Abstract
Global efforts to eliminate tuberculosis (TB) must address the unique barriers that children (ages 0 through 9 years) and adolescents/young adults (AYA; ages 10 through 24 years) face in adhering to treatment for TB infection and disease. We conducted a narrative review to summarize current knowledge on the social determinants of treatment adherence among these age groups to guide efforts and policy to address their unique needs. Our findings revealed that research on TB treatment adherence among children and AYA is still in its nascent stage. The current literature revealed structural/community-, health system-, household-, and individual-level factors that influence treatment adherence and varied with developmental stage. There is a need to develop multilevel interventions to address the unique challenges that children and AYA face in adhering to TB treatment.
Keywords: tuberculosis, medication adherence, adolescents, children, social determinants
Children, adolescents, and young adults face challenges to adherence in the treatment of tuberculosis (TB) infection and disease. Interventions need to account for multilevel factors and their developmental stage to provide supportive TB care and increase treatment completion.
INTRODUCTION
Globally, an estimated 45 million children (0 through 9 years) and 217 million adolescents and young adults (AYA; 10 through 24 years) are infected with tuberculosis (TB) [1]. Of these individuals, an estimated 800 000 children and 1.8 million AYA develop TB disease each year [1, 2]. There is growing recognition that TB control programs need to focus more on children and AYA. Young children are at high risk of disease progression after TB exposure, and AYA have adult-type diseases that can contribute to ongoing community transmission [3]. At the same time, children and AYA have unique barriers to TB care [1, 4], and global efforts to eliminate TB require specific approaches that meet the needs of these populations. Adherence to treatment of TB infection, also known as TB preventive treatment (TPT), and to the treatment of TB disease remains a particular challenge for children and AYA. Studies have found that children have suboptimal adherence to treatment for TB infection and disease [5–8], and AYA with TB disease have higher rates of loss to follow-up compared to children and older adults [9–11], as well as higher TB-related mortality than children [12]. The social determinants of TB treatment adherence in adults have been well characterized [13–15], and several large-scale interventions have been developed to address and evaluate these factors, including the use of digital adherence technologies (DATs) and conditional cash-transfer programs [16–19]. This narrative review summarizes the current knowledge on the social determinants of adherence to treatment of TB infection and disease among children and AYA, with the aim of guiding efforts to address the unique needs of these populations.
METHODS
We use the World Health Organization (WHO)’s definitions of childhood as the period before 10 years of age and adolescence/young adulthood as the period from 10 through 24 years of age. According to the WHO, adolescence spans from 10 through 19 years of age, and young adulthood, from 20 through 24 years of age [20]. We included young adults in this review because the transitions that begin in adolescence, including completion of education and establishment of an independent household, now extend into young adulthood in many different settings [21].
We searched five databases—PubMed, PsycInfo, CINAHL, Web of Science, and Google Scholar—for relevant articles using the following search terms (and variations): child, adolescent, young adult, TB, and treatment adherence. We summarized relevant studies and developed a conceptual model of the existing evidence on the social determinants of TB treatment adherence among children and AYA.
Results
Few studies have examined the social determinants of adherence to treatment for TB infection and disease among children and AYA. Most of the relevant research was conducted in sub-Saharan Africa, followed by South America, South and Southeast Asia, and the United States. Studies among AYA assessed adherence to treatment for both TB infection and disease, but studies among children primarily focused on adherence to treatment for TB infection. An additional limitation was that studies have traditionally defined children as those less than 15 years old and adults as those 15 years and older. This limitation prevented further stratification of factors between children and AYA and created gaps in the literature for older adolescents.
