Abstract
Background
Though the association between air pollution and incident type 2 diabetes (T2D) has been well documented, evidence on the association with development of subsequent diabetes complications and post-diabetes mortality is scarce. We investigate whether air pollution is associated with different progressions and outcomes of T2D.
Methods
Based on the UK Biobank, 398,993 participants free of diabetes and diabetes-related events at recruitment were included in this analysis. Exposures to particulate matter with a diameter ≤ 10 μm (PM10), PM2.5, nitrogen oxides (NOx), and NO2 for each transition stage were estimated at each participant’s residential addresses using data from the UK’s Department for Environment, Food and Rural Affairs. The outcomes were incident T2D, diabetes complications (diabetic kidney disease, diabetic eye disease, diabetic neuropathy disease, peripheral vascular disease, cardiovascular events, and metabolic events), all-cause mortality, and cause-specific mortality. Multi-state model was used to analyze the impact of air pollution on different progressions of T2D. Cumulative transition probabilities of different stages of T2D under different air pollution levels were estimated.
Results
During the 12-year follow-up, 13,393 incident T2D patients were identified, of whom, 3791 developed diabetes complications and 1335 died. We observed that air pollution was associated with different progression stages of T2D with different magnitudes. In a multivariate model, the hazard ratios [95% confidence interval (CI)] per interquartile range elevation in PM2.5 were 1.63 (1.59, 1.67) and 1.08 (1.03, 1.13) for transitions from healthy to T2D and from T2D to complications, and 1.50 (1.47, 1.53), 1.49 (1.36, 1.64), and 1.54 (1.35, 1.76) for mortality risk from baseline, T2D, and diabetes complications, respectively. Generally, we observed stronger estimates of four air pollutants on transition from baseline to incident T2D than those on other transitions. Moreover, we found significant associations between four air pollutants and mortality risk due to cancer and cardiovascular diseases from T2D or diabetes complications. The cumulative transition probability was generally higher among those with higher levels of air pollution exposure.
Conclusions
This study indicates that ambient air pollution exposure may contribute to increased risk of incidence and progressions of T2D, but to diverse extents for different progressions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02573-0.
Keywords: Air pollution, Type 2 diabetes, Diabetes complication, Diabetes mortality, Multi-state model
Background
Type 2 diabetes (T2D), caused by insulin resistance and pancreatic beta cell dysfunction, accounts for approximately 90% of all diabetes cases [1, 2]. Approximately 463 million people worldwide suffered from diabetes in 2019, and the prevalence continues to rise [3]. More than half of patients will develop any one of complications, and these diabetes-related complications represent the major causes of disability and mortality due to T2D [4, 5]. Owing to the advances in the treatment of T2D, many individuals will survive longer with these conditions, leading to an increased economic burden in the presence of diabetes complications [4, 6].
Existing evidence supports an association between the risk of T2D and ambient air pollution exposure, especially for fine particulate matter pollution (PM2.5) and nitrogen dioxide (NO2) [7, 8]. The biological mechanisms underlying this association include immune activation, endoplasmic reticulum stress, central nervous system inflammation, and oxidative stress [9, 10]. Additionally, previous studies have found that ambient air pollution was associated with some complications of diabetes, including diabetic retinopathy, incident cardiovascular diseases, and chronic kidney diseases [11–13]. However, it remained unknown whether ambient air pollution was associated with the dynamic progression of T2D, such as from baseline to incident T2D, further to diabetes complications, and subsequent to death. Based on the previous studies, it is reasonable to hypothesize that ambient air pollution could increase the risk of diabetes complications and subsequent mortality, in other words, exposure to higher levels of ambient air pollution could be associated with an increased risk of different progressions of T2D. The investigation on the effect of risk factors in T2D trajectory would be important for specific interventions at different stages of T2D.
We utilized a multi-state model to assess the association between air pollution exposure and different progressions of T2D. We further predicted the transition probabilities of different stages for participants exposed to different levels of air pollution. The findings from this analysis will add to the evidence to prioritize action to reduce ambient air pollution and optimize prevention and management strategies of T2D.
Methods
Study design
The UK Biobank is a large population-based cohort study. Details of the UK Biobank have been described previously [14]. In brief, the cohort included more than 500,000 participants aged 37–73 years at baseline (2006–2010) in 22 sites across England, Scotland, and Wales, and each participant was followed up. All participants completed a baseline survey, including socioeconomic factors, lifestyle factors, and the history of medication.
In this analysis, we included 398,993 participants who were free of diabetes and any diabetes-related events (cardiovascular diseases, diabetes eye diseases, diabetes kidney diseases, and diabetes neuropathy diseases) at baseline (Additional file 1: Fig. S1). Participants with occurrences of the diabetes-related events before the diagnosis of T2D were excluded (Additional file 1: Fig. S1). Participants with missing exposure data were also excluded, whose residential addresses were geocoded outside the range of assessment (Additional file 1: Fig. S1). The prevalent cases of diabetes and any diabetes-related events at baseline were assessed based on self-reported information, medication history, and hospital inpatient records (supplemental material).
This study was approved by the North West Multicenter Research Ethics Committee, and informed consent was obtained from all participants.
Environmental exposure assessment
The mean annual concentrations of particulate matter with a diameter ≤10 μm (PM10), PM2.5, nitrogen oxides (NOx), and NO2 were collected from the UK’s Department for Environment, Food and Rural Affairs (DEFRA), a platform providing high-resolution near-surface air pollution data in the UK from 2002 to 2020 [15]. Annual concentration maps of various air pollutants were modeled on 1 km × 1 km grid using an air dispersion model based on different sources from the National Atmospheric Emissions Inventory, a combination of measurement data for secondary inorganic aerosol, and models for sources including resuspension of dust. These concentrations were calibrated using measured concentrations taken from background sites in Defra's Automatic Urban and Rural Network [15].
