Abstract
To characterize patients with mumps vaccine failure, avidity testing was performed with the Enzygnost Anti-Parotitis Virus/IgG kit using a single-dilution–6 M urea denaturation method. Five groups of patients were tested. Group 1 consisted of 29 patients with primary mumps infections; group 2 was 20 children and adults with a definite history of natural infection; group 3 was 7 patients with a recent mumps vaccination, 1 of whom developed parotid gland swelling and aseptic meningitis; group 4 was 14 patients with mumps vaccine failure; and group 5 was 6 patients with recurrent episodes of parotitis in addition to a history of vaccination. On the basis of the results of groups 1 and 2, an avidity of ≦31% was determined to be low, and ≧32% was determined to be high. Avidity maturation from low to high appears to occur around 180 days after the acute illness. The results of group 3 showed that the vaccine-induced immunoglobulin G (IgG) had very low avidity. Among the 14 patients in group 4, 12 patients, including 7 with a positive IgM response, were diagnosed as having secondary vaccine failures. The results of group 5 suggested the possibility that the avidity of the mumps vaccine-induced IgG remains low or borderline. These results showed that secondary mumps vaccine failure occurs not infrequently, even among school age children under condition in which the vaccine coverage is low (i.e., 33% in our study population), and therefore, vaccinees are prone to be exposed to wild-type viruses. Avidity testing should provide information useful for the analysis of mumps virus infections.
In recent years, increasing attention has been paid to mumps epidemics which occur in highly as well as partially vaccinated populations (1a, 4, 6, 9). In fact, in the Hokkaido district, where this study was done, the mumps vaccine coverage in recent years was around 33% and the vaccine failure rate was reported to be 9.8% (1). Since the current mumps vaccine has been believed to be highly effective (14, 21) and most of the patients with vaccine failures had a detectable serum immunoglobulin M (IgM) antibody (1a, 4, 6), mumps vaccine failures have been attributed mainly to primary vaccine failure (PVF). The relative contribution of waning immunity to vaccine failures (secondary vaccine failure [SVF]) is controversial (1a, 9).
In this context, a question has been raised about the validity of detectable IgM in serum as a marker for primary infection. For this, several lines of evidence have been advanced concerning the superiority of avidity testing for virus-specific IgG over the detection of IgM in distinguishing a primary from a secondary immune response in a number of viral infections, such as those with tick-borne encephalitis virus (5), rubella virus (8, 10, 18), varicella-zoster virus (11), measles virus (15), cytomegalovirus (16), B19 parvovirus (17), human herpesvirus 6 (19), and hepatitis C virus (20). The development of enzyme-linked immunosorbent assay (ELISA) methodology to determine avidity has contributed to this generalization.
In determining avidity by using an ELISA system, a titration curve shift method is the most accurate but is rather cumbersome and costly. Therefore, it will be restricted to problematic cases with borderline avidity. In this respect, a single-dilution method is simple to perform and cost saving, although subject to a few limitations which are of concern. A major problem is that this method is restricted to an appropriate range of linearity between optical density (OD) and antibody titer. Under- or overestimation of avidity can occur at very low or high antibody titers (5, 8, 20). For practical purposes, however, the latter is an acceptable and reliable method for routine testing of clinical samples when the limitations are taken into consideration (5, 17).
The purpose of this study was to characterize patients with mumps vaccine failures on the basis of IgG avidity testing, which was performed by means of the single-dilution method.
MATERIALS AND METHODS
Patients.
The clinical diagnosis of mumps was based on general criteria (3); that is, an illness with acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary gland lasting ≧2 days and without another apparent cause. Whether parotid swelling was unilateral or bilateral was not stringently evaluated because bilateral swelling is not necessarily a clinical hallmark of a primary mumps virus infection (2).
Patients were grouped into five categories according to mumps virus infection and immunization status.
Group 1 (acute group, cases A-1 through A-29, ages 3 to 13 years) consisted of 29 patients with primary mumps virus infection. They fitted the clinical criteria, with a positive IgM response as a marker of current mumps virus infection and without a history of previous natural infection or vaccination. In cases A-1 through -18, blood was drawn within 7 days of the onset of parotid gland swelling, while in A-19 through -29, blood was drawn for reasons other than mumps several weeks or months after the acute episode of mumps. Cases A-4, -5, and -14 were complicated by aseptic meningitis.