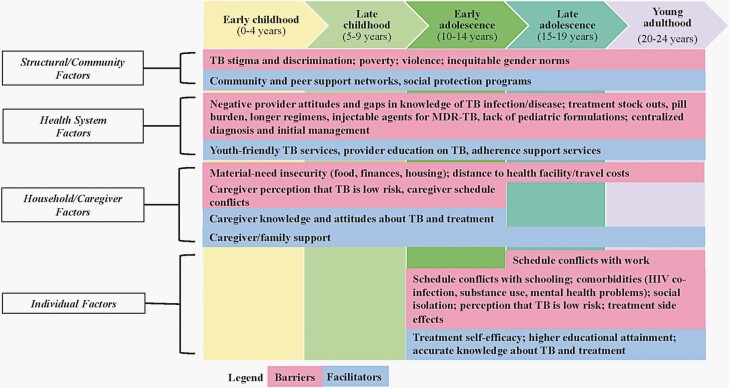
Our review revealed structural/community-, health system-, household-, and individual-level factors that influenced TB treatment adherence for infection and disease. We synthesized these findings into a conceptual model depicting the multi-level factors at different stages of childhood and adolescence/young adulthood (Figure 1). This conceptual model maps onto the socio-ecological model, which posits that individual health behaviors, such as treatment adherence, are shaped by multi-level factors [22].
Figure 1.
Barriers and facilitators to adherence to treatment for TB infection and disease among children, adolescents, and young adults, as identified in the literature. Abbreviations: HIV, human immunodeficiency virus; MDR-TB, multidrug-resistant tuberculosis; TB, tuberculosis.
Structural and Community Factors
Anticipated stigma and discrimination were found to be critical barriers to treatment adherence for TB disease and infection among children and AYA [5, 23–25], as has been previously documented among adults [26]. In a qualitative study from Botswana, caregivers described stigma associated with frequently queuing at the TB clinic, which made it difficult for them to take their child to the clinic for daily directly observed therapy [25]. In Eswatini, adolescents with HIV had increased odds of suboptimal adherence to isoniazid preventive therapy if they believed it would lead to HIV-related stigma from their friends or neighbors [23]. AYA can also be concerned about TB-related stigma from romantic or sexual partners, with young women expressing depression and anxiety on the prospects for marriage [27–29]. Still, the association of this with adherence has not been described.
Poverty and violence are important determinants of anti-TB medication adherence. Community-level poverty shapes the resources available to patients to be able to access and receive appropriate TB care [30, 31]. Community-based violence, either as a direct effect or a reflection of underlying factors such as poverty, may also impact TB care; in Brazil, adolescents with TB living in areas with high rates of informal settlements and homicides had higher odds of unfavorable outcomes, particularly loss to follow-up [31]. Exposure to violence can be either witnessed or experienced [32], and its effect on treatment adherence needs to be further explored but is likely multifactorial. Community violence may impact adherence both directly (eg, families may be hesitant to go to the health center due to safety concerns) and indirectly (eg, violence may lead to mental health issues, which, in turn, impede adherence) [33].
Gender norms can affect TB care across the age spectrum. A study in India found that female children with multidrug-resistant (MDR)-TB did not receive equal TB care as male children and had worse outcomes [24]. Other research, primarily among people living with HIV, has demonstrated that hegemonic masculine norms, which value male toughness [34], contribute to male avoidance in seeking healthcare [35]. It is possible that such norms explain why AYA males in some settings have suboptimal TB treatment adherence compared to females [8, 36].
On the other hand, community and peer support networks may mitigate the negative effects of TB stigma and facilitate adherence to TB treatment among children and AYA [37]. Social protection programs can also promote adherence to treatment for TB disease [37, 38]; for example, in a national retrospective cohort study of AYA from Brazil, receipt of government cash transfers was associated with a reduced likelihood of an unfavorable treatment outcome [38].
Health System Factors
Provider beliefs, experience, and knowledge in the management of TB infection and disease can further influence treatment adherence [5, 39, 40]. For example, providers may believe that treatment of TB infection can increase the risk of drug resistance [41]; as a result, they may not convey the importance of treatment for TB infection to caregivers and patients. In addition, limited adherence monitoring and provider support has been linked to poor adherence among children and adolescents with TB infection and disease [5, 24, 25, 39, 42–44]. Meanwhile, studies from Kenya and Rwanda reported that strong provider counseling and support facilitated adherence to treatment for TB infection among young children [40, 43].