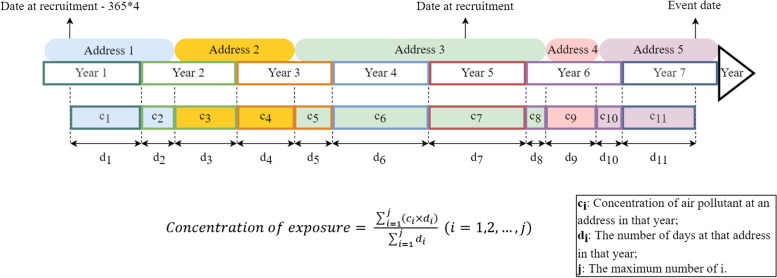
Given the exposures at baseline may not accurately represent the air pollution exposure over the long follow-up period, we estimated exposures to four air pollutants from the date of 4 years before recruitment to the dates when any outcome occurred. To assess the air pollutant exposures for each participant, we collected participants’ residential address history including the dates that participants lived in each location. The annual mean air pollution concentration for each residential location was estimated by the following processes. First, the annual mean concentration of air pollutants of the current year was assigned to the participant by 1km × 1km grid cells in which they resided. Second, we counted the number of days of residence at that address in the calendar year. Third, we calculated average levels of the air pollutant exposures for each participant by weighting the time spent at each residential address. The formula can be specified as:
where ci is the annual mean concentration at an address in that year, di is the days at that address in the calendar year, and j is the number of combinations of different addresses and corresponding days in a calendar year. The exposure estimation strategy was presented in Fig. 1.
Fig. 1.
The air pollution exposure estimation strategy. The colorful, rounded rectangles represent the participant’s address. The rectangles with colorful borders represent each year during the follow-up period. The color-filled rectangles with colorful borders represent the concentration of air pollutants at that address in that current year. The fill-in color corresponds to that of the address, and the border color matches that of the current year
Follow-up and ascertainment of outcomes
All the participants were followed up since the date when the participants consented to join the UK Biobank study, and the end date was either death, loss to follow-up, or the last date of follow-up on 31 March 2021, whichever occurred first.
The key outcomes of interest in this study were the incident T2D, diabetes complications events, and mortality. During the follow-up, T2D and diabetes complications were identified through gathering information from hospital admissions, primary care, and death records, provided by the UK National Health Service. Diabetes complications were defined as the first occurrence of any diabetes complications events after the diagnosis of incident T2D [16]. Diabetes complications in this study included diabetic eye diseases, diabetic kidney diseases, diabetic neuropathy diseases, cardiovascular diseases (CVD), peripheral vascular diseases, and metabolic events. All-cause mortality and cause-specific mortality were obtained from the national death registry. Cause-specific mortality included cancer, CVD, and respiratory diseases, which accounted for 62.1%, 10.2%, and 6.2% of the overall deaths, respectively. The detailed information on codes and data fields of the key outcomes was presented in the Additional file 1: Details of outcomes.
Covariates
We considered a series of important covariates that have been shown to be associated with either T2D or ambient air pollution in this analysis, including demographics, socioeconomic factors, lifestyle, and comorbidities [1, 2, 8]. Demographic, socioeconomic factors and lifestyle factors included age at recruitment (continuous variable), sex, ethnicity (white and non-white), living area (urban and rural), smoking status (never, previous, and current), healthy diet (including intake of fish, meat, vegetables, fruit, and alcohol consumption), and physical activity (low, moderate, and high). Obesity was evaluated by a body mass index higher than 30 kg/m2. Comorbidities were assessed based on self-reported data, medication history, and hospital inpatient data, including hypertension, high cholesterol, and cancer. Details of the assessment of covariates are displayed in Additional file 1: Table S1. The missing data on covariates were imputed with multivariate imputation via a chained equation.
Statistical analysis
The correlation between air pollutant exposures was assessed by Spearman’s correlation coefficients. We utilized a multi-state regression model to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the associations between per interquartile range (IQR) increase in air pollutants and progression trajectories of T2D from healthy to incident T2D, further to complication, and ultimately to death. We also assessed the associations between air pollution and cause-specific mortality risk from baseline, T2D, and diabetes complications. The multi-state model is an extension of the competing risk model to assess the impact of certain exposures on different stages of disease progression [17, 18], and has been applied in several studies on the associations between lifestyle and the risk of cardiometabolic multimorbidity [19, 20].
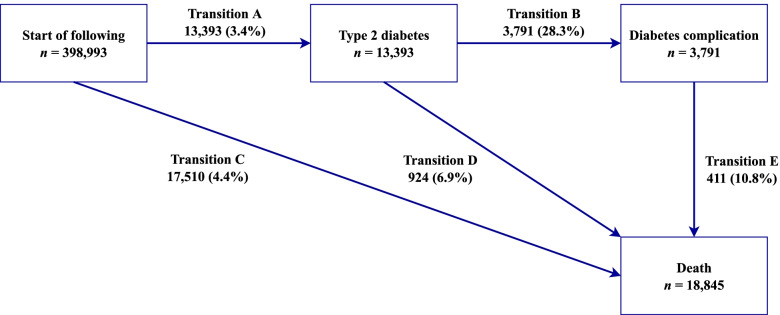
In this study, five transition phases were considered based on the natural history of T2D (Fig. 2) [2]: (A) baseline to T2D (n = 13,393); (B) T2D to any one of diabetes complications (n = 3791); (C) baseline to death without T2D (n = 17,510); (D) T2D to death from any cause (n = 924); (E) diabetes complications to death from any cause (n = 411). Moreover, transition patterns from T2D to cause-specific mortality were presented in Additional file 1: Fig. S2. For participants who experienced multiple states on the same date (n = 2075), the entry date of the first state was calculated as the entry date of the later state minus the median interval time of each stage in this study (598 days for transition B; 564 days for transition D; 485 days for transition E). For example, for participants diagnosed with diabetes and related complications on the same day, the date of onset of T2D was equal to the date of complications minus 598 days.
Fig. 2.
Transitions from baseline to T2D, diabetes complications, and all-cause death. Diabetes complications included diabetic eye diseases, diabetic kidney diseases, diabetic neuropathy diseases, cardiovascular diseases, peripheral vascular diseases, and metabolic events. State-specific number of events was reported in boxes, and the transition-specific number of events and percentages (within brackets) were reported on arrows
We constructed 3 models to examine the associations between ambient air pollutants and five transitions of T2D trajectory. In the basic model, we adjusted for age, sex, and ethnicity. In model 2, we additionally adjusted for the potential confounders listed above. As obesity and chronic disease history could be on the causal pathway between air pollution and T2D, we excluded obesity and chronic diseases in model 3. We then used model 3 as the primary model for the subsequent analyses to avoid the potential bias.
We used restricted cubic splines with three knots to estimate deviations from linearity in exposure-response of the association between air pollutants and different stages of T2D trajectory and used likelihood ratio tests to examine the nonlinear associations.
