Abstract
The global climate has been changing over the last century due to greenhouse gas emissions and will continue to change over this century, accelerating without effective global efforts to reduce emissions. Ticks and tick-borne diseases (TTBDs) are inherently climate-sensitive due to the sensitivity of tick lifecycles to climate. Key direct climate and weather sensitivities include survival of individual ticks, and the duration of development and host-seeking activity of ticks. These sensitivities mean that in some regions a warming climate may increase tick survival, shorten life-cycles and lengthen the duration of tick activity seasons. Indirect effects of climate change on host communities may, with changes in tick abundance, facilitate enhanced transmission of tick-borne pathogens. High temperatures, and extreme weather events (heat, cold, and flooding) are anticipated with climate change, and these may reduce tick survival and pathogen transmission in some locations. Studies of the possible effects of climate change on TTBDs to date generally project poleward range expansion of geographical ranges (with possible contraction of ranges away from the increasingly hot tropics), upslope elevational range spread in mountainous regions, and increased abundance of ticks in many current endemic regions. However, relatively few studies, using long-term (multi-decade) observations, provide evidence of recent range changes of tick populations that could be attributed to recent climate change. Further integrated ‘One Health’ observational and modeling studies are needed to detect changes in TTBD occurrence, attribute them to climate change, and to develop predictive models of public- and animal-health needs to plan for TTBD emergence.
Keywords: Ixodidae, ticks, tick-borne pathogen, climate change, distribution
The global climate has been changing over the last century under the influence of emissions of greenhouse gases (particularly carbon dioxide and methane) from an expanding and energy-hungry human population, and will continue to change over this century, accelerating markedly without effective global efforts to reduce emissions (Field et al. 2014). If greenhouse gas emissions are not reduced enough to keep warming below 2°C, effects on the planet may be catastrophic. With greater warming there is greater likelihood that climatic ‘tipping points’ will be crossed resulting in abrupt and irreversible changes to the planet. An example is the possible complete loss of the Greenland ice sheet resulting in a global 7-m rise in sea level flooding most coastal cities around the world, including those of the eastern United States (Overpeck and Weiss 2009, Field et al. 2014). In this article, we consider the possible effects of climate change on ticks and tick-borne diseases (TTBDs) this century, but we do not consider possible extreme effects of climate change that may arise after tipping points are passed as the consequences cannot be predicted and at that juncture tick-borne disease may be the least of our concerns.
Impacts of climate change on vector-borne diseases have been thought possible since impacts of climate change on health began to be explored (Kovats and Haines 1995, Githeko et al. 2000). Early studies in this field focused on the human-to-human mosquito-transmitted diseases, such as dengue and malaria (Martens et al. 1995, Patz et al. 1998), but they generated much controversy. The models projected widespread global spread of these diseases, but many scientists, particularly entomologists and epidemiologists, criticized these studies. Apart from criticism of the models used (Rogers and Randolph 2000), the main basis for criticism was the relatively limited impact of climate on risk from these diseases at present, due to control programs and socioeconomic factors; i.e., factors associated with the rate at which people can acquire infection, and then transmit them again to other people via mosquitoes (Reiter 2001, Reiter et al. 2004). We have previously made the point that human pathogens transmitted by hard ticks, which are wildlife-associated zoonoses, may be more directly impacted by climate change and less directly impacted by human activity (Ogden et al. 2014).
Our capacity to understand, detect, and attribute effects of climate change on TTBD are enhanced by applying a One Health approach. The One Health concept postulates that human, animal, and ecosystem health are interrelated and interdependent, and that preparatory or reactionary responses to threats to human well-being demand holistic, transdisciplinary approaches encompassing all three components (Cunningham et al. 2017, Ogden et al. 2019). While in this review individual effects of climate change on TTBD are explored, climate change will likely impact TTBD via an interaction of effects on animal, human, and ecosystem health.
In this article, we explore three aspects of climate change and tick-borne diseases: 1) how climate and weather could impact the occurrence and abundance of ticks and the transmission of tick-borne pathogens by direct and indirect effects on tick and pathogen life-cycles; 2) how projected changes to climate may alter geographical ranges and abundance of TTBDs; and 3) evidence to date for changes to patterns of occurrence of TTBDs that could be attributable to climate change. The article is illustrative of the possible effects of climate change, but, given the enormity of the biological and animal- and human-health fields of ticks and tick-borne disease, it does not aim to be exhaustive.
Weather- and Climate-Sensitivity of Ticks and Tick-Borne Diseases
In this section, we consider the effects of weather and climate on survival and abundance of ticks, and on the presence and efficiency of tick-borne pathogen transmission cycles. Throughout we consider ‘weather’ to mean measures of temperature and rainfall over short time periods (days to a few months). In contrast, ‘climate’ refers to long-term averages (usually >3 mo) in measures of temperature and rainfall relevant to the lifecycles of ticks which are often multiple years (https://www.nasa.gov/mission_pages/noaa-n/climate/climate_weather.html). The change to climate projected by climate models are regionally variable; we do not provide details here, which have been summarized in the most recent Intergovernmental Panel on Climate Change report (IPCC 2013). However, in broad terms, projected changes to the climate are as follows. Mean temperatures are expected to increase globally but will increase faster in the northern regions of the northern hemisphere. Precipitation is expected to increase in the northern regions of the northern hemisphere and much of Asia, and to decrease in southern North America, northern South America, the Mediterranean region, and Australasia. These changes are expected to be accompanied by increasing climate variability and extreme weather events including hurricanes and cyclones, severe storms, extreme heat events, droughts, and flooding. The changes to climate (in reality and in model-based projections) are anthropogenic in origin and depend on socioeconomic pathways, and changes in land use and greenhouse gas emissions are expected to go hand-in-hand (Harrison et al. 2016). Consequently, it is increasingly recognized that the climate change effects and land use changes underlying the greenhouse gas emissions need to be considered simultaneously in projecting change to occurrence and risk from tick-borne diseases (e.g., Li et al. 2019).
Weather and Climate Sensitivity of Tick Populations
Climate may determine tick population presence and abundance either by direct effects on per-capita rates of mortality and reproduction, or by indirect effects on tick survival and reproduction via effects on tick activity, host populations, and tick habitat. In the laboratory, ticks exposed to far subzero temperatures (less than −5°C) die rapidly (Lindsay 1995, Burks et al. 1996, Vandyk et al. 1996, Ogden et al. 2004). However, some species can tolerate low subzero temperatures by virtue of glycoprotein antifreeze in the hemolymph (Neelakanta et al. 2010). In nature, it appears that in woodland habitats, the duff layer can provide a refuge for ticks from far subzero temperatures, and in some studies, the daily per-capita mortality rate of unfed ticks in winter remained similar to those in the summer even though air temperatures fell to less than −20°C for extended periods (Lindsay et al. 1995; Brunner et al. 2012; Burtis et al. 2016a, 2019). Ticks are physically and physiologically resistant to desiccation, but will of course die if they are dehydrated (Lees 1946, Rodgers et al. 2007). Ticks rehydrate by secreting hygroscopic saliva onto the hypostome in humid environments. The saliva absorbs water from the humid atmosphere and, once saturated, the saliva is re-ingested by the tick (Alarcon-Chaidez 2014). It is thought though that low ambient humidity has indirect effects on survival of questing exophilic ticks (i.e., those species/instars that quest for hosts on the surface rather than in host burrows or nests) by causing them to make more frequent descents to the duff layer to rehydrate, which hastens exhaustion of fat reserves, and reduces the likelihood of successful host finding (Lees and Milne 1951). Low levels of environmental moisture may affect tick populations through effects on host-seeking behavior and success (Berger et al. 2014, Burtis et al. 2016b).
