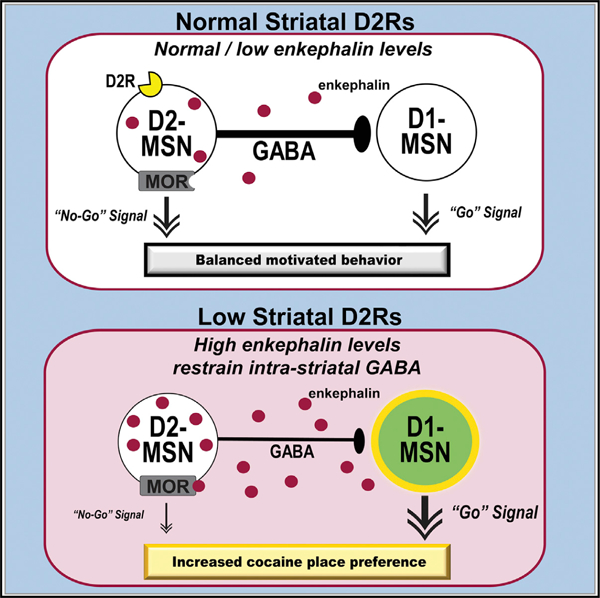
SUMMARY
Low dopamine D2 receptor (D2R) availability in the striatum can predispose for cocaine abuse; though how low striatal D2Rs facilitate cocaine reward is unclear. Overexpression of D2Rs in striatal neurons or activation of D2Rs by acute cocaine suppresses striatal Penk mRNA. Conversely, low D2Rs in D2-striatal neurons increases striatal Penk mRNA and enkephalin peptide tone, an endogenous mu-opioid agonist. In brain slices, met-enkephalin and inhibition of enkephalin catabolism suppresses intra-striatal GABA transmission. Pairing cocaine with intra-accumbens met-enkephalin during place conditioning facilitates acquisition of preference, while mu-opioid receptor antagonist blocks preference in wild-type mice. We propose that heightened striatal enkephalin potentiates cocaine reward by suppressing intra-striatal GABA to enhance striatal output. Surprisingly, a mu-opioid receptor antagonist does not block cocaine preference in mice with low striatal D2Rs, implicating other opioid receptors. The bidirectional regulation of enkephalin by D2R activity and cocaine offers insights into mechanisms underlying the vulnerability for cocaine abuse.
Graphical abstract
In brief
Low striatal D2 receptor levels are associated with cocaine abuse. Dai et al. bidirectionally alter striatal D2 receptor levels to probe the downstream mechanisms underlying this abuse liability. They provide evidence that enhanced enkephalin tone resulting from low D2 receptors is associated with suppressed intra-striatal GABA and potentiated cocaine reward.
INTRODUCTION
Cocaine addiction is a prevalent substance use disorder (SUD) that not only incurs substantial costs for the user’s physical health, but also for interpersonal relationships and society at large. With no clinically approved efficacious treatments available, identifying the neurobiological underpinnings that predispose individuals to develop a cocaine SUD is a critical step toward advancing preventive medicine. One well-reported vulnerability factor for cocaine addiction is low levels of the dopamine D2 receptor (D2R) within the striatum, a key region in the brain’s mesolimbic reward pathway (Dalley et al., 2007; Michaelides et al., 2012; Volkow et al., 2006). We previously generated a mouse model of low striatal D2Rs and found that selective deletion of the Drd2 gene from indirect pathway striatal medium spiny neurons (MSN-Drd2KO) enhances cocaine place preference and facilitates cocaine locomotor sensitization (Dobbs et al., 2016, 2019). While this cell-type specific knockout animal has become a useful model for studying addiction vulnerability, the exact downstream mechanisms by which low striatal D2Rs predispose individuals to cocaine abuse still remain relatively unknown. One potential mechanism is through D2R-mediated regulation of enkephalins, which are endogenous opioid peptides implicated in cocaine reward and locomotor sensitization.
Enkephalins are mainly derived from the preproenkephalin gene (Penk), which generates both met- and leu-enkephalin, and to a lesser extent from the preprodynorphin gene (Pdyn), which produces leu-enkephalin after cleavage of dynorphin (Akil et al., 1984; Day et al., 1998; Gianoulakis, 2005; Weisinger, 1995). Enkephalins have high affinity for the μ- and δ-opioid receptors (MOR and DOR, respectively), and are expressed throughout the brain, with the striatum being one of the highest expressing regions. In the striatum, D2R-containing MSNs express Penk and are the primary source of enkephalin tone in this region (Gerfen and Surmeier, 2011; Lemos et al., 2016). Multiple reports indicate that the activity level of D2Rs regulates the expression levels of enkephalin in the striatum. Dopamine depletion using 6-OHDA leads to a marked enhancement in striatal Penk mRNA expression, which can be reversed by chronic administration of the D2-like receptor agonist, quinpirole (Gerfen et al., 1990). This suggests that low activity of D2Rs upregulates Penk mRNA in the striatum, while activation of D2Rs downregulates it. Other experiments using global, constitutive Drd2 knockout mice or the D2-like receptor antagonist, haloperidol, provide further independent evidence that loss of D2R signaling causes upregulation of striatal enkephalin expression (Baik et al., 1995; Romano et al., 1987). Since these previous studies manipulated D2R activity in a global fashion, it remains unknown which type of D2R-expressing cells are responsible for regulating striatal enkephalin expression. This is an important consideration since D2Rs are expressed not only in MSNs, but also in cholinergic interneurons and on axonal projections from midbrain dopamine neurons and cortical and hippocampal glutamatergic neurons (Bamford et al., 2004; Bello et al., 2011; Higley and Sabatini, 2010; Maurice et al., 2004; Sesack et al., 1994).
Cocaine, administered repeatedly at moderate doses or at a ‘‘binge-like’’ high dose, has also been reported to increase Penk mRNA levels in the striatum (Crespo et al., 2001; Mantsch et al., 2004; Mathieu-Kia and Besson, 1998; Mongi-Bragato et al., 2016), but see two reports showing the opposite (Branch et al., 1992; Daunais et al., 1997). It is speculated that a history of cocaine taking induces an enhancement in striatal enkephalin tone that is responsible for opioid receptor-dependent long-term depression (LTD) of the striatopallidal pathway (Creed et al., 2016; Kupchik et al., 2014). Modulation of this striatopallidal pathway activity has been shown to provoke cocaine seeking (Heinsbroek et al., 2017); however, whether heightened striatal enkephalin actually drives cocaine seeking remains to be determined.
The activity of MORs is important in signaling the motivational and affective aspects of rewards, which, through careful dissection of regional differences, have been mapped as hedonic hotspots and coldspots in the ventral aspect of the striatum, also known as the nucleus accumbens (NAc) (Castro and Berridge, 2014; Castro and Bruchas, 2019; Peciña and Berridge, 2005). Considering the high expression level of enkephalin in the NAc and the rest of the striatum, and high affinity of this endogenous peptide for MORs, it is reasonable to hypothesize that enkephalin contributes to MOR-mediated reward. In support of this hypothesis, a previous study showed that enkephalin is reinforcing in its own right, as rats will self-administer met-enkephalin into the NAc, as well as selective MOR or DOR agonists (Goeders et al., 1984; Simmons and Self, 2009).
Other studies provide further evidence that endogenous opioids, likely enkephalin, and opioid receptors in the NAc, play an important role in mediating cocaine reward and reinforcement. For example, microinjection of the MOR antagonist, D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), into the NAc suppressed cocaine-induced reinstatement of drug seeking (Simmons and Self, 2009) and cocaine place preference (Soderman and Unterwald, 2008). While this CTAP effect could have been mediated by β-endorphins, which also have high affinity for MORs, studies using genetic manipulations point to enkephalin as being an important player in cocaine reinforcement. Global deletion of Penk or the DOR gene (Oprd1) suppresses acquisition of cocaine self-administration and the breakpoint for cocaine, while knockout of the DOR or MOR gene (Oprm1) suppresses cue-induced reinstatement of cocaine seeking (Gutiérrez-Cuesta et al., 2014). However, since these effects are the result of global Penk, Oprd1, Oprm1 knockout, it is unclear whether enkephalin signaling in the striatum, per se, is sufficient to potentiate cocaine reward. Further, understanding how D2Rs modulate enkephalin expression is likely to unveil the mechanism by which low striatal D2Rs contribute to the addiction vulnerable phenotype and also provide new targets for preventive therapeutics that address vulnerability.
Here, we tested the hypothesis that low levels of D2R activation in the striatum triggers increased enkephalin expression, release, and functional tone, which potentiates cocaine conditioned place preference and eventually contributes to increased vulnerability for abuse. We manipulated the activity levels of striatal D2Rs using viral and genetic techniques and measured enkephalin expression levels and functional tone in the striatum using quantitative PCR and ex vivo electrophysiology, respectively. In a separate set of experiments, we tested whether heightened striatal enkephalin tone facilitates cocaine reward in a place conditioning procedure.
RESULTS
Dopamine D2 receptor expression in striatal medium spiny neurons regulates preproenkephalin expression
We bidirectionally manipulated D2R expression in the striatum (overexpression and knockdown) using two approaches that allow for temporal and cell-type specificity. First, we used an inducible tet-Off system to transiently overexpress the human Drd2 gene in adult mice (Kellendonk et al., 2006). This approach was previously shown to direct Drd2 expression to striatal MSNs and increase striatal D2R binding by 15% (Kellendonk et al., 2006). Even though this approach is not designed to have cell-type specificity of transgene expression, overexpression of Drd2 was enriched in Penk-positive D2-MSNs (40%) versus D1R-expressing MSNs (25%) (Labouesse et al., 2018). Here, we measured levels of Penk RNA in the striatum of Drd2 overexpressing (D2R-OE) mice and their control littermates.
Expression of the Drd2 transgene in D2R-OE mice was switched off by feeding with doxycycline-supplemented chow for 2 weeks (ON Dox) (Kellendonk et al., 2006). During ON Dox we found no difference in levels of Penk RNA between D2R-OE and control mice (Figures 1A and 1B). In absence of doxycycline treatment when mice were fed normal chow (OFF Dox), the relative levels of striatal Penk RNA were reduced by 50% in D2R-OE mice compared with controls (0.50 ± 0.06 versus 1.02 ± 0.09; t7 = 4.3, p = 0.0036; Figure 1B). In addition, gene chip microarray analysis showed that D2R-OE mice have an 18% decrease in cRNA for Penk in the striatum compared with controls (7626 ± 110 versus 9266 ± 145, respectively; t8 = 9.0, p < 0.0001; Figure 1C). Together, this indicates that overexpression of D2Rs in striatal MSNs results in a reliable reduction of Penk gene transcription (OFF Dox: Spearman’s r = 0.82, p < 0.01; Figure 1D).
Figure 1. Dopamine D2 receptor activity in medium spiny neurons bidirectionally regulates striatal preproenkephalin expression.
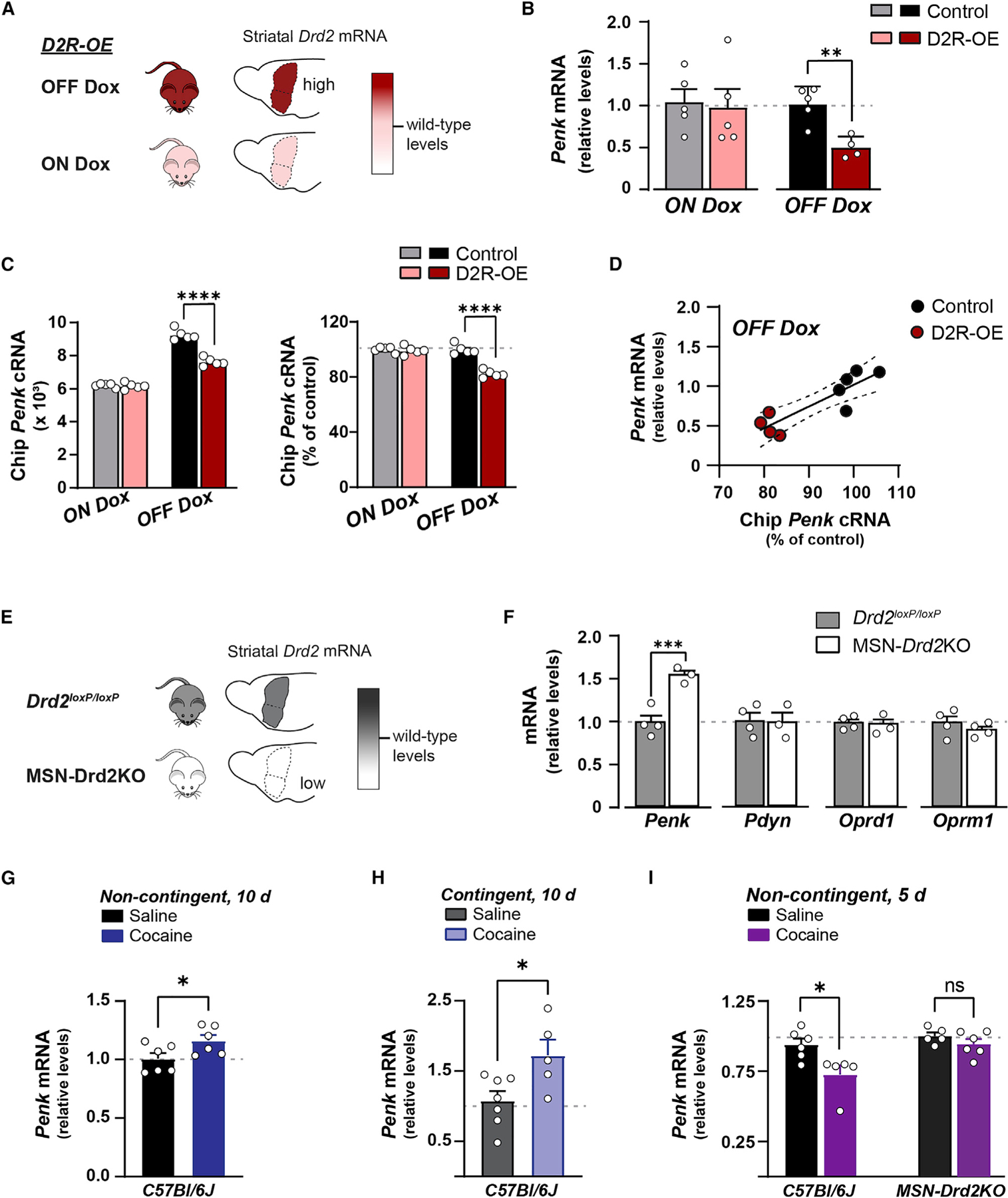
Expression of specific genes is quantified with qPCR and gene chip microarray for mice overexpressing D2Rs (A–D) or following selective loss of D2Rs from D2-MSNs (E and F).
