Abstract
This article describes a case of healthcare-associated monkeypox infection in France during the 2022 outbreak. A female medical resident accidently pricked herself with a soiled subcutaneous needle used to harvest a vesicle of a patient infected by monkeypox virus and developed 4 days later a unique skin lesion, positive for monkeypox virus.
Keywords: healthcare-associated infection, healthcare worker, monkeypox
A healthcare worker developed a unique skin lesion positive for Monkeypox virus, 4 days after having accidently pricked herself with a soiled needle. To our knowledge, this is the first case of healthcare-acquired Monkeypox infection during the 2022 outbreak.
Since May 2022, European and North American healthcare workers have been facing an outbreak of monkeypox virus human infections with >25 000 cases worldwide and 2239 cases in France reported as of 4 August 2022 [1, 2]. This article describes a case of monkeypox healthcare-associated infection in a French healthcare worker during the 2022 outbreak.
Monkeypox is a zoonotic disease caused by an orthopoxvirus, endemic in Central and West Africa since 1970, mostly affecting young adults through direct animal-to-human but also human-to-human contacts. Apart from an outbreak in the United States in 2003 due to infected animals from Africa, monkeypox infections had been limited to the southern hemisphere until now [3].
Monkeypox clinical features described in Africa are similar to smallpox presentation, apart from a severe lymphadenopathy. However, unlike smallpox, monkeypox was associated with low human-to-human transmissibility. Monkeypox virus transmission is possible by human-to-human contact or contaminated objects and through respiratory droplet transmission routes. However, risk factors for transmission and infection, zoonotic hosts, and vectors remain unclear [4, 5].
The 2022 outbreak has different characteristics than previous ones, with human-to-human transmission mainly among men who have sex with men, suggesting that monkeypox virus spreads during sexual intercourse with promiscuous mucocutaneous contact [6]. To date in France, only 15 women and 2 children have been infected since the beginning of this outbreak [1]. Monkeypox-infected people inconstantly present fever, skin lesions as vesicles, pustules, ulcers, and lymphadenopathy affecting the whole body and especially anogenital zone with proctitis and anorectal pain but also pharyngitis and acute tonsillitis [2].
Since 21 May 2022, the Infectious Diseases Department of Bichat Hospital in Paris (France) has been one of the main medical centers in charge of testing the patients suspected of monkeypox infection in order to confirm the diagnosis and manage the complicated cases in the Paris metropolitan region.
CASE REPORT
On 7 July 2022, a 30-year-old patient presented to our department with skin lesions and significant anal pain. This patient had a history of multiple sexual partners and unprotected sex with insertive and receptive anal intercourse. Pustules appeared on his pubis and penis 7 days before the consultation. Six days later, vesicles and pustules appeared on his face and wrist along with perianal ulcers. Clinical examination found a pharyngitis. He had no fever. To confirm monkeypox infection, monkeypox virus–specific polymerase chain reaction was performed on mucocutaneous and oropharynx swabs using a previously described protocol [7]. The patient’s samples were positive for monkeypox virus on cutaneous (cycle threshold value [Ct] = 22), anal (Ct = 20), and pharyngeal (Ct = 29) swabs and plasma (Ct = 33). Human immunodeficiency virus (HIV) rapid antibody test was negative.
While harvesting a vesicle on the right ankle of this patient, a 25-year-old female medical resident accidently pricked her right thumb with the subcutaneous needle (Braun Sterican Safety Needle 25G) that she used to collect the patient’s vesicle. She did not activate the safety needle after puncture of the vesicle and kept the needle unprotected in hand while sampling the vesicle. She was wearing appropriate personal protective equipment (PPE) consisting of disposable gown, disposable gloves, FFP2 mask, and goggles. Although she wore gloves, she bled after the needle prick. She immediately immersed her thumb in sodium hypochlorite for approximately 15 minutes. The same day, she had a medical consultation with an infectious diseases physician. Postexposure prophylaxis for HIV was not indicated in this situation. However, she received a dose of third-generation smallpox vaccine (1 subcutaneous injection of 0.5 mL Imvanex) within 3 hours. She had no medical history and no history of smallpox vaccine.