We predicted the cumulative probabilities of the later state from the prior state during the follow-up among participants living with different levels of air pollution. Cumulative predicted probabilities were calculated for men and women aged 55 years old, with the maximized distribution of the observed adjusted factors (ethnicity, residential area, healthy diets, physical activity, smoking status, family history of diabetes).
Sensitivity analysis
To assess the robustness of the results, we further conducted several sensitivity analyses: (1) 2-pollutant models for each of the 4 air pollutants by including 2 air pollutants in the same model. Because PM10 and PM2.5 were strongly correlated (Spearman’s correlation coefficient > 0.9), and NOx and NO2 were also strongly correlated, we did not include them in the same model [21]; (2) further adjustment for traffic noise as traffic noise may be the potential confounder of the association between air pollution and incidence of T2D [22, 23]; (3) using different time intervals (1 day, 1 year, and 3 years) to calculate the entry date of the previous state for participants entering different states on the same day; (4) restricting the mortality due to T2D or diabetes complications; (5) excluding participants without complete data of covariates; and (6) excluding the participants who were diagnosed with T2D or diabetes complications on the same date.
Stratified analysis
We further conducted several analyses stratified by age, residential area, smoking status, physical activity, and healthy diet. We calculated p values using likelihood ratio tests comparing models with and without multiplicative interaction terms.
All the statistical analyses were performed using R software (version 4.0.3). The missing data were imputed using the ‘mice’ package. The multi-state model was run using the “mstate” package [17]. Results with a 2-sided p value < 0.05 were considered statistically significant.
Results
Descriptive results
A total of 398,993 participants included in the study were younger than those excluded and more likely to be female and white (Additional file 1: Table S2). The mean age of participants included was 55.49 years (SD 8.06 years). Over half were female (57.1%) and most participants were white (94.4%). During a mean follow-up of 12.0 (SD = 1.5) years [4,768,126 person-years (PYs)], 13,393 (28.1/10,000 PYs) participants experienced T2D (Fig. 2). Among these participants, 3791 (555.2/10,000 PYs) developed complications, and 924 (135.3/10,000 PYs) died without experiencing complications. Of the 3791 cases with diabetes complications, 411 died with a crude mortality rate of 237.2 per 10,000 PYs. The primary cause of death was cancer followed by CVD and respiratory disease (Additional file 1: Fig. S2).
Table 1 presents the baseline characteristics of participants by the top and bottom quartiles of exposure to PM2.5 and NO2 in the transition from baseline to T2D. Participants with higher exposure to ambient air pollution were younger and more likely to be living in urban area and current smokers. The mean exposures to PM10, PM2.5, NOx, and NO2 in the whole stage were 15.04, 10.01, 27.27, and 18.23 μg/m3, respectively (Additional file 1: Table S3). These four exposures were highly positively correlated with pairwise correlation coefficients higher than 0.78 (p < 0.05, Additional file 1: Fig. S3).
Table 1.
Characteristics of 398,993 participants
| Overall (n = 398,993) |
PM2.5 (μg/m3) | NO2 (μg/m3) | |||
|---|---|---|---|---|---|
| Q1: 1.69–8.74 (n = 99,752) |
Q4: 11.05–18.70 (n = 99,747) |
Q1: 2.86–14.17 (n = 99,750) |
Q4: 21.25–57.92 (n = 99,748) |
||
| Age [years, mean (SD)] | 55.49 (8.06) | 55.86 (7.93) | 55.20 (8.24) | 56.18 (7.85) | 54.75 (8.25) |
| Sex (%) | |||||
| Male | 171,055 (42.9) | 41,922 (42.0) | 42,957 (43.1) | 42,212 (42.3) | 43,543 (43.7) |
| Female | 227,938 (57.1) | 57,830 (58.0) | 56,790 (56.9) | 57,538 (57.7) | 56,205 (56.3) |
| Ethnicity (%) | |||||
| White | 376,743 (94.4) | 98,181 (98.4) | 85,835 (86.1) | 98,301 (98.5) | 85,601 (85.8) |
| Non-white | 22,250 (5.6) | 1571 (1.6) | 13,912 (13.9) | 1449 (1.5) | 14,147 (14.2) |
| Residential area (%) | |||||
| Urban | 343,011 (86.0) | 78,809 (79.0) | 97,350 (97.6) | 58,586 (58.7) | 98,935 (99.2) |
| Rural | 55,982 (14.0) | 20,943 (21.0) | 2397 (2.4) | 41,164 (41.3) | 813 (0.8) |
| Smoking status (%) | |||||
| Never | 227,700 (57.1) | 59,195 (59.3) | 54,593 (54.7) | 59,787 (59.9) | 53,265 (53.4) |
| Previous | 131,114 (32.9) | 31,749 (31.8) | 33,344 (33.4) | 32,536 (32.6) | 33,135 (33.2) |
| Current | 40,179 (10.1) | 8808 (8.8) | 11,810 (11.8) | 7427 (7.4) | 13,348 (13.4) |
| Obese (%) | |||||
| Yes | 110,765 (27.8) | 20,755 (20.8) | 20,420 (20.5) | 19,748 (19.8) | 21,832 (21.9) |
| No | 387,917 (97.2) | 78,997 (79.2) | 79,327 (79.5) | 80,002 (80.2) | 77,916 (78.1) |
| Physical activity (%) | |||||
| Low | 72,398 (18.1) | 17,204 (17.2) | 17,645 (17.7) | 17,443 (17.5) | 18,077 (18.1) |
| Moderate | 164,071 (41.1) | 40,976 (41.1) | 42,436 (42.5) | 40,854 (41.0) | 41,698 (41.8) |
| High | 162,524 (40.7) | 41,572 (41.7) | 39,666 (39.8) | 41,453 (41.6) | 39,973 (40.1) |
| Healthy diet (%) | |||||
| Yes | 284,828 (71.4) | 70,418 (70.6) | 72,492 (72.7) | 72,301 (72.5) | 71,609 (71.8) |
| No | 114,165 (28.6) | 29,334 (29.4) | 27,255 (27.3) | 27,449 (27.5) | 28,139 (28.2) |
| Family history of diabetes (%) | |||||
| Yes | 83,680 (21.0) | 19,848 (19.9) | 21,617 (21.7) | 19,748 (19.8) | 22,202 (22.3) |
| No | 315,313 (79.0) | 79,904 (80.1) | 78,130 (78.3) | 80,002 (80.2) | 77,546 (77.7) |
| Chronic diseases (%) | |||||
| High cholesterol | 41,841 (10.5) | 9998 (10.0) | 11,129 (11.2) | 9515 (9.5) | 11,518 (11.5) |
| Hypertension | 161,003 (40.4) | 39,475 (39.6) | 38,540 (38.6) | 40,945 (41.0) | 38,778 (38.9) |
| Cancer | 42,225 (10.6) | 10,669 (10.7) | 10,516 (10.5) | 10,900 (10.9) | 10,248 (10.3) |
Association between air pollution and different progressions of type 2 diabetes
The associations between air pollution and risk of different progressions of T2D were presented in Table 2. In the basic model, all four air pollutants were significantly associated with an increased risk of dynamic progressions of T2D. The results in model 2 were stable when we additionally adjusted for lifestyle factors, obesity, and history of chronic diseases.