Group 2 (past group, cases P-1 through P-20, ages 3 to 38 years) consisted of 20 children and adults with a definite history of mumps at least 1 year before the sampling.
Group 3 (vaccine group, cases V-1 through V-7, ages 1 to 7 years) consisted of seven patients with a recent mumps vaccination. Patients V-1 and -7 received a trivalent mumps-measles-rubella vaccine, and V-2 through -6 received a monovalent mumps vaccine. In Japan during 1989 to 1991, the Urabe strain was used exclusively in the trivalent vaccine (cost free, abolished in 1993), and the Urabe or some other strain was used in the trivalent vaccine during 1991 to 1993 and has been used as a monovalent vaccine since 1993. In addition, since mumps vaccination must be done at one’s own expense (about $30) in Japan, it is usually administered only once at any age after the first birthday. Case V-7 developed parotid gland swelling 17 days after vaccination and then, 4 days later, aseptic meningitis. The Urabe mumps vaccine virus was detected in the cerebrospinal fluid of this patient by PCR (by Akio Yamada using a method described elsewhere [13]).
Group 4 (failure group, cases VF-1 through VF-14, ages 5 to 11 years) consisted of 14 patients with vaccine failure. All of the patients had clinical symptoms indistinguishable from those of group 1 (acute group) but had a history of vaccination with either a trivalent mumps-measles-rubella vaccine or a monovalent mumps vaccine 2 or more years before. Cases VF-5, -9, -13, and -14 were complicated by aseptic meningitis; moreover, case VF-9 involved permanent right neuronal hearing loss. The wild-type mumps virus genome was detected by PCR in the cerebrospinal fluid of this patient (by Tetsuo Nakayama using a method described elsewhere [12]).
Group 5 (recurrent group, cases R-1 through R-6, ages 4 to 12 years) consisted of a rather heterogeneous population of six patients with recurrent parotitis and a history of vaccination. Several years after vaccination, all of these patients experienced at least one episode of a febrile illness with parotitis which had been diagnosed at that time as mumps, but the validity of the previous diagnosis of mumps could not be verified. Case R-1 had two episodes of parotitis during the past 6 months. Any other apparent cause for the parotid gland swelling of these patients has not been established.
Methods.
For ELISA, an Enzygnost Anti-Parotitis Virus/IgM or IgG kit (Dade-Behring, Marburg, Germany) was used. Procedures for IgM detection were performed in accordance with the instructions of the manufacturer, by manual handling with a Vmax microplate reader (Molecular Devices, Sunnyvale, Calif.). For a long time, a positive response by IgM has been equated with a primary response. However, with the recent advent of more sensitive assay systems for the detection of IgM and the introduction of the concept of IgG avidity into clinical practice, the equation needs some modifications. That is, IgM can be detected after reinfection or reactivation of several kinds of viruses, although it may be less frequent and lower in intensity than that detected after a primary infection (5, 15, 16, 18, 19). Accordingly, in this study, a positive IgM response was regarded as a marker of a current infection but not in itself of a primary infection. According to the manufacturer, the IgM kit does not permit quantitative analysis.
For determination of IgG titer and avidity, a pair of samples (initial dilution, 1:231) were placed in the antigen and control wells and incubated at 37°C for 1 h or at 4°C for 16 h. After this, one was washed with an ordinary washing buffer provided by the manufacturer and the other was washed with a washing buffer supplemented with urea (Nakalai Tesque, Kyoto, Japan), twice for 5 min each time (for determination of the optimal concentration of urea, see Results). After being washed twice for 2 min each time with the ordinary washing buffer, the subsequent procedures were done in accordance with the instructions of the manufacturer. Percent avidity was calculated as (urea-treated OD ÷ untreated OD) × 100.
According to the information given by the supplier (Hoechst Japan, Tokyo, Japan), OD values between 0.100 and 2.500 allow calculation of IgG titers, which corresponds approximately to titers between ×300 and ×18,000. Thus, we determined avidity within this OD range. Samples with OD values below 0.100 were regarded as not detectable, and samples with OD values beyond 2.500 were diluted appropriately and retested.
