Abstract
INTRODUCTION/OBJECTIVES
Non-albicans Candida species such as C. parapsilosis and C. glabrata have emerged as prevalent pathogens in premature infants. The aim of this study was to systematically delineate the histopathologic findings in neonatal non-albicans candidiasis.
METHODS
We performed a retrospective clinicopathologic analysis of extremely premature (23-28 weeks gestation) infants diagnosed with invasive candidiasis. Archival autopsy tissues were subjected to periodic acid-Schiff, methenamine-silver and anti-Candida (immuno)histochemical stains, as well as dual anti-Candida and anti-cytokeratin or anti-CD31 immunofluorescence assays. In addition, we studied the prevalence of intestinal Candida colonization in a consecutive autopsy series of extremely premature infants.
RESULTS
Based on positive postmortem blood and/or lung cultures, invasive candidiasis (3 non-albicans, 11 C. albicans) was diagnosed in 14/187 extremely premature infants examined between 1995 and 2017. In contrast to the well-known inflammatory and tissue-destructive phenotype of congenital C. albicans infection, invasive non-albicans candidiasis/candidemia caused by C. parapsilosis and C. glabrata was inconspicuous by routine hematoxylin-eosin-based histopathologic analysis despite a heavy fungal presence detected in intestines, lungs and blood by targeted (immuno)histochemical assays. Intestinal colonization by Candida species was identified in 16/26 (61%) extremely premature neonates who had lived for at least one week, as assessed by anti-Candida immunostaining.
CONCLUSION
Invasive neonatal non-albicans candidiasis/candidemia appears to have no distinct histopathologic signature. Based on the notoriously low sensitivity of fungal blood cultures and the observed high frequency of Candida intestinal colonization (>50%), it is likely that non-albicans candidiasis/candidemia may be underdiagnosed in (deceased) preterm infants. Routine inclusion of targeted (immuno)histochemical fungal detection strategies in the perinatal autopsy may lead to deeper insight into the prevalence and clinical relevance of neonatal non-albicans candidiasis.
Keywords: Candida parapsilosis, Candida glabrata, yeast, prematurity, newborn
INTRODUCTION
Candida species are the leading cause of invasive fungal disease (clinically defined as candidemia (blood stream infection) and/or infection of deep organs (1)) in premature infants (2, 3). The risk for invasive candidiasis is particularly high in extremely-low-birth-weight neonates (birth weight < 1000 g), with mortality rates up to 30% (4). The susceptibility of very preterm infants to Candida infections has been attributed to the immaturity of their skin and immune system, as well as multiple risk factors inherent in their intensive care and prolonged hospitalization [summarized in (2)].
Whereas C. albicans has historically been the most prevalent cause of fungal infections, infections with non-albicans Candida species have increased dramatically over the last two decades (5-7). At present, C. parapsilosis, C. glabrata (formerly Torulopsis glabrata), C. tropicalis, and C. krusei together represent about half of all Candida species isolated from blood cultures. This epidemiological shift has been attributed, in part, to the increased use of azoles and caspofungin (8).
While originally considered to be non-virulent, non-albicans Candida species such as C. parapsilosis and C. glabrata have emerged as significant pathogens, even outranking C. albicans as the leading organism in invasive neonatal candidiasis in some centers (9). Candida albicans and non-albicans Candida species show important differences in morphology, virulence factors, and host interaction. Like most Candida species, C. parapsilosis and C. glabrata exist mainly as spherical or ovoid blastospores or yeast cells and are further capable of producing chains of elongated blastospores termed pseudohyphae (10). Candida albicans also exists as elongated filamentous cells known as true hyphae (11). This true hyphal morphogenesis is implicated in the capacity of C. albicans to invade and injure epithelial and endothelial cells (12). Interspecies morphological differences also extend to differences in biofilm formation (13, 14) secretory activity (15), and response of the host immune system (16-20).
The aim of this study was to delineate the pathologic findings in premature newborns with invasive non-albicans Candida infections. Based on their differences in virulence factors and host response, we speculated that the pathologic findings in invasive neonatal non-albicans candidiasis might be distinct from the better-known, tissue-destructive neonatal C. albicans infection phenotype.