(A) Schematic shows Drd2 mRNA levels are increased over wild type (pink) in mice expressing the human Drd2 transgene (D2R-OE) and in the absence of doxycycline-supplemented chow (dark red).
(B) Striatal preproenkephalin (Penk) mRNA levels in D2R-OE mice (n = 4–5) or controls (n = 5). When Drd2 transgene expression was suppressed (ON Dox, pink) Penk mRNA was similar to controls. Turning on Drd2 transgene (OFF Dox, dark red) reduced Penk mRNA (B) and cRNA (C) compared with controls (t test).
(D) Correlation of Penk levels between gene chip microarray and qPCR when the transgene is expressed (OFF Dox, red) in D2R-OE and control mice.
(E) Schematic shows decreased Drd2 mRNA levels in mice with a homozygous deletion of Drd2 from D2-MSNs (MSN-Drd2KO, white) relative to wild type (gray).
(F) MSN-Drd2KO mice (n = 3) show increased Penk mRNA, but no change in dynorphin (Pdyn) or δ- and μ -opioid receptors (Oprd1, Oprm1) compared with littermate controls (gray, n = 4; t test).
(G–I) Ten days of non-contingent (G; 20 mg/kg, intraperitoneally) or contingent (H; mean: 17 ± 3 mg/kg, intravenous self-administration) cocaine followed by 14 days of withdrawal increased striatal Penk mRNA in C57Bl/6J. (I) Five days of non-contingent (15 mg/kg, intraperitoneally) cocaine followed by 14 days of withdrawal reduced Penk mRNA in C57Bl/6J, but not MSN-Drd2KO mice (J). ns = not significant;*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Mean ± SEM and/or individual values shown.
Conversely, reducing striatal D2R levels with targeted deletion of the Drd2 gene from D2-MSNs increased Penk expression. Striatal samples from mice with deletion of both Drd2 alleles in D2-MSNs have >80% reduction of Drd2 RNA (Figure 1E; Dobbs et al., 2016; Lemos et al., 2016) and a 56% ± 5.3% increase in Penk RNA relative to littermate controls, which have unaltered D2R levels (t5 = 5.19, p < 0.01; Figure 1F). Next, we investigated whether there were adaptive alterations in MORs, DORs, or dynorphin (Pdyn), an opioid peptide expressed by D1R-expressing MSNs. We found no difference in expression levels of Pdyn (t4 = 0.08, p > 0.05), the MOR gene Oprm1 (t6 = 1.2, p > 0.05), or the DOR gene Oprd1 (t5 = 0.17, p > 0.05) in the striatum of MSN-Drd2KO mice compared with littermate controls.
Cocaine bidirectionally alters striatal Penk mRNA via D2Rs in MSNs depending on length of treatment
Long-term cocaine treatment (≥15 mg/kg × ≥ 10 days) is reported to increase Penk mRNA expression in the striatum (Crespo et al., 2001; Mantsch et al., 2004; Mathieu-Kia and Besson, 1998; Mongi-Bragato et al., 2016). Evidence suggests this effect is through long-term cocaine treatment downregulating D2R activity (Baik et al., 1995; Moore et al., 1998; Nader et al., 2002, 2006; Romano et al., 1987; Steiner and Gerfen, 1998) Moore et al., 1998a,b. Given our data showing the connection between striatal Drd2 and Penk expression, we hypothesized the length of cocaine treatment would similarly bidirectionally regulate striatal Penk expression by targeting D2Rs in MSNs.
We replicated reports in the literature and found that long-term, non-contingent cocaine administration (20 mg/kg × 10 days) followed by 14 days of withdrawal significantly increased Penk expression in the striatum of C57Bl/6J mice compared with saline-treated controls (t10 = 2.24, p < 0.05; Figure 1G). We observed a similar increase in Penk expression in wild-type mice following 14-day withdrawal from 10 days of cocaine self-administration (17.4 ± 2.8 mg/kg cocaine per day) compared with those that self-administered saline (t10 = 2.62, p < 0.05; Figure 1H). Conversely, wild-type mice exposed to a short-term, non-contingent cocaine regimen (15 mg/kg × 5 days) followed by 14 days of withdrawal showed significantly less striatal Penk expression compared with saline-treated controls (t9 = 2.85, p < 0.05, Figure 1I). Thus, long-term and short-term cocaine exposure followed by withdrawal bidirectionally regulates striatal Penk expression in a similar manner to striatal D2R activity. We next tested whether D2Rs specifically in MSNs were mediating cocaine’s effect by replicating the short-term cocaine experiment in MSN-Drd2KO mice. As expected, short-term cocaine followed by withdrawal failed to decrease striatal Penk expression in MSN-Drd2KO mice as it did in wild type. MSN-Drd2KO mice showed no change in Penk expression after short-term cocaine treatment compared with saline-treated MSN-Drd2KO mice (t9 = 1.25, p = 0.2). Together, these data indicate that activity of MSN D2Rs bidirectionally regulate striatal Penk expression, and that cocaine impinges upon these D2Rs to similarly regulate Penk expression.
Met-enkephalin and MOR agonist suppress intra-striatal GABA transmission
We next assessed the physiological response to met-enkephalin in the nucleus accumbens (NAc) by testing how it affects collateral GABA transmission using ex vivo electrophysiological recordings. Since Gi-coupled dopamine receptors potently suppress collateral GABA transmission in the NAc (Dobbs et al., 2016; Gallo et al., 2018), we hypothesized that met-enkephalin would have a similar effect via activation of Gi-coupled opioid receptors. Met-enkephalin has high affinity for the DOR and MOR, and both are expressed in MSNs (Gracy et al., 1997; Mansour et al., 1994; Svingos et al., 1996, 1997).
We expressed channelrhodopsin-2 (ChR2) in D2-MSNs using Cre-dependent vectors injected in the NAc of MSN-Drd2KO mice and Adora2aCre controls. This enabled optogenetic stimulation of collateral axons from D2-MSNs while recording from neighboring ChR2-negative MSNs to measure GABA-A receptor-mediated inhibitory post-synaptic currents (IPSCs).
We first tested the selective MOR agonist DAMGO (1 μM) in Adora2a-Cre controls (Figure 2A). DAMGO robustly suppressed the amplitude of optogenetic-evoked IPSCs (oIPSCs) to 35% ± 4% of baseline (1W-RMANOVA, F1.04, 4.18 = 38.59, p < 0.01; baseline versus DAMGO: t4 = 16.43, p < 0.001). This was reversed by the non-selective opioid receptor antagonist naloxone (5 μM; baseline versus naloxone: t4 = 1.56, p = 0.4). Similarly, bath application of met-enkephalin (1 μM), in the presence of peptidase inhibitors to slow met-enkephalin degradation (2 μM thiorphan, 10 μM bestatin), robustly inhibited oIPSC amplitude relative to peptidase inhibitor baseline (2W-Mixed Model, main effect of drug: F2,30 = 83.4, p < 0.0001) (Figures 2B and 2C). Maximal inhibition was similar between genotypes (MSN-Drd2KO: 42% ± 8% of baseline; Adora2aCre: 33% ± 5% of baseline; t49 = 1.2, p = 0.6), suggesting no apparent alterations in the function of MORs or DORs in MSN-Drd2KO mice. Naloxone (5 μM) reversed inhibition by met-enkephalin similarly between MSN-Drd2KO and Adora2aCre controls (t49 = 1.7, p = 0.2).
Figure 2. Enkephalin and MOR agonist suppress intra-striatal GABA transmission.
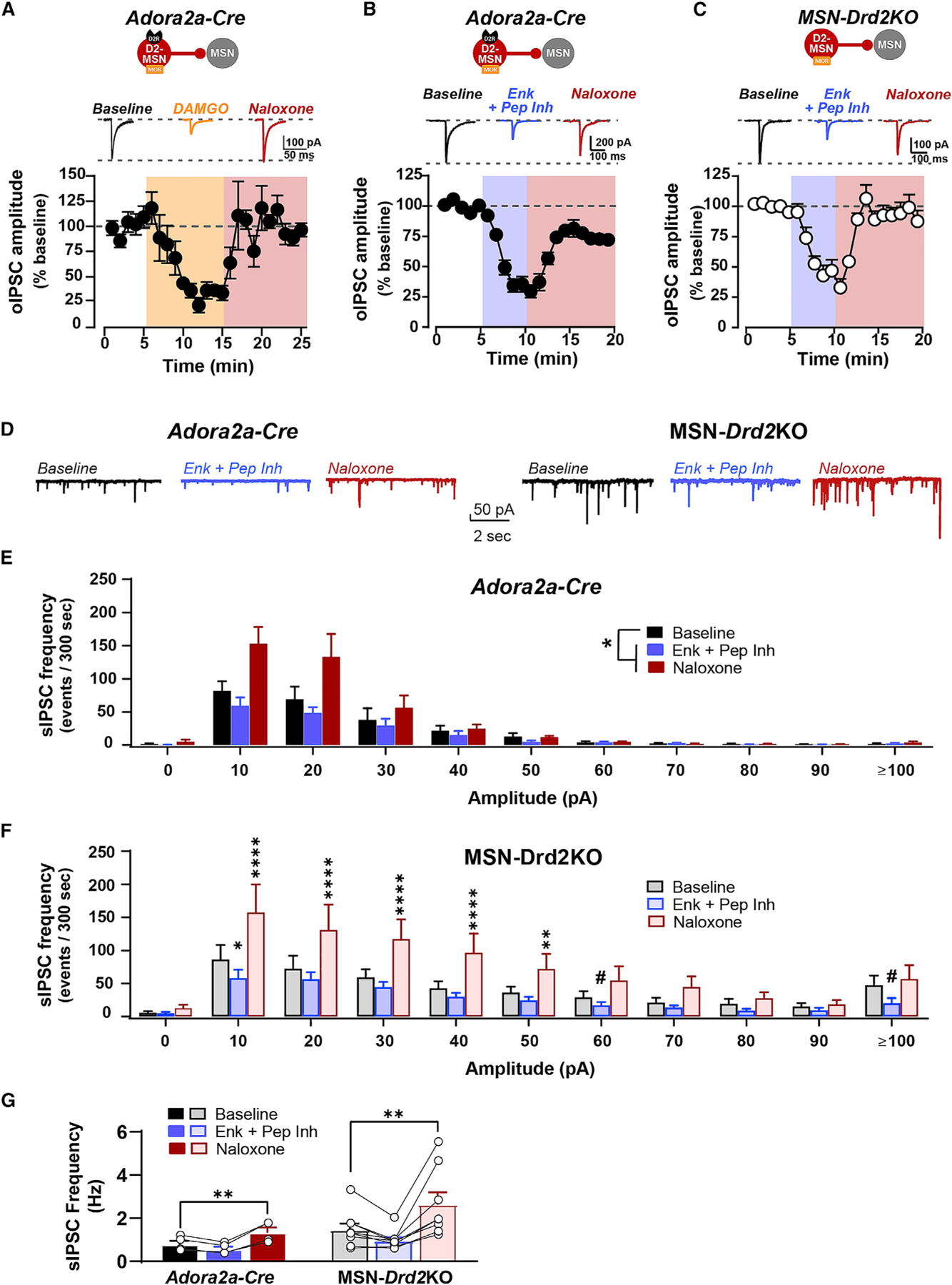
(A–C) Top, Representative oIPSC traces recorded from MSNs in Adora2a-Cre or MSN-Drd2KO in presence of the μ-opioid receptor agonist DAMGO (1 μM, orange), enkephalin (1 μM) + peptidase inhibitors (2 μM thiorphan + 10 μM bestatin; blue), or the opioid receptor antagonist naloxone (5 μM; red). Bottom, Time course of oIPSC amplitude expressed as percent of baseline recorded from ChR2-negative MSNs from Adora2a-Cre (n = 5 cells/2 mice) and MSN-Drd2KO mice (C, n = 10 cells/3 mice).
