Abstract
Coronavirus disease 2019 (COVID-19) is a multi-system disease that has led to a pandemic with unprecedented ramifications. The pandemic has challenged scientists for the past 2 years and brought back previously abandoned research topics. COVID-19 infection causes a myriad of symptoms ranging from mild flu-like symptoms to severe illness requiring hospitalization. Case reports showed multiple systemic effects of COVID-19 infection, including acute respiratory distress syndrome, fibrosis, colitis, thyroiditis, demyelinating syndromes, and mania, indicating that COVID-19 can affect most human body systems. Unsurprisingly, a major concern for women all over the globe is whether a COVID-19 infection has any long-term effects on their menstrual cycle, fertility, or pregnancy. Published data have suggested an effect on the reproductive health, and we hypothesize that the reported reproductive adverse effects are due to the robust immune reaction against COVID-19 and the associated cytokine storm. While the COVID-19 receptor (angiotensin converting enzyme, ACE2) is expressed in the ovaries, uterus, vagina, and placenta, we hypothesize that it plays a less important role in the adverse effects on the reproductive system. Cytokines and glucocorticoids act on the hypothalamo–pituitary gonadal axis, arachidonic acid pathways, and the uterus, which leads to menstrual disturbances and pregnancy-related adverse events such as preterm labor and miscarriages. This hypothesis is further supported by the apparent lack of long-term effects on the reproductive health in females, indicating that when the cytokine storm and its effects are dampened, the reproductive health of women is no longer affected.
Keywords: COVID-19, fertility, menstrual cycle, pregnancy, reproductive health
COVID-19 affects the female reproductive system.
Introduction
According to the World Health Organization, around 418 million cases of COVID-19 infection have been reported with more than 5 million deaths as of February 2022. The most common symptoms of COVID-19 were a flu-like illness including fever, chills, cough, shortness of breath, fatigue, headache, loss of smell, nausea, and diarrhea [1]. However, several case reports have been published on multiple systemic effects of COVID-19, indicating that COVID-19 can affect many systems and organs in the human body [1]. The rapid spread of the COVID-19 infection raised several questions about the effect of the viral infection on women’s reproductive health with particular concern on its effect on fertility and pregnancy.
There was anecdotal evidence of women reporting menstrual cycle irregularity after their COVID-19 infection. Unfortunately, menstruation is an understudied research topic, and there is a gap in published research about menstrual changes after COVID-19 infection [1]. Recent articles highlighted this effect with a recent study showing that 16% of women reported menstrual disturbances after their infection [2]. Another emerging burden that was studied is the increased pregnancy adverse events associated with COVID-19 infection [3]. A recent meta-analysis concluded that COVID-19 infection was associated with preterm birth, stillbirth, and lower birth weight [3].
Sex differences in disease prevalence, pathogenesis, and modulation have been frequently reported [4], and the fluctuation of hormones throughout the menstrual cycle and pregnancy are potential reasons for the sex differences. This has been studied in chronic autoimmune diseases that are more common in women such as rheumatoid arthritis and systemic lupus erythematosus. Moreover, sex differences were noted in immune response to multiple respiratory viruses. For example females of reproductive age mount a greater cytokine storm after influenza infection, and thus take longer to recover from the infection [5]. These fluctuations might also have implications on progression of acute diseases such as COVID-19 infection. While the effect of female sex hormones on the immune system are fairly understood, the reciprocal relationship is less understood.
In this review paper, we will analyze the relationship between the female reproductive system and the immune system, focusing on the effects of acute immune responses associated with COVID-19 infection on the reproductive system. We will build on the current evidence on this relationship to extrapolate from the possible mechanisms by which COVID-19 infection impacts the menstrual cycle, pregnancy, and fertility in COVID-19 infected female patients.
Search methods
A comprehensive search of PubMed up to May 2022 was conducted to identify peer-reviewed literature. We used the following keywords: COVID-19, reproductive health, pregnancy, pregnant, menstrual cycle, menstrual disturbances, fertility, COVID-19 vaccine, immune, immune reaction, and immunity. We only included articles in the English language. This review included 83 selected studies, conducted in both humans and animals.
The immune response against COVID-19 infection
COVID-19 is a single-stranded positive sense RNA virus that first infects the pulmonary epithelial cells by the binding of its viral S glycoprotein to its receptor, the angiotensin converting enzyme (ACE2), on these cells [6]. The pulmonary epithelial cells are the first cell type to initiate the innate immune response against COVID-19 by secreting interleukin 8 (IL-8) and attracting neutrophils [6–8]. The immune cells (neutrophils and macrophages) activate several pathways to initiate the immune response, one of which is the Janus kinase-signal transducer and activator of transcription pathway (JAK–STAT). In COVID-19 infection, JAK–STAT pathway induces the transcription of nuclear factor kappa B (NF-κB), among other factors which enhance the production of proinflammatory cytokines, including interleukins (IL-1, IL-6), monocyte chemo-attractant protein-1 (MCP-1), tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1A, and type 1 interferon (IFN) [9]. Recent studies have shown that increased disease severity has been associated with greater cytokine storm with increased levels of IL-1 and IL-6 and decreased levels of type 1 IFN [6, 10].