RESULTS
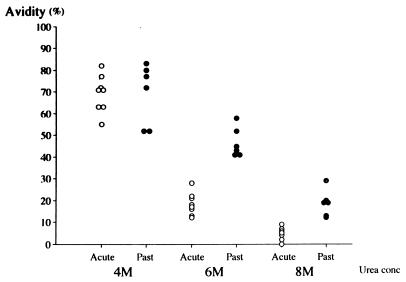
First, to determine an optimal concentration of urea, we tested some representative samples from groups 1 and 2 washed with urea at three different concentrations (4, 6, and 8 M). As shown in Fig. 1, because the best separation in terms of avidity between the two groups was observed without any overlap when 6 M urea was used, we subsequently tested samples by means of 6 M urea washing.
FIG. 1.
Results of avidity testing using different concentrations of urea. Symbols: ○, acute-phase samples of primary infection; •, samples of past infection.
The results of avidity testing with the 20 samples from the 18 patients in group 1 (acute group, cases A-1 through -18) are shown in Table 1. As shown in Table 1, while the IgG titers were quite variable, regardless of the number of days from the onset of parotid gland swelling to blood sampling, avidity was uniformly low. These 20 samples gave a mean avidity of 10.3% ± 7.3% (mean ± 3 standard deviations [SD] = 32.2%). Thus, we settled on an avidity of ≧32% as high.
TABLE 1.
Mumps virus-specific IgG titers and avidities of patients with primary mumps infections
| Case no. | Age (yr)/sexa | Day of samplingb | IgM | IgG
|
|
|---|---|---|---|---|---|
| Titer (102) | Avidity (%) | ||||
| A-1 | 3/M | 0 | Posc | 8 | 8 |
| A-2 | 6/F | 0 | Pos | 34 | 1 |
| A-3 | 11/M | 0 | Pos | 10 | 2 |
| A-4 | 7/F | 0 | Pos | 124 | 17 |
| A-4 | 7/F | 5 | Pos | 141 | 18 |
| A-5 | 6/M | 0 | Pos | 48 | 12 |
| A-5 | 6/M | 8 | Pos | 105 | 16 |
| A-6 | 11/M | 1 | Pos | 47 | 18 |
| A-7 | 3/M | 1 | Pos | 5 | 0 |
| A-8 | 5/M | 1 | Pos | 20 | 7 |
| A-9 | 13/M | 1 | Pos | 31 | 3 |
| A-10 | 5/M | 2 | Pos | 41 | 9 |
| A-11 | 7/F | 3 | Pos | 171 | 22 |
| A-12 | 5/M | 3 | Pos | 152 | 19 |
| A-13 | 4/M | 3 | Pos | 27 | 0 |
| A-14 | 5/M | 5 | Pos | 20 | 5 |
| A-15 | 5/M | 5 | Pos | 94 | 21 |
| A-16 | 7/F | 5 | Pos | 19 | 2 |
| A-17 | 8/M | 7 | Pos | 86 | 13 |
| A-18 | 5/M | 7 | Pos | 112 | 12 |
M, male; F, female.
The day on which parotid gland pain and/or swelling was first noticed was designated day 0.
Pos, positive.
As shown in Table 2, the results obtained with the 20 samples from the 20 persons from group 2 (past group) were relatively homogeneous in terms of both IgG titer and avidity compared with those of group 1 (acute group). These gave a mean avidity of 46.6% ± 5.4% (mean − 3 SD = 30.4%). Thus, we settled on an avidity of ≦31% as low. Consequently, all of the samples from group 1 (acute group) were classified as low avidity and all of the samples from group 2 (past group) were classified as high avidity according to this criterion.
TABLE 2.
Mumps virus-specific IgG titers and avidities of individuals with past mumps infections
| Case no. | IgG
|
|
|---|---|---|
| Titer (102) | Avidity (%) | |
| P-1 | 51 | 39 |
| P-2 | 51 | 39 |
| P-3 | 27 | 40 |
| P-4 | 26 | 41 |
| P-5 | 76 | 41 |
| P-6 | 20 | 42 |
| P-7 | 51 | 43 |
| P-8 | 26 | 45 |
| P-9 | 42 | 46 |
| P-10 | 18 | 46 |
| P-11 | 20 | 47 |
| P-12 | 15 | 47 |
| P-13 | 99 | 47 |
| P-14 | 66 | 48 |
| P-15 | 65 | 48 |
| P-16 | 75 | 52 |
| P-17 | 31 | 53 |
| P-18 | 87 | 53 |
| P-19 | 56 | 56 |
| P-20 | 31 | 58 |
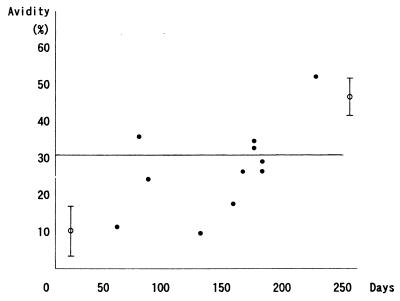
In Fig. 2, the results of cases A-19 through -29, which represent the time course of avidity maturation following primary infection, are shown. Three of the six samples which were obtained more than 165 days after the acute illness showed high avidity, and the remaining three samples showed borderline low avidity (≧27%).
