Outcomes 2021;14:e007098. 10.1161/CIRCOUTCOMES.120.007098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang S, Zhong Y, Wang Let al. Anxiety, home blood pressure monitoring, and cardiovascular events among older hypertension patients during the COVID-19 pandemic. Hypertens Res 2022;45:856–65. 10.1038/s41440-022-00852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Çelik M, Yılmaz Y, Karagöz Aet al. Anxiety disorder associated with the COVID-19 pandemic causes deterioration of blood pressure control in primary hypertensive patients. Medeni Med J 2021;36:83–90. 10.5222/MMJ.2021.08364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi K, Chin K, Umezawa Set al. Influence of stress induced by the first announced state of emergency due to coronavirus disease 2019 on outpatient blood pressure management in Japan. Hypertens Res 2022;45:675–85. 10.1038/s41440-021-00832-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laffin LJ, Kaufman HW, Chen Zet al. Rise in blood pressure observed among US adults during the COVID-19 pandemic. Circulation 2022;145:235–7. 10.1161/CIRCULATIONAHA.121.057075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah NP, Clare RM, Chiswell Ket al. Trends of blood pressure control in the U.S. during the COVID-19 pandemic. Am Heart J 2022;247:15–23. 10.1016/j.ahj.2021.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fosco MJ, Silva P, Taborda GAet al. [Association between mandatory lockdown due to COVID-19 and severe arterial hypertension]. Medicina (B Aires) 2020;80(Suppl 6):25–9. [PubMed] [Google Scholar]
- 29. Pengo MF, Albini F, Guglielmi Get al. Home blood pressure during COVID-19-related lockdown in patients with hypertension. Eur J Prev Cardiol 2022;29:e94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Girerd N, Meune C, Duarte Ket al. Evidence of a blood pressure reduction during the COVID-19 pandemic and associated lockdown period: insights from e-Health data. Telemed J E Health 2022;28:266–70. 10.1089/tmj.2021.0006 [DOI] [PubMed] [Google Scholar]
- 31. Feitosa FGAM, Feitosa ADM, Paiva AMGet al. Impact of the COVID-19 pandemic on blood pressure control: a nationwide home blood pressure monitoring study. Hypertens Res 2022;45:364–8. 10.1038/s41440-021-00784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. European Society of Hypertension . ESH ABPM COVID-19 Study. https://www.eshonline.org/spotlights/esh-abpm-covid-19-study/ (3 August 2022, date last accessed). [Google Scholar]
- 33. Williams B, Mancia G, Spiering Wet al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953–2041. https://www.ncbi.nlm.nih.gov/pubmed/30234752 [DOI] [PubMed] [Google Scholar]
- 34. Boutou AK, Georgopoulou A, Pitsiou Get al. Changes in the respiratory function of COVID-19 survivors during follow-up: a novel respiratory disorder on the rise? Int J Clin Pract 2021;75:e14301. 10.1111/ijcp.14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tantisattamo E, Imhof C, Jager KJet al. Association of obesity with 3-month mortality in kidney failure patients with COVID-19. Clin Kidney J 2022;15:1348–60. 10.1093/ckj/sfac083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakaloudi DR, Barazzoni R, Bischoff SCet al. Impact of the first COVID-19 lockdown on body weight: a combined systematic review and a meta-analysis. Clin Nutr 2021; 10.1016/j.clnu.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sideli L, Lo Coco G, Bonfanti RCet al. Effects of COVID-19 lockdown on eating disorders and obesity: a systematic review and meta-analysis. Eur Eat Disord Rev 2021;29:826–41. 10.1002/erv.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khan MA, Menon P, Govender Ret al. Systematic review of the effects of pandemic confinements on body weight and their determinants. Br J Nutr 2022;127:298–317. 10.1017/S0007114521000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. La Fauci G, Montalti M, Di Valerio Zet al. Obesity and COVID-19 in children and adolescents: reciprocal detrimental influence—systematic literature review and meta-analysis. Int J Environ Res Public Health 2022;19:7603. 10.3390/ijerph19137603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang T-H, Chen Y-C, Chen W-Yet al. Weight gain associated with COVID-19 lockdown in children and adolescents: a systematic review and meta-analysis. Nutrients 2021;13:3668. 10.3390/nu13103668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daniels NF, Burrin C, Chan Tet al. A systematic review of the impact of the first year of COVID-19 on obesity risk factors: a pandemic fueling a pandemic? Curr Dev Nutr 2022;6:nzac011. 