(D) Representative spontaneous IPSC (sIPSC) traces recorded from MSNs in Adora2a-Cre (left) or MSN-Drd2KO (right) are shown in the presence of enkephalin + peptidase inhibitors (blue) or naloxone (red).
(E and F) Number of sIPSCs plotted by amplitude for Adora2a-Cre (left, n = 4 cells/2 mice) or MSN-Drd2KO (right, n = 8 cells/3 mice) during enkephalin + peptidase inhibitors (E) or naloxone (F) relative to peptidase inhibitor baseline (black).
(G) sIPSC frequency was enhanced by naloxone in both genotypes. Parametric and non-parametric tests performed (see text). #p = 0.05–0.07, *p < 0.05, **p < 0.01, ****p < 0.0001. Mean ± SEM and/or individual values/traces shown.
We next measured whether met-enkephalin also inhibits spontaneously generated IPSCs (sIPSCs) in this same preparation. As we observed previously, MSN-Drd2KOs have a higher striatal GABA tone than controls (Lemos et al., 2016), which was reflected by a greater baseline sIPSC amplitude (K-S test, D = 0.64, p < 0.05; Figures 2D–2F). Met-enkephalin attenuated the overall mean sIPSC amplitude in MSN-Drd2KOs, but not controls, when compared with peptidase inhibitor baseline (data not shown); however, we suspected that averaging the amplitude data might mask an effect in the controls. Thus, we built frequency histograms of sIPSC amplitude size and compared the amplitudes between drug washes and genotypes. Met-enkephalin suppressed sIPSCs in controls relative to baseline (Wilcoxon signed rank test: p < 0.05; Figure 2E), an effect likely masked in the analysis of the overall mean. Met-enkephalin also suppressed sIPSCs in MSN-Drd2KOs compared with baseline (2W-RMANOVA, amplitude × drug: F20,140 = 3.42, p < 0.0001; Figure 2F). Further, comparison of the sIPSC distributions using a K-S test revealed that met-enkephalin suppressed sIPSCs significantly more for MSN-Drd2KOs than controls (D = 0.64, p < 0.05). This effect is supported by the wider range of met-enkephalin suppression at both small (10 pA: t140 = 2.3, p < 0.05) and large (≥100 pA: t140 = 2.2, p = 0.05) amplitude responses for the MSN-Drd2KOs.
Subsequent application of naloxone (5 μM) resulted in an overshoot of sIPSC amplitude over baseline, thus revealing the presence of a baseline opioid peptide tone. In MSN-Drd2KOs, naloxone enhanced GABA transmission for a wide range of amplitudes (10 pA–60pA; post hoc t tests: t’s = 2.1–5.8, p’s < 0.01–0.0001). However, while naloxone enhanced sIPSCs in controls (Wilcoxon signed rank test: p < 0.05), comparison of the distributions revealed that naloxone enhanced GABA transmission significantly more in MSN-Drd2KOs than controls (K-S test: D = 0.64, p < 0.05). The larger naloxone effect in the MSN-Drd2KO mice was the first indication of an enhanced functional tone of enkephalin in mice with low striatal D2Rs.
Met-enkephalin did not suppress the sIPSC frequency (2W-RMANOVA, drug: F2,20 = 13.18, p < 0.001; t20 = 1.5, p = 0.3). However, similar to the effect on amplitude, naloxone significantly increased sIPSC frequency relative to baseline for both genotypes (t20 = 3.5, p < 0.01; Figure 2G). Together, these data indicate that exogenously applied met-enkephalin acts on opioid receptors to inhibit small amplitude synaptic GABA responses. Further, opioid receptor antagonist revealed the presence of an opioid peptide tone in the striatum that appears stronger in animals with low D2Rs.
Evidence of heightened endogenous tone of enkephalin in MSN-Drd2KO mice
The presence of a higher tone of striatal met-enkephalin is consistent with the elevated Penk mRNA expression observed in MSN-Drd2KOs. To address this further, we carried out two sets of experiments to test for evidence of this electrophysiologically and behaviorally. Electrophysiologically, we assessed the ability of a peptidase inhibitor cocktail to inhibit collateral GABA on its own. The peptidase inhibitors thiorphan and bestatin raise met-enkephalin extracellular levels in the striatum by inhibiting the enzymes that degrade met-enkephalin (Nam et al., 2019). This peptidase inhibitor cocktail was effective in suppressing glutamate transmission in the amygdala by enhancing enkephalin responses (Gregoriou et al., 2020). We therefore hypothesized peptidase inhibitors would be more effective at suppressing striatal GABA transmission in MSN-Drd2KO mice than controls. We found that application of peptidase inhibitors suppressed the amplitude of oIPSCs and the frequency and amplitude of sIPSCs recorded from NAc MSNs (Figures 3 and S1). The suppression of oIPSC amplitude was only significant in MSN-Drd2KO mice (76% ± 4% from baseline versus 96% ± 5% of baseline in controls; 2W-Mixed Model, drug × genotype: F1,35 = 11.75, p < 0.01; MSN-Drd2KO versus Adora2aCre: t35 = 4.8, p < 0.0001; Figures S1A–S1D). The suppression of overall sIPSC mean amplitude was also only significant in MSN-Drd2KOs and not in controls (data not shown); however, comparison of the distributions of sIPSC amplitude size showed that peptidase inhibitors had a similar effect on both controls and MSN-Drd2KOs (K-S test: D = 0.55, p = 0.08; Figures 3A and 3B). Peptidase inhibitors reduced sIPSC amplitude in controls (Wilcoxon signed rank test: p < 0.001) and MSN-Drd2KOs (2W-RMANOVA, drug: F1,77 = 50.1, p < 0.0001). Similarly, peptidase inhibitors reduced sIPSC frequency equally between genotypes (2W-RMANOVA, drug: F1,10 = 6.24, p < 0.05; Figure 3D). Pre-incubation with the opioid receptor antagonist naloxone (5 μM) blocked the effect of peptidase inhibitors to inhibit sIPSC frequency (baseline: 21 ± 6, peptidase inhibitors: 32 ± 6 sIPSCs/15 s; t5 = 2.9, p < 0.05). However, to our surprise, pre-incubation with naloxone did not block the inhibition of oIPSC amplitude, suggesting the possibility that another neuropeptide is involved. Receptor antagonists for Substance P and nociceptin, two peptides highly expressed in the striatum, also did not block the suppression of oIPSCs by peptidase inhibitors. Pre-incubation with the neurokinin1 antagonist Aprepitant (1 μM) and nociceptin antagonist J 113397 (1 μM) failed to block oIPSC suppression induced by peptidase inhibitors (one-sample t tests, baseline versus antagonists: t’s = 4.5–6.7, p’s < 0.01; Figure S1D). Taken together, the data indicate that while peptidase inhibitors suppressed GABA transmission in controls and MSN-Drd2KOs, the effect is more robust in MSN-Drd2KO mice. Further, it appears that peptidase inhibitors exert their effect by raising levels of opioid and non-opioid peptides.
Figure 3. Endogenous peptides suppress spontaneously evoked GABA in the striatum.
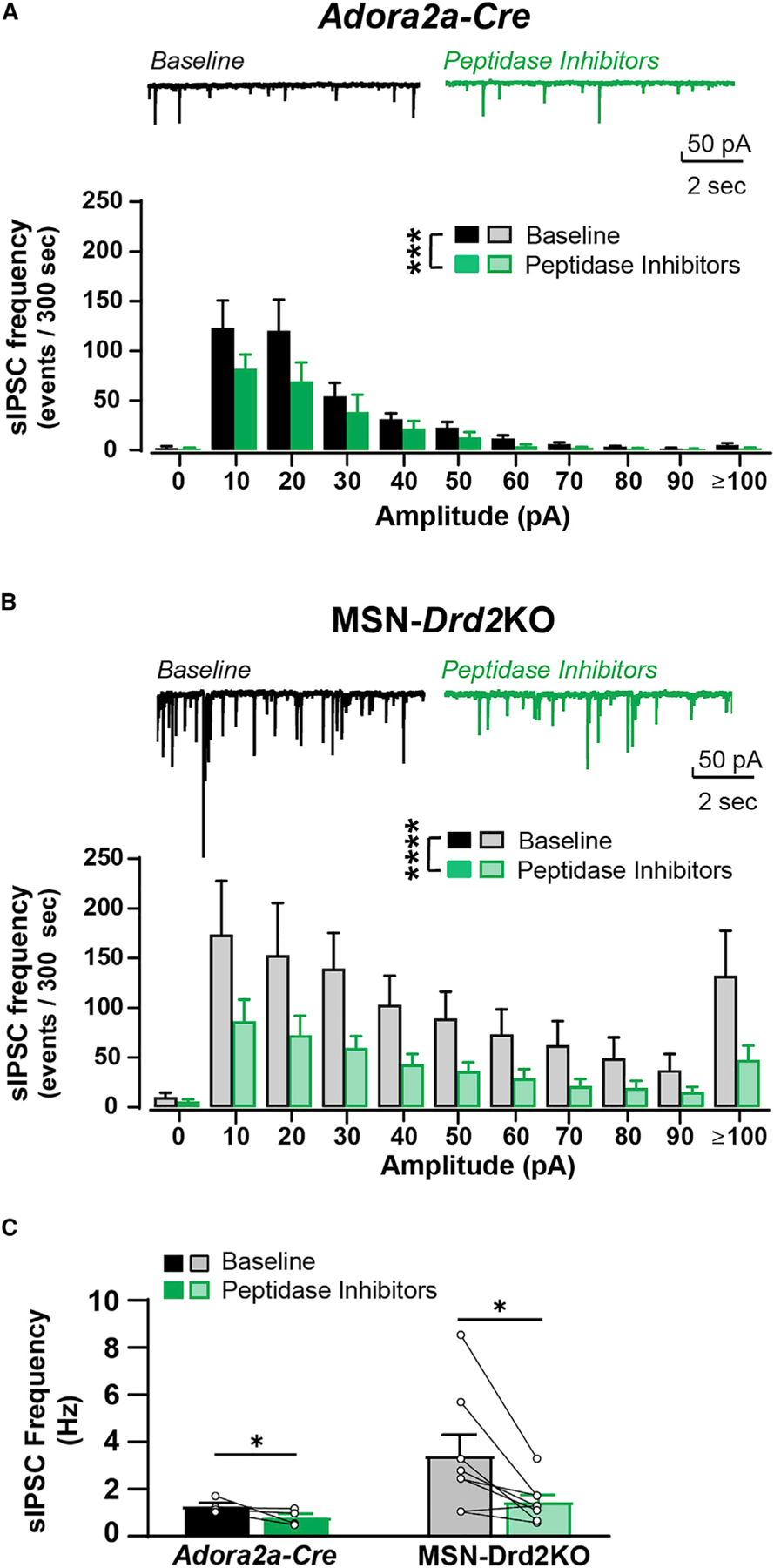
(A and B) Top, Representative spontaneous IPSC (sIPSC) traces recorded from MSNs in Adora2a-Cre (A) or MSN-Drd2KO (B) in presence of peptidase inhibitors (2 μM thiorphan, 10 μM bestatin; green). Bottom, sIPSCs plotted by amplitude for Adora2a-Cre (A, n = 4 cells/2 mice) or MSN-Drd2KO (B, n = 8 cells/3 mice) at baseline (black) and during peptidase inhibitors.
(C) sIPSC frequency is inhibited by peptidase inhibitors for both genotypes. Parametric and non-parametric tests performed (see text) p < 0.05, ***p < 0.001, ****p < 0.0001. Mean ± SEM and/or individual values/traces shown. See also Figure S1.
We next probed for in vivo evidence of heightened enkephalin tone in mice with low D2Rs and sought to determine the consequences for motivated behavior. Endogenous met-enkephalin tone is important for maintaining a basal hedonic set-point. Administration of naloxone during place conditioning induces a place aversion in mice (Cunningham et al., 1995; Solecki et al., 2009). Evidence from Penk and Oprm1 constitutive knockout mice suggests naloxone induces place aversion by blocking the action of enkephalin on MORs (Skoubis et al., 2001, 2005). We reasoned that if MSN-Drd2KO mice have higher endogenous enkephalin tone, they might be more sensitive to developing and expressing place aversion to a low dose of naloxone (Figure 4A). As we observed previously (Dobbs et al., 2016, 2019), MSN-Drd2KO mice showed less locomotor activity compared with littermate controls during saline and naloxone (1 mg/kg, intraperitoneally) conditioning trials (3W-RMANOVA, genotype: F1,13 = 27.3, p < 0.001; Figure 4B). We assessed naloxone place aversion by comparing the time spent on the grid floor between the conditioning subgroups (Grid + Nal versus Hole + Nal). In this approach, a place aversion is expressed as less time spent on the grid floor for the Grid + Nal group than the Hole + Nal group. We predicted MSN-Drd2KO mice would spend less time on the naloxone paired floor compared with controls. However, neither genotype showed evidence of naloxone place aversion during saline-primed preference tests (Tests 1 and 2: one-sample t test versus 50%: p’s > 0.6; Figure 4C). This was confirmed by additional analysis of the grid time data (Test 1: 2W-ANOVA, conditioning: F1,9 = 0.57, p = 0.5; Test 2: 2W-ANOVA, conditioning: F1,11 = 0.39, p = 0.5; Figure 4D). Though, when tested in the presence of naloxone, MSN-Drd2KO mice spent significantly less percent time on the naloxone paired floor than controls (t13 = 2.5, p < 0.05). Analysis of time spent on the grid floor showed the same effect in MSN-Drd2KO mice (2W-ANOVA, genotype × conditioning: F1,11 = 5.49, p < 0.05; MSN-Drd2KOs: t11 = 2.3, p = 0.08; Controls: t11 = 1.0, p = 0.3). Importantly, naloxone had no effect on locomotion, indicating the place aversion was not due to locomotion suppression (Figure S2).
