Abstract
Despite recent advances in DNA-based genotyping, the microcytotoxicity test is still broadly used for the determination of human leukocyte class I antigens in patients as well as organ donors and also for the detection of HLA antibodies. Excellent purity and viability of peripheral blood mononuclear cells (PBMC) are essential for reliable HLA typing results. Background staining and cell loss can contribute to impaired typing results or even cause misinterpretations. A novel isolation procedure using cell preparation tubes (CPT) with prefilled Ficoll was compared with the standard Ficoll gradient. We determined the recovery, purity, and viability of the PBMC after several periods of storage. Finally, the isolated cells were used for HLA class I typing, and background reactivities were scored. By using the CPT method, the recovery of PBMC was significantly higher than recovery with the standard technique (P ≤ 0.001). Contamination by granulocytes increased considerably during the storage time for the standard protocol, whereas purity remained stable when CPT were used (P ≤ 0.001). With both methods, lymphocyte viability declined markedly over time. We found significantly more dead cells by using the CPT methods. Due to high background scores, HLA typing was impossible after 48 h. The isolation of PBMC by the CPT method resulted in a higher yield and improved purity compared to those obtained with the standard gradient technique. The decreasing viability after 48 h limits the use of both methods for HLA typing and HLA antibody screening.
Since new technologies based on the invention of the PCR by Mullis (10) have become available, serological HLA typing has been partially replaced. Included among these new technologies is the precise DNA typing of HLA class II alleles by amplification with sequence-specific oligonucleotides (14), by sequence-specific priming (2, 11), or by sequenced-based typing (3); all of these have led to considerable improvements in organ and bone marrow transplantation. Nevertheless, the microcytotoxicity test (18) has still remained the standard for HLA class I typing in patients and organ donors and for crossmatch techniques. The isolation of peripheral blood mononuclear cells (PBMC) from blood samples is an important preanalytical step, not only for HLA typing, but also for other routine laboratory procedures such as flow cytometry (1, 12, 16, 17). Alternatively, when only erythrocyte depletion is called for, lysis procedures using ammonium chloride or hypotonic solutions are frequently used (9, 19); these have the advantage of bypassing the overall cell loss and the well-known depletion of specific subpopulations (1, 12, 16, 17) that occur when density gradients are used. With these methods, depletions of erythrocytes and granulocytes are accomplished with a relative enrichment of PBMC, as first described by Böyum (4, 5). Additional lymphocyte enrichment strategies using nylon wool columns or immunomagnetic beads (21) are also used. Conventional techniques demand substantial manual skills for blood layering and interface removal; they are also time-consuming and sometimes imprecise due to selective cell loss and impure segregation (1, 12, 16, 17).
The purpose of our study was to compare the Ficoll density gradient technique with a newly developed method using Ficoll prefilled cell preparation tubes (CPT). We evaluated the recovery and purity of PBMC by flow cytometry, which allowed for precise cell quantification by using fluorochrome-containing microparticles. We further assessed the viabilities of lymphocytes by propidium iodide staining. Studies to determine the influence of storage time before or after PBMC isolation on cell recovery and quality were also performed (6, 8, 13, 22). Subsequently, the isolated cells were subjected to HLA class I typing. The typing quality was assessed microscopically by using the score recommended by the International Histocompatibility Workshop (IHW).
MATERIALS AND METHODS
Samples and study design.
Peripheral blood samples (2.7 ml of K-EDTA) were obtained from 10 healthy volunteers after informed consent was given. Leukocyte subsets were quantified immediately after donation by flow cytometry. Three 8-ml samples were collected from each donor in tubes containing anticoagulant citrate-dextrose solution, formula A (ACD-A) (Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.) and stored at 20°C for either 2, 24, or 48 h before the Ficoll gradient procedure was performed. In parallel, five 8-ml samples were instilled directly into CPT (Becton Dickinson Vacutainer Systems), which contained 1.0 ml of 0.1 M sodium citrate, 1 ml of Ficoll-Hypaque, and a gel barrier. Three of these CPT were left at 20°C for either 2, 24, or 48 h, whereas the other two samples were centrifuged immediately after collection. PBMC were mixed with the plasma supernatants in the original CPT and stored at 20°C for either 24 or 48 h.
Isolation of PBMC by the standard density gradient technique.