10.1093/cdn/nzac011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mason TB, Barrington-Trimis J, Leventhal AM.. Eating to cope with the COVID-19 pandemic and body weight change in young adults. J Adolesc Health 2021;68:277–83. 10.1016/j.jadohealth.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radwan H, Al Kitbi M, Hasan Het al. Indirect health effects of COVID-19: unhealthy lifestyle behaviors during the lockdown in the United Arab Emirates. Int J Environ Res Public Health 2021;18:1964. 10.3390/ijerph18041964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bérard E, Huo Yung Kai S, Coley Net al. Lockdown-related factors associated with the worsening of cardiovascular risk and anxiety or depression during the COVID-19 pandemic. Prev Med Rep 2021;21:101300. 10.1016/j.pmedr.2020.101300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clemente-Suárez VJ, Ramos-Campo DJ, Mielgo-Ayuso Jet al. Nutrition in the actual COVID-19 pandemic. A narrative review. Nutrients 2021;13:1924. 10.3390/nu13061924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bakaloudi DR, Jeyakumar DT, Jayawardena Ret al. The impact of COVID-19 lockdown on snacking habits, fast-food and alcohol consumption: a systematic review of the evidence. Clin Nutr 2021. 10.1016/j.clnu.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Błaszczyk-Bębenek E, Jagielski P, Bolesławska Iet al. Nutrition behaviors in polish adults before and during COVID-19 lockdown. Nutrients 2020;12:3084. 10.3390/nu12103084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cushman WC, Cutler JA, Hanna Eet al. Prevention and Treatment of Hypertension Study (PATHS): effects of an alcohol treatment program on blood pressure. Arch Intern Med 1998;158:1197–207. 10.1001/archinte.158.11.1197 [DOI] [PubMed] [Google Scholar]
- 49. Holmes MV, Dale CE, Zuccolo Let al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014;349:g4164. 10.1136/bmj.g4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murthy P, Narasimha VL.. Effects of the COVID-19 pandemic and lockdown on alcohol use disorders and complications. Curr Opin Psychiatry 2021;34:376–85. 10.1097/YCO.0000000000000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avery AR, Tsang S, Seto EYWet al. Stress, anxiety, and change in alcohol use during the COVID-19 pandemic: findings among adult twin pairs. Front Psychiatry 2020;11:571084. 10.3389/fpsyt.2020.571084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chodkiewicz J, Talarowska M, Miniszewska Jet al. Alcohol consumption reported during the COVID-19 pandemic: the initial stage. Int J Environ Res Public Health 2020;17:4677. 10.3390/ijerph17134677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ingram J, Maciejewski G, Hand CJ.. Changes in diet, sleep, and physical activity are associated with differences in negative mood during COVID-19 lockdown. Front Psychol 2020;11:588604. 10.3389/fpsyg.2020.588604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. López-Bueno R, Calatayud J, Casaña Jet al. COVID-19 confinement and health risk behaviors in Spain. Front Psychol 2020;11:1426. 10.3389/fpsyg.2020.01426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barbosa C, Cowell AJ, Dowd WN.. Alcohol consumption in response to the COVID-19 pandemic in the United States. J Addict Med 2021;15:341–4. 10.1097/ADM.0000000000000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pollard MS, Tucker JS, Green HD.. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open 2020;3:e2022942. 10.1001/jamanetworkopen.2020.22942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Niedzwiedz CL, Green MJ, Benzeval Met al. Mental health and health behaviours before and during the initial phase of the COVID-19 lockdown: longitudinal analyses of the UK Household Longitudinal Study. J Epidemiol Community Health 2021;75:224–31. https://www.ncbi.nlm.nih.gov/pubmed/32978210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol Hepatol 2020;5:625. 10.1016/S2468-1253(20)30159-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colbert S, Wilkinson C, Thornton Let al. COVID-19 and alcohol in Australia: industry changes and public health impacts. Drug Alcohol Rev 2020;39:435–40. 10.1111/dar.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bremner J. U.S. alcohol sales increase 55 percent in one week amid coronavirus pandemic. Newsweek 2020. https://www.newsweek.com/us-alcohol-sales-increase-55-percent-one-week-amid-coronavirus-pandemic-1495510 (18 September 2022, date last accessed). [Google Scholar]