Figure 4. MSN-Drd2KO mice are more sensitive to aversive properties of naloxone.
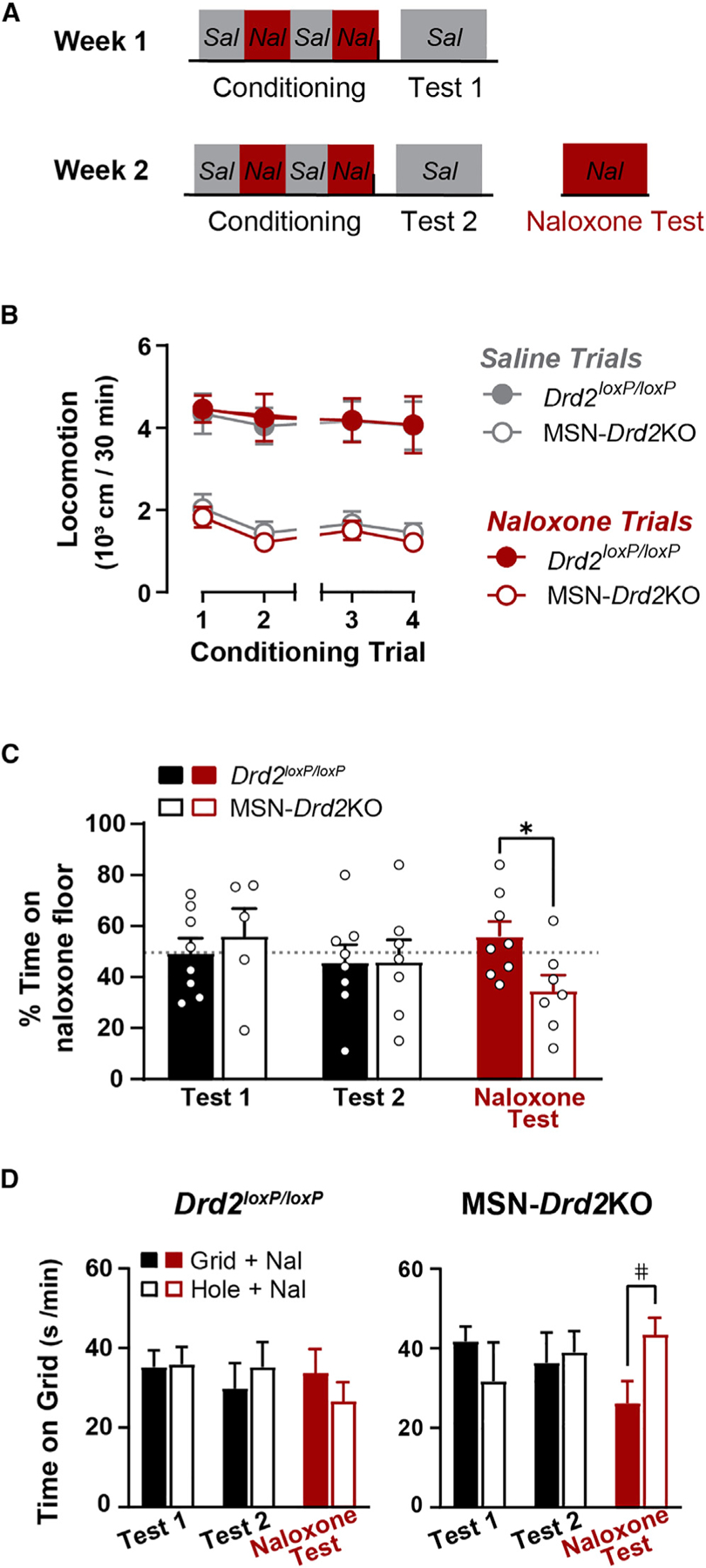
(A) Timeline of behavioral experiment.
(B) Locomotor activity during saline (gray) and naloxone (red) conditioning trials is shown for Drd2loxP/loxP (filled) and MSN-Drd2KOs (open) (3W-RMANOVA).
(C) Percent time on the naloxone paired floor for Drd2loxP/loxP (filled, n = 8) and MSN-Drd2KO (open, n = 5–7) at Test 1, Test 2, and naloxone test (red) (t tests).
(D) Time on grid floor is shown across preference tests for Drd2loxP/loxP (left) and MSN-Drd2KO (right) that had naloxone paired with grid floor (filled, Grid + Nal, n = 3–4) or hole floor (open, Hole + Nal, n = 3–4) (2W-ANOVAs). #p = 0.08, *p < 0.05. Mean ± SEM and/or individual values/traces shown. See also Figure S2.
Together, the electrophysiology and place preference data provide evidence for heightened met-enkephalin tone in the striatum of MSN-Drd2KO mice, and suggest this tone has consequences for motivated behavior. Further, these data suggest that met-enkephalin and peptidase inhibitors suppress collateral GABA transmission through activation of MORs.
Nucleus accumbens opioid receptors facilitate cocaine reward
MSN-Drd2KO mice acquire cocaine place preference faster than littermate controls (Dobbs et al., 2016). We wondered whether the heightened enkephalin tone and signaling through MORs could be contributing to the enhanced cocaine place preference (CPP), since blockade of MORs in the NAc of rats attenuates cocaine place preference (Soderman and Unterwald, 2008). To this end, we tested whether MOR signaling in the NAc facilitates expression of CPP in wild-type mice and whether it underlies the enhanced acquisition of cocaine preference in MSN-Drd2KO mice.
Control mice (Drd2loxP/loxP) received cocaine place conditioning trials over 8 days, with preference measured before and after conditioning (Pre-test and Post-test; Figure 5A). A second Post-test was performed following intra-NAc shell CTAP administration to determine the contribution of MORs to the expression of cocaine CPP (CTAP test). Mice acquired cocaine CPP as indicated by a significant increase in the percent time spent on the cocaine-paired floor at Post-test compared with the Pre-test (1W-Mixed Model: F1.61,16.91 = 4.3, p < 0.05; Pre-test versus Post-test: t13 = 2.98, p < 0.05; Figure 5B). Blockade of MORs in the NAc shell (CTAP 300 ng/side) reduced the percent time spent on the cocaine paired floor (Post-test: 60% ± 4%, CTAP test: 53% ± 6%). In addition, the percent time spent on the cocaine floor during the CTAP test was not significantly different from at Pre-test (t8 = 1.04, p = 0.7), suggesting that CTAP blocked the expression of CPP. This effect is further supported by analysis of the grid time data (2W-Mixed Model, conditioning × test: F2,31 = 4.49, p < 0.05; Pretest: t31 = 0.82, p = 0.8; Post-test: t31 = 3.42, p < 0.01; CTAP test: t31 = 0.99, p = 0.7; Figure 5C). Importantly, locomotor activity during the CTAP test was not higher than the Post-test, which is an effect that can mask a place preference, and instead was slightly lower (Mixed Model: F1.9, 19.9 = 3.64, p < 0.05; t8 = 3.82, p < 0.05; Figure S3). Together, these data indicate that activation of MORs in the NAc shell are necessary for the full expression of CPP and extend findings from rats to a mouse model.
Figure 5. Mu-opioid receptor antagonist in the nucleus accumbens blocks cocaine place preference in wild-type, but not MSN-Drd2Kos.
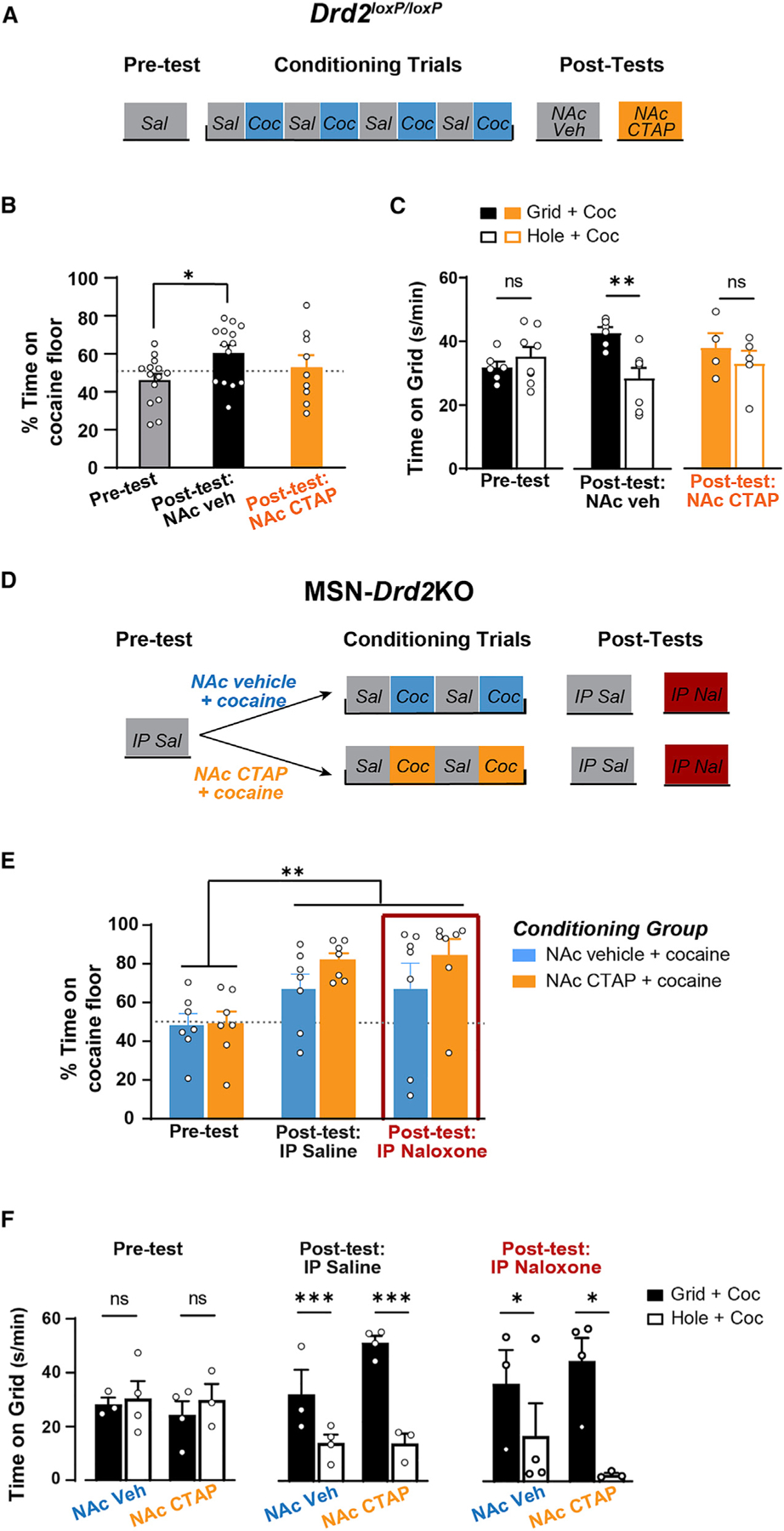
(A) Timeline of behavioral experiment in Drd2loxP/loxP mice.
(B) Percent time on cocaine (15 mg/kg) paired floor is shown for Pre-test (gray, n = 14), Post-test (black, n = 14), and CTAP test (300 ng/side; orange, n = 9) (RMANOVA).
(C) Time on grid floor is shown for mice that had cocaine paired with grid floor (filled, Grid + Cocaine, n = 4–6) or with hole floor (open, Hole + Cocaine, n = 5–8) during Pre-test, Post-test, and CTAP test (2W-ANOVAs).
(D) Timeline of behavioral experiment in MSN-Drd2KO mice.
(E) Percent time on cocaine (15 mg/kg) paired floor is shown for Pre-test (n = 7/group), Post-test (n = 7/group), and naloxone test (10 mg/kg; red box, n = 7/group) (2W-RMANOVA). Mice had cocaine paired with either intra-NAc vehicle (blue, n = 7) or intra-NAc CTAP (300 ng/side; orange, n = 7).
(F) Time on grid floor is shown for mice that had cocaine paired with grid floor (filled, Grid + Cocaine, n = 3–4/group) or with hole floor (open, Hole + Cocaine, n = 3–4/group) during Pre-test, Post-test, and naloxone test (2W-ANOVAs). Ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001. Mean ± SEM and/or individual values shown. See also Figure S3.