Table 2.
Associations between air pollution and risk of five progressions of T2D using the multi-state model
| Transition | Cases | PM10 | PM2.5 | NOx | NO2 |
|---|---|---|---|---|---|
| Basic model | |||||
| Baseline → T2D | 13,393 | 1.66 (1.63, 1.70) | 1.63 (1.60, 1.67) | 1.39 (1.37, 1.41) | 1.47 (1.44, 1.49) |
| T2D → complication | 3791 | 1.14 (1.09, 1.19) | 1.09 (1.05, 1.14) | 1.11 (1.07, 1.15) | 1.16 (1.11, 1.20) |
| Baseline → death | 17,510 | 1.54 (1.51, 1.57) | 1.52 (1.49, 1.55) | 1.33 (1.31, 1.35) | 1.39 (1.37, 1.41) |
| T2D → death | 924 | 1.43 (1.31, 1.56) | 1.51 (1.38, 1.65) | 1.22 (1.14, 1.31) | 1.27 (1.18, 1.37) |
| Complication → death | 411 | 1.49 (1.31, 1.71) | 1.57 (1.38, 1.80) | 1.39 (1.25, 1.55) | 1.44 (1.28, 1.62) |
| Model 2 | |||||
| Baseline → T2D | 13,393 | 1.67 (1.63, 1.71) | 1.64 (1.61, 1.68) | 1.40 (1.38, 1.42) | 1.49 (1.46, 1.52) |
| T2D → complication | 3791 | 1.13 (1.08, 1.18) | 1.08 (1.03, 1.13) | 1.10 (1.06, 1.15) | 1.15 (1.11, 1.20) |
| Baseline → death | 17,510 | 1.51 (1.48, 1.54) | 1.50 (1.47, 1.53) | 1.32 (1.30, 1.34) | 1.39 (1.37, 1.41) |
| T2D → death | 924 | 1.42 (1.29, 1.55) | 1.50 (1.37, 1.64) | 1.22 (1.14, 1.31) | 1.28 (1.18, 1.38) |
| Complication → death | 411 | 1.45 (1.27, 1.65) | 1.53 (1.34, 1.74) | 1.35 (1.21, 1.51) | 1.41 (1.24, 1.59) |
| Model 3 | |||||
| Baseline → T2D | 13,393 | 1.66 (1.62, 1.69) | 1.63 (1.59, 1.67) | 1.39 (1.37, 1.42) | 1.49 (1.46, 1.51) |
| T2D → complication | 3791 | 1.13 (1.08, 1.18) | 1.08 (1.03, 1.13) | 1.10 (1.06, 1.15) | 1.15 (1.11, 1.20) |
| Baseline → death | 17,510 | 1.51 (1.48, 1.54) | 1.50 (1.47, 1.53) | 1.32 (1.30, 1.34) | 1.39 (1.36, 1.41) |
| T2D → death | 924 | 1.41 (1.29, 1.54) | 1.49 (1.36, 1.64) | 1.22 (1.13, 1.31) | 1.27 (1.17, 1.37) |
| Complication → death | 411 | 1.46 (1.28, 1.67) | 1.54 (1.35, 1.76) | 1.36 (1.22, 1.52) | 1.42 (1.25, 1.60) |
HRs (95% CI) are results for per IQR increase from multi-state models. IQR increase was 3.25 μg/m3 for PM10, 2.31 μg/m3 for PM2.5, 12.43 μg/m3 for NOx, and 7.08 μg/m3 for NO2. All values are statistically significant (p < 0.05)
Basic model was adjusted for age, sex, and ethnicity
Model 2 was based on a basic model and additionally adjusted for residential area, smoking status, healthy diet, physical activity, family history of diabetes, obesity, history of hypertension, high cholesterol, and cancer
Model 3 was adjusted for age, sex, ethnicity, residential area, smoking status, healthy diet, physical activity, and family history of diabetes
Abbreviations: CI confidence interval, HR hazard ratio, IQR interquartile
In model 3, results persisted and remained robust (Table 2). For example, the HRs (95% CI) per IQR elevation (2.31 μg/m3) in PM2.5 were 1.63 (1.59, 1.67) and 1.08 (1.03, 1.13) for transitions from healthy to T2D and from T2D to complications, and 1.50 (1.47, 1.53), 1.49 (1.36, 1.64), and 1.54 (1.35, 1.76) for mortality risk from baseline, T2D, and diabetes complications, respectively. The associations of four air pollutants on incident T2D were generally stronger than those on other transitions, and the HRs (95% CI) per IQR increase in PM10, NOx, and NO2 exposure were 1.66 (1.62, 1.69), 1.39 (1.37, 1.42), and 1.49 (1.46, 1.51), respectively. Generally, the estimates for transition from diabetes complications to death were slightly stronger than that from T2D directly to death. For example, the HR (95% CI) of mortality from diabetes complications per IQR (7.08 μg/m3) increase in NO2 exposure was 1.42 (1.25, 1.60), and the estimate was 1.27 (1.17, 1.37) for mortality from T2D.
The positive associations between four exposures and dynamic progressions of T2D were observed to be monotonic across the range of ambient air pollution exposures, except for the association between particulate matter and transition from T2D to diabetes complications (Additional file 1: Fig. S4).
The associations between air pollution and risk of cause-specific mortality were shown in Additional file 1: Table S4. Four air pollution exposures were positively associated with increased risks of cancer, CVD, and respiratory diseases mortality from T2D or diabetes complication. The associations between air pollution and the risk of cancer mortality from T2D were stronger than those for mortality due to other causes. The estimates for transition from diabetes complications to CVD mortality were stronger than those for other cause-specific mortality.