- 61. Rodriguez LM, Litt DM, Stewart SH.. Drinking to cope with the pandemic: the unique associations of COVID-19-related perceived threat and psychological distress to drinking behaviors in American men and women. Addict Behav 2020;110:106532. 10.1016/j.addbeh.2020.106532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bartoszek A, Walkowiak D, Bartoszek Aet al. Mental well-being (depression, loneliness, insomnia, daily life fatigue) during COVID-19 related home-confinement—a study from Poland. Int J Environ Res Public Health 2020;17:7417. 10.3390/ijerph17207417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Virdis A, Giannarelli C, Neves MFet al. Cigarette smoking and hypertension. Curr Pharm Des 2010;16:2518–25. 10.2174/138161210792062920 [DOI] [PubMed] [Google Scholar]
- 64. Groppelli A, Giorgi DM, Omboni Set al. Persistent blood pressure increase induced by heavy smoking. J Hypertens 1992;10:495–9. 10.1097/00004872-199205000-00014 [DOI] [PubMed] [Google Scholar]
- 65. Sheppard JP, Fletcher B, Gill Pet al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens 2016;29:614–25. 10.1093/ajh/hpv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun Y, Li Y, Bao Yet al. Brief report: increased addictive internet and substance use behavior during the COVID-19 pandemic in China. Am J Addict 2020;29:268–70. 10.1111/ajad.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen L, Li J, Xia Tet al. Changes of exercise, screen time, fast food consumption, alcohol, and cigarette smoking during the COVID-19 pandemic among adults in the United States. Nutrients 2021;13:3359. 10.3390/nu13103359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Di Renzo L, Gualtieri P, Pivari Fet al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med 2020;18:229. 10.1186/s12967-020-02399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. López-Bueno R, Calatayud J, Casaña Jet al. COVID-19 confinement and health risk behaviors in Spain. Front Psychol 2020;11:1426. 10.3389/fpsyg.2020.01426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Klemperer EM, West JC, Peasley-Miklus Cet al. Change in tobacco and electronic cigarette use and motivation to quit in response to COVID-19. Nicotine Tob Res 2020;22:1662–3. 10.1093/ntr/ntaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Elling JM, Crutzen R, Talhout Ret al. Tobacco smoking and smoking cessation in times of COVID-19. Tob Prev Cessat 2020;6:39. 10.18332/tpc/122753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guignard R, Andler R, Quatremère Get al. Changes in smoking and alcohol consumption during COVID-19-related lockdown: a cross-sectional study in France. Eur J Public Health 2021;31:1076–83. 10.1093/eurpub/ckab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sidor A, Rzymski P.. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients 2020;12:1657. 10.3390/nu12061657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bar-Zeev Y, Shauly M, Lee Het al. Changes in smoking behaviour and home-smoking rules during the initial COVID-19 lockdown period in Israel. Int J Environ Res Public Health 2021;18:1931. 10.3390/ijerph18041931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stanton R, To QG, Khalesi Set al. Depression, anxiety and stress during COVID-19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health 2020;17:4065. 10.3390/ijerph17114065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vanderbruggen N, Matthys F, Van Laere Set al. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: results from a web-based survey. Eur Addict Res 2020;26:309–15. 10.1159/000510822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cardoso CG, Gomides RS, Queiroz ACCet al. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics (Sao Paulo) 2010;65:317–25. 10.1590/S1807-59322010000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saco-Ledo G, Valenzuela PL, Ruiz-Hurtado Get al. Exercise reduces ambulatory blood pressure in patients with hypertension: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2020;9:e018487. 10.1161/JAHA.120.018487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Whelton PK, Carey RM, Aronow WSet al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–115. [DOI] [PubMed] [Google Scholar]