Next, we tested whether blocking MORs during cocaine conditioning would attenuate acquisition of cocaine CPP in MSN-Drd2KO mice (Figure 5D). Intra-NAc CTAP or vehicle was administered immediately prior to cocaine conditioning trials. After only two cocaine and two saline conditioning trials, both the intra-NAc vehicle and intra-NAc CTAP groups showed significantly greater percent time on the cocaine-paired floor compared with Pre-test (2W-RMANOVA, test: F2,24 = 7.02, p < 0.001; Pre-test versus Saline Post-test: t24 = 3.2, p < 0.01; Figure 5E). This was confirmed by analysis of the time spent on the grid floor (2W-ANOVA, conditioning: F1,10 = 35.0, p < 0.001; Figure 5F). Thus, blockade of MORs in the NAc did not attenuate acquisition of cocaine CPP in MSN-Drd2KO mice. However, since these MSN-Drd2KO mice have enhanced enkephalin tone throughout the entire striatum, it is possible that just blocking MORs in the NAc is not sufficient to attenuate CPP acquisition. Further, enkephalin also has high affinity for DORs, and activation of these receptors may contribute to the enhanced CPP acquisition. To address these possibilities, we tested these mice following a systemic injection of the non-selective opioid receptor antagonist naloxone (10 mg/kg). Surprisingly, naloxone did not block expression of cocaine CPP in either group, as indicated by greater percent time spent on the cocaine floor on the naloxone test versus the Pre-test (t24 = 3.3, p < 0.01), as well as by analysis of time spent on the grid floor for each treatment group (2W-ANOVA, conditioning: F1,10 = 9.14, p < 0.05). These data indicate that while MOR signaling sustains CPP in wild-type control mice, blocking MOR signaling is not sufficient to attenuate the faster acquisition of cocaine preference previously reported in MSN-Drd2KO mice.
Heightened enkephalin in the nucleus accumbens facilitates cocaine reward
Evidence from global enkephalin knockout mice and pharmacological manipulation of MORs and DORs suggest that striatal enkephalin contributes to the rewarding and locomotor sensitizing effects of cocaine (Gutiérrez-Cuesta et al., 2014; Mongi-Bragato et al., 2016). Although blockade of MORs does not attenuate CPP acquisition in MSN-Drd2KOs, these mice also have enhanced D1R signaling, which could mask the potentiating effects of enkephalin or even act synergistically with enhanced enkephalin to potentiate cocaine CPP. To test whether heightened striatal enkephalin alone is sufficient to potentiate the conditioned rewarding effects of cocaine, we paired cocaine with intra-NAc shell administration of either enkephalin or vehicle in wild-type Drd2f/f mice during conditioning (Figure 6A). Intra-NAc shell enkephalin did not affect locomotion during conditioning (3W-Mixed Model, no enkephalin main effect or interaction), but cocaine increased locomotion compared with saline (trial × cocaine: F2.56, 64.87 = 4.74, p < 0.01; post hoc t tests: t = 4.3–11.3, p < 0.001; Figure S4).
Figure 6. Enkephalin in the nucleus accumbens facilitates acquisition of cocaine place preference.
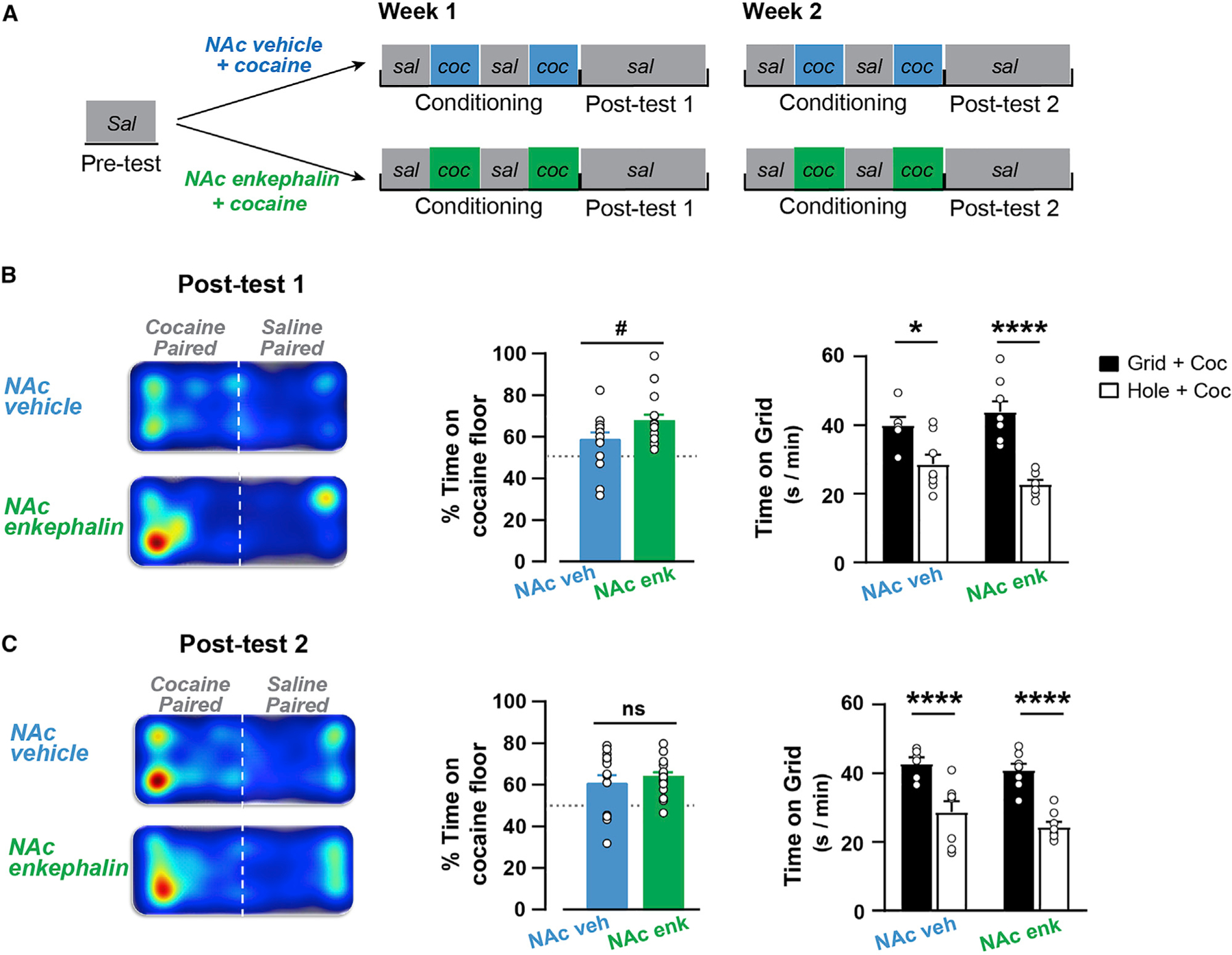
(A) Timeline of behavioral experiment in Drd2loxP/loxP mice.
(B and C) Left, Representative heatmaps of animal position on cocaine- or saline-paired floors of the conditioning chamber are shown for NAc vehicle group and NAc enkephalin group during Post-test 1 (B) and Post-test 2 (C). Middle, Percent time on cocaine-paired floor for NAc vehicle group (blue, n = 9–14) and NAc enkephalin group (orange, n = 11–16) at Post-test 1 (B) and Post-test 2 (C) (t tests). Right, Time on grid floor is shown for mice that had cocaine paired with grid floor (filled, Grid + Cocaine, n = 6–8) or with hole floor (open, Hole + Cocaine, n = 8) for NAc vehicle group and NAc enkephalin group (2W-ANOVA). ns = not significant, #p = 0.06, *p < 0.05, ****p < 0.0001. Mean ± SEM and/or individual values shown. See also Figures S4 and S5.
Neither the vehicle nor enkephalin paired groups showed a significant pre-conditioning preference (vehicle: 46 ± 3, enkephalin: 51% ± 4% time on cocaine-paired floor; one-sample t tests, p’s > 0.3; unpaired t test vehicle versus enkephalin: t27 = 0.87, p = 0.4; Figure S4). After 1 week of conditioning, both groups spent greater than 50% time on the cocaine-paired floor (2W-ANOVA, test: F2,83 = 10.7, p < 0.0001; Pre-test versus Test 1: t83 = 4.13, p < 0.001). However, when compared with the NAc vehicle group, the NAc enkephalin group showed a strong trend to spend more percent time on the cocaine floor (t28 = 1.94, p = 0.06; Figure 6B). This enhanced acquisition was also observed when analyzing the time spent on the grid floor. While both the NAc vehicle and NAc enkephalin groups showed preference for the cocaine-paired floor (conditioning: F1,26 = 40.57, p < 0.0001), there was a strong trend for greater preference in the NAc enkephalin group (conditioning × enkephalin: F1,26 = 3.67, p = 0.06; vehicle: t26 = 3.03, p < 0.05, enkephalin: t26 = 6.1, p < 0.0001). The potentiated cocaine preference in the enkephalin group, though, did not persist after another week of conditioning, as indicated by no difference between groups in the percent time on the cocaine floor at Post-test 2 (enkephalin versus vehicle: t28 = 0.75, p = 0.5; Figure 6C).
In Figure 1, we showed that short-term cocaine exposure followed by withdrawal reduces striatal Penk expression. We wondered whether short-term cocaine without withdrawal, like in our CPP experiments, would also lower striatal Penk and could potentially be biasing our results. C57Bl/6J mice were treated with cocaine (15 mg/kg) for 5 days, and striatal Penk expression was measured immediately after the final treatment. In contrast to the effect of short-term cocaine followed by withdrawal, short-term cocaine in absence of withdrawal did not alter Penk expression relative to saline-treated controls (t7 = 0.78, p = 0.5; Figure S5). This was not due to poor injections, as we observed a robust cocaine-induced locomotion that sensitized across days compared with saline-treated controls (2W-Mixed Model, day × drug: F6,197 = 60.01, p < 0.0001; day 1 versus day 5 for cocaine: t17 = 7.95, p < 0.0001; Figure S5). Thus, it is unlikely that the short-term cocaine exposure in our CPP experiments lowered Penk expression levels in vehicle-treated controls and accounted for the differences between treatment groups.
DISCUSSION
A large literature implicates low availability of D2Rs in the striatum as a vulnerability factor for developing SUDs; however, the downstream circuit mechanisms driving this vulnerability have remained elusive. Here, we provide evidence that both the levels of Drd2 mRNA expression and the degree of D2R activation by cocaine in striatal neurons bidirectionally regulate expression of enkephalin within the striatum. Overexpression of D2Rs in striatal neurons led to decreased Penk mRNA, while selective loss of D2Rs from D2-MSNs increased Penk mRNA. Further, naloxone more potently enhanced striatal GABA transmission and conditioned a place aversion in MSN-Drd2KOs relative to controls, indicating that low striatal D2Rs increase the tone of enkephalin in the striatum. The length of cocaine treatment was associated with a similar bidirectional change in striatal Penk mRNA and was dependent on the activation of D2Rs expressed in D2-MSNs.
Increasing the enkephalin tone within the slice inhibited GABA transmission between striatal neurons. This may be an important mechanism for promoting cocaine seeking because we previously showed that suppressed collateral GABA transmission decreases lateral inhibition onto D1-MSNs, facilitates striatal output, and promotes cocaine behavioral responses (Dobbs et al., 2016, 2017, 2019). In agreement with this, artificially enhancing striatal enkephalin tone during cocaine place conditioning trials potentiated the acquisition of CPP, while antagonizing striatal μ-opioid receptors blocked expression of CPP in wild-type mice. Together, this suggests enhanced striatal enkephalin tone is a mechanism that contributes to heightened cocaine reward and could promote risk to develop a cocaine SUD.
Previous reports indicate that global D2R knockout mice or treatment with non-selective D2-like antagonists increase Penk expression in the striatum (Baik et al., 1995; Romano et al., 1987). Here, we show that selective deletion of D2Rs expressed in D2-MSNs is sufficient to enhance striatal Penk expression, while, conversely, overexpression of D2Rs in striatal neurons decreases Penk. Interestingly, withdrawal from long-term and short-term cocaine treatment had a similar bidirectional influence over striatal Penk expression. We suspect long-term cocaine increased Penk by downregulating striatal D2R availability or decreasing D2R sensitivity, as previously reported (Gong et al., 2021; Moore et al., 1998; Nader and Czoty, 2005). Conversely, withdrawal from short-term cocaine decreases Penk expression in the striatum of wild-type mice, but has no effect on Penk levels in mice with low striatal D2Rs. These data recapitulate the striatal D2R overexpression findings and further support the mechanism that cocaine is affecting Penk expression by altering the activity and expression of D2Rs expressed in D2-MSNs.
Factors determining whether cocaine increases or decreases Penk expression likely include duration of exposure, total cocaine received, and withdrawal time. Previous studies found that longer cocaine exposure, either via self-administration (Crespo et al., 2001; Mantsch et al., 2004) or non-contingent injections (Mathieu-Kia and Besson, 1998; Mongi-Bragato et al., 2016), increased Penk mRNA in the striatum. We propose that longer cocaine exposure, higher cumulative dose, and/or longer withdrawal would downregulate or desensitize D2Rs selectively in D2-MSNs in response to the chronically heightened dopamine levels in the striatum, and this would in turn increase Penk expression. Thus, the activity of striatal D2Rs appears to tightly regulate the function of Penk gene. While it is unclear what is driving this interaction, experiments using cell culture suggest that elevated levels of cAMP-dependent protein kinases facilitate Penk transcription (Eiden and Hotchkiss, 1983; Eiden et al., 1984; Quach et al., 1984).
This cell-intrinsic adaptation in response to altered dopamine transmission has been previously suggested in reports with dopamine-depleted mice. Enhanced Penk expression following 6-OHDA lesions is proposed as an adaptive mechanism to maintain balance in striatal output, as it correlates with partial recovery of motor impairment (Steiner and Gerfen, 1998). However, this Penk enhancement may also generate vulnerability to cocaine seeking by suppressing collateral GABA transmission onto D1-MSNs and ultimately promoting their output. This is supported by our electrophysiology data showing that bath application of met-enkephalin or the m-opioid agonist DAMGO, or potentiation of endogenous opioids suppresses GABA transmission onto putative D1-MSNs.