Treatment stock outs [25, 39, 45], pill burden [24, 43], the length of TB treatment [46, 47], and lack of pediatric formulations [41, 48] also influence adherence among children and AYA. Two large multinational trials found that shorter regimens for TB infection had significantly higher levels of adherence compared to longer regimens [46, 47]. Qualitative studies have reported the difficulty of administering TB treatment to children who cannot swallow pills. Even children who can swallow pills find it challenging to take the large number required to treat TB disease [41, 48]. Fixed-dose combinations may reduce this burden, but there is limited evidence of its role to improve adherence [49]. MDR-TB creates additional challenges for adherence [50], as its treatment involves a higher pill burden, additional adverse effects, longer duration, and the need to receive injectable agents at a clinical facility [24, 51]. The implementation of shorter all-oral MDR-TB regimens may improve adherence in children and AYA [52, 53], but this hypothesis needs to be evaluated.
The centralized model of TB care for children and young AYA also impacts adherence. In many settings, children and young AYA (typically under age 15 years) who present to a primary care facility for TB evaluation often are referred to hospitals or other tertiary facilities because front-line providers have not been trained in the diagnosis and initial management of childhood TB [54, 55]. These referrals lead to further obstacles, including transportation costs and the need for parents to take additional time off work [41]. Moreover, without the appropriate linkage back to their primary care facility, children can be lost to follow-up [56]. Several efforts to decentralize TB management for children and AYA address these barriers to care [23, 57–59].
The lack of AYA-friendly TB services has been identified as a critical barrier to treatment adherence among this population [4, 24, 57]. Healthcare workers have reported that they lack dedicated protocols for AYA, who generally are treated the same as older adults [57]. The WHO has called for AYA-centered services, including age-appropriate TB education, adherence counseling, and support; AYA-friendly clinic spaces separated from areas for older adult patients; and alternative approaches for adherence monitoring that does not conflict with school or work hours [4].
Household/Caregiver Factors
Food, financial, and housing insecurity have been associated with poor adherence to treatment for TB infection and disease among both children and AYA [37, 38, 40, 42, 60]. Heads of households experiencing financial insecurity may prioritize getting a job instead of traveling to the clinic to collect TB medications for their children [40]. In addition, food insecurity contributes to nonadherence due to the belief that taking TB treatment without food is harmful [37, 40]. Limited money to travel to the clinic also has been cited as a barrier to accessing TB treatment and adherence among children and AYA [23, 37, 40]. These challenges may be further exacerbated when other household members have TB [61], but this link has not been evaluated in the literature.
Caregiver knowledge and attitudes about TB and its treatment play an important role in treatment adherence, particularly among younger children. For example, some caregivers have cited beliefs that children do not need treatment for TB infection since they have no symptoms of TB, while other caregivers believe that treatment for TB disease is no longer important once symptoms have resolved [5, 7, 39, 41, 45]. In contrast, children are more likely to complete treatment for TB infection if their caregivers believe in the importance of such treatment, which often occurs when another family member has had TB [5, 40, 62]. Additionally, caregivers have reported that they do not feel that they have sufficient education about TB infection or disease or training in how to give treatment to children [5, 25, 39, 63]. Education, counseling, and support to caregivers and families are facilitators that have been shown to improve treatment adherence [5, 40, 43].
Individual Patient Factors
As AYA gain more autonomy and independence, individual patient factors become more important for treatment adherence in this age group. Few studies have investigated the reasons why AYA have lower adherence to TB treatment as compared to younger children or older adults [11, 38, 44, 64]. The growing need for autonomy, reduced reliance on caregivers, greater dependence on peers and romantic/sexual partners, and increased focus on short-term gains over long-term health outcomes may all play a role, but these factors have not been evaluated in AYA with TB [1, 60, 65, 66]. Educational attainment among AYA TB patients and their caregivers impact adherence to treatment for TB infection and disease [23, 38, 42]. AYA knowledge and attitudes about TB and its treatment likely influence adherence, as has been observed in adults [26], but we were unable to find specific studies in AYA that assessed this association.