Cumulative transition probability of type 2 diabetes trajectories
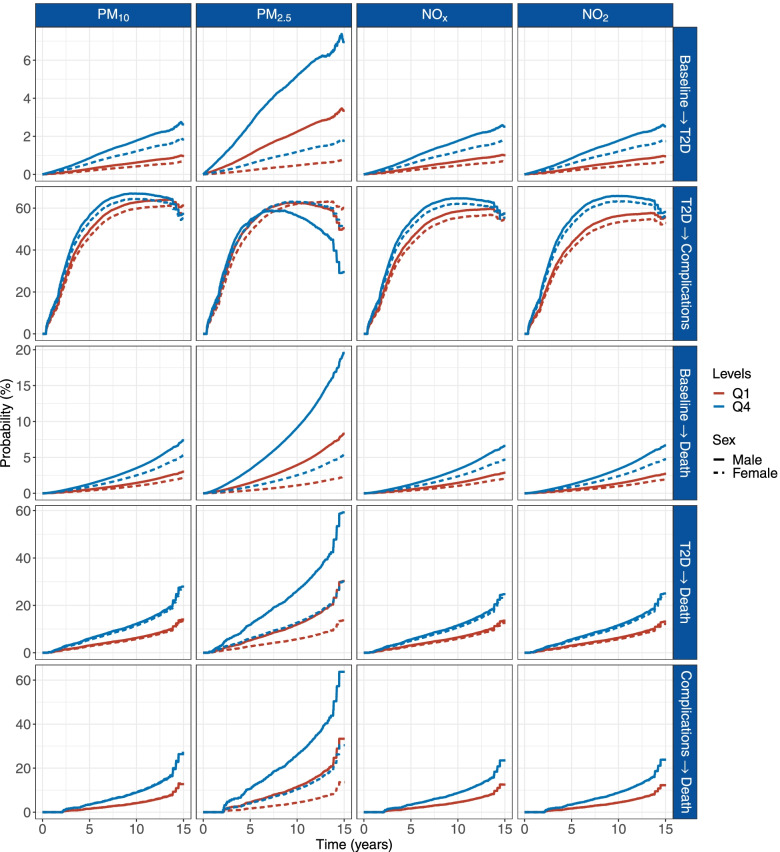
Figure 3 shows the cumulative transition probabilities of different progressions for participants aged 55 years old during the follow-up period. The transition probabilities of development of T2D, diabetes complications, and post-diabetes mortality were generally higher in participants living in a higher level of air pollution. For example, the cumulative probabilities for T2D in healthy people at 14.7 years of follow-up were 2.7% and 1.9% for men and women, respectively, living with the top quartile of PM10, and were 1.0% and 0.7% for men and women, respectively, living with the lowest quartile. Comparing to those living in the lowest quartile of NO2, the cumulative probabilities for diabetes complications in patients with T2D living in the top quartile exposure at 14.9 years of follow-up increased by approximately 3% (58.2% in Q4 vs. 55.6% in Q1 for men and 56.2% in Q4 vs. 52.9% in Q1 for women). The difference in cumulative mortality between the highest quartile of NOx and the lowest quartile in patients with diabetes complications at 14.5 years of follow-up was approximately 11% (23.5% in Q4 vs. 12.5% in Q1 for men; 23.6% in Q4 vs. 12.6% in Q1 for women).
Fig. 3.
Cumulative transition probabilities of T2D for participants exposed to different levels of air pollution. Computed for 55-year-old men (continuous) and women (dotted) for values of air pollution of quartile 1 and quartile 4. Quartile cutoffs were 2.92–13.23 μg/m3 and 16.48–28.48 μg/m3 for PM10, 1.69–8.74 μg/m3 and 11.05–18.70 μg/m3 for PM2.5, 3.75–19.79 μg/m3 and 32.22–111.01 μg/m3 for NOx, and 2.86–14.17 μg/m3 and 21.25–57.92 μg/m3 for NO2, respectively. The model was adjusted for age, sex, ethnicity, living area, smoking status, healthy diet, physical activity, and family history of diabetes. Abbreviation: T2D, type 2 diabetes
Sensitivity analyses
In the 2-pollutant model, the associations between PM10 and PM2.5 and different transitions of T2D remained significant and stable, except for the associations with transition from T2D to diabetes complications. The associations of NOx and NO2 with progressions of T2D remained significant, except for those with mortality risk from T2D or diabetes complications (Additional file 1: Table S5). In the model including traffic noise, we observed stable associations between the four air pollutants and transitions from baseline to T2D, further to diabetes complication, and subsequent to death (Additional file 1: Table S6). The associations in sensitivity analyses using different time intervals did not significantly change (Additional file 1: Table S7). In sensitivity analyses excluding deaths not due to T2D or diabetes-related events, the estimates decreased and associations of four exposures with transition from T2D to death became insignificant (Additional file 1: Table S8). The results after excluding participants with missing data were similar to those from our main multivariable models (Additional file 1: Table S9). When excluding the participants who were diagnosed with T2D or diabetes complications on the same date, the estimates increased for transitions from baseline to T2D and subsequently to diabetes complications but decreased for transitions to death from T2D or diabetes complications (Additional file 1: Table S10).
Stratified analyses
There was a suggestion of effect modification by residential area across different exposures (Additional file 1: Fig. S5–S8). Stronger estimates on increased risk of T2D were found in those living in a rural area (p-for-interaction <0.001). For example, the HRs (95% CI) for incident T2D in participants living in a rural area and an urban area were 1.97 (1.81, 2.14) and 1.38 (1.36, 1.40) per IQR (12.43 μg/m3) increment in NOx, respectively. We also observed stronger associations between air pollution and transition from T2D to diabetes complications in those living in a rural area (p-for-interaction 0.061 for PM10, <0.001 for PM2.5, NOx, and NO2).
Discussion
This study provided some novel findings by showing the positive associations between four air pollutants (PM10, PM2.5, NOx, and NO2) and different transition stages from healthy to T2D, to diabetes complications, and then to death. We also quantified the cumulative probabilities of five transitions and found that participants exposed to higher levels of air pollution may have an increased risk of adverse diabetic outcomes, particularly the risk of diabetes complications and diabetic mortality. Furthermore, there was a consistent evidence of effect modification by residential areas across different exposures.