ACD-A-anticoagulated blood was diluted 1:1 with Hank’s buffer and completely layered on an identical volume of the density gradient (Lymphoflot; Biotest, Dreieich, Germany), which contained 5.6% Ficoll and 9.6% diactrizoate with a density of 1.077 ± 0.001 g/ml and an osmolarity of 300 mosM. Samples were centrifuged for 20 min at 700 × g and 20°C without applying a brake. The PBMC interface was carefully removed by pipetting and was washed twice with Hank’s buffer by stepwise centrifugation for 15 min at 300 × g and for 10 min at 90 × g for platelet removal. PBMC were resuspended in 3 ml of Lymphostabil (Biotest), a Terasaki Park medium which contains several amino acids, d-glucose, HEPES buffer, and 0.5% fetal calf serum.
Isolation of PBMC by using CPT.
Two CPT were centrifuged immediately after collection (protocol A) before the PBMC were mixed with the autologous plasma supernatants. These samples remained unopened for either 24 or 48 h. Three CPT were first stored at 20°C (protocol B) and then centrifuged after either 2, 24, or 48 h. Centrifugation was performed for 20 min at 1,730 × g and 20°C. At the end of each storage interval, PBMC were subjected to the washing procedures with Hank’s buffer that are described above.
Flow cytometric cell quantification and viability determination.
All flow cytometric analyses were performed on an EPICS XL MCL (Coulter Immunotech, Krefeld, Germany) flow cytometer, and System II software was used for data acquisition. The flow cytometer was properly aligned, and fluorochrome compensation for fluorescein isothiocyanate (FITC) and phycoerythin (PE) was correctly tuned with respect to signal amplification. All specific monoclonal antibodies, namely, anti-CD45–FITC (clone DW124-5-2), anti-CD14–PE (clone 116), anti-CD3–FITC (clone UCHT1), and anti-CD19–FITC (clone 89B), and the isotype control antibodies (immunoglobulin G1 [IgG1] and IgG2a) were purchased from Coulter Immunotech. One hundred microliters of the sample and 20 μl of each antibody were incubated for 20 min at 4°C in the dark. Erythrocyte lysis was performed automatically by a Multi-QPrep workstation (Coulter Immunotech). For direct quantitation of lymphocyte subpopulations (15), 100-μl Flow Count Fluorospheres (Coulter Immunotech) were added to the prepared specimens. Cell concentrations were calculated by using the following formula: number of cells/μl = total number of cells of interest/total number of Fluorospheres × assayed concentration of Fluorospheres/μl.
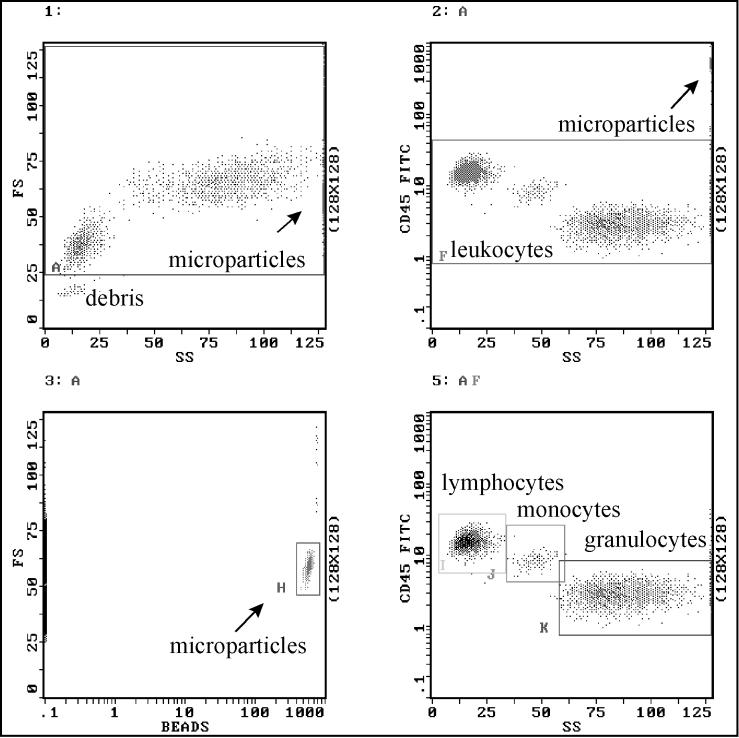
As shown in Fig. 1, the target cells were stained with anti-CD45–FITC and analyzed in a two-parameter dot plot of the first fluorescence channel versus side scatter. Lymphocytes, monocytes, and granulocytes could be distinguished by the density of epitope expression and their side scatter characteristics (20), which allowed each of them to be gated exclusively. The Flow Count Fluorospheres are polystyrene microparticles and contain fluorescence dyes with a broad emission spectrum of 510 to 700 nm. They are easy to detect by using a third fluorescence channel versus forward scatter diagram. Gate H reveals a tight distribution of all Fluorosphere singlets. Cell viabilities were assessed by propidium iodide staining (Sigma, Deisenhofen, Germany). Propidium iodide was added at a final concentration of 10 μg/ml for 10 min.
