Abstract
There are limited data for the clinical efficacy of bebtelovimab in preventing severe coronavirus disease 2019. Among outpatients unable to take nirmatrelvir-ritonavir at a large health system, 10 of 377 (2.7%) patients who received bebtelovimab and 17 of 377 (4.5%) matched untreated patients were hospitalized or died. The 43% observed risk reduction with bebtelovimab was not statistically significant (P = 0.14).
Keywords: bebtelovimab; COVID-19, SARS-CoV-2, monoclonal antibody, pandemic
Prompt treatment of coronavirus disease 2019 (COVID-19) with monoclonal antibodies binding to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protein has been found to reduce hospitalization in clinical trials of unvaccinated patients and observational studies of vaccinated patients [1]. However, ongoing SARS-CoV-2 evolution with substantial amino acid substitutions and deletions in the spike protein have reduced neutralizing activity [1] and clinical effectiveness [2] of monoclonal antibodies authorized by the Food and Drug Administration (FDA). In February 2022, the FDA authorized bebtelovimab [3], a novel monoclonal antibody with retained neutralization activity against Omicron and Omicron subvariants, based on nonclinical data and a trial observing faster nasopharyngeal viral clearance but unable to assess efficacy in preventing severe disease [4]. In April 2022, with the predominance of the Omicron variant BA.2, bebtelovimab became the only SARS-CoV-2 monoclonal antibody authorized for treatment of COVID-19 in the United States.
In response to an Omicron BA.2 wave, Mass General Brigham, a large health care system in Massachusetts and New Hampshire, began providing intravenous antiviral remdesivir or bebtelovimab to high-risk COVID-19 outpatients with contraindications to oral antiviral treatment with nirmatrelvir plus ritonavir. Remdesivir was preferred in accordance with national and local guidance; however, providing the required 3 daily infusions became impracticable in the context of an intense wave among vulnerable patients and frequent patient refusal due to cost and logistical concerns. Consequently, we sought to understand the clinical effectiveness of bebtelovimab in preventing hospitalization and death to inform the approach to the ongoing BA.5 wave.
METHODS
Patients
We utilized electronic health records (EHRs) from the 7 Mass General Brigham hospitals and associated ambulatory care centers for adult patients with incident COVID-19 diagnosis between March 16 and May 31, 2022. We excluded patients at lower risk of severe disease (a score of ≤3 on the Monoclonal Antibody Screening Score [MASS], a comorbidity index predictive of COVID-19 hospitalization) [5], patients diagnosed in the context of hospital admission, patients who received an alternate recommended outpatient therapy for COVID-19 (nirmatrelvir plus ritonavir, remdesivir, or molnupiravir), patients who received bebtelovimab outside of Mass General Brigham, and patients who were not residents of Massachusetts or New Hampshire. All potentially eligible records were individually reviewed by 2 investigators before inclusion.
Analysis
We performed a retrospective matched analysis of high-risk patients who did and did not receive bebtelovimab for outpatient treatment of early COVID-19 to estimate the average treatment effect. Initially we attempted exact matching on age, vaccination status, recent vaccination, and transplant status. However, the resulting cohort was imbalanced by race and ethnicity, and treated patients could not be fully matched. We subsequently utilized exact matching on history of solid organ or stem cell transplant followed by 1:1 nearest neighbor propensity score matching without replacement, which successfully matched all bebtelovimab-treated patients and yielded sufficient balance (Supplementary Table 1). A logistic model included age (18–49, 50–64, 65–79, or ≥80), MASS score (4 and 5 or 6 or greater), vaccination status (unvaccinated, partially vaccinated, vaccinated, or vaccinated and boosted), timing of most recent vaccination (within the last 20 weeks or >20 weeks), self-reported race and ethnicity (White non-Hispanic/Latinx or all other races and ethnicity), known contraindication for nirmatrelvir plus ritonavir, and history of solid organ or stem cell transplant.
The primary end point was composite of all-cause hospital admission within 14 days and/or death within 28 days of their first positive SARS-CoV-2 test (including home antigen tests). We used a modified Poisson model using robust error variance [6] and general estimating equations [7, 8] to estimate relative risk reduction with bebtelovimab compared with no treatment. Two-sided tests using a significance threshold of P < .05 were used. We estimated >80% power to detect an 85% reduction in risk, similar to that observed in the trial of sotrovimab [9].