Several systematic reviews have summarized the association between air pollution exposure and the risk of T2D [7, 24], and these studies support a positive association. For example, one recent meta-analysis reviewed 86 studies and reported the HRs (95% CI) of incident T2D associated with each 10 μg/m3 increment in PM2.5 and PM10 were 1.10 (1.04, 1.17) and 1.11 (1.00, 1.22), respectively [7]. Consistent with these studies, our study found the positive associations of PM10, PM2.5, NOx, and NO2 with transition from baseline to T2D. The difference in estimates may be due to ambient air pollution exposures among the UK Biobank participants being much lower than those in previous studies conducted in developing countries. Furthermore, variations in pollution characteristics and susceptability differences among different populations cannot be excluded.
Despite several studies that have explored the associations of ambient T2D and heart failure with ambient air pollution using the UK Biobank [25, 26], we attempted to use this study to uncover the associations between air pollution and dynamic progression of T2D, rather than just one disease state. In the current study, we observed the significantly adverse associations between air pollution and risk of incident T2D, diabetes complications, and mortality from T2D or diabetes complications. The estimates of incident T2D risk in our analyses were stronger than those reported in previous study, possibly due to the mulit-state model adjusted for competing risk from mortality [25].
Although air pollution is linked to T2D, the association with the dynamic transitions is not clear. Previous studies reported a significantly positive association between ambient air pollutants (PM2.5 and NO2) and risk of cardiovascular disease risk among patients with diabetes, diabetic retinopathy, microalbuminuria, and hospitalization of acute complications [13, 27–29], whereas other studies did not [30, 31]. In the present study, high exposure to PM10, PM2.5, NO2, and NOx was found to be positively associated with risk of any one of diabetes complications from T2D, and the associations were relatively weaker than other transitions.
In line with previous studies, our results showed a positive association between ambient air pollutants and the transition from baseline to death [32]. Previously studies conducted in different countries found a positive association between long-term exposure to air pollution and diabetes mortality [33–36]. Beyond the evidence mentioned above, we hypothesized that air pollution were associated with mortality from T2D and diabetes complications. In the present study, significant positive associations was observed between ambient air pollution exposure and transition stages for all cause mortality and cause-specific mortality (cancer and CVD mortality) from T2D or diabetes complications. Generally, the estimates for the transition from T2D to death were slightly weaker than those for the transition from diabetes complications to death and were attenuated when excluding the mortality, not due to T2D or diabetes complications. This may support evidence that the association of air pollution with diabetes mortality may be explained in large part by the fact that air exposures might increase risk of fatal events in patients with diabetes complications. The differences in our results may be due to several methodological issues. First, previous studies explored the transition from healthy to diabetes mortality. As such, this does not reflect whether air pollution effects were different on the dynamic transition. Second, although diabetes has been found to be a major cause of mortality, most patients with diabetes may die mainly from diabetes complications [37]. Previous studies collected diabetes-associated mortality according to the ICD code for diabetes, which may omit potential premature mortality due to diabetes complications. Lastly, the competing risk from death may lead to a violation of the independent censoring assumption and could alter the risk estimates.
There are some studies elucidating the biological mechanisms of relationship between air pollution and T2D. Both epidemiological studies and animal experiments have shown that exposure to air pollution can induce vascular insulin resistance and metabolic disturbances by elevating pulmonary oxidative stress [38, 39]. Exposure to PM2.5 might involve hypothalamic inflammation via an active sympathetic nervous system [40]. Long-term exposures to PM10 and NO2 were also associated with elevated levels of systematic subclinical inflammation, and adipokines [10]. Moreover, recent studies also found that air pollution was associated with chronic hyperglycemia, dyslipidemia, and blood pressure variation in diabetes patients [41–43]. These factors may trigger the release of inflammatory factors, generate reactive oxygen species, exacerbate abnormalities in endothelial function, and increase the risk of both micro- and macrovascular diseases in diabetes [44–46].
In this study, we observed some notable evidence for effect modification. Our results found stronger associations between air pollution exposure and incidence of T2D among participants residing in a rural area, consistent with previous findings [47]. This may be associated with some specific community types, such as healthy food access and the construction of primary health care.
Although ambient air pollution is considered as a leading risk factor for diabetic morbidity and mortality [48], this cannot reflect the association on the dynamic progression. Our findings provide evidence that ambient air pollution, including particulate matter and nitrogen oxides, is associated with both the incidence and the subsequent progressions of T2D. This suggests ambient air pollution may contribute to both the long-term progression of T2D and the mortality risk of existing disease. In addition, the estimates of air pollution were generally stronger on transition stage from baseline to T2D than in other transition stages, and the associations with diabetes mortality were also noteworthy. Our results inform that more attention should be paid not only to the primary prevention of T2D, but also to the prevention of air pollution after the diagnosis of T2D, so as to potentially mitigate the development of diabetes complications and reduce premature mortality.
The major strengths of our study were a large population-based cohort and the detailed information on socioeconomic, behavioral, and clinical profile data in the UK Biobank, which enabled us to structure a multi-state model of T2D development and to control for potential confounding factors. Compared with traditional Cox regression models, we used the multi-state model to distinguish the impacts of air pollution on the five transition phases. Thus, we were able to detect the sensitive stages of diabetes development and how progression can be affected by the pollution. A series of sensitivity analyses confirmed the robustness of the results.
Our study had some limitations. The participants in the UK Biobank were healthy-volunteer. Second, the outcome identification relied on the ICD-10 codes, and some of the complications are not specific to T2D. The identification of diabetes complications may be open to potential misclassification. Third, some participants were simultaneously diagnosed with diabetes and its complications, which can also lead to outcome misclassification. We then excluded the participants diagnosed with T2D and diabetes complications on the same date, and the associations between air pollution and different progressions of T2D remained significant and positive. Lastly, some information (e.g., work environment) was unavailable from the UK Biobank which would result in exposure misclassifications.
Conclusions
In conclusion, air pollution was differentially positively associated with the progression trajectories of T2D. Reducing air pollution exposure prior to T2D and diabetes complications may also contribute to a favorable longevity of lifetime by reducing the risk of premature mortality. These findings provide the evidence for adhering to public health recommendations to control air pollution.