Both ambient temperature and humidity can affect tick questing activity in quadratic relationships, i.e., exophilic ticks quest less frequently when temperatures are low and high. They similarly spend more time in the duff layer re-hydrating when humidity is low and may be repelled from activity by heavy rainfall (reviewed in Eisen et al. 2016). Assuming ambient humidity is not suboptimal, temperature can affect tick survival by determining the proportion of each day, and the duration of each year, that they can spend questing, and therefore the likelihood of host acquisition. Although the effects of climate change on temperature and humidity would have immediate effects on tick activity and survival, they also may affect evolutionary trajectories of tick populations by changing the environmental determinants of fitness. An example is provided by the variation in per-capita mortality rates of Ixodes scapularis Say (Acari: Ixodidae) in different parts of the United States. Per-capita mortality rates are greater due to greater desiccation stress under hotter conditions in the southern United States. Dessication stress may have acted as a selective pressure that resulted in the evolution of host-seeking behavior in which immatures remain below the leaf litter surface in southern I. scapularis populations, thereby avoiding desiccating conditions at the surface (Ginsberg et al. 2017).
Temperature affects the rate of development from one life stage to the next: engorged female to egg-laying female (the preoviposition period), egg development (the pre-eclosion period), and development of engorged larvae and nymphs to questing nymphs and adults, respectively (e.g., Ogden et al. 2004). In general, the relationship of the duration of development to temperature is nonlinear, with development having an increasingly long duration (or stopping altogether) at colder temperatures (e.g., Ogden et al. 2004). Given that cold winter temperatures are not limiting on the survival of tick populations as long as the habitat provides a refuge for the ticks, it has been suggested that the effects of temperature on lifecycle length, via effects on interstadial development rates, are key to the effects of climate warming on tick populations. The logic for this is that the warmer the climate, the shorter the lifecycle, and assuming daily per-capita mortality rates are unaffected by temperature (other than by effects of temperature on activity resulting in exhaustion of energy reserves: Burtis et al. 2019), then more larvae survive to be reproducing adults (Ogden and Lindsay 2016).
Temperature is not, however, the sole determinant of rates of development from one instar to the next. Interstadial development rates, as well as tick activity, are also affected by diapause. Here, we distinguish ‘diapause’ from ‘quiescence’. Effects of low or high temperature and humidity on reducing tick activity is ‘quiescence’ as described above (Belozerov 2009). Quiescent ticks resume activity as soon as temperature and humidity return to suitable levels. In contrast, diapause is a genetic trait for arrested development or activity arising from internal programming of the tick. This is usually switched on and/or off by factors such as day-length (i.e., photoperiod) that may have evolved because they signal approaching unfavorable conditions. However, in some species, temperature may, in fact, have a modulating effect on diapause (Belozerov 1964, Cabrera and Labruna 2009). Two types of diapause may impact the lifecycles of ixodid ticks: 1) behavioral diapause in which unfed ticks delay host-seeking activity even though weather conditions may be favorable; and 2) developmental (or morphogenetic) diapause in which eggs or fed immatures delay their development and fed mated females do not oviposit until some months after feeding, periods of time that are additional to that induced by effects of temperature on development (Oliver 1989, Sonenshine 1993). Therefore, the length and timing of the life cycle can be impacted by both ambient temperature-dependent quiescence of activity and development and temperature-independent, day-length-induced behavioral, or developmental diapause. Not all members of a local tick population may have the capacity to express diapause. In North America, Amblyomma americanum (L.) (Acari: Ixodidae) populations appear to comprise individuals that do and do not exhibit diapause (Pound et al. 1993). Populations of I. scapularis appear to vary similarly, with interpopulation differences in expression of diapause at different points in the lifecycle across the tick’s geographical range (Sonenshine 1993; Belozerov et al. 2002; Belozerov and Naumov 2002; Ogden et al. 2004, 2018). The interplay between temperature-dependent mechanisms and temperature-independent diapause inducement and termination drivers in tick lifecycles needs to be considered in assessing impacts of climate and climate change (Ogden et al. 2006, Ludwig et al. 2016).
Climate change is likely to impact the abundance and distribution of tick populations by indirect effects on 1) the habitat qualities that provide refuges for ticks from extremes of weather, and may or may not protect ticks from pathogens and predators (Samish and Alekeev 2001, MacDonald 2018, Li et al. 2019); and 2) the abundance of hosts, which may also be linked to habitat changes or by direct effects of climate change on survival (Simon et al. 2014, Dawe and Boutin 2016). It is likely that range expansions/contractions and changes in densities/species compositions of host and habitat communities will be highly idiosyncratic for different tick species. Whether or not changes in geographical distributions of suitable climate, habitat, and host communities actually result in changes in tick population ranges will depend on the capacity of the ticks to be dispersed outside existing ranges by hosts.
Weather and Climate Sensitivity of Tick-Borne Pathogen Transmission Cycles
Apart from the effect of climate change on determining the future geographical distribution and abundance of ticks, climate change may impact occurrence and force of infection in pathogen transmission cycles, in three main ways.
Effects on Seasonality of Different Tick Instars
The seasonal activity patterns of ticks depend on the combined effects of climate on development rates from one life stage to the next, coupled with how climate impacts weather conditions suitable for host-seeking activity (e.g., Ogden et al. 2005). In general, warming patterns in temperate regions would be expected to extend (or advance) the period each year when temperatures are suitable for tick activity (e.g., Moore et al. 2014, Monaghan et al. 2015). Clearly, effects of surface atmospheric temperature and humidity will be of more importance for ticks that display exophilic behavior rather than nidicolous ticks that live in the nests or burrows of their hosts, which may have microclimates that allow year-round development and activity (Bown et al. 2003). However, as pointed out above, it is possible that climate change may result in some ticks evolving nidicolous behavior with a consequent reduction (or at least change) in pathogen transmission cycles (Ginsberg et al. 2017).
For tick-borne pathogens maintained by trans-stadial transmission (without a component of transovarial transmission), seasonal synchrony of different tick instars can be an important determinant of the force of infection in transmission cycles. For maintenance of transmission cycles by trans-stadial transmission, the ‘infecting’ stage (either adult or nymph) must infect reservoir hosts, and the reservoir hosts must remain infectious and alive long enough to infect a ‘receiving’ stage (larvae and nymphs if the infecting stage is adults; only larvae if the infecting instar is nymphs). The more synchronous the seasonal activity of the infecting and receiving instars, the more efficient will be the transmission cycle. The combined effects of temperature on tick development and activity may mean that to some extent the synchrony of seasonality of the different tick stages is climate-sensitive. To what extent such effects impact transmission cycles depends on the duration of infection or infectivity in the host and also the life expectancy of short-lived hosts such as rodents. An example is the transmission of tick-borne encephalitis virus (TBEV) by Ixodes ricinus (L.) (Acari: Ixodidae) ticks in Europe. Infection in rodent hosts is exceedingly short (a few days), so precise seasonal synchrony of infecting nymphs and receiving larvae, which is climate sensitive, is an important determinant of the geographical range of risk from TBEV and how that range may change with climate change (Randolph et al. 1999, Randolph and Rogers 2000). A high degree of seasonal synchrony of infecting and receiving life stages also may permit transmission between co-feeding ticks, whether or not the host is systemically infected. In certain circumstances, this can be crucial to the maintenance of transmission cycles (Ogden et al. 1997, Labuda et al. 1996).