- 80. Castañeda-Babarro A, Arbillaga-Etxarri A, Gutiérrez-Santamaría Bet al. Physical activity change during COVID-19 confinement. Int J Environ Res Public Health 2020;17:6878. 10.3390/ijerph17186878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lesser IA, Nienhuis CP.. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health 2020;17:3899. 10.3390/ijerph17113899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng C, Huang WY, Sheridan Set al. COVID-19 pandemic brings a sedentary lifestyle in young adults: a cross-sectional and longitudinal study. Int J Environ Res Public Health 2020;17:6035. 10.3390/ijerph17176035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stockwell S, Trott M, Tully Met al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med 2021;7:e000960. 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wunsch K, Kienberger K, Niessner C.. Changes in physical activity patterns due to the Covid-19 pandemic: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:2250. 10.3390/ijerph19042250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pérez-Gisbert L, Torres-Sánchez I, Ortiz-Rubio Aet al. Effects of the COVID-19 pandemic on physical activity in chronic diseases: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:12278. 10.3390/ijerph182312278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ng TK, Kwok CK, Ngan GYet al. Differential impacts of COVID-19 pandemic on physical activity involvements and exercise habits in people with and without chronic diseases: a systematic review and meta-analysis. Arch Phys Med Rehabil 2022;103:1448–65.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oliveira MR, Sudati IP, Konzen VDMet al. Covid-19 and the impact on the physical activity level of elderly people: a systematic review. Exp Gerontol 2022;159:111675. 10.1016/j.exger.2021.111675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Violant-Holz V, Gallego-Jiménez MG, González-González CSet al. Psychological health and physical activity levels during the COVID-19 pandemic: a systematic review. Int J Environ Res Public Health 2020;17:9419. 10.3390/ijerph17249419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Browne RAV, Macêdo GAD, Cabral LLPet al. Initial impact of the COVID-19 pandemic on physical activity and sedentary behavior in hypertensive older adults: an accelerometer-based analysis. Exp Gerontol 2020;142:111121. 10.1016/j.exger.2020.111121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abouzid M, El-Sherif DM, Eltewacy NKet al. Influence of COVID-19 on lifestyle behaviors in the Middle East and North Africa Region: a survey of 5896 individuals. J Transl Med 2021;19:129. https://www.ncbi.nlm.nih.gov/pubmed/33785043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cross TJ, Isautier JMJ, Stamatakis Eet al. Self-reported physical activity before a COVID-19 ‘lockdown’: is it just a matter of opinion? BMJ Open Sport Exerc Med 2021;7:e001088. 10.1136/bmjsem-2021-001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. He FJ, Tan M, Ma Yet al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:632–47. 10.1016/j.jacc.2019.11.055 [DOI] [PubMed] [Google Scholar]
- 93. Zhang X, Chen B, Jia Pet al. Locked on salt? Excessive consumption of high-sodium foods during COVID-19 presents an underappreciated public health risk: a review. Environ Chem Lett 2021;19:3583–95. 10.1007/s10311-021-01257-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marty L, de Lauzon-Guillain B, Labesse Met al. Food choice motives and the nutritional quality of diet during the COVID-19 lockdown in France. Appetite 2021;157:105005. 10.1016/j.appet.2020.105005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jodczyk AM, Gruba G, Sikora Zet al. PaLS study: how has the COVID-19 pandemic influenced physical activity and nutrition? Observations a year after the outbreak of the pandemic. Int J Environ Res Public Health 2021;18:9632. 10.3390/ijerph18189632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bennett G, Young E, Butler Iet al. The impact of lockdown during the COVID-19 outbreak on dietary habits in various population groups: a scoping review. Front Nutr 2021;8:626432. 10.3389/fnut.2021.626432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Robinson E, Boyland E, Chisholm Aet al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite 2021;156:104853. 10.1016/j.appet.2020.104853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. American Frozen Food Institute. Frozen Food Sales Amid COVID-19: U.S. Consumer Engagement – April 2020. https://affi.org/wp-content/uploads/2020/documents/Frozen%20Food%20Sales%20Amid%20COVID-19%20Consumer%20Research%20-%20FINAL.pdf (4 November 2022, date last accessed). [Google Scholar]
- 99. Lo K, Woo B, Wong Met al. Subjective sleep quality, blood pressure, and hypertension: a meta-analysis. J Clin Hypertens 2018;20:592–605. 10.1111/jch.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Van Ryswyk EM, Mukherjee S, Chai-Coetzer CLet al. Sleep disorders, including sleep apnea, and hypertension. Am J Hypertens 2018;31:857–64. 10.1093/ajh/hpy082 [DOI] [PubMed] [Google Scholar]