RESULTS
Study Population and Treatment
Between March 16 and May 31, 2022, 5451 outpatients with COVID-19 met study criteria as potentially eligible for bebtelovimab (Supplementary Figure 1). A total of 377 outpatients were treated at Mass General Brigham and were matched 1:1 with 377 patients who were not treated. Treated patients received bebtelovimab a median (interquartile range [IQR]) of 3 (2–3) days following diagnosis. Bebtelovimab was well tolerated, and there were no reported adverse events associated with administration of bebtelovimab in this cohort. The characteristics of bebtelovimab recipients and matched nonrecipients were similar in comorbidity score, vaccination receipt, age, race and ethnicity, most individual comorbidities, and date of diagnosis (Table 1). However, patients with heart disease or stroke (P = .007) and those with rheumatologic or inflammatory bowel disease (P = .06) were relatively under-represented among nonrecipients. During the study period, the Omicron subvariants BA.2 and BA.2.12.1 accounted for 90% and B.1.1.529 and BA.1 accounted for 9% of sequenced viruses submitted to GISAID from Massachusetts [10].
Table 1.
Baseline Characteristics of Included COVID-19 Cases (March 16–May 31, 2022)
| Characteristic | Bebtelovimab | No Bebtelovimab | P |
|---|---|---|---|
| No. | 377 | 377 | |
| Age group, No. (%) | .937 | ||
| 18–49 y | 23 (6.1) | 23 (6.1) | |
| 50–64 y | 79 (21.0) | 72 (19.1) | |
| 65–79 y | 185 (49.1) | 189 (50.1) | |
| ≥80 y | 90 (23.9) | 93 (24.7) | |
| Male sex, No. (%) | 180 (47.7) | 170 (45.1) | .511 |
| Race and ethnicity, No. (%) | .404 | ||
| Asian | 6 (1.6) | 6 (1.6) | |
| Black | 9 (2.4) | 13 (3.4) | |
| Hispanic or Latinx | 14 (3.7) | 10 (2.7) | |
| Other or unavailable | 10 (2.7) | 4 (1.1) | |
| White | 338 (89.7) | 344 (91.2) | |
| High SES vulnerability of zip code, No. (%) | 34 (9.0) | 30 (8.0) | .695 |
| Vaccination status, No. (%) | .971 | ||
| Vaccinated and boosted | 310 (82.2) | 306 (81.2) | |
| Vaccinated | 47 (12.5) | 49 (13.0) | |
| Partially vaccinated | 3 (0.8) | 4 (1.1) | |
| Unvaccinated | 17 (4.5) | 18 (4.8) | |
| Last vaccine dose >20 weeks prior, No. (%) | 287 (76.1) | 300 (79.6) | .293 |
| Comorbidity score, MASS, median (IQR) | 8 [6–11] | 8 [6–11] | .802 |
| Age, median (IQR) | 71 [64–79] | 71 [64–79] | .694 |
| Solid organ transplant, No. (%) | 68 (18.0) | 66 (17.5) | .924 |
| Stem cell transplant, No. (%) | 8 (2.1) | 11 (2.9) | .642 |
| BMI, No. (%) | .496 | ||
| <25 kg/m2 or unavailable | 100 (26.5) | 111 (29.4) | |
| 25–30 kg/m2 | 129 (34.2) | 112 (29.7) | |
| 30–35 kg/m2 | 80 (21.2) | 77 (20.4) | |
| >35 kg/m2 | 68 (18.0) | 77 (20.4) | |
| Immunocompromise, No. (%) | 298 (79.0) | 304 (80.6) | .650 |
| Diabetes, No. (%) | 150 (39.8) | 156 (41.4) | .711 |
| Heart disease or stroke, No. (%) | 207 (54.9) | 169 (44.8) | .007 |
| Pulmonary disease, No. (%) | 89 (23.6) | 97 (25.7) | .554 |
| Bipolar, schizophrenia, and other disorders, No. (%) | 23 (6.1) | 17 (4.5) | .417 |
| Depression and anxiety, No. (%) | 120 (31.8) | 100 (26.5) | .128 |
| Hematologic malignancy, No. (%) | 38 (10.1) | 34 (9.0) | .710 |
| Solid tumor malignancy, No. (%) | 214 (56.8) | 226 (59.9) | .416 |
| Rheumatologic or inflammatory bowel disease, No. (%) | 70 (18.6) | 50 (13.3) | .059 |
Immunocompromise includes patients with history of malignancy and patients on immunosuppressive medications.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; MASS, Monoclonal Antibody Screening Score; SES, socioeconomic status.