Many tick-borne pathogens have evolved strategies for persistent infection in reservoir hosts. However, in general, host immune responses result in a relatively short period (days to weeks) of high parasitemia and efficient host-to-tick transmission for most pathogens in most host species (Babesia, Anaplasma, Theileria spp.; Walter 1984, Randolph 1995, Young et al. 1996, Ogden et al. 2003, Levin and Ross 2004). So although seasonal asynchrony of tick instars may not prevent the existence of transmission cycles, greater synchrony likely enhances transmission (Ogden et al. 2008; Fig. 1). It is possible that effects of climate and climate change may operate indirectly on tick populations to produce geographical variations in the phenology of tick activity that impact transmission cycles. In northern North America nymphs and larvae of the tick I. scapularis are seasonally asynchronous (nymphs in spring, larvae in late summer) in the northeast, whereas in the upper Midwest, approximately half of larvae are active at the same time as nymphs in spring, so there appears to be an east-west gradient in the degree to which larvae are active at the same time as nymphs, and this correlates with climatic variables (Gatewood et al. 2009). However, field and modeling studies indicate that the driver for seasonal synchrony in I. scapularis populations in this region is more likely the degree to which the ticks exhibit the capacity for temperature-independent diapause (Ogden et al. 2018). Therefore, it is possible that the impact of climate on seasonal synchrony is via the degree to which the heritable trait of diapause enhances survival in the tick populations under the different climatological conditions in the upper Midwest and the northeast of this region (Ogden et al. 2018).
Fig. 1.
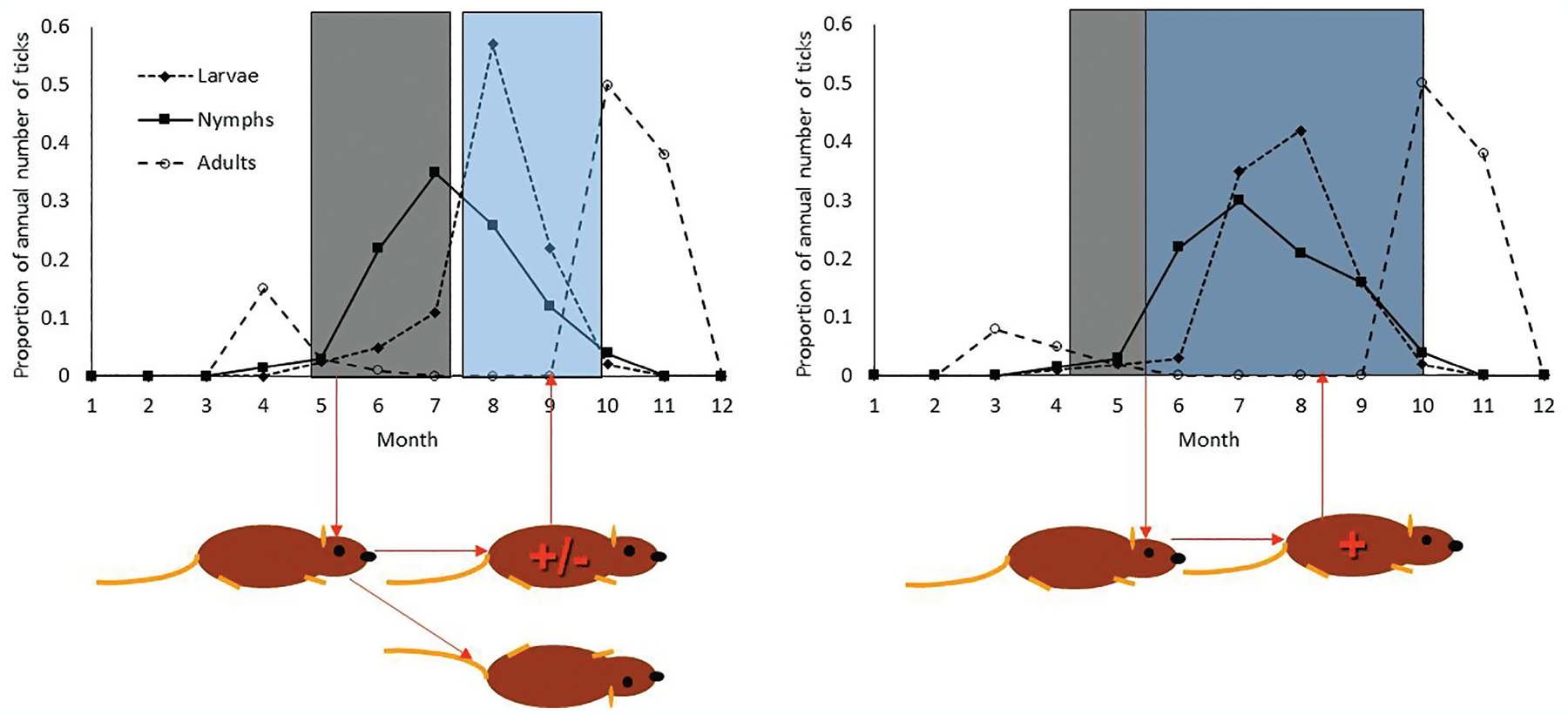
Impacts of seasonality of tick instars on transmission of tick-borne pathogens (after Ogden et al. 2008b). The dark gray and light blue rectangles illustrate differences in the degree of overlap of seasonal activity of larvae and nymphs.
Figure 1 illustrates one of the mechanisms by which the seasonality of tick stages affects transmission of tick-borne pathogens. In this illustration, nymphs (solid lines) and larvae (dotted lines) are seasonally asynchronous in the figure on the left but more synchronous in the figure on the right. In the figure on the left the cartoon of rodents indicates that the temporal separation of peaks of infecting nymphs and receiving larvae means that many rodents may become less infective to ticks, or may die, before they have the chance to host receiving larvae. This would be unlikely when nymphal and larval ticks are seasonally synchronous as in the figure on the right.
Effects on Reservoir Host Communities
It is likely that animal reservoir host populations will be impacted by climate change, albeit in ways that will likely be idiosyncratic for each species (of wildlife), animal production system (for domesticated animals), and geographical location. Climate change-driven geographical range changes, extinctions, and invasions affecting individual species and communities will likely occur, and beyond some broad patterns, these may be difficult to predict (Wilson et al. 2005, Simon et al. 2014, Urban 2015). Among wildlife host communities, it is likely that host species that are generalists in terms of their ecological niches will be those most resilient to change, and this may result in reduced diversity in tropical regions and increased diversity in temperate regions as tropical generalists change their geographical ranges (Davey et al. 2013, Kortsch et al. 2015). Some researchers consider that decreases in host biodiversity are associated with increased risk of tick-borne pathogens, whereas increases in biodiversity are protective (the ‘dilution effect’; Keesing and Ostfeld 2015). However, the extent to which changes in host diversity will impact tick-borne pathogen transmission is much debated (Wood et al. 2014, Levi et al. 2016), may be limited, and could act to increase or decrease risk (Ogden and Tsao 2009).
Effects on Pathogen Survival
For pathogens transmitted by dipteran vectors, the duration of the extrinsic incubation period (EIP: the time it takes for ingested pathogens to multiply and spread from the gut to the salivary glands for onward transmission) is an important temperature-dependent determinant of when and where transmission can occur now and in the future with climate change. However, as the development of the pathogen in hard-bodied ticks happens during feeding on the host, at approximately the host’s body temperature, and because transmission typically occurs in a subsequent life stage (often months later), the EIP is not a determinant of where and when tick-borne pathogen transmission cycles can occur (Ogden and Lindsay 2016). Inhibition of transmission of tick-borne pathogens by low temperatures, and enhancement of transmission by a changing climate, are therefore by virtue of the effects on tick and reservoir host occurrence and abundance. It is possible though that high temperatures can inhibit transmission by effects on pathogen survival in ticks. In vitro growth of Lyme disease-causing spirochaetes was suboptimal at high temperatures (Veinović et al. 2016).