Supplementary Information
Additional file 1: Details of outcomes. Table S1. The UDI, definition and measurements of covariates. Table S2. Characteristics of the participants included or excluded in the study. Table S3. Distributions of the annual average exposures among 398,993 participants. Table S4. Associations between air pollution and risk of cause-specific mortality. Table S5. Results of 2-pollutant models. Table S6. Results of sensitivity analyses in the model including traffic noise (n = 393,515). Table S7. Results of sensitivity analyses using different time intervals. Table S8. Results of sensitivity analyses after excluding deaths not from diabetes or diabetes complications (n = 380,437). Table S9. Results of sensitivity analyses using complete data (n = 318,019). Table S10. Results excluding the participants diagnosed with T2D and complications on the same date (n = 396,961). Figure S1. Flowchart of participants included in this study. Figure S2. Transitions from baseline to T2D, diabetes complications, and cause-specific mortality. Figure S3. Spearman’s correlation coefficients between air pollutant exposures. Figure S4. Exposure-response associations between air pollution exposure and different transitions of T2D. Figure S5. Effect modifications of the association between PM10 and five transitions of T2D. Figure S6. Effect modifications of the association between PM2.5 and five transitions of T2D. Figure S7. Effect modifications of the association between NOx and five transitions of T2D. Figure S8. Effect modifications of the association between NO2 and five transitions of T2D.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 69550. The authors thank Shuming Zhu for the support in setting up and configuring the high-performance computing resource to conduct this study.
Abbreviations
- CVD
Cardiovascular diseases
- DEFRA
Department for Environment, Food and Rural Affairs
- NO2
Nitrogen dioxide
- NOx
Nitrogen oxide
- PM10
Particulate matter with a diameter ≤10 μm
- PM2.5
Particulate matter with a diameter ≤2.5 μm
- T2D
Type 2 diabetes
Authors’ contributions
YW conceived the idea and contributed to statistical analysis, interpretation of data, and the draft of the manuscript. SZ contributed to the analysis of the data for the work and revised the manuscript. SQ contributed to substantively revised the manuscript. MC contributed to data analysis. HTL contributed to the acquisition of the data. CW, HZ, LC, MV, and SEM contributed to the critical revision of the manuscript. HLL conceived the idea and contributed to the acquisition of the data, supervision, interpretation of the work, and edition of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [grant number: INV-016826]. The funders had no role in the study design or implementation; data collection, management, analysis, data interpretation; manuscript preparation, review, approval, or the decision to submit the manuscript for publication.
Availability of data and materials
The datasets are available upon reasonable request to the Access Management System (AMS) through the UK Biobank website (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The air pollution exposure data are available from DEFRA (https://uk-air.defra.gov.uk/data/pcm-data).
Declarations
Ethics approval and consent to participate
The project has approval from the North West Multi-centre Research Ethics Committee (MREC) (REC reference: 21/NW/0157), and informed written consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinglin Wu and Shiyu Zhang contributed equally to this article.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Vijan S. Type 2 Diabetes. Ann Intern Med. 2019;171(9):ITC65–ITC80. doi: 10.7326/AITC201911050. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Barnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, et al. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res. 2020;180:108817. doi: 10.1016/j.envres.2019.108817. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Cai M, Qian ZM, Wang X, Zhang Z, Wang C, et al. Ambient air pollution, lifestyle, and genetic predisposition associated with type 2 diabetes: findings from a national prospective cohort study. Sci Total Environ. 2022;849:157838. doi: 10.1016/j.scitotenv.2022.157838. [DOI] [PubMed] [Google Scholar]
- 9.Gorini F, Sabatino L, Gaggini M, Chatzianagnostou K, Vassalle C. Oxidative Stress Biomarkers in the Relationship between Type 2 Diabetes and Air Pollution. Antioxidants (Basel). 2021;10(8):1234. [DOI] [PMC free article] [PubMed]
- 10.Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, et al. Association Between Long-term Exposure to Air Pollution and Biomarkers Related to Insulin Resistance, Subclinical Inflammation, and Adipokines. Diabetes. 2016;65(11):3314–3326. doi: 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Al-Kindi SG, Brook RD. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(17):2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 12.Wu MY, Lo WC, Chao CT, Wu MS, Chiang CK. Association between air pollutants and development of chronic kidney disease: A systematic review and meta-analysis. Sci Total Environ. 2020;706:135522. doi: 10.1016/j.scitotenv.2019.135522. [DOI] [PubMed] [Google Scholar]
- 13.Pan SC, Huang CC, Chin WS, Chen BY, Chan CC, Guo YL. Association between air pollution exposure and diabetic retinopathy among diabetics. Environ Res. 2020;181:108960. doi: 10.1016/j.envres.2019.108960. [DOI] [PubMed] [Google Scholar]
- 14.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Air . Modelled background pollution data. London: Department for Environment, Food and Rural Affairs; 2022. [Google Scholar]
- 16.Kim J, Jensen A, Ko S, Raghavan S, Phillips LS, Hung A, et al. Systematic Heritability and Heritability Enrichment Analysis for Diabetes Complications in UK Biobank and ACCORD Studies. Diabetes. 2022;71(5):1137–1148. doi: 10.2337/db21-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Prog Biomed. 2010;99(3):261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Hu Y, Yu C, Guo Y, Pei P, Yang L, et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021;42(34):3374–3384. doi: 10.1093/eurheartj/ehab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh-Manoux A, Fayosse A, Sabia S, Tabak A, Shipley M, Dugravot A, et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: A cohort study. PLoS Med. 2018;15(5):e1002571. [DOI] [PMC free article] [PubMed]
- 21.Liu Y, Pan J, Fan C, Xu R, Wang Y, Xu C, et al. Short-Term Exposure to Ambient Air Pollution and Mortality From Myocardial Infarction. J Am Coll Cardiol. 2021;77(3):271–281. doi: 10.1016/j.jacc.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Kupcikova Z, Fecht D, Ramakrishnan R, Clark C, Cai YS. Road traffic noise and cardiovascular disease risk factors in UK Biobank. Eur Heart J. 2021;42(21):2072–2084. doi: 10.1093/eurheartj/ehab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thacher JD, Poulsen AH, Hvidtfeldt UA, Raaschou-Nielsen O, Brandt J, Geels C, et al. Long-Term Exposure to Transportation Noise and Risk for Type 2 Diabetes in a Nationwide Cohort Study from Denmark. Environ Health Perspect. 2021;129(12):127003. [DOI] [PMC free article] [PubMed]