FIG. 1.
Representative flow cytometric analysis of leukocyte subpopulations using anti-CD45–FITC staining and fluorescent microspheres (Flow Count Fluorospheres) for cell quantification.
HLA class I typing by a lymphocytotoxicity test.
PBMC were subjected to HLA class I typing by the microcytotoxicity test with commercial 72-well polystyrene typing trays (Lymphotype AB-72; Biotest), which were primed with 1-μl volumes of antisera. One microliter of PBMC, containing about 3 × 103 cells, was added and incubated for 30 min at 20°C; then 5 μl of freshly prepared rabbit complement was added, and PBMC were further incubated for 60 min at 20°C. The staining and fixing procedures were carried out simultaneously by a softdrop technique using 8 μl of Stain-Fix (One Lambda, Canoga Park, Calif.). The trays were left at 20°C for at least 30 min so as to allow the cells to settle completely. Living and damaged cells were distinguished by visualization on a phase-contrast microscope. Viable PBMC are characterized by their small size and their bright and refractile appearance. Cytotoxicity reactions were quantified as the percentage of complement-damaged cells according to the scoring system of the IHW. Scores 1 and 2, with 0 to 20% dead cells, correspond to a negative result, whereas scores 6 through 8 demonstrate strong positive reactions, with 51 to 100% damaged cells, and score 4 (21 to 50% damaged cells) reflects weak or doubtful positive reactions.
Statistics.
Means and standard deviations were calculated for each subject. The median was given as the descriptive statistic for relative, nonparametrically distributed parameters (viability and background score). Comparisons between different storage times and density gradient techniques were tested for statistical significance by using the Friedman test for related variables from the same population. A P value of <0.05 was considered significant.
RESULTS
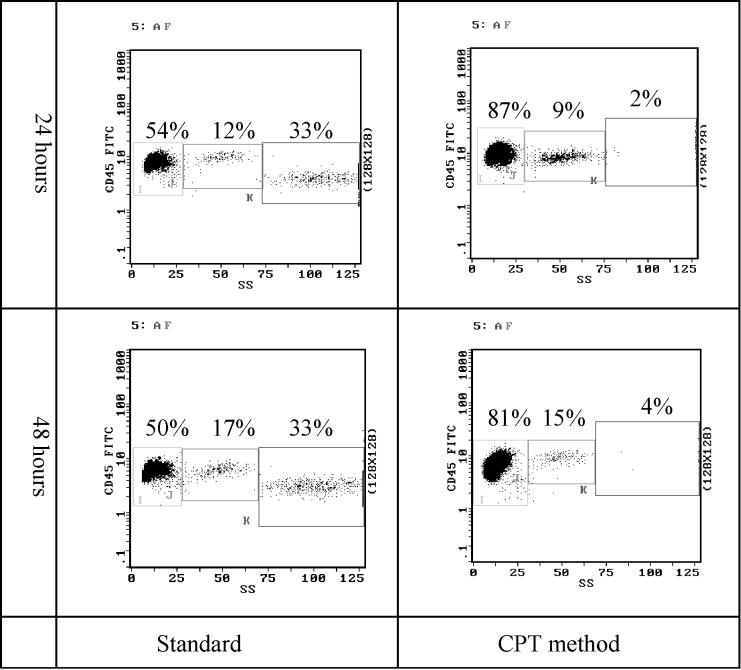
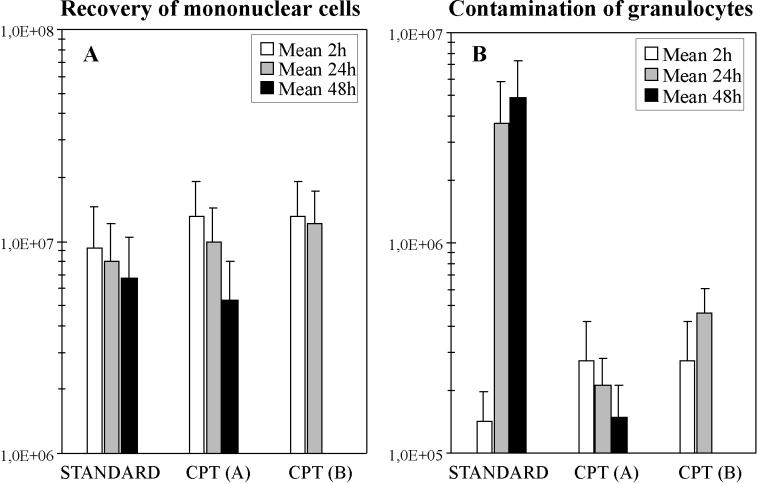
The recovery of PBMC and the extent of granulocyte contamination were assessed by flow cytometric analysis as shown in Fig. 2. The leukocyte subpopulation counts obtained by using the Ficoll gradient technique (standard) or the CPT method with two different protocols are compared in Fig. 3A. With 2 h of storage, the mean yield of PBMC by using CPT (protocol A