FIG. 2.
Time course of avidity maturation in mumps. The day on which parotid gland pain and/or swelling was first noticed was designated day 0. The line at 31% denotes the borderline between low and high avidity. The circle with error bars at the left indicates the mean ± SD of the acute group, and that at the right indicates that of the past group.
The results of avidity testing of the 10 samples from group 3 (vaccine group) are shown in Table 3. Cases V-1 through -6 were characterized by a low titer of very low-avidity IgG. By sharp contrast, a high titer of low-avidity IgG, which was comparable to that of group 1 (acute group), was detected in case V-7, which involved aseptic meningitis following vaccination.
TABLE 3.
Mumps virus-specific IgG titers and avidities of individuals vaccinated against mumps
| Case no. | Age (yr)/sexa | Daysb | IgMc | IgG
|
|
|---|---|---|---|---|---|
| Titer (102) | Avidity (%) | ||||
| V-1 | 1/F | 20 | Neg | NDd | |
| V-1 | 1/F | 45 | Pos | 16 | 1 |
| V-2 | 4/F | 31 | NT | 4 | 3 |
| V-3 | 3/M | 34 | NT | 6 | 1 |
| V-4 | 4/F | 75 | NT | 14 | 1 |
| V-4 | 4/F | 90 | NT | 18 | 2 |
| V-5 | 7/F | 166 | Neg | 12 | 0 |
| V-5 | 7/F | 237 | Neg | 21 | 16 |
| V-6 | 2/M | 349 | NT | 6 | 16 |
| V-7 | 1/M | 36 (19)e | Pos | 231 | 28 |
M, male; F, female.
The day of vaccination was designated day 0.
Neg, negative; Pos, positive; NT, not tested.
ND, not detectable.
This patient developed parotid gland swelling 17 days after vaccination. Day 19 was counted from the onset of disease. The Urabe mumps vaccine virus was detected in the cerebrospinal fluid by PCR.
The results of avidity testing with the 15 samples from the 14 patients from group 4 (failure group) are shown in Table 4. Cases VF-1 and -2 showed a positive IgM response with low-avidity IgG. This indicates a typical primary immune response, suggesting that these cases were PVFs. Cases VF-10 through -14 showed a negative IgM response with a high titer of high-avidity IgG. The IgG titers were clearly distinguishable from those in group 2 (past group). This indicates a typical secondary immune response, suggesting that these cases were SVFs. Cases VF-3 through -9 showed a positive IgM response and a high titer of high-avidity IgG which was comparable to that of the SVF cases. This strongly indicates that these cases were also SVFs.
TABLE 4.
Mumps virus-specific IgG titers and avidities of patients with mumps vaccine failures
| Case no. | Age (yr)/sexa | Age (yr) at vaccination | Day of samplingb | IgMc | IgG
|
|
|---|---|---|---|---|---|---|
| Titer (102) | Avidity (%) | |||||
| VF-1 | 8/F | 3 | 0 | Pos | 30 | 1 |
| VF-2 | 6/F | 3 | 1 | Pos | 31 | 20 |
| VF-3 | 8/M | 3 | 0 | Pos | 99 | 47 |
| VF-4 | 11/M | 3 | 0 | Pos | 202 | 66 |
| VF-5d | 7/M | 4 | 2 | Pos | 170 | 60 |
| VF-6 | 8/M | 2 | 2 | Pos | 131 | 56 |
| VF-7 | 5/F | 3 | 3 | Pos | 195 | 63 |
| VF-8 | 7/F | 1 | 3 | Pos | 115 | 51 |
| VF-9de | 6/F | 1 | 5 | Pos | 351 | 86 |
| VF-10 | 5/M | 2 | 1 | Neg | 69 | 58 |
| VF-10 | 5/M | 2 | 18 | Neg | 337 | 81 |
| VF-11 | 5/M | 4 | 1 | Neg | 134 | 59 |
| VF-12 | 5/F | 1 | 5 | Neg | 169 | 57 |
| VF-13d | 8/M | 1 | 5 | Neg | 198 | 63 |
| VF-14d | 11/M | 2 | 8 | Neg | 219 | 52 |
M, male; F, female.