Effects of Climate Change That May Reduce Tick Populations and Pathogen Transmission
High temperatures may have negative effects on some tick and pathogen populations. High temperatures make the cuticle of arthropods more permeable to water, reducing their resistance to desiccation (Beament 1959), and may have effects on fecundity in some species (Fish 1993), so in general, very high temperatures may limit tick populations in some locations. This could drive ticks to evolve a more nidicolous behavior as seen for I. scapularis in the southern United States (see above) resulting in reduced tick-borne pathogen transmission. For more specialized tick-host–pathogen systems, different effects of climate change on each species could potentially disrupt transmission in some locations (Fernández-Ruiz and Estrada-Peña 2020), while changes to seasonal synchrony of different tick instars may disrupt transmission of some pathogens such as TBEV in some locations (Randolph and Rogers 2000). Chaotic climate and weather extremes associated with a changing climate may impact tick survival. In general, variable weather and climate may be more lethal to ticks than absolute temperatures (Hermann and Gern 2013). Extensive flooding due to heavy rainfall events may kill ticks as long as flooding is prolonged (Bidder et al. 2019) or covers ticks with silt preventing questing (Weiler et al. 2017), whereas droughts will likely reduce tick populations due to desiccation (Berger et al. 2014). Survival of ticks in some habitats, which provide limited refuges from extremes of temperature and humidity, may depend on the stability of current weather patterns. For example I. ricinus, normally a tick of woodland habitats, survives on relatively barren sheep grazed uplands in northern and western United Kingdom and Eire in part due to mild winters and high rainfall throughout the year (Gray 1998). Chaotic weather bringing droughts, and very cold and very hot periods may particularly impact tick populations in such exposed habitats.
Projected Effects of Climate Change on Ticks and Tick-Borne Pathogens
There have been extensive efforts to assess the effects of projected climate change on ticks and tick-borne pathogens and examples are identified in this section. Many have focused on how geographical ranges of ticks may change, by inferring a climate ‘envelope’ for tick species populations from field and laboratory observation (and synthesis in dynamic models) or by ecological niche modeling approaches using presence/absence or presence-only data. Some have explored the effects of climate change on the seasonality of, and duration of seasons for tick activity, again by inferring from observational data the climatic determinants of activity. In our experience, better quality assessments of the possible impacts of climate change have the following characteristics:
Biologically plausible and externally validated associations of aspects of tick lifecycles and/or tick-borne pathogen transmission cycles (or their presence/absence) with climate variables under current climate.
Projections of future climate that come from ensembles of established regional or global climate models, such as the Coupled Model Intercomparison Project 5 (CMIP-5; Taylor et al. 2011), to account for variability among equally plausible futures projected by different models (while noting that some models are designed for particular geographical regions).
Projections for a range of future time slices up to a century ahead. Projections of future climate according to different scenarios for greenhouse gas emissions, which are currently termed representative concentration pathways (RCPs; van Vuuren et al. 2011).
All aspects of the sciences involved in projecting the effects of climate change on TTBDs (acarological, ecological, microbiological, climatological) have evolved over the ~25 yr of study of the effects of climate change on vector-borne diseases, and the literature reflects this. In the following illustrative summary of studies on projected effects of climate change, it is not our intention to score the studies according to their adherence to the characteristics described above, although that may be a worthwhile objective of future studies. It is also not our intention to list these studies exhaustively, and a systematic review is needed to more completely assess the literature. The majority of the studies that project the effects of climate change on ticks and tick-borne pathogens are those exploring possible geographical range changes. The most common method used to establish relationships with climate are ecological niche modeling (e.g., for A. americanum, Dermacentor variabilis Say [Acari: Ixodidae] and Rhipicephalus microplus Canestrini [Acari: Ixodidae]: Marques et al. 2020), and other ‘pattern matching’ approaches such as landscape regression (e.g., I. scapularis in North America: Brownstein et al. 2005). In some studies, dynamic models are used to infer sensitivity of populations to temperature by modeling effects of temperature on tick development and activity obtained from laboratory and/or field data (e.g., I. scapularis and A. americanum in North America, respectively: Ogden et al. 2006, 2014 and Sagurova et al. 2019; and I. ricinus in Europe: Li et al. 2019). In some studies, relatively simple metrics of the climatic determinants of tick population survival such as climate envelopes have been used (e.g., for Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand, I. ricinus in Europe, and Rhipicephalus species in Africa, respectively, Lawrence et al. 2017, Jaenson and Lindgren 2011, Olwoch et al. 2007). In almost all cases projected, changes in tick distributions were considered as proxies for future distributions of tick-borne pathogens, and all have projected pole-ward and upslope (altitudinal) expansion of the range of tick populations in temperate regions, whereas some have projected possible poleward contraction of equatorial limits of tick ranges associated with very high temperatures (e.g., Minigan et al. 2018, Brownstein et al. 2005; Fig. 2). The projected distributions of Rhipicephalus and Amblyomma species ticks in sub-Saharan Africa produced a complex picture of range expansions and contractions depending on species and geographic location associated with interactions among latitude, altitude, and the relative importance of temperature and rainfall on habitat suitability for each species (Olwoch et al. 2007, Estrada-Peña et al. 2008).
Fig. 2.
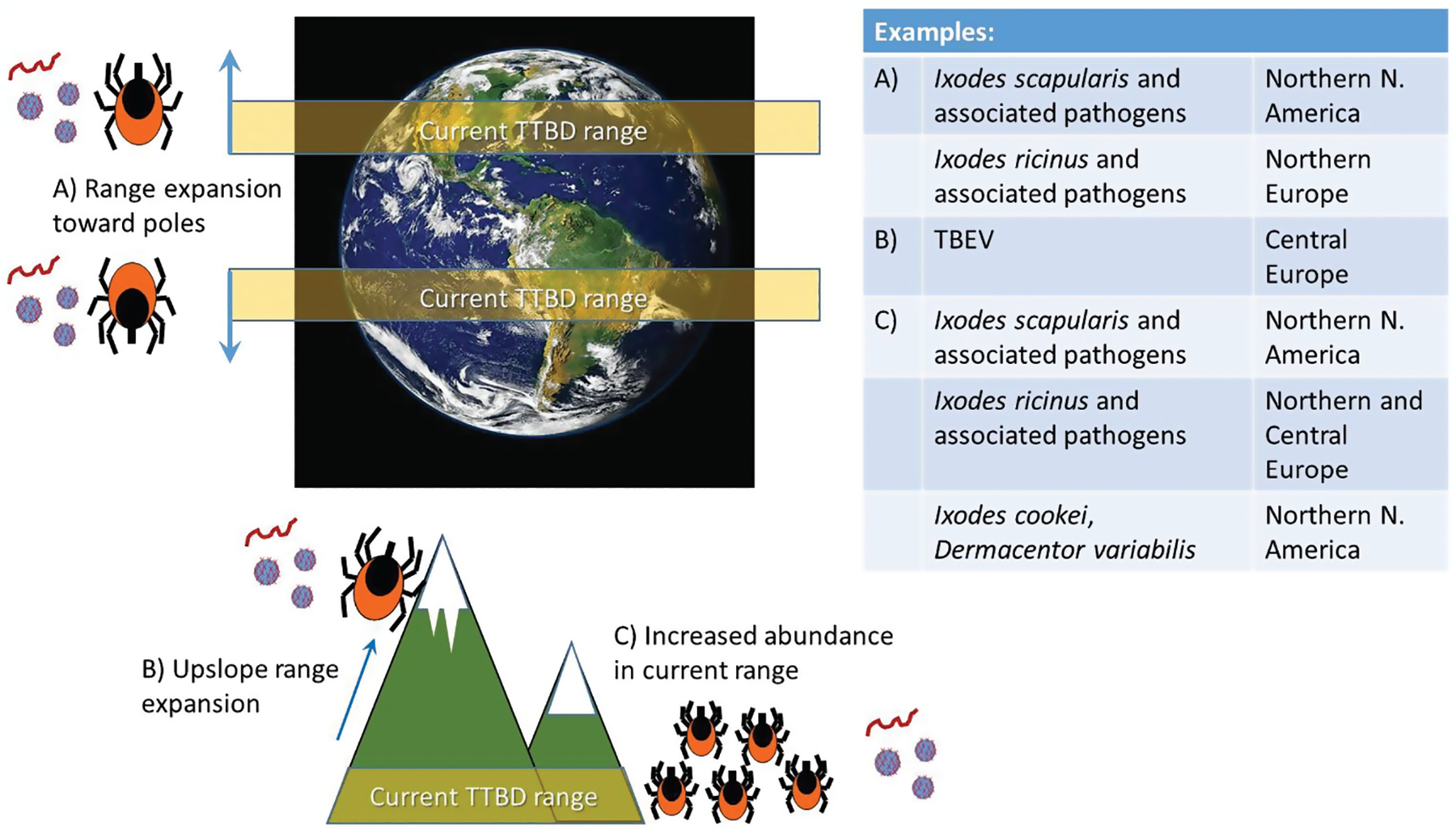
Schematic diagram of projected effects of climate change on tick and tick-borne pathogen populations. In the table to the right selected examples where there is evidence of changes are shown. Details of these changes are described in the text.