- 101. Jahrami HA, Alhaj OA, Humood AMet al. Sleep disturbances during the COVID-19 pandemic: a systematic review, meta-analysis, and meta-regression. Sleep Med Rev 2022;62:101591. 10.1016/j.smrv.2022.101591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Blume C, Schmidt MH, Cajochen C.. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol 2020;30:R795–7. 10.1016/j.cub.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Robillard R, Dion K, Pennestri Met al. Profiles of sleep changes during the COVID-19 pandemic: demographic, behavioural and psychological factors. J Sleep Res 2021;30:e13231. 10.1111/jsr.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cellini N, Canale N, Mioni Get al. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res 2020;29:e13074. 10.1111/jsr.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li Y, Qin Q, Sun Qet al. Insomnia and psychological reactions during the COVID-19 outbreak in China. J Clin Sleep Med 2020;16:1417–8. 10.5664/jcsm.8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lin L, Wang J, Ou-yang Xet al. The immediate impact of the 2019 novel coronavirus (COVID-19) outbreak on subjective sleep status. Sleep Med 2021;77:348–54. 10.1016/j.sleep.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Morin CM, Bjorvatn B, Chung Fet al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med 2021;87:38–45. 10.1016/j.sleep.2021.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cénat JM, Blais-Rochette C, Kokou-Kpolou CKet al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res 2021;295:113599. 10.1016/j.psychres.2020.113599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hassinger AB, Breuer RK, Mishra A.. Sleep patterns of US healthcare workers during the first wave of the COVID-19 pandemic. Sleep Breath 2022;26:1351–61. 10.1007/s11325-021-02515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zare F, Sadeghian F, Alatab Set al. COVID-19 epidemic effects on sleep quality among health sector workers: a follow up study. Chronobiol Int 2022;39:1015–26. [DOI] [PubMed] [Google Scholar]
- 111. Cheshmehzangi A, Chen H, Su Zet al. How does the COVID-19 fuel insomnia? Brain Behav Immun Health 2022;21:100426. 10.1016/j.bbih.2022.100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mazzaglia G, Ambrosioni E, Alacqua Met al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009;120:1598–605. 10.1161/CIRCULATIONAHA.108.830299 [DOI] [PubMed] [Google Scholar]
- 113. Chobanian AV. Impact of nonadherence to antihypertensive therapy. Circulation 2009;120:1558–60. 10.1161/CIRCULATIONAHA.109.906164 [DOI] [PubMed] [Google Scholar]
- 114. Durand H, Hayes P, Morrissey ECet al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens 2017;35:2346–57. 10.1097/HJH.0000000000001502 [DOI] [PubMed] [Google Scholar]
- 115. Fang J, Chang T, Wang Get al. Association between cost-related medication nonadherence and hypertension management among US adults. Am J Hypertens 2020;33:879–86. 10.1093/ajh/hpaa072 [DOI] [PubMed] [Google Scholar]
- 116. Shimels T, Asrat Kassu R, Bogale Get al. Magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Ethiopia during the COVID-19 pandemic. PLoS One 2021;16:e0249222. 10.1371/journal.pone.0249222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. The corona-virus disease 2019 pandemic compromised routine care for hypertension: a survey conducted among excellence centers of the European Society of Hypertension. J Hypertens 2021;39:190–5. 10.1097/HJH.0000000000002703 [DOI] [PubMed] [Google Scholar]
- 118. Kostev K, Kumar S, Konrad Met al. Prescription rates of cardiovascular and diabetes therapies prior to and during the COVID-19 lockdown in Germany. Int J Clin Pharmacol Ther 2020;58:475–81. 10.5414/CP203849 [DOI] [PubMed] [Google Scholar]
- 119. Weber T, Amar J, de Backer Tet al. Covid-19 associated reduction in hypertension-related diagnostic and therapeutic procedures in Excellence Centers of the European Society of Hypertension. Blood Press 2022;31:71–9. 10.1080/08037051.2022.2060182 [DOI] [PubMed] [Google Scholar]
- 120. Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep 2010;12:10–6. 10.1007/s11906-009-0084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu M-Y, Li N, Li WAet al. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol Res 2017;39:573–80. 10.1080/01616412.2017.1317904 [DOI] [PubMed] [Google Scholar]