Naloxone more effectively increased spontaneous GABA transients in MSN-Drd2KO mice compared with controls, suggesting that pre-existing low levels of striatal D2Rs enhance opioid peptide tone. However, the peptidase inhibitor-mediated suppression of optically evoked GABA was not blocked by naloxone, or receptor antagonists for other common striatal peptides like Substance P and nociceptin. Thus, it appears another peptide is upregulated in MSN-Drd2KO mice that is suppressing collateral GABA. One potential candidate is the cocaine-amphetamine regulated transcript (CART) peptide, which is highly expressed in the NAc and associated with blunting the behavioral responses to cocaine (Jaworski et al., 2003; Yoon et al., 2007, 2010).
MSN-Drd2KO mice are also more sensitive to naloxone place aversion, indicating that alterations in striatal enkephalin tone may shift an organism’s hedonic set-point. This is supported by reports that opioid receptors and peptides localized to the NAc shell and ventral pallidum are important regulators of hedonic motivation (Castro and Berridge, 2014; Castro and Bruchas, 2019; Peciña and Berridge, 2005). Consistent with this, antagonizing m-opioid receptors in the NAc shell with CTAP blocked expression of cocaine CPP in wild-type mice. In addition, augmenting enkephalin tone selectively within the NAc shell was sufficient to potentiate the acquisition, but not the maintenance, of CPP. It is important to note that the effect size was modest likely due to the relatively low enkephalin dose (5 μg met-enkephalin acetate salt/side) and restricted infusions to the NAc shell. While this dose was based on the effective concentrations in our electrophysiology experiments, single bolus infusions in the NAc are quite different from continuous bath application in the slice. We speculate that a higher enkephalin dose or an infusion that encompassed a larger striatal region might reveal a larger potentiating effect of enkephalin on cocaine reward. Although we cannot rule out the involvement of the dorsal striatum, these findings are supported by previous studies showing that intra-NAc administration of an MOR agonist or antagonist reinstates or blocks cocaine seeking, respectively (Simmons and Self, 2009; Soderman and Unterwald, 2008), and that rats self-administer met-enkephalin directly into the NAc (Goeders et al., 1984). It is also possible we encountered a ceiling effect for cocaine preference; however, in our experience it is difficult to establish dose-responses for cocaine CPP. Regardless, the combination of place preference experiments presented here provide strong convergent evidence for a role of opioid peptides in the striatum, and enkephalin in particular, in supporting the acquisition and maintenance of conditioned cocaine reward.
The ability of enkephalin to enhance acquisition of CPP is similar to the MSN-Drd2KO mice phenotype, which shows faster acquisition of cocaine preference (Dobbs et al., 2016). Surprisingly, even though MSN-Drd2KO mice have heightened striatal enkephalin and faster acquisition of cocaine CPP, a MOR antagonist in the NAc did not block development of cocaine CPP. It is possible that in the MSN-Drd2KO, enkephalin signaling through MORs in the dorsal striatum was sufficient to facilitate cocaine CPP; however, this is unlikely since systemic naloxone failed to block expression of cocaine CPP. We suspect the heightened enkephalin tone in MSN-Drd2KO mice may have induced plasticity of DORs, or may be acting in concert with other neuroadaptations, to potentiate cocaine reward. Indeed, repeated cocaine exposure induces a DOR-mediated LTD of GABA transmission from D2-MSNs to the ventral pallidum (Creed et al., 2016). In addition, MSN-Drd2KO mice have enhanced D1-mediated signaling (Dobbs et al., 2019), which could mask the effect of opioid receptor antagonists.
Together, these data suggest that low Drd2 levels in D2-MSNs or long-term cocaine use enhances enkephalin tone in the striatum, which suppresses collateral GABA transmission onto D1-MSNs to facilitate direct pathway output and cocaine reward. This is consistent with enkephalin being necessary for the expression of behavioral and molecular plasticity in striatal neurons following repeated cocaine exposure (Mongi-Bragato et al., 2016). In addition, enkephalins have been shown to affect plasticity of striatal circuits and D1-MSN activity by regulating glutamate transmission via MORs (Atwood et al., 2014; Trieu et al., 2022). Our findings expand on this by demonstrating that enkephalins also regulate collateral GABA transmission onto D1-MSNs. In addition, constitutively active MORs expressed in D2-MSNs due to chronically heightened enkephalin may induce LTD of striatopallidal synapses, as previously seen in rats with a history of cocaine self-administration (Kupchik et al., 2014). Whether a similar MOR-mediated LTD is induced within striatal GABAergic collaterals is unknown and remains an interesting question for future studies.
We found that withdrawal from short-term cocaine use, which may be akin to a recreational use pattern, attenuates Penk levels via activation of D2Rs in D2-MSNs. While it is unclear what effect low striatal enkephalin levels have on the circuit and behavior, it is possible this could suppress cocaine reward and protect against developing problematic cocaine use. However, low striatal enkephalin may drive some to escalate cocaine use in the short term in an attempt to regulate their baseline hedonic set-point. In this case, we predict that those who continue to use cocaine chronically or in high doses would develop downregulated striatal D2Rs, enhanced Penk expression, and potentiated cocaine reward. Further, the proclivity toward cocaine use in those with pre-existing low striatal D2R availability might suggest that enkephalin levels are already high and enhancing cocaine’s rewarding properties. Moreover, data from other studies suggest that increased striatal enkephalin due to low striatal D2Rs may be a common mechanism underlying vulnerability to a wide range of SUDs. Low D2R availability in the striatum is reported in individuals with an alcohol (Gleich et al., 2020; Heinz et al., 2005; Volkow et al., 1996, 2007), methamphetamine (Lee et al., 2009; Volkow et al., 2001), nicotine (Fehr et al., 2008), and opiate use disorder (Martinez et al., 2012; Wang et al., 1997), and increased striatal Penk mRNA or enkephalin peptide levels are reported following use of nicotine (Petruzziello et al., 2013), methamphetamine (Cadet et al., 2016), and alcohol (Chang et al., 2010; Meinhardt et al., 2015; Palm et al., 2012). Thus, D2R-mediated changes in striatal enkephalin and the resulting alterations in collateral GABA transmission may represent a unifying neural circuit mechanism mediating the risk for SUD. These data reveal the complex nature of enkephalin signaling in the striatum and suggest that heightened striatal enkephalin due to low D2Rs or extended cocaine use can have long-term effects on MSN activity, striatal output, and drug seeking and taking.
Limitations of the study
Our electrophysiology experiments used pharmacological approaches to infer heightened enkephalin tone in the striatum. We found that bath application of peptidase inhibitors reduced the frequency of spontaneous GABA events, and this was reversed by naloxone. We interpret these findings as evidence for heightened opioid peptide tone acting on presynaptic opioid receptors in D2-MSNs to restrain GABA release. While we found that MSN-Drd2KO mice also have heightened Penk gene expression, we cannot rule out a role for other opioids affecting GABA transmission. Opioid peptides responsible could be met- or leu-enkephalin from D2-MSNs, dynorphins or leu-enkephalin from D1-MSNs, and β-endorphin from striatal afferents. In addition, since recordings were performed in putative (ChR2-negative) D1-MSNs, it remains unclear whether enhancedenkephalin tone similarly inhibits collateral GABA onto D2-MSNs. Future work using genetically encoded fluorophores to label D1-and D2-MSNs will address the complexity of enkephalin’s actions at specific striatal synapses.
In addition, although MSN-Drd2KO mice were more sensitive to develop naloxone place aversion, we cannot rule out a contribution from dynorphin or β-endorphin. However, given these mice have normal Pdyn mRNA and robustly enhanced Penk mRNA, we suspect this effect is mediated by heightened enkephalin. We also observed that enhancing the enkephalin levels selectively in the NAc only modestly potentiated cocaine CPP acquisition. This may indicate that enkephalin in the NAc and dorsal striatum work together to support cocaine reward. Future experiments using MSN-selective Penk knockout mice will directly address the contribution of striatal enkephalin to cocaine seeking and taking and collateral GABA transmission.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information regarding reagents and resource sharing should be directed to and will be fulfilled by the Lead Contact, Dr. Lauren Dobbs (ldobbs@austin.utexas.edu).
Materials availability
This study did not generate new materials.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Adult (8–21 week) mice were used in all experiments. Male and female mice with a homozygous deletion of the D2R from MSNs (MSN-Drd2KO) were generated by crossing Drd2loxP/loxP and Adora2aCre mice, as previously described (Dobbs et al., 2017; Lemos et al., 2016). To overexpress the D2R, transgenic mice expressing the long form of the human Drd2 gene were generated as previously described (Kellendonk et al., 2006; Mayford et al., 1996). All mouse lines are available commercially and listed in the Key resources table. Mice expressing the Drd2 transgene under the tetracycline response element tetO were crossed with mice expressing the tetracycline transactivator (tTA) transgene under the CamKIIα promotor. This induced overexpression of the Drd2 transgene in the striatum of double transgenic tTA/tetO-positive mice (D2R-OE), but not in control mice which carry only one, or neither transgene. Transgene expression was switched off following treatment with the tetracycline analogue doxycycline administered in the chow (40 mg/kg Mutual Pharmaceutical). To test the effect of cocaine on Penk expression, male C57Bl/6J (Jackson Laboratory; #000664) or MSN-Drd2KOs were used. Experiments were performed in accordance with guidelines from the Animal Care and Use Committees at the University of Texas at Austin, the National Institute on Alcohol Abuse and Alcoholism, and the New York State Psychiatric Institute. Mice were group housed, except cannulated mice, and maintained under a 12:12 h light cycle (6:30 ON/18:30 OFF) with food and water ad libitum.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
|
| ||
| rAAV5-EF1-DIO-hChR2(H134R)-EYFP | UNC Vector Core | n/a; https://www.med.unc.edu/genetherapy/vectorcore/in-stockaav-vectors/deisseroth/ |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Cocaine HCl | Millipore Sigma | Cat #: C5776 |
| Saline | Hospira | NDC 0409–4888-06 |
| iScript Reverse Transcription Supermix | Biorad | Cat #: 1708840 |
| Met-enkephalin acetate salt hydrate | Millipore Sigma | Cat #: M6638 |
| DL-thiorphan | Millipore Sigma | Cat #: T6031 |
| Bestatin HCl | Millipore Sigma | Cat #: B8385 |
| Naloxone HCl | Tocris | Cat #: 0599 |
| CTAP | Tocris | Cat #: 1560 |
| Gabazine (SR95531) | Abcam | Cat #: ab120042 |
| NBQX disodium salt | Abcam | Cat #: ab120046 |
| (R)-CPP | Tocris | Cat #: 0247 |
| CGP 55845 HCl | Abcam | Cat #: ab120337 |
| Aprepitant | Tocris | Cat #: 6486 |
| J 113397 | Tocris | Cat #: 2598 |
| RNAlaterStabilization Solution | Ambion | Cat #: AM7020 |
| TaqMan Gene Expression Assays: preproenkephalin | Thermo Fisher | Mm01212875_m1 |
| TaqMan Gene Expression Assays: beta actin | Thermo Fisher | Mm01205647 |
| TaqMan Gene Expression Assays: dopamine D2 receptor | Thermo Fisher | Mm00438541_m1 |
| TaqMan Gene Expression Assays: delta 1 opioid receptor | Thermo Fisher | Mm00443063_m1 |
| TaqMan Gene Expression Assays: mu 1 opioid receptor | Thermo Fisher | Mm01188089_m1 |
| TaqMan Gene Expression Assays: prodynorphin | Thermo Fisher | Mm00457573_m1 |
| PrimePCR Probe Assay: Drd2, mouse | Bio Rad | qMmuCIP0030279 |
| SYBR Green Master Mix | Applied Biosystems | Fisher cat# 4309155 |
| Invitrogen Superscript II First Strand Synthesis System | Thermo Fisher | 11904018 |
| DNase I | Thermo Fisher | 89836 |
|
| ||
| Critical commercial assays | ||
|
| ||
| RNeasy Clean-Up Kit | QIAGEN | 74204 |
| RNeasy Plus Mini Kit | QIAGEN | Cat #: 74136 |
| MessageAmp II Biotin Enhanced Kit | Ambion | AM1791 |
| Affymetrix 430A2.0 microarrays | Affymetrix (acquired by Thermo Fisher | Not available |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mouse: Adora2a-Cre: Tg(Adora2a-cre)KG139Gsat/Mmucd | MMRRC | Cat# 031168-UCD; RRID:MMRRC_031168-UCD |
| Mouse: Drd2loxP/loxP: B6.129S4(FVB)-Drd2tm1.1Mrub/J | JAX | Cat# 020631; RRID:IMSR_JAX:020631 |
| Mouse: C57Bl/6J | JAX | Cat# 000664; RRID:IMSR_JAX:000664 |
| Mouse: B6;CBA-Tg(Camk2a-tTA)1Mmay/J | JAX | Cat# 003010; RRID:IMSR_JAX:003010 |
| Mouse: B6.Cg-Tg(tetO-DRD2)2–5Kndl/J | JAX | Cat# 028294; RRID:IMSR_JAX:028294 |
|
| ||
| Oligonucleotides | ||
|
| ||
| PPE1 Primer: AAGCCCTTTTCCAGCAGTGA | Sigma Custom Oligo | |
| PPE2 Primer: TGTACAGCACAAAGCAGCATGT | Sigma Custom Oligo | |
| GAPDH1 Primer: TGCAGTGGCAAAGTGGAGATT | Sigma Custom Oligo | |
| GAPDH2 Primer: TTGAATTTGCCGTGAGTGGA | Sigma Custom Oligo | |
|
| ||
| Software and algorithms | ||
|
| ||
| DNA-Chip Analyzer (dChip) software package | https://sites.google.com/site/dchipsoft/ | |
| ErmineG Gene expression profiling software | https://erminej.msl.ubc.ca/ | |
METHOD DETAILS
Stereotaxic cannulation and virus injection
Adult male and female Drd2loxP/loxP and MSN-Drd2KO mice were placed in a stereotaxic frame under isoflurane anesthesia and a 26-gauge bilateral guide cannula (Plastics One) was implanted above the NAc shell (AP: +1.34, ML: ± 1.0, DV: —3.5). Two skull screws and dental cement were used to secure the cannula in place. A dummy stylet was placed into the guide and protected with a dust cap when not in use. Mice were given at least one week after surgery before experimental procedures began. For electrophysiology experiments, adult female MSN-Drd2KO mice were stereotaxically infused under isoflurane anesthesia with a Cre-dependent adeno-associated virus (AAV) expressing channelrhodopsin-2-EYFP (rAAV5-EF1-DIO-hChR2(H134R)-EYFP; 4.5 × 1012, UNC; 300nL/side at 100 nL/min) bilaterally into the NAc core (from bregma: AP: + 1.3; ML: ± 1.0; DV: −4.6).