The day on which parotid gland pain and/or swelling was first noticed was designated day 0.
Pos, positive; Neg, negative.
Complicated by aseptic meningitis.
Mumps virus was detected in the cerebrospinal fluid by PCR.
The results of avidity testing with the seven samples from the six patients in group 5 (recurrent group) are shown in Table 5. All of the samples except that from case R-3, which was not tested, were negative for IgM. Also, none showed a high IgG titer, which suggests a secondary immune response. Case R-6 showed no IgG response. Overall, none exhibited a typical primary or secondary immune response, which suggested current mumps virus infection. Cases R-1 and -2 showed just borderline avidity, case R-3 showed borderline high avidity, and cases R-4 and -5 showed low avidity.
TABLE 5.
Mumps virus-specific IgG titers and avidities of patients with recurrent episodes of parotitis
| Case no. | Age (yr)/sexa | Age (yr) at vaccination | Previous parotitis | Day of samplinge | IgMb | IgG
|
|
|---|---|---|---|---|---|---|---|
| Titer (102) | Avidity (%) | ||||||
| R-1 | 7/M | 2 | Repeated | 1 | Neg | 53 | 33 |
| R-1 | 7/M | 2 | Repeated | 1c | Neg | 43 | 30 |
| R-2 | 4/M | 1 | Once | 2 | Neg | 40 | 30 |
| R-3 | 12/M | 4 | Repeated | 0 | NT | 10 | 37 |
| R-4 | 8/M | 3 | Once | 2 | Neg | 20 | 21 |
| R-5 | 9/M | 5 | Repeated | 1 | Neg | 3 | 10 |
| R-6 | 6/M | 2 | Once | 0 | Neg | NDd | |
M, male; F, female.
Neg, negative; Pos, positive; NT, not tested.
This episode occurred 6 months after the previous episode.
ND, not detectable.
The day on which parotid gland pain and/or swelling was first noticed was designated day 0.
Concerning the vaccine strains used to induce antibodies, no significant differences in terms of both titers and avidities were found in any group according to the difference between the trivalent vaccine (the Urabe strain) and a monovalent vaccine (the Urabe strain or some other strain).
DISCUSSION
In this series of experiments, we first sought to determine the optimal concentration of urea. While many previous reports, including ours, have claimed that 8 M urea washing is appropriate with regard to various kinds of viruses and ELISA systems (8, 11, 15, 16, 17, 19, 20), the present study showed the optimal concentration of urea to be 6 M in this system. Nevertheless, the determination of an avidity of ≦31% as low (primary response) and ≧32% as high (secondary response or past immunity) is quite compatible with previous reports (8, 15, 16, 20).
Previous studies concerning other viruses (17, 19, 20) have shown that a significant change in avidity, i.e., low to high, is observed for 100 to 150 days after infection. In this study of mumps, as shown in Fig. 2, the progression from low to high avidity appears to occur around 160 to 180 days after infection. Although the number of samples was limited, the maturation of mumps-specific IgG seems to be similar to that of other viruses.
In the analysis of group 3 (vaccine group), only the patient with clinically overt disease (V-7) showed a remarkable response characterized by a high titer of low-avidity IgG which was comparable to that of a natural primary infection. Although the possibility of an unrecognized double infection by wild-type mumps virus was not ruled out, this case may represent the immune response when a vaccine virus behaves like a wild-type virus. By contrast, the results of the other six vaccinees in this group showed the minimal response of a low titer of low-avidity IgG following immunization. The observation period of up to 349 days after immunization must have been sufficient for the IgG titer and avidity to rise, taking the results of natural infection (Table 1 and Fig. 2) into consideration. Certainly, analysis of many more samples is necessary to delineate a time course of avidity maturation following immunization. But this may be a formidable task, because in Japan, where wild-type mumps viruses still prevail, natural boosters may modify the natural course of immune maturation.