Clearly, changes in the geographical patterns of climatic suitability for tick species will not result in rapid changes in geographical ranges unless there is sufficient long-range movement of ticks by hosts into new areas of suitability. Terrestrial hosts can carry ticks over relatively short distances, producing a ‘pushed’ slow expansion of the ranges of TTBDs, whereas migratory birds appear capable of more rapid ‘pulled’ range expansions (reviewed in Ogden et al. 2013). Migratory passerines seem particularly capable of dispersing exophilic ticks and the pathogens they carry (Scott et al. 2001, Ogden et al. 2008, Dubska et al. 2009, Cohen et al. 2015), as well as bird-specialist nidicolous ticks (e.g., Scott et al. 2001, Gray et al 2014). So in many cases, geographical range changes could be expected to result from a changing climate. One study has identified the impact of climate change on the geographical distribution of TBEV in Europe via the effects on where seasonal synchrony of larval and nymphal I. ricinus activity is expected to occur (Randolph and Rogers 2000).
As well as effects of climate change on geographical ranges of ticks, some studies have assessed how climate change may affect the onset or duration of tick activity and the impact on human cases of tick-borne zoonoses such as Lyme disease. In these studies, the season of tick activity generally begins earlier in the year and is potentially longer (e.g., Levi et al. 2015, Monaghan et al. 2015, MacDonald et al. 2020).
Evidence for the Effects of Climate Change on Ticks and Tick-Borne Disease
Evidence for the effects of climate change is a two-step process—detection in changes to the occurrence of TTBDs and then attribution to climate change (Ebi et al. 2017). There are many surveillance or ecological monitoring systems and studies globally that are capable of detecting changes in occurrence over small time scales (e.g., Clow et al. 2017, Hvidsten et al. 2020), and these can be useful in supporting evidence of a climate signal in tick range changes by virtue of observed geographical patterns of range expansion that correlate with climatic conditions (Ebi et al. 2017). Stronger attribution is, however, provided by surveillance that has been in operation long enough to provide the long-term datasets needed to attribute those changes to climatic change. Examples of cases where datasets are long enough to potentially attribute changes in tick or pathogen occurrence to contemporaneously observed climate change, and in which range expansion occurred logically after temperatures increased, include those that tracked the northward expansion of Ixodes persulcatus Schulze (Acari: Ixodidae) and TBEV occurrence over 20–30 yr in northern Russia (Tokarevich et al. 2011), the expansion of I. ricinus in a region of eastern Russia over 35 yr (Korotkov et al. 2015), the northward expansion in the range of I. scapularis ticks in southern Canada over 25 yr (Leighton et al. 2012), and the altitudinal increase in TBEV over 38 yr in the Czech Republic (Kriz et al. 2012). An increased incidence of TBEV infection in an already endemic area of Sweden was reported over a 38-yr surveillance period and associated with a warming climate (Lindgren and Gustafson 2001). However, each case is essentially a single observation, and there are many other possible climate-independent reasons why ticks and tick-borne pathogens may change their geographical ranges (Randolph 2010, Kilpatrick and Randolph 2012, Kugeler et al. 2015, Eisen et al. 2016), so caution must be taken in interpreting studies and attributing distributional changes to climate change. Nevertheless, there is increasing evidence of changes in the ranges of ticks in multiple locations that are consistent with an impact of climate change.
Knowledge Gaps and Future Research, Surveillance, and Modeling Needed
Inevitably, assessments and observations of the possible effects of climate change on the occurrence of, and level of risk posed (to humans or animals), by TTBDs use simple metrics such as presence/absence and abundance/prevalence as these are what are readily measurable at present. However, clearly there are complexities to tick lifecycles and transmission cycles that may be affected by climate change. Impacts may operate by (or be mitigated by) regulatory mechanisms such as acquired host resistance to ticks and pathogens. High temperatures (and perhaps other weather stressors) may impair expression of acquired immune responses by animal hosts (Jolles et al. 2015, Bagath et al. 2019) and reduce the effects of host immunity on regulation of tick and pathogen populations. Increasing abundance of ticks associated with a warming climate may be limited by, or actually overcome by density-dependent acquired resistance to ticks (Ogden et al. 2002a,b), and at the same time affect pathogen transmission at the tick-host interface (Wikel 1999). Increased tick density may facilitate regulation of pathogen transmission via ‘endemic stability’, i.e., higher densities of ticks increase the likelihood that hosts acquire tick-borne infections as very young animals when they are partially protected from severe disease due to maternal immunity to the pathogens (Norval et al. 1992). Changes in the population dynamics of wild animal host communities, as well as multiple, sympatric ticks and pathogens (Gasmi et al. 2018), may mean that effects of climate change are difficult to detect or predict. Furthermore, climate change is likely to impact evolutionary trajectories of all components of the tick-host–pathogen community. Tick populations often demonstrate genetic heterogeneity in different geographical locations (e.g., for I. scapularis, Qiu et al. 2002, Gulia-Noss et al. 2016, Xu et al. 2020), which may be associated with differences in lifecycle processes, survivorship, and host-seeking behavior (e.g., Ginsberg et al. 2014, Ogden et al. 2018, Arsnoe et al. 2019). To what extent genetic heterogeneity is driven by climate is generally unknown, as is any potential effect of climate change. However, effects on trajectories of tick and pathogen evolution may result in adaptive emergence of tick-borne pathogens that have greater impact on human and/or animal populations in terms of pathogenicity and invasiveness (Ogden et al. 2019).
There is an increasingly urgent need to predict possible emergence and re-emergence of tick-borne diseases for public and animal health planning. Throughout, a One Health approach will be necessary to encompass the complexity of the relationships needed for prediction, detection, and attribution of effects of climate change. Modeling will continue to define ecological niches, their climatic component, and other key determinants that must be accounted for. ‘Pattern matching’ approaches will continue to be relevant, but the capacity for ticks and pathogens to expand their range into ecozones where they have not occurred in human memory or record (e.g., I. scapularis in southern Canada), and to be absent from some suitable locations in areas of emergence (Clow et al. 2017) means that enhanced mechanistic knowledge of tick lifecycles and population processes, host population dynamics and pathogen transmission are increasingly needed to validate pattern matching approaches and to support dynamic modeling approaches.