METHODS
Cases were retrieved from the autopsy files of the Department of Pathology at Women and Infants Hospital (1995–2017) using the search criterion ‘Candida’. Corresponding medical charts were reviewed for relevant perinatal and neonatal information. Archival hematoxylin and eosin (H&E)-stained sections of postmortem tissues were reviewed. Selected sections were further subjected to methenamine-silver nitrate and periodic acid-Schiff staining as well as avidin-biotin-immunoperoxidase staining using a rabbit polyclonal anti-Candida albicans antibody (ab53891, Abcam, Cambridge, MA), which cross reacts with other Candida yeasts including C. parapsilosis and C. glabrata. Controls for specificity consisted of omission of the primary antibody, which abolished all immunoreactivity.
The anatomic localization of Candida organisms was further assessed by combining anti-Candida with anti-cytokeratin (epithelium) and anti-CD31 (endothelium) immunofluorescence staining. Tissue sections were incubated sequentially with polyclonal rabbit anti-Candida, AlexaFluor 594-conjugated anti-rabbit IgG (Jackson, ImmunoResearch Laboratories, Inc., West Grove, PA), monoclonal mouse anti-cytokeratin (AE1/3) or anti-CD31 (DakoCytomation, Glostrup, Denmark), and AlexaFluor 488-conjugated anti-mouse IgG (Jackson). Sections were covered with aqueous mounting medium containing 4′,6-diamidine-2'-phenylindole dihydrochloride (DAPI, Vector Laboratories, Inc., Burlingame, CA). Controls consisted of omission of one or both primary antibodies, which abolished the respective immunoreactivities. The sections were viewed by confocal microscopy. Slice or three-dimensional volume reconstruction and projections were generated, as previously described (21)
Colonization of the gastrointestinal tract is one important pathway to subsequent invasive candidiasis (22, 23). To gain insight in the frequency of intestinal colonization by Candida species in extremely preterm newborns during their NICU stay, we performed a retrospective autopsy study of a consecutive series of infants born between 23 and 28 weeks’ gestation (2006-2017) who had lived for at least one week. Archival sections of lower gastrointestinal tract were subjected to peroxidase-based immunohistochemical analysis using the anti-Candida antibody described above and examined for the presence and morphology of fungal organisms.
RESULTS
Invasive non-albicans candidiasis.
Of 187 extremely premature infants (23-28 weeks’ gestation) autopsied between 1995 and 2017, 14 (7.5%) were diagnosed with invasive candidiasis, as defined by the presence of candidemia (blood stream infection) and/or deep organ involvement. More superficial diseases, such as cutaneous and esophageal candidiasis in the absence of candidemia, were excluded (1). Sixty-six had lived at least one week. There were 3 neonates with non-albicans Candida and 11 neonates with C. albicans infection. The relevant clinical, placental and postmortem findings of the three infants with invasive non-albicans candidiasis are summarized in Table 1. Postmortem examination of the three infants revealed injury patterns associated with complications of extreme prematurity, including early bronchopulmonary dysplasia, pulmonary hemorrhage, intraventricular hemorrhage and necrotizing enterocolitis. Fungal organisms were inconspicuous by hematoxylin-eosin staining. However, targeted (immuno)histochemical stains used for the current study revealed abundant Candida yeast forms in gastrointestinal tract, lungs and most other organs in all three cases. Candida organisms were readily detected by traditional histochemical fungal stains such as periodic acid-Schiff and Gomori-methenamine silver in most organs examined (Fig. 1). In liver and intestines, interpretation of these histochemical stains was compromised by the presence of intrinsic pigments and intraluminal debris, respectively. In these organs, detection of Candida organisms was greatly enhanced by anti-Candida immunohistochemical analysis. A comparative analysis of these various fungal detection methods is illustrated in Figure 2.
Table 1.