Self-efficacy, which refers to individuals’ belief in their own ability to carry out a specific behavior [67, 68], predicts adherence to HIV treatment in AYA [69]. However, limited research has explored the role of self-efficacy in TB treatment adherence. We found one study that demonstrated that self-efficacy for collecting TB medicines monthly was associated with reduced odds of suboptimal treatment adherence among adolescents and caregivers of children with TB infection [23].
Co-morbidities, specifically TB-HIV coinfection [31, 36, 38, 70] and substance use [31, 38], have been associated with poor treatment adherence for TB disease among AYA. TB disease is additionally associated with poor mental health, particularly among AYA, due to prolonged home isolation or hospitalization for infection control purposes, and experienced and/or internalized stigma [23, 24, 37, 66]. In one study from India, adolescent TB patients described feelings of loneliness, anger, and anxiety because they missed school and playing with their friends [24]. More so than children, AYA living with TB disease internalize TB stigma, which likely leads to poor mental health outcomes and suboptimal treatment adherence [37].
Discussion
Our findings indicate several gaps in the literature (Table 1) and the need to develop multi-level interventions to address the unique challenges that children and AYA face in adhering to TB treatment. At the structural level, interventions need to address TB stigma and inequitable gender norms for children and AYA, and foster community and peer support networks for adherence. Social protection programs can further address household material-need insecurities, such as cash transfers and food support [71, 72]. At the health-systems level, greater understanding of child and AYA preferences is needed to develop age-appropriate TB services. These services include greater decentralization and improved linkage to care to primary care facilities to reduce loss to follow-up [56]. Our findings also suggest the need to ensure providers are trained to educate caregivers, children, and AYA about TB infection and disease. Better adherence monitoring and support systems need to be developed; for example, DATs—including mobile phone short messaging service texts or calls, digital pill boxes, and video-based observed therapy [73]—may be a particularly promising option given the high level of technology and mobile phone use in youth around the world [74, 75]. At the same time, these technologies need to be adapted to the unique developmental stages of children and AYA [76]. At the individual level, better integration of TB care with other child and AYA health services is needed, including for HIV, substance use, and mental health [23, 24, 37, 66, 77]. Peer support groups and peer treatment navigators have supported mental health and HIV treatment adherence, and may also be helpful for TB [78, 79]. The goal of this multilevel approach would be to provide caregivers, children, and AYA with the knowledge, resources, support, and self-efficacy to complete treatment for TB infection and disease.
Table 1.
Research Gaps on the Social Determinants of TB Treatment Adherence in Children and Adolescents and Young Adults
| Structural/Community Factors |
| • Pathways of how exposure to violence creates barriers to adherence |
| • Interventions to address TB-related stigma and inequitable gender norms for children and AYA |
| • Role of social protection programs and peer support networks for children and AYA with TB |
| Health System Factors |
| • Child and AYA needs and preferences to develop age-appropriate services |
| • Impact of fixed-dose combinations and all-oral MDR TB regimens on adherence |
| • Influence of novel adherence monitoring tools, including digital adherence technologies, on adherence |
| • Effect of decentralized TB care on adherence |
| Household/Caregiver Factors |
| • Influence of TB illness in additional household members, including the caregiver of the child/AYA, on adherence |
| • Interventions to address household material-need insecurities to improve adherence |
| Individual Factors |
| • Influence on adherence of caregiver and patient knowledge and attitudes about TB disease and treatment |
| • Effect of developmental changes during adolescence and young adulthood on adherence, including a shifting emphasis from family relationships to peer relationships |
| • Role of self-efficacy to support TB treatment adherence |
| • Interventions to support mental health during TB treatment |
| Other Research Needs |
| • Wider geographic representation |
| • Additional studies on adherence to TB disease treatment in children |
| • Greater inclusion of older adolescents |
Abbreviations: AYA, adolescents and young adults; MDR, multidrug-resistant; TB, tuberculosis.
Conclusion
Research on the social determinants of TB treatment adherence among children and AYA is still in its nascent stage. However, the current literature suggests the need for multi-level, developmentally-sensitive interventions to address the unique barriers to TB treatment adherence for children and AYA. Such interventions should be incorporated into global TB elimination strategies.