A coordinated research and surveillance program that iteratively links field and laboratory observations with in-silico synthesis is needed for these purposes. However, the foundation of such endeavor will be long-term One Health observational studies that record as many aspects of TTBD ecology and epidemiology as possible. These studies need to 1) be strategically placed along ecological, climatological, altitudinal, and latitudinal transects, 2) run for timescales long enough to detect changes in occurrence patterns and attribute these to climate change, 3) be equally capable of exploring and accounting for climate change-independent drivers of changing patterns; and 4) detect reductions as well as increases in risk from TTBD.
Acknowledgments
We thank E. Hofmeister for constructive comments on an early draft of the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
References Cited
- Alarcon-Chaidez FJ 2014. Salivary glands—structure, physiology and molecular biology. pp. 163–205. In Sonenshine DE and Rowe RM (eds.). The Biology of Ticks, 2nd edn. Oxford University Press, New York. [Google Scholar]
- Arsnoe I, Tsao JI, and Hickling GJ. 2019. Nymphal Ixodes scapularis questing behavior explains geographical variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 10: 553–563. [DOI] [PubMed] [Google Scholar]
- Bagath M Krishnan G, Devaraj C, Rashamol VP, Pragna P, Lees AM, and Sejian V. 2019. The impact of heat stress on the immune system in dairy cattle: a review. Res. Vet .Sci 126: 94–102. [DOI] [PubMed] [Google Scholar]
- Beament J 1959. The waterproofing mechanism of arthropods: I. The effect of temperature on cuticle permeability in terrestrial insects and ticks. J. Exp. Biol 36: 391–422. [Google Scholar]
- Belozerov VN 1964. The diapause of the larvae of the tick Ixodes ricinus L. and its dependence on external conditions. Zool. Zhurnal 43: 1626–1637. [Google Scholar]
- Belozerov VN 2009. Diapause and quiescence as two main kinds of dormancy and their significance in life cycles of mites and ticks (Chelicerata: Arachnida: Acari). Part 2. Parasitiformes. Acarina 17: 3–32. [Google Scholar]
- Belozerov VN, and Naumov RL. 2002. Nymphal diapause and its photoperiodic control in the tick Ixodes scapularis (Acari: Ixodidae). Folia Parasitol. 49: 314–318. [DOI] [PubMed] [Google Scholar]
- Belozerov VN, Fourie LJ, and Kok DJ. 2002. Photoperiodic control of developmental diapause in nymphs of prostriate ixodid ticks (Acari: Ixodidae). Exp. Appl. Acarol 28: 163–168. [DOI] [PubMed] [Google Scholar]
- Berger KA, Ginsberg HS, Dugas KD, Hamel L and Mather TN. 2014. Adverse moisture events predict seasonal abundance of Lyme disease vector ticks (Ixodes scapularis). Parasit. Vectors 7: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidder LA, Asmussen KM, Campbell SE, Goffigan KA, and Gaff HD. 2019. Assessing the underwater survival of two tick species, Amblyomma americanum and Amblyomma maculatum. Ticks Tick. Borne. Dis 10: 18–22. [DOI] [PubMed] [Google Scholar]
- Bown KJ, Bennett M, Woldehiwet Z, Begon M, and Ogden NH, 2003. Seasonal dynamics of Anaplasma (Ehrlichia) phagocytophila infection in a rodent-Ixodes trianguliceps system in the UK. Emerging Infect. Dis 9, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein JS, Holford TR, and Fish D. 2005. Effect of climate change on Lyme disease risk in North America. Ecohealth. 2: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner JL, Killilea M, and Ostfeld RS. 2012. Overwintering survival of nymphal Ixodes scapularis (Acari: Ixodidae) under natural conditions. J. Med. Entomol 49: 981–987. [DOI] [PubMed] [Google Scholar]
- Burks CS, Stewart RL, Needham GR, and Lee RE. 1996. The role of direct chilling injury and inoculative freezing in cold tolerance of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis. Physiol. Entomol 21: 44–50. [Google Scholar]
- Burtis JC, Ostfeld RS, Yavitt JB, and Fahey TJ. 2016a. The relationship between soil arthropods and the overwinter survival of Ixodes scapularis (Acari: Ixodidae) under manipulated snow cover. J. Med. Entomol 53: 225–229. [DOI] [PubMed] [Google Scholar]
- Burtis JC, Sullivan P, Levi T, Oggenfuss K, Fahey TJ, and Ostfeld RS. 2016b. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in emerging regions. Parasit. Vectors 9: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis JC, Fahey TJ, and Yavitt JB. 2019. Survival and energy use of Ixodes scapularis nymphs throughout their overwintering period. Parasitology. 146: 781–790. [DOI] [PubMed] [Google Scholar]
- Cabrera RR, and Labruna MB. 2009. Influence of photoperiod and temperature on the larval behavioral diapause of Amblyomma cajennense (Acari: Ixodidae). J. Med. Entomol 46: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Clow K, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl D, and Jardine C. 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One 12: e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EB, Auckland LD, Marra PP, and Hamer SA. 2015. Avian migrants facilitate invasions of neotropical ticks and tick-borne pathogens into the United States. Appl. Environ. Microbiol 81: 8366–8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AA, Scoones I, and Wood JN. 2017. Introduction: One Health for a changing world: new perspectives from Africa. Phil. Trans. R. Soc. B 372(pii): 20160162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CM, Devictor V, Jonzén N, Lindström A, and Smith HG. 2013. Impact of climate change on communities: revealing species’ contribution. J. Anim. Ecol 82: 551–561. [DOI] [PubMed] [Google Scholar]
- Dawe KL, and Boutin S. 2016. Climate change is the primary driver of white-tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecol. Evol 6: 6435–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubska L, Literak I, Kocianova E, Taragelova V, and Sychra O. 2009. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl. Environ. Microbiol 75: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL, Ogden NH, Semenza JC, and Woodward A. 2017. Detecting and attributing health burdens to climate change. Environ. Health Perspect 125: 085004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, and Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53: 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Horak IG, and Petney T. 2008. Climate changes and suitability for the ticks Amblyomma hebraeum and Amblyomma variegatum (Ixodidae) in Zimbabwe (1974–1999). Vet. Parasitol 151: 256–267. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, de la Fuente J, Latapia T, and Ortega C. 2015. The impact of climate trends on a tick affecting public health: a retrospective modeling approach for Hyalomma marginatum (Ixodidae). PLoS One 10: e0125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz N, and Estrada-Peña A. 2020. Could climate trends disrupt the contact rates between Ixodes ricinus (Acari, Ixodidae) and the reservoirs of Borrelia burgdorferi s.l.? PLoS One. 15: e0233771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CB, Barros VR, Mach KJ, Mastrandrea MD, van Aalst M, Adger WN, Arent DJ, Barnett J, Betts R, Bilir TE, et al. 2014. Technical summary, pp. 35–94. In Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, et al. (eds.). Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Fish D 1993. Population ecology of Ixodes dammini, pp. 25–42. In Ginsberg HS (ed.), Ecology and environmental management of Lyme disease. Rutgers University Press, New Brunswick, NJ. [Google Scholar]
- Gasmi S, Bouchard C, Ogden NH, Adam-Poupart A, Pelcat Y, Rees EE, Milord F, Leighton PA, Lindsay RL, Koffi JK, et al. 2018. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One. 13: e0201924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, et al. 2009. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl. Environ. Microbiol 75: 2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Rulison EL, Azevedo A, Pang GC, Kuczaj IM, Tsao JI, and LeBrun RA. 2014. Comparison of survival patterns of northern and southern genotypes of the North American tick Ixodes scapularis (Acari: Ixodidae) under northern and southern conditions. Parasit. Vectors 7: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Albert M, Acevedo L, Dyer MC, Arsnoe IM, Tsao JI, Mather TN, and LeBrun RA. 2017. Environmental factors affecting survival of immature Ixodes scapularis and implications for geographical distribution of Lyme Disease: the climate/behavior hypothesis. PLoS One. 12: e0168723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Lindsay SW, Confalonieri UE, and Patz JA. 2000. Climate change and vector-borne diseases: a regional analysis. Bull. World Health Organ 78: 1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Gray JS 1998. The ecology of ticks transmitting Lyme borreliosis. Exper. Appl. Acarol 22: 249–258. [Google Scholar]