Clinical, placental and postmortem findings.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age at birth | 23 wks | 24 wks | 25 wks |
| Birth weight | 510 g | 640 g | 730 g |
| Delivery mode | C-section (umbilical cord prolapse) | Vaginal (preterm labor) | C-section (placental abruption) |
| Invasive procedures | Intubation, umbilical A/V catheters, PICC line | Intubation, umbilical A/V catheters, PICC line | Intubation, umbilical A/V and central catheters |
| Neonatal complications | Necrotizing enterocolitis, multi-system organ failure | Intraventricular hemorrhage, hypertrophic cardiomyopathy, segmental intestinal necrosis | S. aureus sepsis, intraventricular hemorrhage, hydrocephaly |
| Age at death | 22 days | 13 days | 24 days |
| Cause of death | Sepsis, necrotizing enterocolitis, extreme prematurity | Sepsis, pneumonia, necrotizing enterocolitis, extreme prematurity | Sepsis, extreme prematurity |
| Antemortem blood cultures | C. glabrata, C. parapsilosis, S. epidermidis | Yeast (day 12) | C. parapsilosis. S. aureus |
| Postmortem cultures | C. glabrata, coagulase-negative Staphylococci (blood); C. parapsilosis (lung) | C. glabrata (blood, lung, spleen), S. capitis (blood) | C. parapsilosis (lung, catheter tip), coagulase-negative Staphylococci (blood) |
| Postmortem interval | 16 h | 12 h | 8 h |
| Placenta | Moderate acute chorioamnionitis, vasculitis and funisitis of cord | Evidence of maternal vascular malperfusion | Evidence of chronic abruption |
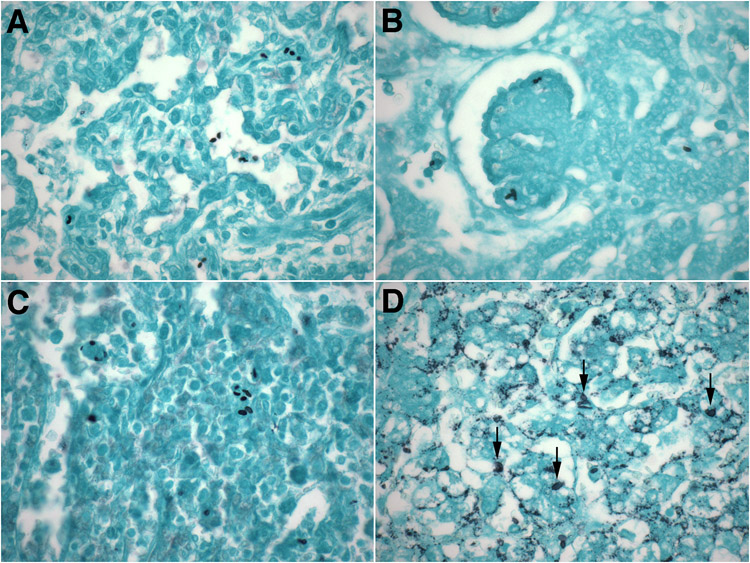
Figure 1. Anatomic distribution of yeasts in invasive neonatal non-albicans candidiasis.
A-D. Representative micrographs of lungs (A), kidneys (B), spleen (C) and liver (D) of extremely preterm neonates with invasive non-albicans candidiasis showing the ubiquitous presence of Candida yeast forms (2-5 μm ovals) in these organs. While readily visible by Gomori-methenamine silver staining in most organs (A-C), the Candida yeasts were obscured by intrinsic lipochrome pigment in liver (D, arrows).
(A-D: Gomori-methenamine silver, original magnification: x600).
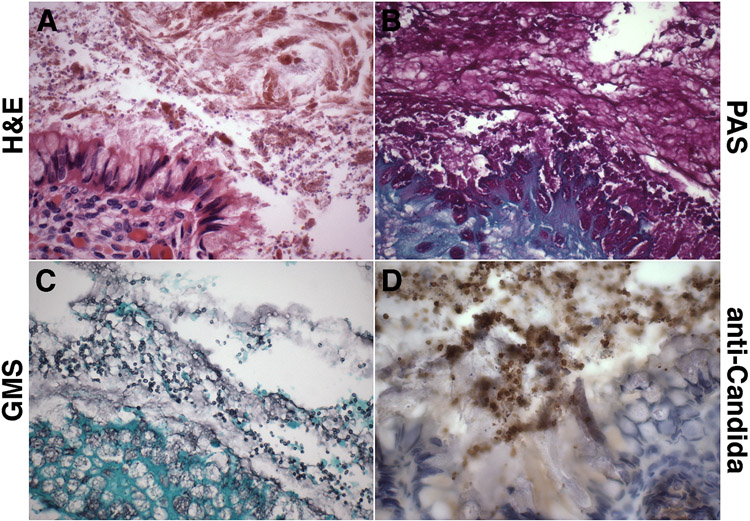
Figure 2. Comparison of (immuno)histochemical fungal detection methods (intestine).