- 24.Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123(5):381–389. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Wang M, Song Y, Ma H, Zhou T, Liang Z, et al. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: A cohort study in UK Biobank. PLoS Med. 2021;18(8):e1003767. doi: 10.1371/journal.pmed.1003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang MY, Zhou T, Song YZ, Li X, Ma H, Hu YH, et al. Joint exposure to various ambient air pollutants and incident heart failure:a prospective analysis in UK Biobank. Eur Heart J. 2021;42(16):1582–1591. doi: 10.1093/eurheartj/ehaa1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su PF, Sie FC, Yang CT, Mau YL, Kuo S, Ou HT. Association of ambient air pollution with cardiovascular disease risks in people with type 2 diabetes: a Bayesian spatial survival analysis. Environ Health. 2020;19(1):110. doi: 10.1186/s12940-020-00664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin WS, Chang YK, Huang LF, Tsui HC, Hsu CC, Guo YLL. Effects of long-term exposure to CO and PM2.5 on microalbuminuria in type 2 diabetes. Int J Hyg Environ Health. 2018;221(4):602–608. doi: 10.1016/j.ijheh.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Dales RE, Cakmak S, Vidal CB, Rubio MA. Air pollution and hospitalization for acute complications of diabetes in Chile. Environ Int. 2012;46:1–5. doi: 10.1016/j.envint.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Herder C, Schneider A, Zhang S, Wolf K, Maalmi H, Huth C, et al. Association of Long-Term Air Pollution with Prevalence and Incidence of Distal Sensorimotor Polyneuropathy: KORA F4/FF4 Study. Environ Health Perspect. 2020;128(12):127013. doi: 10.1289/EHP7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Song X, Han Y, Ji Y, Gao S, Shang Y, et al. Size-fractioned ultrafine particles and black carbon associated with autonomic dysfunction in subjects with diabetes or impaired glucose tolerance in Shanghai, China. Part Fibre Toxicol. 2015;12:8. doi: 10.1186/s12989-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orellano P, Reynoso J, Quaranta N, Bardach A, Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int. 2020;142:105876. doi: 10.1016/j.envint.2020.105876. [DOI] [PubMed] [Google Scholar]
- 33.Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36(10):3313–3320. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raaschou-Nielsen O, Sorensen M, Ketzel M, Hertel O, Loft S, Tjonneland A, et al. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: a cohort study. Diabetologia. 2013;56(1):36–46. doi: 10.1007/s00125-012-2698-7. [DOI] [PubMed] [Google Scholar]
- 35.Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, et al. Association between long-term exposure to ambient air pollution and diabetes mortality in the US. Environ Res. 2018;165:330–336. doi: 10.1016/j.envres.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan A, Zhang Y, Zhang LW, Chen X, Li X, Wu H, et al. Associations between the incidence and mortality rates of type 2 diabetes mellitus and long-term exposure to ambient air pollution: A 12-year cohort study in northern China. Environ Res. 2020;186:109551. doi: 10.1016/j.envres.2020.109551. [DOI] [PubMed] [Google Scholar]
- 37.Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020;162:108086. doi: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 38.Dang J, Yang M, Zhang X, Ruan H, Qin G, Fu J, et al. Associations of Exposure to Air Pollution with Insulin Resistance: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2018;15(11):2593. [DOI] [PMC free article] [PubMed]
- 39.Haberzettl P, O'Toole TE, Bhatnagar A, Conklin DJ. Exposure to Fine Particulate Air Pollution Causes Vascular Insulin Resistance by Inducing Pulmonary Oxidative Stress. Environ Health Perspect. 2016;124(12):1830–1839. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, et al. Long-Term Exposure to Concentrated Ambient PM2.5 Increases Mouse Blood Pressure through Abnormal Activation of the Sympathetic Nervous System: A Role for Hypothalamic Inflammation. Environ Health Perspect. 2014;122(1):79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Zheng S, Nie Y, Weng J, Cheng N, Hu X, et al. Association between Short-Term Exposure to Air Pollution and Dyslipidemias among Type 2 Diabetic Patients in Northwest China: A Population-Based Study. Int J Environ Res Public Health. 2018;15(4):631. [DOI] [PMC free article] [PubMed]
- 42.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut. 2018;235:576–588. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Wang JB, Yu ZB, Lin HB, Chen K. Air pollution exposures and blood pressure variation in type-2 diabetes mellitus patients: A retrospective cohort study in China. Ecotox Environ Safe. 2019;171:206–210. doi: 10.1016/j.ecoenv.2018.12.069. [DOI] [PubMed] [Google Scholar]
- 44.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539–1549. doi: 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diab Endocrinol. 2019;7(12):938–948. doi: 10.1016/S2213-8587(19)30081-6. [DOI] [PubMed] [Google Scholar]
- 47.McAlexander TP, De Silva SSA, Meeker MA, Long DL, McClure LA. Evaluation of associations between estimates of particulate matter exposure and new onset type 2 diabetes in the REGARDS cohort. J Expo Sci Environ Epidemiol. 2022;32(4):563–570. doi: 10.1038/s41370-021-00391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Wang B, Liu S, Li S, Zhang K, Luo B, et al. Type 2 diabetes attributable to PM2.5: A global burden study from 1990 to 2019. Environ Int. 2021;156:106725. doi: 10.1016/j.envint.2021.106725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Details of outcomes. Table S1. The UDI, definition and measurements of covariates. Table S2. Characteristics of the participants included or excluded in the study. Table S3. Distributions of the annual average exposures among 398,993 participants. Table S4. Associations between air pollution and risk of cause-specific mortality. Table S5. Results of 2-pollutant models. Table S6. Results of sensitivity analyses in the model including traffic noise (n = 393,515). Table S7. Results of sensitivity analyses using different time intervals. Table S8. Results of sensitivity analyses after excluding deaths not from diabetes or diabetes complications (n = 380,437). Table S9. Results of sensitivity analyses using complete data (n = 318,019). Table S10. Results excluding the participants diagnosed with T2D and complications on the same date (n = 396,961). Figure S1. Flowchart of participants included in this study. Figure S2. Transitions from baseline to T2D, diabetes complications, and cause-specific mortality. Figure S3. Spearman’s correlation coefficients between air pollutant exposures. Figure S4. Exposure-response associations between air pollution exposure and different transitions of T2D. Figure S5. Effect modifications of the association between PM10 and five transitions of T2D. Figure S6. Effect modifications of the association between PM2.5 and five transitions of T2D. Figure S7. Effect modifications of the association between NOx and five transitions of T2D. Figure S8. Effect modifications of the association between NO2 and five transitions of T2D.
Data Availability Statement
The datasets are available upon reasonable request to the Access Management System (AMS) through the UK Biobank website (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The air pollution exposure data are available from DEFRA (https://uk-air.defra.gov.uk/data/pcm-data).