Notes
Financial support . D.J. is supported by the U.S. National Institutes of Health (K23HL153581). S.S.C. is supported by the U.S. National Institutes of Health (5K01TW010829).
Supplement sponsorship. This article appears as part of the supplement “What’s New in Childhood Tuberculosis?” sponsored by the Stop TB Partnership.
Potential conflicts of interest . S.S.C. received an honorarium from J&J for a lecture on TB in youth at The Union World Conference on Lung Health in October 2021.
Contributor Information
Anna M Leddy, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Devan Jaganath, Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA; Division of Pediatric Infectious Diseases, University of California, San Francisco, San Francisco, California, USA.
Rina Triasih, Department of Pediatrics, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, Indonesia.
Eric Wobudeya, Mulago National Referral Hospital, Kampala, Uganda.
Marcia C Bellotti de Oliveira, Department of Pediatrics, Souza Marques Medical School, Rio de Janeiro, Brazil.
Yana Sheremeta, All-Ukrainian Network of People Living With HIV/AIDS, Kyiv, Ukraine.
Mercedes C Becerra, Department of Global Health and Social Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Silvia S Chiang, Department of Pediatrics, Alpert Medical School of Brown University, Providence, Rhode Island, USA; Center for International Health Research, Rhode Island Hospital, Providence, Rhode Island, USA.
References
- 1. Snow KJ, Cruz AT, Seddon JA, et al. Adolescent tuberculosis. Lancet Child Adolesc Health 2020;4:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dodd PJ, Gardiner E, Coghlan R, et al. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014; 2:e453–9. [DOI] [PubMed] [Google Scholar]
- 3. Starke JR, Donald PR.. Handbook of Child and Adolescent Tuberculosis. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 4. World Health Organization. Roadmap Towards Ending TB in Children and Adolescents. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 5. Triasih R, Padmawati RS, Duke T, et al. A mixed-methods evaluation of adherence to preventive treatment among child tuberculosis contacts in Indonesia. Int J Tuberc Lung Dis 2016; 20:1078–83. [DOI] [PubMed] [Google Scholar]
- 6. Rutherford ME, Ruslami R, Maharani W, et al. Adherence to isoniazid preventive therapy in Indonesian children: a quantitative and qualitative investigation. BMC Res Notes 2012; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garie KT, Yassin MA, Cuevas LE.. Lack of adherence to isoniazid chemoprophylaxis in children in contact with adults with tuberculosis in Southern Ethiopia. PLoS One 2011; 6:e26452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiang SS, Beckhorn CB, Wong M, et al. Patterns of suboptimal adherence among adolescents treated for tuberculosis. Int J Tuberc Lung Dis 2020; 24:723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snow K, Hesseling AC, Naidoo P, et al. Tuberculosis in adolescents and young adults: epidemiology and treatment outcomes in the Western Cape. Int J Tuberc Lung Dis 2017; 21:651–7. [DOI] [PubMed] [Google Scholar]
- 10. Enane LA, Lowenthal ED, Arscott-Mills T, et al. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis 2016; 20:1320–5. [DOI] [PubMed] [Google Scholar]
- 11. Wobudeya E, Jaganath D, Sekadde MP, et al. Outcomes of empiric treatment for pediatric tuberculosis, Kampala, Uganda, 2010-2015. BMC Public Health 2019; 19:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osman M, du Preez K, Seddon JA, et al. Mortality in South African children and adolescents routinely treated for tuberculosis. Pediatrics 2021; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hargreaves JR, Boccia D, Evans CA, et al. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011; 101:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maciel E, Amancio JS, Castro DB, et al. Social determinants of pulmonary tuberculosis treatment non-adherence in Rio de Janeiro, Brazil. PLoS One 2018; 13:e0190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shargie EB, Lindtjørn B.. Determinants of treatment adherence among smear-positive pulmonary tuberculosis patients in Southern Ethiopia. PLoS Med 2007; 4:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter DJ, Daniel R, Torrens AW, et al. The impact of a cash transfer programme on tuberculosis treatment success rate: a quasi-experimental