A-D. Representative micrographs of intestine of 23-week gestation neonate (case 1) with invasive C. parapsilosis infection showing abundant Candida yeasts in the intestinal lumen. In this comparative analysis of (immuno)histochemical fungal detection methods, anti-Candida immunohistochemistry (D) was found to be superior for detection of Candida yeasts among meconium and intestinal debris, compared with hematoxylin-eosin staining (A), periodic acid-Schiff staining (B), and Gomori-methenamine silver staining (C).
(A: hematoxylin-eosin, B: periodic acid-Schiff, C: Gomori-methenamine silver, D: anti-Candida DAB immunostaining with hematoxylin counterstain. All: original magnification: x600).
Dual anti-Candida and anti-cytokeratin immunofluorescence staining confirmed the striking abundance of intestinal yeast forms in all three cases (Fig. 3A-C). Infiltrating yeast cells were often noted in a linear pattern between intestinal epithelial cells and aggregated along the subcellular basement membrane of the glands/crypts (Fig. 3B,C). Similarly, dual immunofluorescence studies of lung sections demonstrated abundant yeast forms in the pulmonary airspaces, displaying variable degrees of degradation and fragmentation (Fig. 3D,E). Whereas the vast majority of Candida yeasts were confined to the airspaces, scattered organisms were observed within the pulmonary interstitium, suggestive of invasion (Fig. 3D).
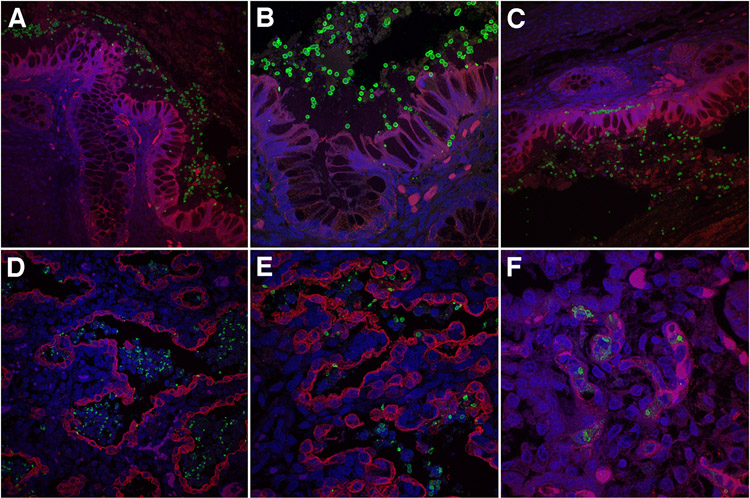
Figure 3. Intestinal and pulmonary features of invasive neonatal non-albicans candidiasis.
A-C. Combined anti-Candida (green) and anti-cytokeratin (red) immunofluorescence staining of representative lower intestinal tract of extremely preterm neonates with invasive non-albicans candidiasis showing abundant Candida yeast forms in the intestinal lumen, lining the colonic epithelium (A). Some yeast cells are seen infiltrating the intestinal epithelium in a single-file pattern (B), while others are arranged in a linear pattern along the epithelial basement membrane (C).
D-E. Representative micrographs of lungs of extremely preterm neonates with invasive non-albicans candidiasis showing abundant, partially degenerated Candida yeast organisms in the distal airspaces and, more sporadically, in the pulmonary interstitium.
F Combined anti-Candida and anti-CD31 immunofluorescence staining demonstrating predominant localization of interstitial Candida yeasts within the pulmonary microvasculature, rather than interstitial stroma.
(A-F: confocal fluorescence microscopy of intestines and lungs subjected to combined anti-cytokeratin (AE1/3) (A-E) or anti-CD31 (F) (red) and anti-Candida (green) immunofluorescence analysis with DAPI counterstain (blue). Original magnification: x400 and x800).
As shown above (Fig. 1), routine histochemical and single anti-Candida immunohistochemical analyses were suggestive of widespread visceral involvement, with particularly heavy fungal burden in lungs, liver, kidneys and spleen. Combining anti-Candida immunofluorescence with the endothelial marker, anti-CD31, revealed that virtually all of these apparently tissue-invasive visceral Candida organisms were confined to the microvasculature, consistent with Candida blood stream invasion (candidemia), rather than systemic visceral invasion in the strictest sense (Fig. 3F).