The next day, blood tests confirmed that the index patient's HIV serology and viral load, as well as his hepatitis C virus (HCV) serology, were negative. He was vaccinated for hepatitis B virus (HBV). The medical resident had also negative HIV and HCV serologies and was vaccinated for HBV.
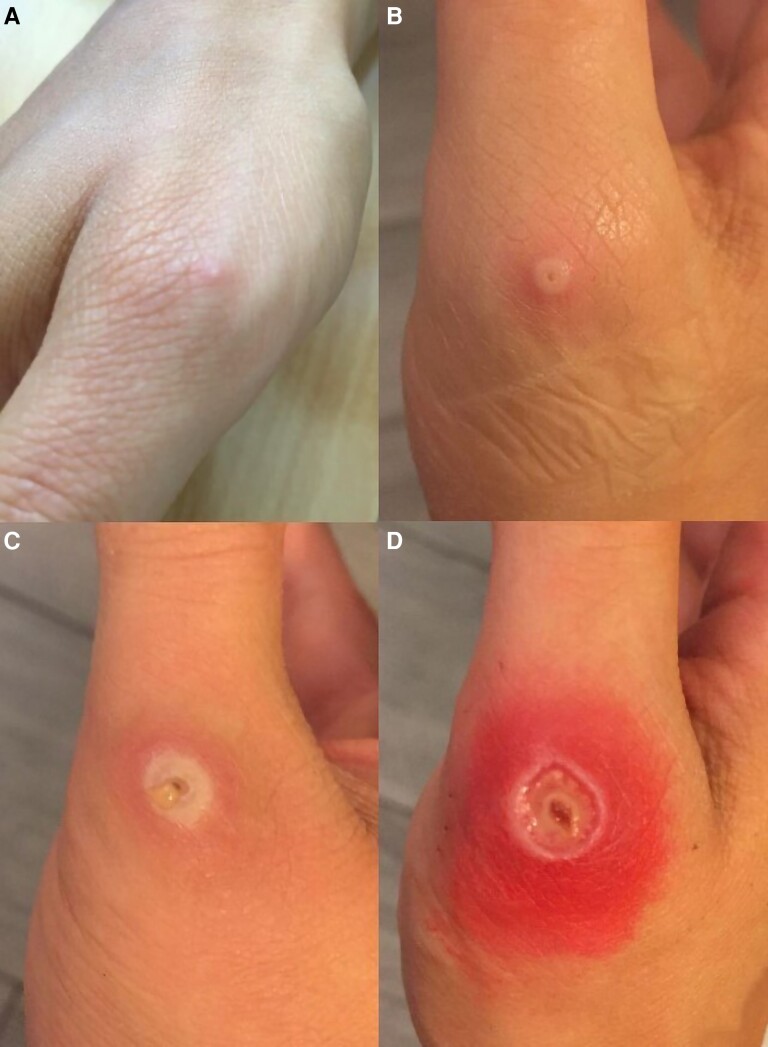
On 11 July (day 1), 4 days after the accident, 1 vesicle of 1 mm diameter appeared on the right thumb of the healthcare worker at the exact site of the prick (Figure 1A). She had only 1 skin lesion and did not have other symptoms, in particular no perianal lesions, fever, pharyngitis, or lymphadenopathy. Liquid inside the vesicle was harvested on cutaneous swab and turned out to be positive (Ct = 29) for monkeypox virus. The pharynx swab was negative. These results confirmed the monkeypox virus infection of the healthcare worker. She did not have another at-risk exposure to monkeypox virus, especially no sexual exposure. To prevent disease spreading, she was asked, as were all confirmed monkeypox cases, to cease work and to isolate herself at home on 12 July for at least 21 days after the beginning of symptoms. We declared the case as a work-related injury for the healthcare worker and reported the healthcare-associated infection to the French health authorities on 12 July. Due to the onset of the disease, the healthcare worker did not receive the second dose of smallpox vaccine, which was planned 28 days after the first dose. Her flatmate was vaccinated with 1 dose of Imvanex and received a second dose 28 days later. Her coworkers were not vaccinated because the transmission risk was considered very low. Indeed, the medical resident covered her 1 mm lesion with a bandage within an hour after its onset, there was no pharyngeal carriage as the pharyngeal swab was negative, and she was rapidly isolated at home within 24 hours after the first symptom.