- Gray JS, Estrada-Pena A, and Vial L. 2014. Ecology of nidicolous ticks, pp. 39–60. In Sonenshine DE, and Roe RM (eds.). Biology of ticks, Vol. 2. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Waterhouse RM, Sattelle DB, de la Fuente J, Ribeiro JM, Megy K, et al. 2016. Genetic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Comm 7: 10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PA, Dunford RW, Holman IP, and Rounsevell M. 2016. Climate change impact modelling needs to include cross-sectoral interactions. Nat. Clim. Change 6: 885–890. [Google Scholar]
- Herrmann C, and Gern L. 2013. Survival of Ixodes ricinus (Acari: Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks Tick. Borne. Dis 4: 445–451. [DOI] [PubMed] [Google Scholar]
- Hvidsten D, Frafjord K, Gray JS, Henningsson AJ, Jenkins A, Kristiansen BE, Lager M, Rognerud B, Slåtsve AM, Stordal F, et al. 2020. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick. Borne. Dis 11: 101388. [DOI] [PubMed] [Google Scholar]
- IPCC. 2013. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change, 1535 pp. In Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM (eds.) Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Jaenson TG, and Lindgren E. 2011. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick. Borne. Dis 2: 44–49. [DOI] [PubMed] [Google Scholar]
- Jolles AE, Beechler BR, and Dolan BP. 2015. Beyond mice and men: environmental change, immunity and infections in wild ungulates. Parasite Immunol. 37: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, and Ostfeld RS. 2015. Is biodiversity good for your health? Science 349: 235–236. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, and Randolph SE. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380: 1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov Y, Kozlova T, and Kozlovskaya L. 2015. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med. Vet. Entomol 29: 129–136. [DOI] [PubMed] [Google Scholar]
- Kortsch S, Primicerio R, Fossheim M, Dolgov AV, and Aschan M. 2015. Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc Biol Sci. 282: 20151546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, and Haines A. 1995. The potential health impacts of climate change: an overview. Med. War 11: 168–178. [DOI] [PubMed] [Google Scholar]
- Kriz B, Maly M, Benes C, and Daniel M. 2012. Epidemiology of tick-borne encephalitis in the Czech Republic 1970–2008. Vector Borne Zoonotic Dis. 12: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Farley GM, Forrester JD, and Mead PS. 2015. Geographic distribution and expansion of human Lyme disease, United States. Emerg. Infect. Dis 21: 1455–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda M, Austyn JM, Zuffova E, Kozuch O, Fuchsberger N, Lysy J, and Nuttall PA. 1996. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 219: 357–366. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Summers SR, Heath ACG, McFadden AMJ, Pulford DJ, Tait AB, and Pomroy WE. 2017. Using a rule-based envelope model to predict the expansion of habitat suitability within New Zealand for the tick Haemaphysalis longicornis, with future projections based on two climate change scenarios. Vet. Parasitol 243: 226–234. [DOI] [PubMed] [Google Scholar]
- Lees AD 1946. The water balance in Ixodes ricinus L. and certain other species of ticks. Parasitology. 37: 1–20. [DOI] [PubMed] [Google Scholar]
- Lees AD, and Milne A. 1951. The seasonal and diurnal activities of individual sheep ticks (Ixodes ricinus L). Parasitology. 41: 189–208. [DOI] [PubMed] [Google Scholar]
- Leighton P, Koffi J, Pelcat Y, Lindsay LR, and Ogden NH. 2012. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol 49: 457–464. [Google Scholar]
- Levi T, Keesing F, Oggenfuss F, and Ostfeld RS. 2015. Accelerated phenology of blacklegged ticks under climate warming. Phil. Trans. Royal Soc. B 370: 20130556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi T, Keesing F, Holt RD, Barfield M, and Ostfeld RS. 2016. Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecol. Appl 26: 484–498. [DOI] [PubMed] [Google Scholar]
- Levin ML, and Ross DE. 2004. Acquisition of different isolates of Anaplasma phagocytophilum by Ixodes scapularis from a model animal. Vector Borne Zoonotic Dis. 4: 53–59. [DOI] [PubMed] [Google Scholar]
- Li S, Gilbert L, Vanwambeke SO, Yu J, Purse BV, and Harrison PA. 2019. Lyme disease risks in Europe under multiple uncertain drivers of change. Environ. Health Perspect 127: 67010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske DJ, and Lloyd VK. 2018. Combining public participatory surveillance and occupancy modelling to predict the distributional response of Ixodes scapularis to climate change. Ticks Tick Borne Dis. 9: 695–706. [DOI] [PubMed] [Google Scholar]
- Lindgren E, and Gustafson R. 2001. Tick-borne encephalitis in Sweden and climate change. Lancet. 358: 16–18. [DOI] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, McEwen SA, Gillespie TJ, and Robinson JT. 1995. Survival and development of Ixodes scapularis (Acari: Ixodidae) under various climatic conditions in Ontario, Canada. J. Med. Entomol 32: 143–152. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Ginsberg HS, Hickling GJ, and Ogden NH. 2016. A dynamic population model to investigate effects of climate and climate-independent factors on the lifecycle of Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 53: 99–115. [DOI] [PubMed] [Google Scholar]
- MacDonald AJ 2018. Abiotic and habitat drivers of tick vector abundance, diversity, phenology and human encounter risk in southern California. Plos One. 13: e0201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, McComb S, O’Neill C, Padgett KA, and Larsen AE. 2020. Projected climate and land use change alter western blacklegged tick phenology, seasonal host-seeking suitability and human encounter risk in California. Global Change Biol 26: 5459–5474. [DOI] [PubMed] [Google Scholar]
- Marques R, Krüger RF, Peterson AT, de Melo LF, Vicenzi N, and Jiménez-García D. 2020. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Vet. Res 51: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens WJM, Niessen L, Rotmans J, Jetten TH, and McMichael AJ. 1995. Potential impact of global climate change on malaria risk. Environ. Health Perspect 103: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan JN, Hager HA, Peregrine AS, and Newman JA. 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick. Borne. Dis 9: 354–362. [DOI] [PubMed] [Google Scholar]
- Monaghan AJ, Moore SM, Sampson KM, Beard CB, and Eisen RJ. 2015. Climate change influences on the annual onset of Lyme disease in the United States. Ticks Tick. Borne. Dis 6: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Eisen RJ, Monaghan A, and Mead P. 2014. Meteorological influences on the seasonality of Lyme disease in the United States. Am. J. Trop. Med. Hyg 90: 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakanta G, Sultana H, Fish D, Anderson JF, and Fikrig E. 2010. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest 120: 3179–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norval RAI, Perry BD, and Young AS. 1992. The epidemiology of theileriosis in Africa. Academic Press, London, United Kingdom. [Google Scholar]
- Ogden NH, and Lindsay LR. 2016. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol. 32: 646–656. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Nuttall PA, and Randolph SE. 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115: 591–599. [DOI] [PubMed] [Google Scholar]