Hospitalization and Deaths
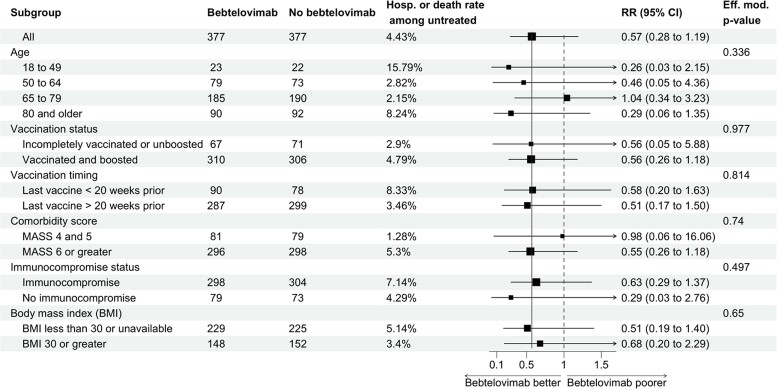
Among the 754 patients included in the analysis, 24 patients (10 bebtelovimab and 14 untreated) were admitted within 14 days of COVID-19 diagnosis. Admissions occurred a median (IQR) of 8.5 (3–12) days following COVID-19 diagnosis among bebtelovimab-treated patients and 2 (1–4.5) days among untreated patients. Three patients died within 28 days (all in the bebtelovimab-untreated group). The primary end point of hospitalization or death occurred in 10 (2.7%) bebtelovimab patients and 17 (4.5%) bebtelovimab-untreated patients. In the primary analytic model, bebtelovimab was associated with a trend toward decreased risk of hospitalization or death (risk ratio, 0.57; 95% CI, 0.28–1.19), but this finding was not statistically significant (P = 0.14). The observed magnitude of reduction in risk of hospitalization and deaths was similar across groups of patients (Figure 1).
Figure 1.
Subgroup analysis of the risk ratio of hospitalization and/or death comparing patients prescribed and not prescribed bebtelovimab. Estimate and confidence interval calculated from a Poisson model using robust error variance [6] performed within each stratum. Effect modification P values were calculated from nested models.
DISCUSSION
In this analysis of observational data from high-risk patients with COVID-19, we identified an estimated 43% reduction in risk of hospitalization or death associated with receipt of the monoclonal antibody bebtelovimab compared with matched patients who did not receive outpatient treatment for COVID-19. Importantly, this observation did not meet the prespecified threshold for statistical significance and could have been observed by chance in the absence of a true association. The estimated magnitude of protection is similar to the 45% reduction estimated for nirmatrelvir plus ritonavir in another study conducted at Mass General Brigham [11].
In vitro assays indicate that bebtelovimab effectively neutralizes the currently prevalent Omicron subvariants including BA.4 and BA.5 [1], but the observed risk reduction was lower than observed in trials and observational studies of other monoclonal antibody therapies. Several reasons may account for the observed decreased risk reduction. First, risk of severe COVID-19 is lower in the context of prevalent vaccination and prior infection even among the high-risk population included in this analysis. Hospitalization or death occurred in 4.5% of untreated patients, whereas in a largely unvaccinated cohort of high-risk COVID-19 patients from the same hospital system in 2020–2021, 12.2% untreated patients were hospitalized or died [12]. The incremental clinical benefit of treatment among lower-risk individuals may be smaller, which is similar to the lower-risk reduction of oral nirmatrelvir plus ritonavir observed in contemporary contexts [11, 13]. One uncontrolled study found similar risk of severe COVID-19 between patients treated with bebtelovimab and those treated with nirmatrelvir plus ritonavir [14]. Second, patients received bebtelovimab a median of 3 days after diagnosis, while trial participants received treatment more promptly [9, 15, 16]. Third, patients with improving COVID-19 symptoms were observed to decline bebtelovimab or cancel infusions, potentially introducing bias. Finally, bebtelovimab could have lower clinical effectiveness than formerly authorized monoclonal antibodies due to treatment-emergent resistant variants (5.5% observed in the BLAZE-4 trial [3]) or other mechanisms. An observational study among 92 solid organ transplant recipients did not detect reduced clinical effectiveness compared with 269 patients who had received sotrovimab [17], but the study was not designed to establish equivalence and was conducted during a period when the efficacy of sotrovimab could have been compromised by resistant variants.