study in Brazil. BMJ Glob Health 2019; 4:e001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nery JS, Rodrigues LC, Rasella D, et al. Effect of Brazil’s conditional cash transfer programme on tuberculosis incidence. Int J Tuberc Lung Dis 2017; 21:790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis-Santos B, Shete P, Bertolde A, et al. Tuberculosis in Brazil and cash transfer programs: a longitudinal database study of the effect of cash transfer on cure rates. PLoS One 2019; 14:e0212617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cattamanchi A, Crowder R, Kityamuwesi A, et al. Digital adherence technology for tuberculosis treatment supervision: A stepped-wedge cluster-randomized trial in Uganda. PLoS Med 2021; 18:e1003628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Who Consolidated Guidelines on Tuberculosis Module 5: Management of Tuberculosis in Children and Adolescents. Geneva, Switzerland: World Health Organization; 2022. [PubMed] [Google Scholar]
- 21. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health 2018; 2:223–8. [DOI] [PubMed] [Google Scholar]
- 22. Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- 23. Kay AW, Thivalapill N, Skinner D, et al. Predictors of suboptimal adherence to isoniazid preventive therapy among adolescents and children living with HIV. PLoS One 2020; 15:e0243713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das M, Mathur T, Ravi S, et al. Challenging drug-resistant TB treatment journey for children, adolescents and their care-givers: A qualitative study. PLoS One 2021; 16:e0248408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stillson CH, Okatch H, Frasso R, et al. “That’s when I struggle” … Exploring challenges faced by care givers of children with tuberculosis in Botswana. Int J Tuberc Lung Dis 2016; 20:1314–9. [DOI] [PubMed] [Google Scholar]
- 26. Munro SA, Lewin SA, Smith HJ, et al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007; 4:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hatherall B, Newell JN, Emmel N, et al. “Who will marry a diseased girl?” Marriage, gender, and tuberculosis stigma in Asia. Qual Health Res 2019; 29:1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukerji R, Turan JM.. Exploring manifestations of TB-related stigma experienced by women in Kolkata, India. Ann Glob Health 2018; 84:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barua M, Van Driel F, Jansen W.. Tuberculosis and the sexual and reproductive lives of women in Bangladesh. PLoS One 2018; 13:e0201134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva AP, Hill P, Belo MT, et al. Non-completion of latent tuberculous infection treatment among children in Rio de Janeiro State, Brazil. Int J Tuberc Lung Dis 2016; 20:479–86. [DOI] [PubMed] [Google Scholar]
- 31. de Oliveira MCB, Sant’Anna CC, Raggio Luiz R, et al. Unfavorable outcomes in tuberculosis: multidimensional factors among adolescents in Rio de Janeiro, Brazil. Am J Trop Med Hyg 2020; 103:2492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cluver L, Meinck F, Toska E, et al. Multitype violence exposures and adolescent antiretroviral nonadherence in South Africa. AIDS 2018; 32:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarke A, Olive P, Akooji N, Whittaker K.. Violence exposure and young people’s vulnerability, mental and physical health. Int J Public Health 2020; 65:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Connell RW, Messerschmidt JW.. Hegemonic masculinity – Rethinking the concept. Gend Soc. 2005; 19:829–59. [Google Scholar]
- 35. Sileo KM, Fielding-Miller R, Dworkin SL, et al. A scoping review on the role of masculine norms in men’s engagement in the HIV care continuum in sub-Saharan Africa. AIDS Care 2019; 31:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulongeni P, Hermans S, Caldwell J, et al. HIV prevalence and determinants of loss-to-follow-up in adolescents and young adults with tuberculosis in Cape Town. PLoS One 2019; 14:e0210937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franck C, Seddon JA, Hesseling AC, et al. Assessing the impact of multidrug-resistant tuberculosis in children: an exploratory qualitative study. BMC Infect Dis 2014; 14:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chenciner L, Annerstedt KS, Pescarini JM, et al. Social and health factors associated with unfavourable treatment outcome in adolescents and young adults with tuberculosis in Brazil: a national retrospective cohort study. Lancet Glob Health 2021; 9:e1380–90. [DOI] [PubMed] [Google Scholar]