- Ogden NH, and Tsao JI. 2009. Biodiversity and Lyme disease: Dilution or amplification? Epidemics 1: 196–206. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Casey AN, French NP, Adams JD, and Woldehiwet Z. 2002a. Field evidence for density-dependent facilitation amongst Ixodes ricinus ticks feeding on sheep. Parasitology. 124: 117–125. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Case AN, Lawrie CH, French NP, Woldehiwet Z, and Carter SD. 2002b. IgG responses to salivary gland extract of Ixodes ricinus ticks vary inversely with resistance in naturally exposed sheep. Med. Vet. Entomol 16: 186–192. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Casey AN, Woldehiwet Z, and French NP. 2003. Transmission of Anaplasma phagocytophilum to Ixodes ricinus ticks from sheep in the acute and post-acute phases of infection. Infect. Immun 71: 2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toews D, and Barker IK. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol 41: 622–633. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Bigras-Poulin M, OCallaghan CJ, Barker IK, Lindsay LR, Maarouf A, Smoyer-Tomic KE, Waltner-Toews D, and Charron D. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol 35: 375389. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O’callaghan CJ, Ramay F, Waltner-Toews D, and Charron DF. 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol 36: 63–70. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Bigras-Poulin M, Hanincová K, Maarouf A, OCallaghan CJ, and Kurtenbach K. 2008. Projected effects of climate change on tick phenology and their influence on fitness of pathogens transmitted by the tick Ixodes scapularis. J. Theor. Biol 254: 621–632. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Mechai S, and Margos G. 2013. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Frontiers Cell. Inf. Microbiol 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, and Wu J. 2014. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ. Health Perspect 122: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Pang G, Ginsberg HS, Hickling GJ, Burke RL, Beati L, and Tsao JI. 2018. Evidence for geographic variation in life-cycle processes affecting phenology of the Lyme disease vector Ixodes scapularis (Acari: Ixodidae) in the United States. J. Med. Entomol 55: 1386–1401. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Wilson JRU, Richardson DM, Hui C, Davies SJ, Kumschick S, Le Roux JJ, Measey J, Saul W-C, and Pulliam JRC. 2019. Emerging infectious diseases and biological invasions—a call for a One Health collaboration in science and management. Royal Soc. Open Sci 6: 181577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JH Jr. 1989. Lyme disease: tick vectors, distribution, and reservoir hosts. J. Med. Assoc. Ga 78: 675–678. [PubMed] [Google Scholar]
- Olwoch JM, Van Jaarsveld AS, Scholtz CH, and Horak IG. 2007. Climate change and the genus Rhipicephalus (Acari: Ixodidae) in Africa. Onderstepoort J. Vet. Res 74: 45–72. [DOI] [PubMed] [Google Scholar]
- Overpeck JT, and Weiss JL. 2009. Projections of future sea level becoming more dire. Proc. Natl. Acad. Sci. U. S. A 106: 21461–21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Martens WJ, Focks DA, and Jetten TH. 1998. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect 106: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound JM, Hilburn LR, and George JE. 1993. Implications of selection and hybridization studies on the mode of inheritance of photoperiodically induced developmental diapause in laboratory strains of the lone star tick (Acari: Ixodidae). J. Med. Entomol 30: 100–106. [DOI] [PubMed] [Google Scholar]
- Qiu WG, Dykhuizen DE, Acosta MS, and Luft BJ. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics. 160: 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Peterson AT, Cobos ME, Ganta R, and Foley D. 2019. Current and future distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One 14: e02–09082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE 1995. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus). Parasitology. 110 (Pt 3): 287–295. [DOI] [PubMed] [Google Scholar]
- Randolph SE 2010. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Vet. Parasitol 167: 92–94. [DOI] [PubMed] [Google Scholar]
- Randolph SE, and Rogers DJ. 2000. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc. Biol. Sci 267: 1741–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Miklisová D, Lysy J, Rogers DJ, and Labuda M. 1999. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 118 (Pt 2): 177–186. [DOI] [PubMed] [Google Scholar]
- Reiter P 2001. Climate change and mosquito-borne disease. Environ. Health Perspect 109(Suppl 1): 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P Thomas CJ, Atkinson PM, Hay SI, Randolph SE, Rogers DJ, Shanks GD, Snow RW, and Spielman A. 2004. Global warming and malaria: a call for accuracy. Lancet Infect. Dis 4: 323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, and Randolph SE. 2000. The global spread of malaria in a future, warmer world. Science. 289: 1763–1766. [DOI] [PubMed] [Google Scholar]
- Rodgers SE, Zolnik CP, and Mather TN. 2007. Duration of exposure to suboptimal atmospheric moisture affects nymphal blacklegged tick survival. J. Med. Entomol 44: 372–375. [DOI] [PubMed] [Google Scholar]
- Sagurova I, Ludwig A, Ogden NH, Pelcat Y, Dueymes G, and Gachon P. 2019. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under Future Climate Conditions. Environ. Health Perspect 127: 107014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samish M, and Alekseev E. 2001. Arthropods as predators of ticks (Ixodoidea). J. Med. Entomol 38: 1–11. [DOI] [PubMed] [Google Scholar]
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, and Morshed MG. 2001. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol 38: 493–500. [DOI] [PubMed] [Google Scholar]
- Simon JA, Marrotte RR, Desrosiers N, Fiset J, Gaitan J, Gonzalez A, Koffi JK, Lapointe F-J, Leighton PA, Lindsay LR, et al. 2014. Climate change, habitat fragmentation, ticks and the white-footed mouse drive occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northern limit of its distribution. Evol. Appl 7: 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE 1993. Biology of ticks, vol. 2, Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Taylor KE, Stouffer RJ, and Meehl GA. 2011. An overview of CMIP5 and the experiment design. Bull. Amer. Meteor. Soc 93: 485–498. [Google Scholar]
- Tokarevich NK, Tronin AA, Blinova OV, Buzinov RV, Boltenkov VP, Yurasova ED, and Nurse J. 2011. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob. Health Action 4: 8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MC 2015. Accelerating extinction risk from climate change. Science 348: 571–573. [DOI] [PubMed] [Google Scholar]
- Vandyk JK, Bartholomew DM, Rowley WA, and Platt KB. 1996. Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. J. Med. Entomol 33: 6–10. [DOI] [PubMed] [Google Scholar]
- Van Vuuren DP, Edmonds J, Thomson A, Riahi K, Kainuma M, Matsui T, Hurtt GC, Lamarque J-F, Meinshausen M, Smith S, et al. 2011. Representative concentration pathways: an overview. Climatic Change 109: 5–31. [Google Scholar]
- Veinović G, Ružić-Sabljić E, Strle F, and Cerar T. 2016. Comparison of growth of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto at five different temperatures. PLoS One. 11: e0157706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G 1984. Transmission and course of parasitemia of Babesia microti (Hannover I strain) in the bank vole (Clethrionomys glareolus) and field vole (Microtus agrestis). Acta Trop. 41: 259–264. [PubMed] [Google Scholar]
- Weiler M, Duscher GG, Wetscher M, and Walochnik J. 2017. Tick abundance: a one year study on the impact of flood events along the banks of the river Danube, Austria. Exp. Appl. Acarol 71: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol 29: 851–859. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Gutiérrez D, Gutiérrez J, Martínez D, Agudo R, and Monserrat VJ. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett 8: 1138–1146. [DOI] [PubMed] [Google Scholar]
- Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, and Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95: 817–832. [DOI] [PubMed] [Google Scholar]
- Xu G, Wielstra B, and Rich SM. 2020. Northern and southern blacklegged (deer) ticks are genetically distinct with different histories and Lyme disease infection rates. Sci. Rep 10: 10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Dolan TT, Morzaria SP, Mwakima FN, Norval RA, Scott J, Sherriff A, and Gettinby G. 1996. Factors influencing infections in Rhipicephalus appendiculatus ticks fed on cattle infected with Theileria parva. Parasitol. 113: 255–266. [DOI] [PubMed] [Google Scholar]