On the basis of the previously accepted concept, mumps vaccine failures have been classified mainly as PVFs. According to previous reports, a positive IgM response was observed in 7 (100%) of 7 vaccine failure cases (4), 26 (90%) of 29 cases (6), and 10 (77%) of 13 cases (1a). In fact, 9 (64%) of our 14 cases had a positive reaction for IgM and therefore would have been classified as PVFs. Avidity determination, however, strongly suggested that as many as seven of the nine cases with a positive IgM response were actually SVFs because of the high-avidity IgG. This indicates that, in total, 12 (86%) of the 14 vaccine failure cases in our study were SVFs. Certainly, this was not a strictly controlled epidemiological study. Nevertheless, the results suggest that SVF is a major form of mumps vaccine failure, at least in our study population. In addition, the fact that many of the SVF cases involved school age children was unexpected.
One explanation for the early occurrence of SVF must be waning immunity, which is expressed as antibody titers falling over a relatively short time period.
In this respect, the results of group 5 (recurrent group) are suggestive. All of the patients showed neither an IgM response nor high-titer IgG, which is comparable to that of SVF cases in group 4, indicating that the cause of parotid gland swelling was other than mumps virus infection. More noticeably, although the IgG titer itself is no different from that in group 2 patients (past natural infection), avidity remained borderline or even low. This may represent a qualitative limitation of vaccine-induced humoral immunity in mumps, that is, a failure to induce a sufficiently mature antibody. The results of group 3 (vaccine group) support this speculation. This might result in the early occurrence of SVF. This phenomenon may be specific to mumps vaccines, because cases R-1, -3, -5, and -6, with a history of measles vaccination (R-1 and -5, a monovalent measles vaccine; R-3 and -6, the trivalent vaccine), showed a high-avidity measles virus-specific IgG which was comparable to that of case R-4, which involved a history of natural measles virus infection (data not shown). It would be interesting to know whether these patients would develop mumps (i.e., SVF) if they come into contact with mumps patients.
To date, immunity to mumps, once obtained, has been believed to be lifelong, regardless of whether it is acquired by natural infection or by immunization. In this context, Gut et al., using an avidity method, recently reported that symptomatic mumps virus reinfections can occur among adults (7). Our study of vaccine failure cases also revealed that SVF occurs not infrequently, even among school age children, under conditions in which the vaccine coverage is low and vaccinees are therefore prone to exposure to wild-type viruses. Given that primary failure is not a main cause of mumps vaccine failure in areas like Japan, it is crucial, first of all, to raise the vaccine coverage to prevent vaccine failures. At the same time, it would be interesting to know whether a booster immunization would further facilitate avidity maturation. Assessment of mumps cases on the basis of avidity testing will add further insights into mumps immunity and provide information useful for the improvement of our mumps immunization strategy.
ACKNOWLEDGMENTS
We thank Kunihiko Kobayashi of the Department of Pediatrics, Hokkaido University, for his critical review of the manuscript. We thank Rie Shimahara, Akira Tsuchida, and Ken-ichi Izeki of the Department of Pediatrics, Fukagawa City Hospital, and Takashi Iwai and Akihiro Okuno of the Department of Pediatrics, Kuchan Kousei Hospital, for providing us with the necessary samples. We also thank Motoi Nishi of the Department of Public Health, Sapporo Medical College, for his kind advice on the analysis of the data.
REFERENCES
- 1. Anakura, M. (Pediatric Society of Sapporo, Sapporo, Japan). Personal communication.