Intestinal colonization by Candida species.
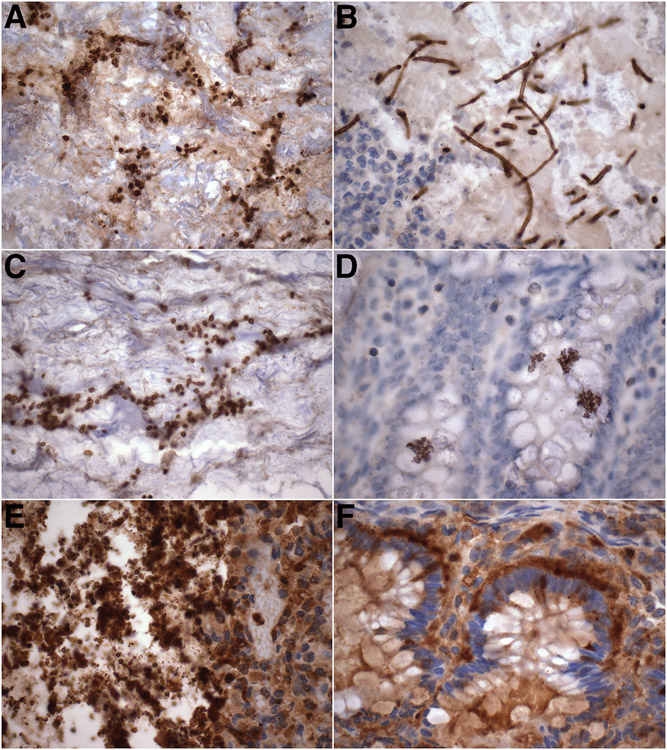
We assessed the prevalence of Candida organisms in archival sections of intestine obtained from preterm infants born between 23 and 28 weeks’ gestation who had lived for at least one week. Candida-immunoreactive organisms were identified in intestinal sections of 16/26 (61%) neonates examined. In three cases, the yeast organisms were relatively large-sized and accompanied by true hyphae (Fig. 4A-B), consistent with C. albicans commensals. In the remaining 13 cases, variable numbers of smaller yeasts lacking hyphal forms were identified (Fig. 4C-F).
Figure 4. Intestinal colonization by Candida species in extremely preterm neonates.
A-B. Micrograph of luminal mucus and debris showing relatively large-sized Candida-immunoreactive yeast forms and scattered pseudohyphae (A, arrows) in association with true hyphae (mycelia) (B), consistent with C. albicans.
C-D. Representative micrographs showing smaller yeast forms in intestinal lumen (C) and glands (D), not accompanied by hyphal forms, suggestive of non-albicans Candida species.
E-F. Intestinal lumen entirely occupied by masses of relatively small Candida yeasts (E), linearly arranged around the base of the colonic glands in areas with intact epithelium.
(A-F: anti-Candida DAB immunostaining with hematoxylin counterstain. All: original magnification: x600).
Infants with or without intestinal Candida colonization were equivalent with respect to gestational age at birth (median gestational age: 24.5 weeks (range: 23 - 28 weeks) for infants with intestinal Candida versus 26 weeks (range: 23 - 28 weeks) for infants without intestinal Candida), birth weight (709 g ± 149 g versus 668 g ± 212 g), length of life (39.5 days (range: 14 - 158 days) versus 42.5 days (range: 20 - 289 days)), or presence of histologic acute chorioamnionitis (6/16 versus 5/10). Placental candidiasis (peripheral funisitis associated with fungal elements) was diagnosed in one case from each group.
DISCUSSION
A 22-year-spanning data search of 187 extremely premature infants from our neonatal autopsy archives yielded 11 diagnosed cases of invasive albicans and 3 cases of invasive non-albicans candidiasis, the latter involving C. parapsilosis and C. glabrata. In contrast to the distinct inflammatory and tissue-destructive histopathologic changes seen in the majority of neonatal C. albicans cases, infection with non-albicans Candida organisms was remarkably inconspicuous by routine hematoxylin-based histologic analysis. Using targeted (immuno)histochemical approaches for the purpose of this study, large numbers of Candida yeasts were localized in gastrointestinal tract, respiratory tract, and circulation.