or B) was 77.6% (1.32 × 107 ± 0.61 × 107) of the original content and significantly higher (P = 0.04) than that with the standard Ficoll method, where a mean recovery of 64.1% (1.09 × 107 ± 0.52 × 107) was seen. The mean recovery decreased to 55.6% (0.94 × 107 ± 0.40 × 107) at 24 h and further decreased significantly (P = 0.007) to 47.6% (0.81 × 107 ± 0.37 × 107) at 48 h with the standard technique. When CPT protocol A was used, a depletion of the PBMC over time was also observed. Mean leukocyte recovery dropped to 58.7% (0.99 × 107 ± 0.44 × 107) and significantly to 31.2% (0.53 × 107 ± 0.28 × 107) after 24 and 48 h, respectively (P ≤ 0.001). However, when CPT protocol B was used, 71.2% (1.21 × 107 ± 0.51 × 107) of the total cells were isolated at 24 h, which was slightly lower than the original value. In contrast, CPT protocol B was totally ineffective for isolating PBMC after 48 h of storage due to the fact that the erythrocytes did not pass the gel barrier of the CPT. In comparison to the standard Ficoll technique, both of the CPT protocols produced significantly higher yields of PBMC after 24 h of storage (P ≤ 0.001).
FIG. 2.
Flow cytometric analysis of leukocyte differential using anti-CD45–FITC versus side-angle light scatter. The recovery of mononuclear cells and the granulocyte contamination are determined in a representative sample. The standard gradient technique is compared with the Ficoll-prefilled tubes (CPT protocols A and B) at different storage times.
FIG. 3.
Determination of cell recovery and granulocyte contamination after the isolation of mononuclear cells by using different gradient techniques and storage times.
The impurity of the PBMC was assessed by measuring the level of contamination by granulocytes (Fig. 3B). When PBMC isolation was performed 2 h after blood collection, no difference was observed between the standard method and the CPT method, with granulocyte contaminations of 0.6% (1.4 × 105 ± 0.5 × 105) and 1.2% (2.7 × 105 ± 1.5 × 105), respectively. With the standard technique the extent of contamination was significantly higher (P ≤ 0.001) at 16.0% (3.7 × 106 ± 2.2 × 106) after 24 h and 19.8% (4.9 × 106 ± 2.4 × 106) after 48 h. In contrast, when the CPT method was used, the purity of the PBMC always remained stable, and a maximum of 2.0% (4.6 × 105 ± 1.5 × 105) granulocytes was observed (P ≤ 0.001).