- 39. Singh AR, Kharate A, Bhat P, et al. Isoniazid preventive therapy among children living with tuberculosis patients: Is it working? A mixed-method study from Bhopal, India. J Trop Pediatr 2017; 63:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Birungi FM, Graham SM, Uwimana J, et al. Adherence to isoniazid preventive therapy among child contacts in Rwanda: a mixed-methods study. PLoS One 2019; 14:e0211934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiang SS, Roche S, Contreras C, et al. Barriers to the treatment of childhood tuberculous infection and tuberculosis disease: a qualitative study. Int J Tuberc Lung Dis 2017; 21:154–60. [DOI] [PubMed] [Google Scholar]
- 42. Laghari M, Talpur BA, Sulaiman SAS, et al. Assessment of adherence to anti-tuberculosis treatment and predictors for non-adherence among the caregivers of children with tuberculosis. Trans R Soc Trop Med Hyg 2021; 115:904–13. [DOI] [PubMed] [Google Scholar]
- 43. Ngugi SK, Muiruri P, Odero T, et al. Factors affecting uptake and completion of isoniazid preventive therapy among HIV-infected children at a national referral hospital, Kenya: a mixed quantitative and qualitative study. BMC Infect Dis 2020; 20:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hovell M, Blumberg E, Gil-Trejo L, et al. Predictors of adherence to treatment for latent tuberculosis infection in high-risk Latino adolescents: a behavioral epidemiological analysis. Soc Sci Med 2003; 56:1789–96. [DOI] [PubMed] [Google Scholar]
- 45. Kibirige L, Izudi J, Okoboi S.. Discontinuation of tuberculosis treatment among children in the Kampala Capital City Authority health facilities: a mixed-methods study. BMC Infect Dis 2021; 21:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diallo T, Adjobimey M, Ruslami R, et al. Safety and side effects of rifampin versus isoniazid in children. New Engl J Med. 2018; 379:454–63. [DOI] [PubMed] [Google Scholar]
- 47. Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015; 169:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirsch-Moverman Y, Mantell JE, Lebelo L, et al. Tuberculosis preventive treatment preferences among care givers of children in Lesotho: a pilot study. Int J Tuberc Lung Dis 2018; 22:858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Albanna AS, Smith BM, Cowan D, et al. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J 2013; 42:721–32. [DOI] [PubMed] [Google Scholar]
- 50. Moyo S, Furin JJ, Hughes Jet al. Outcomes in adolescents undergoing treatment for drug-resistant tuberculosis in Cape Town, South Africa, 2008–2013. Arch Pediatr Infect Dis. 2015; 3:e17934. [Google Scholar]
- 51. Isaakidis P, Paryani R, Khan S, et al. Poor Outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLoS One 2013; 8:e68869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohr-Holland E, Reuter A, Furin J, et al. Injectable-free regimens containing bedaquiline, delamanid, or both for adolescents with rifampicin-resistant tuberculosis in Khayelitsha, South Africa. EClinicalMedicine 2020; 20:100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ndjeka N, Campbell JR, Meintjes G, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study [published online ahead of print May 2, 2022]. Lancet Infect Dis. doi: 10.1016/S1473-3099(21)00811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zawedde-Muyanja S, Reuter A, Tovar MAet al. Provision of decentralized TB care services: a detect-treat-prevent strategy for children and adolescents affected by TB. Pathogens 2021; 10:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chiang SS, Cruz AT, Del Castillo H, et al. Evaluation of health-care providers’ knowledge of childhood tuberculosis in Lima, Peru. Paediatr Int Child Health 2015; 35:29–35. [DOI] [PubMed] [Google Scholar]