Jugular catheterization
Male and female C57Bl/6J mice were surgically implanted with indwelling jugular catheters by inserting the catheter into a hole in the vein made using vannas spring scissors. The catheter was held in place with silk suture thread and fed subcutaneously so the inlet port was fixed between the scapulae. Catheters were constructed from micro-renathane tubing (Braintree Scientific) attached to a 26-gauge pedestal inlet port surrounded by polyester mesh (Plastics One). Catheter patency was tested at the beginning of the experiment by infusing Brevital into the catheter (2% solution, 20 uL). Mice that failed patency were removed from the study.
Drugs
Doxycycline was administered ad libitum as a supplemented chow (40 mg/kg, Mutual Pharmaceutical) for 2 weeks to switch the transgene off. Cocaine (15 mg/kg, Sigma) and saline were administered intraperitoneally (i.p.) at 10 m//kg body weight. Naloxone, met-enkephalin acetate salt, DL-thiorphan, and bestatin HCl were supplied by Millipore Sigma. Gabazine (SR95531) was supplied by Abcam and CTAP and naloxone were from Tocris. Doses for cocaine and naloxone for place conditioning were selected based on previous experiments (Cunningham et al., 1995; Dobbs et al., 2016; Skoubis et al., 2005; Solecki et al., 2009). The in vivo met-enkephalin dose was selected based on our ex vivo slice physiology experiments. The CTAP dose was selected based off efficacy in blocking cocaine preference and opioid analgesia in mice and rats (Kramer et al., 1989; Soderman and Unterwald, 2008). Drugs were dissolved in aCSF (electrophysiology) or saline (CPP).
Electrophysiology
Due to limited availability of animals during the COVID-19 pandemic, only males were available for the electrophysiology experiments. Sagittal slices (240 μm) from adult male Adora2aCre or MSN-Drd2KO mice were prepared in cold cutting solution (in mM: 225 sucrose, 119 NaCl, 2.5 KCl, 0.1 CaCl2, 4.9 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, 1.25 glucose, and 3 kynurenic acid). Artificial cerebrospinal fluid (aCSF) contained (in mM): 124 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, and 20 glucose. Slices were maintained at 31–33°C for the duration of recordings. Whole-cell voltage clamp recordings were measured from ChR2-negative MSNs in the NAc core. We excluded cells that had a ChR2 current, were spontaneously active, had an Ri > 500 MΩ, or a resting membrane potential > −75 mV. Cells were held at −55 mV and GABA-A receptor-mediated synaptic responses were isolated with synaptic blockers (5 μM NBQX, 10 μM CPP, and 2 μM CGP). Recordings were made using glass electrodes (2.5–3.5 MΩ) filled with an internal solution containing (in mM): 60 CsMS, 60 CsCl, 10 HEPES, 0.2 Cs-EGTA, 4 Na-ATP, 0.4 Na-GTP, and 10 phosphocreatine (pH ~ 7.2, ~300 mOsm). Optically-evoked and spontaneously generated inhibitory post-synaptic currents (IPSCs) were measured during bath application of peptidase inhibitors (5 min; 2 μM thiorphan + 10 μM bestatin), met-enkephalin + peptidase inhibitors (5 min; 1 μM), naloxone (10 min; 5 μM) and gabazine (5 min; 5 μM). Optically-evoked IPSCs were triggered every 20 s by a single light pulse (473 nm; 0.2–1ms duration) delivered through a fiber optic (200 μm/0.22 NA, ThorLabs) connected to a laser (25 mW, CrystaLaser). Data were acquired using Multiclamp 700B (Molecular Devices), filtered at 1 kHz, and digitized at 5 kHz. All data were analyzed using pClamp (Clampfit, v. 10.3) and Mini Analysis (Synaptosoft).
Place conditioning
Male and female mice received either a 5-min habituation session (naloxone place aversion experiments) or a 30-min pre-conditioning preference test (cocaine place preference experiments) before undergoing eight days of an unbiased conditioning procedure in a two-chamber apparatus (Med Associates) with distinct tactile floor cues (Grid or Hole). For naloxone place aversion conditioning, mice received saline (CS-) or naloxone (CS+, 1 mg/kg) and were confined to either the Grid or Hole floor for 30 min. For cocaine place conditioning, mice received saline (CS-) or cocaine (CS+; 15 mg/kg) and were confined to the Grid or Hole floor for 15 min. Floor orientation (Grid on left or right), drug order (cocaine on first or second day), and drug-cue pairing (cocaine with Grid or Hole) were counterbalanced across mice. Saline-primed preference tests were administered after 4 and/or 8 days of conditioning, wherein mice have access to both floor types for 30 min. Additional drug-primed tests were also performed on the last experiment day in the naloxone place aversion experiment and the CTAP experiments. Preference is calculated as the percent time on the cocaine-paired floor, and also as the time spent on the grid floor for each conditioning subgroup (Grid + Drug or Hole + Drug). A significant difference in the time spent on the grid floor between these two conditioning groups indicates place conditioning (Cunningham et al., 2003). For example, if mice that had cocaine paired with the grid floor spend significantly more time on the grid floor than mice that had cocaine paired with the hole floor, this would be indicative of place preference.
To determine the role of enkephalin and MORs in the acquisition and expression of cocaine CPP, we infused the MOR antagonist CTAP or a met-enkephalin/peptidase inhibitor cocktail into the NAc shell during cocaine conditioning trials or preference tests in two different experiments. In the first experiment, Drd2loxP/loxP mice were conditioned with cocaine over 8 days. Mice then received a preference test, which involved an intra-NAc vehicle infusion immediately followed by saline (i.p.). The next day, mice were tested again after receiving an intra-NAc CTAP infusion immediately followed by saline (i.p.). In the second experiment, MSN-Drd2KO mice were conditioned with cocaine over 8 days. On CS+ days, each mouse received cocaine (15 mg/kg, i.p.) immediately after an intra-NAc infusion of either saline (NAc Veh + Coc group) or CTAP (NAc CTAP + Coc group). On CS- days, all mice received saline (10 mL/kg, i.p.) immediately following a sham microinjection into the NAc, wherein a shortened bilateral injector was inserted into the cannula that did not extend into the tissue. Saline-primed preference tests were administered after 4 (Test 1) and 8 (Test 2) conditioning days, and a naloxone-primed test was given the last experimental day. Mice received sham intra-NAc injections followed by i.p. injections on preference test days. In the third experiment, Drd2loxP/loxP mice were conditioned with cocaine over 8 days. On CS+ days, cocaine was given immediately after intra-NAc infusion of met-enkephalin cocktail (NAc Enk + Coc group). CS- days and saline-primed preference test were administered as in Experiment 2.
Intra-NAc microinjection procedure
Intra-NAc vehicle, enkephalin or CTAP was administered by gently restraining mice and inserting a bilateral microinjector into the NAc shell that extended 1.5 mm beyond the end of the cannula. Saline, CTAP (300 ng/side), or a met-enkephalin cocktail (5 μg met-enkephalin acetate salt + the peptidase inhibitors: 150 ng DL-thiorphan, 1 μg bestatin HCl) was infused over 3 min (100 nL/min) via a dual syringe pump. Peptidase inhibitors were included with met-enkephalin to prevent premature degradation of met-enkephalin. Microinjectors were left in place 2 min after infusion to allow for drug diffusion. Stylets and dust caps were replaced and then mice were administered either saline or cocaine and were placed into the preference chamber.
Contingent and non-contingent cocaine treatment
To determine the effect of withdrawal from non-contingent long-term and short-term cocaine exposure on striatal Penk expression, C57Bl/6J mice and MSN-Drd2KO mice were administered saline or cocaine injections for either 5 days (15 mg/kg, i.p.) or 10 days (20 mg/kg, i.p.). For these experiments, injections occurred in the animal’s light cycle and mice were immediately returned to their home cage after injection. To determine whether contingently administered cocaine affects striatal Penk expression, mice were trained to self-administer cocaine intravenously in an operant chamber equipped with two retractable levers and cue lights positioned over each lever (Med Associates). Mice were allowed to lever press for cocaine under a fixed ratio 1 schedule of reinforcement, wherein a single press of the active lever resulted in illumination of the cue light and delivery of a cocaine infusion (1 mg/kg/infusion). A separate group of mice were yoked saline controls, which received saline infusions in the same pattern as a yoked cocaine-administering animal. Self-administration occurred in the animal’s dark cycle over 10 days in daily 2-h sessions. For these contingent and non-contingent experiments, brains were extracted for qPCR analysis 14 days after their last injection. To determine how short-term cocaine in absence of withdrawal affects striatal Penk expression, mice first had 3 days of saline injections (i.p., 10mL/kg) followed by 5 days of saline or cocaine (15 mg/kg, i.p.). Locomotor activity was measured in an open field apparatus (28 cm × 28 cm; Med Associates) for 1 h after each injection in the animal’s light cycle. Brains were extracted for qPCR analysis immediately after the last injection.
Quantitative polymerase chain reaction
Mice were euthanized with isoflurane, decapitated and brains were extracted. The striatum was dissected on ice and stored in RNA-later Stabilization Solution (ThermoFisher) for 24 h at 4°C, then at −80°C until extraction. RNA was purified using a RNeasy Plus Mini kit (Qiagen) and cDNA was synthesized using iScript Reverse Transcription Supermix (Biorad) or the Invitrogen Superscript II First Strand Synthesis System (Thermo Fisher). For real time PCR of samples from MSN-Drd2KO or C57BL/6J mice, TaqMan Gene Expression Assays (Thermo Fisher) or PrimePCR Probe Assays (Bio-Rad) were used to quantify relative gene expression using a StepOnePlus Real-Time PCR system (Applied Biosystems) or a CFX384 (Bio-Rad). The specific probes used were: from Thermo Fisher, beta actin (Mm01205647), preproenkephalin (Mm01212875_m1), dopamine D2 receptor (Mm00438541_m1), delta opioid receptor (Mm00443063_m1), and mu opioid receptor (Mm01188089_m1), prodynorphin (Mm00457573_m1), and from Bio-Rad, dopamine D2 receptor (qMmuCIP0030279). For the D2R-OE experiment, SYBR Green PCR Master Mix (Applied Biosystems) was used to quantify Penk expression and real time PCR was carried out on a DNA engine with chromo4 real time detection system (Bio-Rad). Primer sequences used included: PPE1: AAGCCCTTTTCCAGCAGTGA, PPE2: TGTACAGCACAAAGCAGCATGT, GAPDH1: TGCAGTGGCAAAGTGGAGATT, GAPDG2: TTGAATTTGCCGTGAGTGGA. All real time PCR reactions were run in triplicate with GAPDH or beta actin run in parallel as a reference. Relative expression of each gene of interest was calculated using the 2−∆∆Ct method (Livak and Schmittgen, 2001).
Gene chip analysis
Total RNA from each of 10 mice (5 control, 5 D2R-OE) was amplified and transcribed into cRNA using the MessageAmp II Biotin Enhanced Kit (Ambion) and hybridized with 10 Affymetrix 430A2.0 microarrays in the Gene Chip Facility of the Columbia Genome Center. The raw data was then analyzed using dChip (https://sites.google.com/site/dchipsoft/) and ErmineG (https://erminej.msl.ubc.ca/).
QUANTIFICATION AND STATISTICAL ANALYSIS
Analyses were performed using Prism (v.8, GraphPad). In consideration of sex as a biological variable (SABV), power and effect sizes were calculated from datasets that included both sexes to determine whether the datasets were sufficiently powered (≥0.8) to detect differences between sex. While males and females were used in most experiments, none of these datasets were sufficiently powered to detect sex differences, and thus data were not analyzed with sex as a statistical factor (Table S1).