Intestinal colonization by Candida species is an important risk factor for subsequent disseminated disease, whereby the density of colonization correlates with the risk for candidemia (23-26). Whereas invasion of intestinal epithelial cells by C. albicans is mediated, in part, by active, physical penetration by hyphae (12), the mechanisms underlying intestinal penetration by non-albicans Candida yeast forms remain incompletely understood. In the present study, confocal microscopy of combined anti-Candida and anti-cytokeratin-stained tissue sections revealed a distinct single-file pattern of infiltrating non-albicans yeasts (C. parapsilosis and C. glabrata) in the intestinal epithelium, unaccompanied by histologic evidence of cellular injury or inflammation. This transepithelial migration was associated with linear aggregation of yeast cells along the basement membrane of the intestinal crypts/glands, suggesting this structure, when intact, provides a natural barrier against deeper, systemic invasion by Candida yeast forms. These findings are concordant with recent studies in murine models of gut colonization following intragastric inoculation with various Candida species. Candida albicans readily invaded the epithelium and disseminated from the gut, attributed to its capacity to produce hyphae. Inoculation with yeast forms such as C. parapsilosis, in contrast, resulted in persistent colonization without systemic invasion. (27).
Deeper invasion and subsequent hematogenous spread by Candida yeast forms are associated with disruption of the intestinal epithelial basement membrane by concomitant traumatic, ischemic or infectious events, such as necrotizing enterocolitis, spontaneous intestinal perforation, and abdominal surgery (24, 28, 29). In the present study, 2/3 neonates with invasive non-albicans candidiasis had a history of overt intestinal or abdominal insults, usually in the form of necrotizing enterocolitis.
Fungal-specific stains further revealed the presence of numerous Candida yeasts in the pulmonary airways and airspaces in all three neonates with invasive non-albicans candidiasis. As in the gastrointestinal tract, the often-massive pulmonary involvement was deceptively insidious by hematoxylin-eosin-based routine histologic examination. This bronchopulmonary (‘air space-invasive’) pattern of pulmonary candidiasis, analogous to that previously described in preterm infants with disseminated C. albicans infection (30, 31), may be caused by downward transfer of oropharyngeal commensals by endotracheal intubation or similar oropharyngeal manipulation. None of the cases of non-albicans candidiasis displayed a true miliary pattern of pulmonary parenchymal necrosis and inflammation, as may be seen in C. albicans pneumonia (30, 31).
The reported frequency of intestinal Candida colonization in NICU patients, based on stool cultures, ranges widely from 10% to 60% (32, 33), with C. albicans and C. parapsilosis as most prevalent commensals (33-36). In the present study, about 60% of deceased extremely preterm neonates displayed evidence of intestinal colonization by Candida organisms, as determined by anti-Candida immunohistochemical analysis of archival intestinal tissues of a consecutive series of neonates who had lived for at least one week. This result likely underestimates the actual frequency of Candida intestinal colonization in extremely premature NICU patients as the available archival material only represented a limited portion of the gastrointestinal tract (estimated less than 5% of the total length), and the sensitivity of the anti-Candida immunohistochemical assay may have been affected by autolysis-associated loss of antigenicity.
In addition to allowing culture-independent identification of fungal organisms as Candida species, the anti-Candida immunohistochemical assays utilized in this study have several advantages over traditional histochemical approaches. Immunostaining, whether peroxidase- or fluorescence-based, facilitated detection of the yeast forms, especially in debris- and pigment-rich backgrounds such as – partially autolyzed – intestines, liver and lungs. Improved visualization by immunostaining further allowed tentative speculation about the type of Candida species involved based on their relative size. Among the more common Candida species, C. albicans yeasts are largest (4-6 x 6-10 μm), followed by C. parapsilosis (2.5-4 x 2.5-9 μm) and C. glabrata (1-4 μm) (37). In some intestinal samples, the presence of relatively large-sized yeast forms in combination with true hyphae was strongly suggestive of C. albicans colonization. In the remaining cases, the exact Candida species remained undetermined, although the smaller size in the absence of true hyphae may be suggestive of non-albicans Candida species.