- 1a.Briss P A, Fehrs L J, Parker R A, Wright P F, Sannella E C, Hutcheson R H, Schaffner W. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis. 1994;169:77–82. doi: 10.1093/infdis/169.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Brunell P A, Brickman A, Steinberg S, Allen E. Parotitis in children who had previously received mumps vaccine. Pediatrics. 1972;50:441–444. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Mumps. Morbid Mortal Weekly Rep. 1990;39/RR-13:24–25. [Google Scholar]
- 4.Cheek J E, Baron R, Atlas H, Wilson D L, Crider R D., Jr Mumps outbreak in a highly vaccinated school population. Arch Pediatr Adolesc Med. 1995;149:774–778. doi: 10.1001/archpedi.1995.02170200064010. [DOI] [PubMed] [Google Scholar]
- 5.Gassmann C, Bauer G. Avidity determination of IgG directed against tick-borne encephalitis virus improves detection of current infections. J Med Virol. 1997;51:242–251. [PubMed] [Google Scholar]
- 6.Germann D, Ströhle A, Eggenberger K, Steiner C-A, Matter L. An outbreak of mumps in a population partially vaccinated with the rubini strain. Scand J Infect Dis. 1996;28:235–238. doi: 10.3109/00365549609027163. [DOI] [PubMed] [Google Scholar]
- 7.Gut L P, Lablache C, Behr S, Kirn A. Symptomatic mumps virus reinfections. J Med Virol. 1995;45:17–23. doi: 10.1002/jmv.1890450104. [DOI] [PubMed] [Google Scholar]
- 8.Hedman K, Seppälä I. Recent rubella virus infection indicated by a low avidity of specific IgG. J Clin Immunol. 1988;8:214–221. doi: 10.1007/BF00917569. [DOI] [PubMed] [Google Scholar]
- 9.Hersh B S, Fine P E M, Kent W K, Cochi S L, Kahn L H, Zell E R, Hays P L, Wood C L. Mumps outbreak in a highly vaccinated population. J Pediatr. 1991;119:187–193. doi: 10.1016/s0022-3476(05)80726-7. [DOI] [PubMed] [Google Scholar]
- 10.Inouye S, Hasegawa A, Matsuno S, Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984;20:525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kangro H O, Manzoor S, Harper D R. Antibody avidity following varicella-zoster virus infections. J Med Virol. 1991;33:100–105. doi: 10.1002/jmv.1890330207. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi K, Kawashima H, Takekuma K, Hoshika A, Mori T, Nakayama T. Detection of mumps virus genome directly from clinical samples and a simple method for genetic differentiation of the Hoshino vaccine strain from wild strains of mumps virus. J Med Virol. 1997;52:195–199. [PubMed] [Google Scholar]
- 13.Katayama K, Oya A, Tanabayashi K, Okazaki K, Hishiyama M, Yamazaki S, Yamada A. Differentiation of mumps vaccine strain from wild viruses by single-strand conformation polymorphism of the P gene. Vaccine. 1993;11:621–623. doi: 10.1016/0264-410x(93)90306-i. [DOI] [PubMed] [Google Scholar]
- 14.Kim-Farley R, Bart S, Stetler H, Orenstein W, Bart K, Sullivan K, Halpin T, Sirotkin B. Clinical mumps vaccine efficacy. Am J Epidemiol. 1985;121:593–597. doi: 10.1093/oxfordjournals.aje.a114037. [DOI] [PubMed] [Google Scholar]
- 15.Narita M, Yamada S, Matsuzono Y, Itakura O, Togashi T, Kikuta H. Immunoglobulin G avidity testing in serum and cerebrospinal fluid for analysis of measles virus infection. Clin Diagn Lab Immunol. 1996;3:211–215. doi: 10.1128/cdli.3.2.211-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruellan-Eugene G, Barjot P, Campet M, Vabret A, Herlicoviez M, Muller G, Levy G, Guillois B, Freymuth F. Evaluation of virological procedures to detect fetal human cytomegalovirus infection: avidity of IgG antibodies, virus detection in amniotic fluid and maternal serum. J Med Virol. 1996;50:9–15. doi: 10.1002/(SICI)1096-9071(199609)50:1<9::AID-JMV3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Söderlund M, Brown C S, Cohen B J, Hedman K. Accurate serodiagnosis of B19 parvovirus infections by measurements of IgG avidity. J Infect Dis. 1995;171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 18.Thomas H I J, Morgan-Capner P. Rubella-specific IgG subclass avidity ELISA and its role in the differentiation between primary rubella and rubella reinfection. Epidemiol Infect. 1988;101:591–598. doi: 10.1017/s0950268800029459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward K N, Gray J J, Joslin M E, Sheldon M J. Avidity of IgG antibodies to human herpesvirus-6 distinguishes primary from recurrent infection in organ transplant recipients and excludes cross-reactivity with other herpesviruses. J Med Virol. 1993;39:44–49. doi: 10.1002/jmv.1890390109. [DOI] [PubMed] [Google Scholar]
- 20.Ward K N, Dhaliwal W, Ashworth K L, Clutterbuck E J, Teo C G. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol. 1994;43:367–372. doi: 10.1002/jmv.1890430409. [DOI] [PubMed] [Google Scholar]
- 21.Wharton M, Cochi S L, Hutcheson R H, Bistowish J M, Schaffner W. A large outbreak of mumps in the postvaccine era. J Infect Dis. 1988;158:1253–1260. doi: 10.1093/infdis/158.6.1253. [DOI] [PubMed] [Google Scholar]