Figure 1.
Evolution of the skin lesion of the healthcare worker between day 1 and day 13. A, Day 1. B, Day 4. C, Day 8. D, Day 13.
On 15 July (day 4), clinical examination found the same lesion but slightly bigger (5 mm) with an umbilical aspect (Figure 1B). The cutaneous swab was still positive (Ct = 36), whereas the pharyngeal swab and blood sample were both negative.
On 19 July (day 8), the lesion was an umbilicated induration surrounded by a red areola with a serous fluid flow (Figure 1C). The control of cutaneous swab on 22 July (day 11) was still positive (Ct = 20). Due to an increase in wound size and the apparition of a serous-purulent fluid flow, bacterial superinfection was suspected and feared especially because of the proximity of the metacarpophalangeal joint of the thumb; thus, antibiotic treatment with amoxicillin/clavulanate was started on 22 July.
DISCUSSION AND CONCLUSIONS
To the best of our knowledge, this is the first case of human monkeypox healthcare-associated infection with direct inoculation of the monkeypox virus by accidental cutaneous prick during a healthcare procedure. This accident could have been avoided if the safety of the subcutaneous needle had been activated immediately after vesicle puncture. This argues for education of young physicians in training before high-risk practice.
Before 2022, only 1 previous case of human-to-human healthcare-associated infection without direct cutaneous contact was reported in England, in 2018 in a healthcare worker after having changed the bed linens of a monkeypox-infected patient with standard PPE (no mask), suggesting transmission through respiratory droplets or contaminated fomites [8].
In our case, the severity of the infection was mild; the healthcare worker had only 1 skin lesion at the site of the wound and has not developed new symptoms so far. This mild infection could be related to the specific route of exposure by direct inoculation in addition to rapid immunization with postexposure vaccination a few hours after the prick. The clinical aspect and time-lapse evolution of the skin reaction was similar to the so-called “Jennerian pustule” described after primary vaccination back in the 1970s [9].
Vaccinia immunization, which has eradicated smallpox, is cross-protective against other orthopoxvirus infections, including monkeypox. This vaccine can be used as postexposure prophylaxis as it is known to modify or prevent onset of clinical disease if given optimally within 4 days of infection [10].
This case questions the systematic preventive vaccination of healthcare workers against monkeypox to prevent the disease for particularly exposed professionals, especially in the absence of specific treatment available for monkeypox infection. The United States Advisory Committee on Immunization Practices recommends preexposure prophylaxis against orthopoxvirus infection among laboratory personnel and healthcare workers who care for patients infected with orthopoxviruses [11], whereas French health authorities suggest only postexposure vaccination [12]. The World Health Organization recommended on 23 July the vaccination of healthcare workers who may be in contact with monkeypox-infected people. However, the risk of healthcare worker contamination during care seems to be very low if compliance with preventive measures is adequate. Finally, tecovirimat might prove efficacious as postexposure prophylaxis after an occupational exposure in healthcare workers and should be assessed in this setting.
Contributor Information
Diane Le Pluart, Assistance Publique–Hôpitaux de Paris, Infectious and Tropical Diseases Department, Bichat–Claude Bernard Hospital, Paris, France.
Mirabelle Ruyer-Thompson, Assistance Publique–Hôpitaux de Paris, Dermatology Department, Bichat–Claude Bernard Hospital, Paris, France.