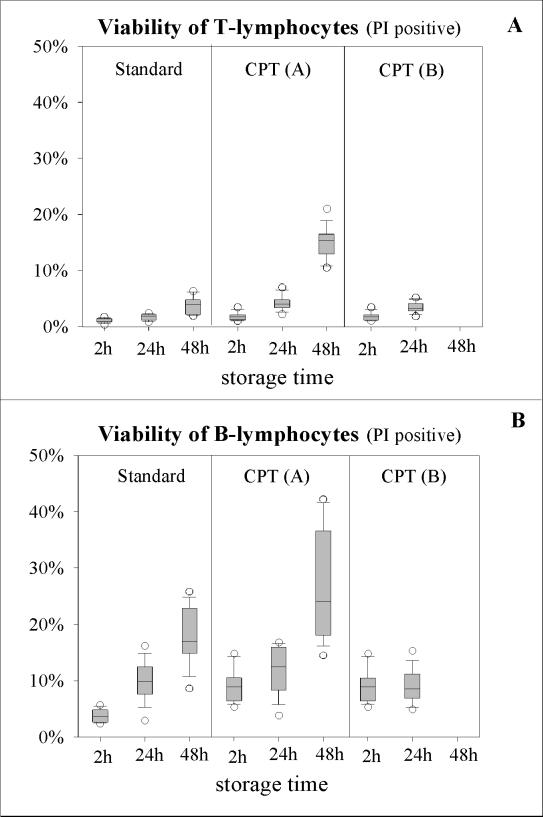
In general, viabilities decreased when the storage time was prolonged, and B lymphocytes were more vulnerable than T lymphocytes (Fig. 4). For the three storage times, median amounts of nonviable T and B lymphocytes with the standard Ficoll technique were 1.0, 1.9, and 4.0% and 3.6, 9.9, and 17.0%, respectively. The differences between the values after 2 and 48 h were significant for both cell types (P ≤ 0.001). When CPT protocol A was used, a significant increase (P ≤ 0.001) in dead cells over time was also observed, with medians of 1.7, 4.0, and 15.4% for T lymphocytes and 8.9, 12.5, and 24.0% for B lymphocytes. In contrast with these findings, a lower percentage of damaged cells was observed with CPT protocol B. Dead T lymphocytes amounted to 1.7 and 3.3%, and dead B lymphocytes amounted to 8.9 and 8.6%, after 2 and 24 h of storage, respectively, whereas after 48 h, whole blood could not be processed. A comparison of lymphocyte viabilities revealed that with the standard method, 1.0% of the T lymphocytes and 3.6% of the B lymphocytes were dead after 2 h of storage. Values for the CPT method, however, were significantly higher at 1.7% (P ≤ 0.001) and 8.9% (P = 0.002), respectively. After 24 h, the median of 1.9% dead T lymphocytes obtained by using the standard protocol was also significantly lower (P ≤ 0.001) than that obtained with CPT protocol A or B (4.0 or 3.3%). With the B lymphocytes, however, none of the viabilities were found to be significantly different from the others. Medians were 9.9, 12.5, and 8.6% with the standard protocol, CPT protocol A, and CPT protocol B, respectively. After 48 h of storage, cell deaths for both T and B lymphocytes were significantly lower (P ≤ 0.001) with the standard procedure (4.0 and 17.0%) than they were with CPT protocol A (15.4 and 24.0%).
FIG. 4.
Determination of cell viability by using propidium iodide (PI) staining. Three density gradient techniques and three storage times are compared.
After storage for the shortest period of time, HLA typing could be accurately performed. HLA typing with lymphocytes isolated after 24 h of storage revealed a lower median background with CPT protocol B (score 1) than with CPT protocol A (score 2) or the standard Ficoll technique (score 2). After 48 h the background scores increased to 6 (standard technique) and 8 (CPT protocol A), and it was thus impossible to perform proper HLA typing by using any of these protocols.
DISCUSSION
For serological HLA class I typing and the detection of specific HLA antibodies, the complement-mediated microcytotoxicity technique first described by Terasaki and McClelland (18) is still the standard procedure and the basic diagnostic tool for decision making in donor-recipient matching. In order to avoid HLA typing errors, it is of major importance to obtain pure and viable mononuclear cells. Several factors can affect background scores and the strength of cytotoxicity reactions, whereby in a worst-case scenario misinterpretations can occur. These include insufficient standardization of the cell preparation procedure due to storage or transport conditions (6, 8, 13, 22), patient health and treatment status, the quantity of cells, and the quality of cadaveric organ donors. Because specialized HLA typing laboratories are often requested to perform such tests by peripheral authorities, the storage and transportation conditions for both whole-blood samples and PBMC suspensions were also assessed.