In summary, we reported our autopsy experience with neonatal invasive non-albicans candidiasis and neonatal intestinal Candida colonization. In view of its insidious histopathologic presentation, the postmortem diagnosis of invasive neonatal non-albicans candidiasis rests entirely on positivity of blood and/or lung cultures. However, blood cultures are notoriously insensitive in diagnosing disseminated candidiasis, with false-negative rates of up to 50%, especially in small children and following prophylactic or empiric antifungal therapy (38, 39). We speculate that many cases of invasive non-albicans candidiasis in (deceased) extremely preterm neonates may go undiagnosed. More generous application of (immuno)histochemical fungal stains in perinatal autopsies may provide important insights into the prevalence, pathophysiology, and clinical relevance of the various forms of candidiasis in the neonatal population, both as commensals and (blood-) invasive organisms.
ACKNOWLEDGMENTS
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under grant number P30GM114750 and the Kilguss Research Core at Women & Infants Hospital of Rhode Island. It was also supported by the William and Mary Oh-William and Elsa Zopfi Professorship in Pediatrics for Perinatal Research (S.K.S. and J.M.B.). J.M.B. also receives support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the NIH (R21HD089278).
REFERENCES
- 1.Pappas PG. Invasive candidiasis. Infect Dis Clin of North Am 2006;20:485–506. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault AB, Bliss JM. Neonatal candidiasis: New insights into an old problem at a unique host-pathogen interface. Curr Fungal Infect Rep 2015;9:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in us hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- 4.Testoni D, Hayashi M, Cohen-Wolkowiez M, Benjamin DK Jr., Lopes RD, Clark RH, Benjamin DK, Smith PB. Late-onset bloodstream infections in hospitalized term infants. Pediatric Infect Dis J 2014;33:920–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyth CC, Chen SC, Slavin MA, Serena C, Nguyen Q, Marriott D, Ellis D, Meyer W, Sorrell TC. Not just little adults: Candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics 2009;123:1360–1368. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int J Infect Dis 2010;14:e954–966. [DOI] [PubMed] [Google Scholar]
- 7.Guinea J Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 2014;20 Suppl 6:5–10. [DOI] [PubMed] [Google Scholar]
- 8.Forrest GN, Weekes E, Johnson JK. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J Infect 2008;56:126–129. [DOI] [PubMed] [Google Scholar]
- 9.Pammi M, Holland L, Butler G, Gacser A, Bliss JM. Candida parapsilosis is a significant neonatal pathogen: A systematic review and meta-analysis. Pediatr Infect Dis J 2013;32:e206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laffey SF, Butler G. Phenotype switching affects biofilm formation by candida parapsilosis. Microbiology 2005;151:1073–1081. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DS, Carlisle PL, Kadosh D. Coevolution of morphology and virulence in candida species. Eukaryot Cell 2011;10:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruere C, Bonnin A, Hube B. Cellular interactions of candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 2010;12:248–271. [DOI] [PubMed] [Google Scholar]
- 13.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: An update. Eukaryot Cell 2005;4:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-candida albicans candida species: Quantification, structure and matrix composition. Med Mycol 2009;47:681–689. [DOI] [PubMed] [Google Scholar]
- 15.Silva NC, Nery JM, Dias AL. Aspartic proteinases of candida spp.: Role in pathogenicity and antifungal resistance. Mycoses 2014;57:1–11. [DOI] [PubMed] [Google Scholar]
- 16.Keppler-Ross S, Douglas L, Konopka JB, Dean N. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot cell 2010;9:1776–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth R, Toth A, Papp C, Jankovics F, Vagvolgyi C, Alonso MF, Bain JM, Erwig LP, Gacser A. Kinetic studies of candida parapsilosis phagocytosis by macrophages and detection of intracellular survival mechanisms. Front Microbiol 2014;5:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann AK, Jacobson K. A novel pseudopodial component of the dendritic cell anti-fungal response: The fungipod. PLoS Pathog 2010;6:e1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth A, Csonka K, Jacobs C, Vagvolgyi C, Nosanchuk JD, Netea MG, Gacser A. Candida albicans and candida parapsilosis induce different