Valentine Marie Ferré, Assistance Publique–Hôpitaux de Paris, Department of Virology, Bichat–Claude Bernard Hospital, Paris, France; Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France.
Morgane Mailhe, Assistance Publique–Hôpitaux de Paris, Infectious and Tropical Diseases Department, Bichat–Claude Bernard Hospital, Paris, France.
Diane Descamps, Assistance Publique–Hôpitaux de Paris, Department of Virology, Bichat–Claude Bernard Hospital, Paris, France; Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France.
Fabrice Bouscarat, Assistance Publique–Hôpitaux de Paris, Infection Control Unit, Bichat–Claude Bernard Hospital, Paris, France.
François-Xavier Lescure, Assistance Publique–Hôpitaux de Paris, Infectious and Tropical Diseases Department, Bichat–Claude Bernard Hospital, Paris, France; Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France.
Jean-Christophe Lucet, Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France; Assistance Publique–Hôpitaux de Paris, Infection Control Unit, Bichat–Claude Bernard Hospital, Paris, France.
Yazdan Yazdanpanah, Assistance Publique–Hôpitaux de Paris, Infectious and Tropical Diseases Department, Bichat–Claude Bernard Hospital, Paris, France; Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France.
Jade Ghosn, Assistance Publique–Hôpitaux de Paris, Infectious and Tropical Diseases Department, Bichat–Claude Bernard Hospital, Paris, France; Université Paris Cité, Institut national de la santé et de la recherche médicale, Unité mixte de recherche 1137, Infection, Antimicrobials, Modelling, Evolution, Paris, France.
Notes
Author contributions. D. L. P. and M. R.-T. drafted the manuscript. V. M. F., D. D., F. B., F.-X. L., J.-C. L., Y. Y., and J. G. revised the manuscript. D. L. P., M. R.-T., and M. M. involved in the clinical management of patients. D. L. P. and M. M. involved in the clinical management of the healthcare worker. V. M. F. and D. D. carried out the monkeypox virus polymerase chain reaction assays of the patients. J.-C. L. gave his expert opinion to manage the healthcare-associated infection. F. B. gave his expert opinion in dermatology. All authors read, revised, and approved the final manuscript.
Acknowledgments. We wish to thank the whole team of the Infectious and Tropical Diseases Department, Virology Department, Dermatology Department, and Infection Control Unit at Bichat Hospital for their involvement in the care of patients during this epidemic period.
Patient consent. The source patient and the healthcare worker gave their consent for the publication of their data and photographs. There was no intervention in this study.
Financial support. This case received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention . 2022 monkeypox outbreak global map data.https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed 3 August 2022.
- 2. Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med 2022; 387:679–691. [DOI] [PubMed] [Google Scholar]
- 3. Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med 2004; 350:342–50. [DOI] [PubMed] [Google Scholar]
- 4. Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 2022; 22:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen BW, Damon IK, Bennett JE, Dolin R, Blaser MJ. Orthopoxviruses vaccinia (smallpox vaccine), variola (smallpox), monkeypox, and cowpox. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, USA: Elsevier. 2019. [Google Scholar]
- 6. Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill 2022; 27:2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Test procedure: monkeypox virus generic real-time PCR test. https://www.cdc.gov/poxvirus/monkeypox/pdf/pcr-diagnostic-protocol-508.pdf. Accessed 6 June 2022.
- 8. Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis 2020; 26:782–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breman JG. Smallpox. J Infect Dis 2021; 224(12 Suppl 2):S379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson K, Heymann D, Brown CS, Edmunds WJ, Elsgaard J, Fine P, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine 2020; 38:5077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao AK, Petersen BW, Whitehill F, Razeq JH, Isaacs SN, Merchlinsky MJ, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haute Autorité de Santé . Monkeypox: vacciner les adultes et professionnels de santé après une exposition à la maladie. https://www.has-sante.fr/jcms/p_3340419/fr/monkeypox-vacciner-les-adultes-et-professionnels-de-sante-apres-une-exposition-a-la-maladie. Accessed 24 May 2022.