- 56. du Preez K, Schaaf HS, Dunbar R, et al. Closing the reporting gap for childhood tuberculosis in South Africa: improving hospital referrals and linkages. Public Health Action 2020; 10:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Laycock KM, Eby J, Arscott-Mills T, et al. Towards quality adolescent-friendly services in TB care. Int J Tuberc Lung Dis 2021; 25:579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dongo JP, Graham SM, Nsonga J, et al. Implementation of an effective decentralised programme for detection, treatment and prevention of tuberculosis in children. Trop Med Infect Dis 2021; 6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zawedde-Muyanja S, Nakanwagi A, Dongo JP, et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis 2018; 22:1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laycock KM, Enane LA, Steenhoff AP.. Tuberculosis in adolescents and young adults: Emerging data on TB transmission and prevention among vulnerable young people. Trop Med Infect Dis 2021; 6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ghazy RM, El Saeh HM, Abdulaziz S, et al. A systematic review and meta-analysis of the catastrophic costs incurred by tuberculosis patients. Sci Rep 2022; 12:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zeladita-Huaman J, Yuen CM, Zegarra-Chapoñan R, et al. Caregivers’ knowledge and perceptions are associated with children’s TB preventive treatment completion. Public Health Action 2021; 11:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hirsch-Moverman Y, Mantell JE, Lebelo L, et al. Provider attitudes about childhood tuberculosis prevention in Lesotho: a qualitative study. BMC Health Serv Res 2020; 20:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chang SH, Eitzman SR, Nahid P, et al. Factors associated with failure to complete isoniazid therapy for latent tuberculosis infection in children and adolescents. J Infect Public Health 2014; 7:145–52. [DOI] [PubMed] [Google Scholar]
- 65. Hergovich A, Sirsch U, Felinger M.. Self-appraisals, actual appraisals and reflected appraisals of preadolescent children. Soc Behav Pers 2002; 30:603–12. [Google Scholar]
- 66. Moscibrodzki P, Enane LA, Hoddinott Get al. The impact of tuberculosis on the well-being of adolescents and young adults. Pathogens 2021; 10:1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977; 84:191–215. [DOI] [PubMed] [Google Scholar]
- 68. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991; 50:179–211. [Google Scholar]
- 69. Kim MH, Mazenga AC, Yu X, et al. High self-reported non-adherence to antiretroviral therapy amongst adolescents living with HIV in Malawi: barriers and associated factors. J Int AIDS Soc 2017; 20:21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reif LK, Rivera V, Bertrand R, et al. Outcomes across the tuberculosis care continuum among adolescents in Haiti. Public Health Action 2018; 8:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ukwaja KN. Social protection interventions could improve tuberculosis treatment outcomes. Lancet Glob Health 2019; 7:e167–8. [DOI] [PubMed] [Google Scholar]
- 72. Klein K, Bernachea MP, Irribarren S, et al. Evaluation of a social protection policy on tuberculosis treatment outcomes: A prospective cohort study. PLoS Med 2019; 16:e1002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Subbaraman R, de Mondesert L, Musiimenta A, et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health 2018; 3:e001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Swahn MH, Braunstein S, Kasirye R.. Demographic and psychosocial characteristics of mobile phone ownership and usage among youth living in the slums of Kampala, Uganda. West J Emerg Med 2014; 15:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Morse RM, Myburgh H, Reubi D, et al. Opportunities for mobile app-based adherence support for children with tuberculosis in South Africa. JMIR mHealth uHealth 2020; 8:e19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bezabih AM, Gerling K, Abebe W, et al. Behavioral theories and motivational features underlying e-health interventions for adolescent antiretroviral adherence: Systematic review. JMIR mHealth uHealth 2021; 9:e25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. du Preez K, Gabardo BMA, Kabra SK, et al. Priority activities in child and adolescent tuberculosis to close the policy-practice gap in low- and middle-income countries. Pathogens 2022; 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reif LK, Abrams EJ, Arpadi S, et al. Interventions to improve antiretroviral therapy adherence among adolescents and youth in low- and middle-income countries: a systematic review 2015–2019. AIDS Behav 2020; 24:2797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mavhu W, Willis N, Mufuka J, et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. Lancet Glob Health 2020; 8:e264–75. [DOI] [PubMed] [Google Scholar]