t-cell responses in human peripheral blood mononuclear cells. J Infect Dis 2013;208:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden JR, Maccani MA, Laforce-Nesbitt SS, Bliss JM. High efficiency opsonin-independent phagocytosis of candida parapsilosis by human neutrophils. Med Mycol 2010;48:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Paepe ME, Mao Q, Ghanta S, Hovanesian V, Padbury JF. Alveolar epithelial cell therapy with human cord blood-derived hematopoietic progenitor cells. Am J Pathol 2011;178:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coates EW, Karlowicz MG, Croitoru DP, Buescher ES. Distinctive distribution of pathogens associated with peritonitis in neonates with focal intestinal perforation compared with necrotizing enterocolitis. Pediatrics 2005;116:e241–246. [DOI] [PubMed] [Google Scholar]
- 23.Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, Blumberg HM, Patterson JE, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. Risk factors for candidemia in neonatal intensive care unit patients. The national epidemiology of mycosis survey study group. Pediatr Infect Dis J 2000;19:319–324. [DOI] [PubMed] [Google Scholar]
- 24.Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol 2003;27:357–364. [DOI] [PubMed] [Google Scholar]
- 25.Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediat Crit Care Med 2010;11:240–245. [DOI] [PubMed] [Google Scholar]
- 26.Manzoni P, Farina D, Galletto P, Leonessa M, Priolo C, Arisio R, Gomirato G. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. J Perinat Med 2007;35:220–226. [DOI] [PubMed] [Google Scholar]
- 27.Mellado E, Cuenca-Estrella M, Regadera J, Gonzalez M, Diaz-Guerra TM, Rodriguez-Tudela JL. Sustained gastrointestinal colonization and systemic dissemination by candida albicans, candida tropicalis and candida parapsilosis in adult mice. Diagn Microbiol Infect Dis 2000;38:21–28. [DOI] [PubMed] [Google Scholar]
- 28.Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, Della-Latta P, Haas J, Cimiotti J, Saiman L. Risk factors for candidemia in critically ill infants: A matched case-control study. J Pediatr 2005;147:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Hornik CP, Benjamin DK Jr., Herring AH, Clark RH, Cohen-Wolkowiez M, Smith PB. Risk factors for invasive candidiasis in infants >1500 g birth weight. Pediatr Infect Dis J 2013;32:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassner EG, Kauffman SL, Yoon JJ, Semiglia M, Kozinn PJ, Goldberg PL. Pulmonary candidiasis in infants: Clinical, radiologic, and pathologic features. AJR Am J Roentgenol 1981;137:707–716. [DOI] [PubMed] [Google Scholar]
- 31.Pasqualotto AC. Candida and the paediatric lung. Paediatr Respir Rev 2009;10:186–191. [DOI] [PubMed] [Google Scholar]
- 32.Manzoni P, Mostert M, Castagnola E. Update on the management of candida infections in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2015;100:F454–459. [DOI] [PubMed] [Google Scholar]
- 33.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, Blumberg HM, Pfaller M, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. Risk factors for candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J 2001;20:1119–1124. [DOI] [PubMed] [Google Scholar]
- 34.Heisel T, Podgorski H, Staley CM, Knights D, Sadowsky MJ, Gale CA. Complementary amplicon-based genomic approaches for the study of fungal communities in humans. PLoS One 2015;10:e0116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman DA, Gurka MJ, Hazen KC, Boyle R, Robinson M, Grossman LB. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. Pediatr Infect Dis J 2006;25:733–737. [DOI] [PubMed] [Google Scholar]
- 36.Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabro A, Jousson O, Donati C, Cavalieri D, De Filippo C. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol 2016;7:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, candida parapsilosis and candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 2012;36:288–305. [DOI] [PubMed] [Google Scholar]
- 38.Thorn JL, Gilchrist KB, Sobonya RE, Gaur NK, Lipke PN, Klotz SA. Postmortem candidaemia: Marker of disseminated disease. J Clin Pathol 2010;63:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Florl C, Richardson MD, Akova M, Bassetti M, Calandra T, Castagnola E, Cornely OA, Garbino J, Groll AH, Herbrecht R, Hope WW, Kullberg BJ, Lortholary O, Meersseman W, Petrikkos G, Roilides E, Viscoli C, Ullmann AJ. Escmid* guideline for the diagnosis and management of candida diseases 2012: Diagnostic procedures. Clin Microbiol Infect 2012;18 Suppl 7:9–18. [DOI] [PubMed] [Google Scholar]