The recovery of PBMC by the novel CPT method was significantly superior to recovery by the standard Ficoll technique after 2 and 24 h of storage (P ≤ 0.001). This result reflects the necessary restrictions made upon the manual pipetting of PBMC from the interface. A sufficient yield prior to HLA typing is important for patients with hematological malignancies or immunosuppressive treatment, so that low absolute lymphocyte counts may be apparent. With CPT protocol B, sufficient recoveries of PBMC were achieved until 24 h of storage, whereas after 48 h, erythrocyte clumping completely impaired PBMC isolation, since the cells could not pass through the gel barrier. In contrast, with CPT protocol A, the isolated PBMC were resuspended in autologous plasma after centrifugation. The recovery rates fell to 58.7 and 31.2% after 24 and 48 h of storage, which might be caused by cell sedimentation on the top of the gel barrier or by early contact with the Ficoll solution and incomplete removal.
The purity of the PBMC was equal to or greater than 98% in all analyses using the CPT technique regardless of the time of storage. With fresh blood samples, the PBMC isolated by the conventional density gradient technique were more than 99% pure. In contrast, this purity progressively decreased to 80% after 48 h of storage. This was mainly caused by a strong increase in granulocyte contamination, which influenced the HLA typing quality. The differences observed with the CPT method were always found to be significant (P ≤ 0.001). Granulocytes were also shown to be present by Dzik (7), even when cells were stored for 12 h before Ficoll centrifugation, but the reasons for this remain unknown. The granulocytes’ integrity or density may have become altered, and they may have aggregated with or even become engulfed by the PBMC.
The viability results obtained by propidium iodide staining did not correlate well with those obtained by cytotoxicity testing, indicating that a partial loss of cell integrity may start to disturb HLA typing. In general, regardless of the density gradient technique, the percentage of dead B lymphocytes was always higher than that of dead T lymphocytes. With all protocols used, a more marked reduction in median viability was observed after 48 h of storage, and as such the isolated PBMC were not suitable for cytotoxicity testing. With either CPT or Ficoll centrifugation after both 2 and 24 h, the measured changes in cell viability were acceptable, although the standard procedure resulted in significantly more viable T lymphocytes (P ≤ 0.001). This beneficial effect was probably caused by the washing steps performed in the traditional procedure immediately after Ficoll centrifugation, whereas with CPT protocol A, all cells, including dead ones, were retained in the original CPT after exposure to the Ficoll solution. In our hands both cell separation techniques were shown to be sufficient for HLA typing, but sample storage and transportation time was limited to 24 h with respect to cell viability. The CPT technique enriches pure and viable PBMC, which allows for sensitive and specific HLA ABC typing with a low level of background staining. These results are comparable to those obtained by immunomagnetic HLA typing techniques, and the CPT technique might partly replace the requirement for immunomagnetic techniques (21). In conclusion, the novel, ready-to-use isolation procedure using Vacutainer CPT offers the opportunity to isolate pure and viable PBMC. The procedure is easier to perform and is much less time-consuming than the conventional technique. Furthermore, the CPT method is more standardized and resulted in a higher PBMC yield without any relevant contamination by granulocytes. It was advantageous with regard to cell recovery and viability to store blood samples in CPT for 24 h before centrifugation. Both methods are able to support sufficient cell viability for at least 24 h of storage or transportation, which would permit their use in serological HLA typing and HLA antibody detection or various other applications.
ACKNOWLEDGMENTS
We gratefully acknowledge Martin Saballus for technical assistance and Una Doherty for editing the manuscript.
We acknowledge the financial support of Becton Dickinson Vacutainer Systems and the technical supervision of Rita Bergendahl.