For data that met assumptions for parametric tests, one-way, two-way, or three-way analysis of variance (ANOVA) was used, with repeated measures when appropriate, to analyze data with more than 2 groups or factors. When repeated measures data had missing values, a mixed-effects model was used. Significant main effects and interactions were followed-up with pairwise t-tests corrected for multiple comparisons (Sidak). Violations to sphericity in 3-way repeated measures ANOVAs (or mixed models) were corrected using Greenhouse-Geisser. Paired, unpaired, or one-sample t-tests, were used to analyze pairwise data, two independent samples, and comparisons against a single value, respectively. Corrections for unequal variance were made using Welch’s correction when appropriate. For data that did not meet assumptions for parametric tests (Adora2a-Cre sIPSC distributions in Figures 2 and 3), non-parametric alternatives were used. Wilcoxon signed rank test was used to compare sIPSCs before and after drug application, and the Kolmogorov-Smirnov test was used to compare distributions between genotypes. Data are presented as the mean ± SEM, and results were considered significant at an alpha of 0.05.
Supplementary Material
Highlights.
Dopamine D2Rs bidirectionally regulate striatal enkephalin expression
Endogenous enkephalin suppresses intra-striatal GABA transmission
Heightened striatal enkephalin levels potentiate cocaine reward in wild-type mice
Opioid receptor antagonists attenuate cocaine reward in wild-type mice
ACKNOWLEDGMENTS
This study was funded by a Rising STARs grant from the University of Texas at Austin and start-up funds from the Dell Medical School to L.K.D., an Undergraduate Research Fellowship Award from the University of Texas at Austin to R.L., a grant from the National Institute of Mental Health to C.K. (R01MH093672), and by the Intramural Programs of NIAAA and NINDS (ZIA-AA000421) to V.A.A.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111440.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, and Walker JM (1984). Endogenous opioids: biology and function. Annu. Rev. Neurosci 7, 223–255. 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Kupferschmidt DA, and Lovinger DM (2014). Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nat. Neurosci 17, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, and Borrelli E (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, and Sulzer D (2004). Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42, 653–663. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, and Rubinstein M (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci 14, 1033–1038. 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, and Kreek MJ (1992). Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res. Mol. Brain Res 14, 231–238. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, Collector D, Torres OV, Terry N, and Jayanthi S (2016). Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci. Rep 6. 10.1038/srep37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, and Berridge KC (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “Wanting”. J. Neurosci 34, 4239–4250. 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, and Bruchas MR (2019). A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron 102, 529–552. 10.1016/j.neuron.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, and Leibowitz SF (2010). Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin. Exp. Res 34, 761–770. 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, and Lüscher C (2016). Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron 92, 214–226. 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, and Ambrosio E (2001). Extinction of cocaine self-administration produces a differential time related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology 25, 185–194. 10.1016/S0893-133X(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, and Okorn DM (1995). Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Experimental and Clinical Psychopharmacology 3 (4), 330–343. 10.1037/1064-1297.3.4.330. [DOI] [Google Scholar]
- Cunningham CL, Ferree NK, and Howard MA (2003). Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 170, 409–422. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, et al. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315, 1267–1270. 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Nader MA, and Porrino LJ (1997). Long-term cocaine self-administration decreases striatal preproenkephalin mRNA in rhesus monkeys. Pharmacol. Biochem. Behav 57, 471–475. [DOI] [PubMed] [Google Scholar]
- Day R, Lazure C, Basak A, Boudreault A, Limperis P, Dong W, and Lindberg I (1998). Prodynorphin processing by proprotein convertase 2: cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J. Biol. Chem 273, 829–836. 10.1074/jbc.273.2.829. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, and Alvarez VA (2016). Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90. 10.1016/j.neuron.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Lemos JC, and Alvarez VA (2017). Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Gene Brain Behav 16. 10.1111/gbb.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME, Eberhart L, Ron D, and Alvarez VA (2019). D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 44, 805–816. 10.1038/s41386-018-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE, and Hotchkiss AJ (1983). Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides 4, 1–9. 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Giraudt P, Affolter H-U, Herbertt E, and Hotchkiss AJ (1984). Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc. Natl. Acad. Sci. USA 81, 3949–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Kläger M, Smolka MN, Scheurich A, et al. (2008). Association of low striatal dopamine D2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am. J. Psychiatr 165, 507–514. 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, Moore H, Javitch JA, and Kellendonk C (2018). Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat. Commun 9, 1086. 10.1038/s41467-018-03272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, and Surmeier DJ (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466. 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr., and Sibley DR (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C (2005). Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr. Top. Med. Chem 4, 39–50. 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Gleich T, Spitta G, Butler O, Zacharias K, Aydin S, Sebold M, Garbusow M, Rapp M, Schubert F, Buchert R, et al. (2020). Dopamine D2/3 receptor availability in alcohol use disorder and individuals at high risk: towards a dimensional approach. Addiction Biol 26, e12915. 10.1111/adb.12915. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Lane JD, and Smith JE (1984). Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol. Biochem. Behav 20, 451–455. 10.1016/0091-3057(84)90284-3. [DOI] [PubMed] [Google Scholar]
- Gong S, Fayette N, Heinsbroek JA, Correspondence CPF, and Ford CP (2021). Cocaine shifts dopamine D2 receptor sensitivity to gate conditioned behaviors. Neuron 109, 3421–3435. 10.1016/j.neuron.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Svingos AL, and Pickel VM (1997). Dual ultrastructural localization of m-opioid receptors and NMDA-type glutamate receptors in the shell of the rat nucleus accumbens. J. Neurosci 17, 4839–4848. 10.1523/jneurosci.17-12-04839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GC, Patel SD, Winters BL, and Bagley EE (2020). Neprilysin controls the synaptic activity of neuropeptides in the intercalated cells of the amygdala. Mol. Pharmacol 98, 454–461. 10.1124/mol.119.119370. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martín-García E, and Maldonado R (2014). Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology 39, 2974–2988. 10.1038/npp.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla AC, Kupchik YM, and Kalivas PW (2017). Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J. Neurosci 37, 757–767. 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rösch F, et al. (2005). Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am. J. Psychiatr 162, 1515–1520. 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Higley MJ, and Sabatini BL (2010). Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci 13, 958–966. 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, and Kuhar MJ (2003). Intra-accumbal injection of CART (Cocaine-Amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J. Pharmacol. Exp. Therapeut 307, 1038–1044. 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Jonathan Polan H, Malleret G, Vronskaya S, Winiger V, Moore H, and Kandel ER (2006). Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49, 603–615. 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kramer TH, Shook JE, Kazmierski W, Ayres EA, Wire WS, Hruby VJ, and Burks TF (1989). Novel peptidic Mu opioid antagonists: pharmacologic characterization in vitro and in vivo. J. Pharmacol. Exp. Therapeut 249, 544–551. [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, and Kalivas PW (2014). Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J. Neurosci 34, 1057–1066. 10.1523/JNEUROSCI.4336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse MA, Sartori AM, Weinmann O, Simpson EH, Kellendonk C, and Weber-Stadlbauer U (2018). Striatal dopamine 2 receptor upregulation during development predisposes to diet-induced obesity by reducing energy output in mice. Proc. Natl. Acad. Sci. USA 115, 10493–10498. 10.1073/pnas.1800171115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, et al. (2009). Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci 29, 14734–14740. 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, and Alvarez VA (2016). Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90, 824–838. 10.1016/j.neuron.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 C T method. Methods 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, and Watson SJ (1994). Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol 350, 412–438. 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mantsch JRR, Yuferov V, Mathieu-Kia A-MM, Ho A, and Kreek MJ (2004). Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 175, 26–36. 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, and Comer SD (2012). Deficits in dopamine D 2 receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol. Psychiatr 71, 192–198. 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Kia AM, and Besson MJ (1998). Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res. Mol. Brain Res 54, 141–151. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, and Surmeier DJ (2004). D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J. Neurosci 24, 10289–10301. 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang Y-Y, Wang L, Hawkins RD, and Kandel ER (1996). Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Sévin DC, Klee ML, Dieter S, Sauer U, and Sommer WH (2015). The neurometabolic fingerprint of excessive alcohol drinking. Neuropsychopharmacology 40, 1259–1268. 10.1038/npp.2014.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang G-J, and Volkow ND (2012). PET imaging predicts future body weight and cocaine preference. Neuroimage 59, 1508–1513. 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongi-Bragato B, Zamponi E, García-Keller C, Assis MA, Virgolini MB, Mascó DH, Zimmer A, and Cancela LM (2016). Enkephalin is essential for the molecular and behavioral expression of cocaine sensitization. Addiction Biol 21, 326–338. 10.1111/adb.12200. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, and Friedman DP (1998). Effect of Cocaine Self-Administration on Dopamine D 2 Receptors in Rhesus Monkeys. Synapse 30, 88–96. . [DOI] [PubMed] [Google Scholar]
- Nader MA, and Czoty PW (2005). PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am. J. Psychiatr 162, 1473–1482. 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, and Porrina LJ (2002). Effects of Cocaine Self-administration on Striatal Dopamine Systems in Rhesus Monkeys: Initial and Chronic Exposure. Neuropsychopharmacology 27 (1), 35–46. 10.1016/s0893-133x(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, and Mach RH (2006). PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience 9 (8), 1050–1056. 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nam H, Chandra R, Francis TC, Dias C, Cheer JF, and Lobo MK (2019). Reduced nucleus accumbens enkephalins underlie vulnerability to social defeat stress. Neuropsychopharmacology 44, 1876–1885. 10.1038/s41386-019-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Roman E, and Nylander I (2012). Differences in basal and ethanol-induced levels of opioid peptides in Wistar rats from five different suppliers. Peptides 36, 1–8. 10.1016/j.peptides.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Peciña S, and Berridge KC (2005). Hedonic hot spot in nucleus accumbens shell: where do m-Opioids cause increased hedonic impact of sweetness? J. Neurosci 25, 11777–11786. 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzziello F, Falasca S, Andren PE, Rainer G, and Zhang X (2013). Chronic nicotine treatment impacts the regulation of opioid and non-opioid peptides in the rat dorsal striatum. Mol. Cell. Proteomics 12, 1553–1562. 10.1074/mcp.M112.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach TT, Tang F, and Schwartz JP (1984). Enkephalin biosynthesis in adrenal medulla modulation of proenkephalin mRNA content of cultured chromaffin cells by 8-bromo-adenosine 3’, 5’-Monophosphate. Mol. Pharmacol 26, 255–260. [PubMed] [Google Scholar]
- Romano GJ, Shivers BD, Harlan RE, Howells RD, and Pfaff DW (1987). Haloperidol increases proenkephalin mRNA levels in the caudate-putamen of the rat: a quantitative study at the cellular level using in situ hybridization. Mol. Brain Res 2, 33–41. 10.1016/0169-328X(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, and Pickel VM (1994). Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J. Neurosci 14, 88–106. 10.1523/jneurosci.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, and Self DW (2009). Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 34, 1946–1957. 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, and Maidment NT (2005). Endogenous enkephalins, not endorphins, modulate basalhedonic state in mice. European Journal of Neuroscience 21, 1379–1384. 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Mathes HW, Walwyn WM, Kieffer BL, and Maidment NT (2001). Naloxone fails to produce conditioned place aversion in m-opioid receptor knock-out mice. Neuroscience 106 (4), 757–763. 10.1016/S0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Soderman AR, and Unterwald EM (2008). Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience 154, 1506–1516. 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki W, Turek A, Kubik J, and Przewlocki R (2009). Motivational effects of opiates in conditioned place preference and aversion paradigm-a study in three inbred strains of mice. Psychopharmacology 207, 245–255. 10.1007/s00213-009-1672-7. [DOI] [PubMed] [Google Scholar]
- Steiner H, and Gerfen CR (1998). Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp. Brain Res 123, 60–76. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Unl GR, and Pickel VM (1996). Ultrastructural immunocytochemical localization of m-opioid receptors in rat nucleus accumbens: extrasynaptic plasmalemmal distribution and association with Leu5-enkephalin. J. Neurosci 16, 4162–4173. 10.1523/jneurosci.16-13-04162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Unl GR, and Pickel VM (1997). m-opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J. Neurosci 17, 2585–2594. 10.1523/jneurosci.17-07-02585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu BH, Remmers BC, Toddes C, Brandner DD, Lefevre EM, Kocharian A, Retzlaff CL, Dick RM, Mashal MA, Gauthier EA, et al. (2022). Angiotensin-converting enzyme gates brain circuit–specific plasticity via an endogenous opioid. Science 375, 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, and Piscani K (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin. Exp. Res 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-JJ, Fowler JS, Ding Y-SS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, et al. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am. J. Psychiatr 158, 2015–2021. 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, and Wong C (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci 26, 6583–6588. 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, and Wong C (2007). Behavioral/Systems/cognitive profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci 27, 12700–12706. 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, and Pascani K (1997). Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 16, 174–182. 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Weisinger G (1995). The transcriptional regulation of the preproenkephalin gene. Biochem. J 307, 617–629. 10.1042/bj3070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, and Kim JH (2007). Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology 53, 344–351. 10.1016/J.NEUROPHARM.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim WY, and Kim JH (2010). Microinjection of CART peptide 55–102 into the nucleus accumbens core inhibits the expression of conditioned hyperactivity in a cocaine-associated environment. Behav. Brain Res 207, 169–173. 10.1016/J.BBR.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