- 122. Saha K, Torous J, Caine EDet al. Psychosocial effects of the COVID-19 pandemic: large-scale quasi-experimental study on social media. J Med Internet Res 2020;22:e22600. 10.2196/22600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zainudeen ZT, Abd Hamid IJ, Azizuddin MNAet al. Psychosocial impact of COVID-19 pandemic on Malaysian families: a cross-sectional study. BMJ Open 2021;11:e050523. 10.1136/bmjopen-2021-050523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kujawa A, Green H, Compas BEet al. Exposure to COVID-19 pandemic stress: associations with depression and anxiety in emerging adults in the United States. Depress Anxiety 2020;37:1280–8. 10.1002/da.23109 [DOI] [PubMed] [Google Scholar]
- 125. Makarem N, Shechter A, Carnethon MRet al. Sleep duration and blood pressure: recent advances and future directions. Curr Hypertens Rep 2019;21:33. 10.1007/s11906-019-0938-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang Y, Mei H, Jiang Y-Ret al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med 2015;11:1047–56. 10.5664/jcsm.5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang Q, Xi B, Liu Met al. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012;35:1012–8. 10.1038/hr.2012.91 [DOI] [PubMed] [Google Scholar]
- 128. Gao C, Scullin MK.. Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Med 2020;73:1–10. 10.1016/j.sleep.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Leone MJ, Sigman M, Golombek DA.. Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr Biol 2020;30:R930–1. 10.1016/j.cub.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wright KP, Linton SK, Withrow Det al. Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr Biol 2020;30:R797–8. 10.1016/j.cub.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Münzel T, Schmidt FP, Steven Set al. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018;71:688–97. 10.1016/j.jacc.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 132. Hahad O, Daiber A, Münzel T.. Reduced aircraft noise pollution during COVID-19 lockdown is beneficial to public cardiovascular health: a perspective on the reduction of transportation-associated pollution. Hypertension 2022;79:335–7. 10.1161/HYPERTENSIONAHA.121.18607 [DOI] [PubMed] [Google Scholar]
- 133. Chen F, Fu W, Shi Oet al. Impact of exposure to noise on the risk of hypertension: a systematic review and meta-analysis of cohort studies. Environ Res 2021;195:110813. 10.1016/j.envres.2021.110813 [DOI] [PubMed] [Google Scholar]
- 134. Wojciechowska W, Januszewicz A, Drożdż Tet al. Blood pressure and arterial stiffness in association with aircraft noise exposure: long-term observation and potential effect of COVID-19 lockdown. Hypertension 2022;79:325–34. 10.1161/HYPERTENSIONAHA.121.17704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rojek M, Rajzer MW, Wojciechowska Wet al. Relationship among long-term aircraft noise exposure, blood pressure profile, and arterial stiffness. J Hypertens 2019;37:1350–8. 10.1097/HJH.0000000000002060 [DOI] [PubMed] [Google Scholar]
- 136. Yang B-Y, Qian Z, Howard SWet al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut 2018;235:576–88. [DOI] [PubMed] [Google Scholar]
- 137. Bhatnagar A. Cardiovascular effects of particulate air pollution. Annu Rev Med 2022;73:393–406. 10.1146/annurev-med-042220-011549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Afsar B, Elsurer Afsar R, Kanbay Aet al. Air pollution and kidney disease: review of current evidence. Clin Kidney J 2019;12:19–32. 10.1093/ckj/sfy111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. He P, Chen R, Zhou Let al. Higher ambient nitrogen dioxide is associated with an elevated risk of hospital-acquired acute kidney injury. Clin Kidney J 2022;15:95–100. 10.1093/ckj/sfab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Regidor E, Vallejo F, Granados JATet al. Mortality decrease according to socioeconomic groups during the economic crisis in Spain: a cohort study of 36 million people. Lancet 2016;388:2642–52. 10.1016/S0140-6736(16)30446-9 [DOI] [PubMed] [Google Scholar]
- 141. Chen K, Wang M, Huang Cet al. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. Lancet Planet Health 2020;4:e210–2. 10.1016/S2542-5196(20)30107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pérez-Gisbert L, Torres-Sánchez I, Ortiz-Rubio Aet al. Effects of the COVID-19 pandemic on physical activity in chronic diseases: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:12278. 10.3390/ijerph182312278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Oliveira MR, Sudati IP, Konzen VDMet al. Covid-19 and the impact on the physical activity level of elderly people: a systematic review. Exp Gerontol 2022;159:111675. 10.1016/j.exger.2021.111675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a systematic review paper with no new data generated in support of it.