REFERENCES
- 1.Ashmore L M, Shopp G M, Edwards B S. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J Immunol Methods. 1989;118:209–215. doi: 10.1016/0022-1759(89)90008-2. [DOI] [PubMed] [Google Scholar]
- 2.Bein G, Gläser R, Kirchner H. Rapid HLA-DRB1 genotyping by nested PCR amplification. Tissue Antigens. 1992;39:68–73. doi: 10.1111/j.1399-0039.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 3.Blasczyk R, Hahn U, Wehling J, Huhn D, Salama A. Complete subtyping of the HLA-A locus by sequence-specific amplification followed by direct sequencing or single-strand conformation polymorphism analysis. Tissue Antigens. 1995;46:86–95. doi: 10.1111/j.1399-0039.1995.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 4.Böyum A. Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 5.Böyum A. Separation of blood leukocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4:269. [PubMed] [Google Scholar]
- 6.De Paoli P, Villalta D, Battistin S, Gasparollo A, Santini G. Selective loss of OKT8 lymphocytes on density gradient centrifugation separation of blood mononuclear cells. J Immunol Methods. 1983;61:259–260. doi: 10.1016/0022-1759(83)90170-9. [DOI] [PubMed] [Google Scholar]
- 7.Dzik W H. Granulocytes contaminate Ficoll preparations of stored blood. Transfusion. 1983;23:538–539. doi: 10.1046/j.1537-2995.1983.23684074285.x. [DOI] [PubMed] [Google Scholar]
- 8.Dzik W H, Neckers L. Mononuclear cell-surface antigens during storage of banked blood. Transplantation. 1984;38:67–71. doi: 10.1097/00007890-198407000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Mansour I, Bourin P, Rouger P, Doinel C. A rapid technique for lymphocyte preparation prior to two-color immunofluorescence analysis of lymphocyte subsets using flow cytometry. J Immunol Methods. 1990;127:61–70. doi: 10.1016/0022-1759(90)90341-r. [DOI] [PubMed] [Google Scholar]
- 10.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 11.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours—an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–235. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 12.Pelegrí C, Rodríguez-Palmero M P, Morante M, Comas J, Castell J C M, Franch A. Comparison of four lymphocyte isolation methods applied to rodent T cell subpopulations and B cells. J Immunol Methods. 1995;187:265–271. doi: 10.1016/0022-1759(95)00193-1. [DOI] [PubMed] [Google Scholar]
- 13.Prince H E, Arens I. Effect of storage on lymphocyte surface markers in whole blood units. Transplantation. 1986;41:235–238. doi: 10.1097/00007890-198602000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Saiki R K, Bugawan T L, Horn G T, Mullis K B, Erlich H A. Analysis of enzymatically amplified β-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- 15.Schlenke, P., C. Frohn, H. Klüter, M. Saballus, H. J. Hammers, S. R. Zajac, and H. Kirchner. Evaluation of a flow cytometric method for simultaneous leukocyte phenotyping and quantification by fluorescent microparticles. Cytometry, in press. [DOI] [PubMed]
- 16.Tamul K R, O’Gorman R G, Donovan M, Schmitz J L, Folds J D. Comparison of a lysed whole blood method to purified cell preparations for lymphocyte immunophenotyping: differences between healthy controls and HIV-positive specimens. J Immunol Methods. 1994;167:237–243. doi: 10.1016/0022-1759(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 17.Tamul K R, Schmitz J L, Kane K, Folds J D. Comparison of the effects of Ficoll-Hypaque separation and whole blood lysis on results of immunophenotypic analysis of blood and bone marrow samples from patients with hematologic malignancies. Clin Diagn Lab Immunol. 1995;2:337. doi: 10.1128/cdli.2.3.337-342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki P I, McClelland J D. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 19.Terstappen L W M M, Meiners H, Loken M R. A rapid sample preparation technique for flow cytometric analysis of immunofluorescence allowing absolute enumeration of cell subpopulations. J Immunol Methods. 1989;123:103–112. doi: 10.1016/0022-1759(89)90034-3. [DOI] [PubMed] [Google Scholar]
- 20.Terstappen L W, Hollander Z, Meiners H, Loken M R. Quantitative comparison of myeloid antigen on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990;48:138–148. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
- 21.Vartdal F, Gaudernach G, Funderud S, Bratlie A, Lea T, Ugelstad J, Thorsby E. HLA class I and II typing using cells positively selected from blood by immunomagnetic isolation—a fast and reliable technique. Tissue Antigens. 1986;28:301–312. doi: 10.1111/j.1399-0039.1986.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 22.Weiblen B J, Debell K, Valeri C R. “Acquired immunodeficiency” of blood stored overnight. N Engl J Med. 1983;309:793. doi: 10.1056/NEJM198309293091311. [DOI] [PubMed] [Google Scholar]