The findings of this analysis should be considered in the context of the study limitations. While matching resulted in cohorts with balanced predictors of progression to severe COVID-19, the factors guiding clinician decision to recommend monoclonal antibodies and the patient's willingness and ability to accept treatment are incompletely captured in the available data and may contribute to residual bias. Additionally, receipt of bebtelovimab or hospitalizations outside of Mass General Brigham and not captured in the EHR would contribute to misassignment of exposure and outcome. The sample size was selected with the hypothesis of an 85% reduction in the primary end point, composite of all-cause hospitalization within 14 days and/or death within 28 days. This magnitude of risk reduction was not observed.
In the context of rapid emergence of novel SARS-CoV-2 variants, trials evaluating the efficacy of monoclonal antibodies in preventing severe disease are infeasible. Use of observational data to emulate these trials is expected to remain important to direct clinical care, but future analyses should plan for lower incidence of severe disease, and potentially lower risk reduction, when planning sample size.
In conclusion, among high-risk patients unable to receive the recommended oral option for COVID-19, bebtelovimab was safe and appeared to offer a similar level of protection as nirmatrelvir plus ritonavir against hospitalization and death.
Supplementary Material
Acknowledgments
Financial support. This work was made possible with help from the Harvard University Center for AIDS Research (CFAR), a funded program of the National Institutes of Health (P30 AI060354) and the National Cancer Institute (R01 CA236546).
Disclaimer. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the institutions with which the authors are affiliated. The funding source had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author contributions. S.D.P. and A.E.W. designed the study. S.D.P., A.K., M.J., and A.E.W. collected and adjudicated the data. S.D.P., J.A.J., A.Y.K., L.R.B., and A.E.W. provided scientific interpretation of the data. S.D.P. and A.E.W. performed the statistical analysis. S.D.P., A.K., M.J., and A.E.W. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.D.P. and A.E.W. drafted the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Patient consent. The authors attest that they are in compliance with the ethical standards of the Helsinki Declaration and human studies committees of the authors’ institutions. The study was approved by the Mass General Brigham Human Research Committee institutional review board, and informed consent was waived.
Contributor Information
Scott Dryden-Peterson, Brigham and Women's Hospital, Boston, Massachusetts, USA; Dana Farber Cancer Institute, Boston, Massachusetts, USA; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard AIDS Institute, Gaborone, Botswana; Harvard Medical School, Boston, Massachusetts, USA.
Andy Kim, Brigham and Women's Hospital, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Mary-Ruth Joyce, Brigham and Women's Hospital, Boston, Massachusetts, USA.
Jennifer A Johnson, Brigham and Women's Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Arthur Y Kim, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Lindsey R Baden, Brigham and Women's Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Ann E Woolley, Brigham and Women's Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. New Engl J Med 2022; 387:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Ginde AA. Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during the omicron BA.1 and BA.1.1-predominant phase [published online ahead of print October 10, 2022]. Int J Infect Dis. doi: 10.1016/j.ijid.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration . Fact sheet for healthcare providers: Emergency Use Authorization for bebtelovimab. Published September 16, 2022. Available at:https://www.fda.gov/media/156152/download. Accessed September 30, 2022.
- 4. Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. MedRxiv 2022.03.10.22272100 [Preprint]. March 12, 2022. Available at: 10.1101/2022.03.10.22272100. Accessed August 19, 2022. [DOI] [Google Scholar]
- 5. Ganesh R, Philpot LM, Bierle DM, et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis 2021; 224:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 7. Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15:1–11. [Google Scholar]
- 8. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22. [Google Scholar]
- 9. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. New Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 10. Khare S, Gurry C, Freitas L, et al. GISAID's role in pandemic response. China CDC Wkly 2021; 3:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv 2022.06.14.22276393 [Preprint]. 2022. Available at: 10.1101/2022.06.14.22276393. Accessed August 19, 2022. [DOI] [Google Scholar]
- 12. Rubin EB, Liu M, Giobbie-Hurder A, et al. Performance of a triage protocol for monoclonal antibodies in a mixed vaccinated and unvaccinated cohort of COVID-19 patients treated with intravenous infusion or subcutaneous injection. Open Forum Infect Dis 2022; 9:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19 [published online ahead of print August 20, 2022]. Clin Infect Dis. doi: 10.1093/cid/ciac673. [DOI] [Google Scholar]
- 14. Razonable RR, O’Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus disease-2019 during severe acute respiratory syndrome coronavirus 2 BA.2 Omicron epoch. J Infect Dis 2022; 226:1683–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. New Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. New Engl J Med 2020; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yetmar ZA, Beam E, O’Horo JC, et al. Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate COVID-19 during the Omicron epoch. Transpl Infect Dis 2022; 24:e13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.