Like other viral infections and stressful situations to the body, the hypothalamic–pituitary–adrenal (HPA) axis is the first neuroendocrine axis to be affected [11]. Although hypothalamic corticotropin-releasing hormone (CRH) is considered a primary mechanism by which cytokines stimulate glucocorticoid release, cytokines have a direct action at the level of the pituitary and adrenal glands as well [11]. These cytokines are also produced in the brain, anterior pituitary gland, and the adrenals [11]. IL-1, IL-6, and TNF-α are lipid soluble; they can cross the blood-brain barrier and induce the production of the CRH from the hypothalamus [11]. Moreover, they influence the release of arginine vasopressin which is a peptide that acts synergistically with CRH to increase the production of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, thus leading to increased glucocorticoid production [11]. While these cytokines exert their effects on the HPA axis mainly through the hypothalamus, they can directly enhance the activity of the anterior pituitary gland and the adrenals [11]. In some in vitro studies, IL-1 has been shown to induce proopiomelanocortin transcription and directly stimulate ACTH release from the pituitary gland. Also, IL-1 has been shown to directly act on adrenal cortex (zona glomerulosa) and medulla to release glucocorticoids [11]. The net effect of these cytokines on the HPA axis is the increased production of glucocorticoids as a feedback mechanism to reduce the cytokine storm. In many cases, the overwhelming cytokine storm and the debilitating state of inflammation are the direct cause of death in COVID-19 patients rather than the virus itself [12]. So, increased glucocorticoid production is a protective mechanism produced by the host to decrease morbidity and mortality. However, it is at the price of the glucocorticoid systemic symptoms, including their effect on the reproductive system. Glucocorticoids are known to have systemic symptoms that are very well known from studies of Cushing syndrome and adrenal hyperplasia and adenomas, which are known to affect the reproductive system [13] such as causing anovulatory infertility.
Interplay between the menstrual cycle and the immune system
Effect of estrogen and progesterone on immune cells
The hormonal fluctuation of estrogen and progesterone during the menstrual cycle affects the immune system in multiple ways. This has been observed in studies of autoimmune disease progression throughout the reproductive milestones of women and throughout the menstrual cycle itself [4]. While progesterone receptors are mainly found on CD4+ lymphocytes, natural killer (NK) cells, dendritic cells, and macrophages; estrogen receptors (ERs) are found on essentially all immune cells [4, 14]. The type of ERs present on the different immune cells influences the effect of estrogen on these cells [14].
The menstrual cycle is divided into four phases: the menstrual phase, follicular phase, ovulation phase, and luteal phases. The follicular phase has an estrogen dominance which induces the proliferation of the endometrium after menstrual shedding and the expression of progesterone receptors [15]. The luteal phase occurs after ovulation and has high estrogen and progesterone levels which induces the decidualization of the endometrium in preparation for pregnancy [15]. Generally, progesterone has anti-inflammatory effects, while estrogen has bipotential effects [14]. At relatively low concentrations (i.e., follicular phase), estrogen has pro-inflammatory effects, such as increasing neutrophil count, enhancing production of pro-inflammatory cytokines, increasing the expression of pattern recognition receptors, and increasing the somatic hypermutation and class switch recombination in B-cells [14]. At higher concentrations (i.e., luteal phase or pregnancy), estrogen has anti-inflammatory effects including downregulation of proinflammatory cytokines and expansion of T regulatory (Treg) cell population [14]. On the other hand, progesterone decreases proinflammatory cytokine production and suppresses T helper1 (Th1) response [14]. Moreover, high levels of estrogen and progesterone are known to induce T helper 2 (Th2) response [4]. Th1 cells are a lineage of CD4+ effector T cell that stimulates cell-mediated immune responses and is necessary for host defense against viral infections [16]. Th1 cells secrete important cytokines such IFN- γ, IL-2, IL-10, and TNF-α [16]. Thus, the menstrual and early follicular phases are characterized by a Th1-predominant cellular and cytokine milieu and the late follicular and mid to late luteal phase by a Th2 milieu [4].
Estrogen receptors specifically disrupt NF-κB transactivation, thus inhibiting IL-6 expression, one of the most important cytokines in the COVID-19 related cytokine storm [17]. So, estrogen may be a protective factor against multiple infections, including COVID-19, and this explains the more robust immune response against COVID-19 in females and the increased rate of mortality from COVID-19 in men [18]. Moreover, premenopausal women were reported to have milder COVID-19 symptoms and less complications than their male cohorts and post-menopausal women, which is mainly due to the sex hormones estrogen and anti-Mullerian hormone (AMH) [19]. There is no correlation between the AMH levels and COVID-19 infection severity; however, there is a negative correlation between the estrogen levels and the inflammatory cytokines levels of IL-6, IL-8, IL-2R, and TNF-α in the luteal phase and C3 in the follicular phase [19]. This might be a protective mechanism in females against the cytokine storm and the complications of COVID-19 infection that leads to acute respiratory distress syndrome [19].
Local role of immune cells in the endometrium
The menstrual cycle is an inflammatory process [20]. Immune cells are present in the endometrium, and their concentrations change across the menstrual cycle [21]. It is unclear yet whether endometrial leukocytes migrate from peripheral blood or if they proliferate locally, and it is probable that both processes occur [22]. A highly active cytokine network is present in the endometrium, and it can be involved in recruiting and/or activating leukocyte subtypes in the endometrium [23]. There is also evidence of local upregulation of IL8 and macrophage-derived chemokine in blood vessels prior to menstruation suggesting that these cytokines can trigger neutrophils influx to the endometrium [23]. For instance, while the total number of cells increase in the proliferative phase, the percentage of leukocyte increases significantly in the secretory phase of the menstrual cycle [21].
Endometrial leukocytes are mainly NK cells, neutrophils, and macrophages that are thought to protect from microbial invasion in states of epithelial barrier disruption (secretory phase) [21]. B-lymphocytes increase significantly in this phase indicating that the endometrium exhibits innate and adaptive immunological activation [21]. On the other hand, T-lymphocytes activity decreases in the secretory phase, and increases (specifically CD8+) in the early to mid-proliferative phase, and this is regulated by progesterone levels [21]. CD8+ activation is important in the early proliferative phase for clearance of residual endometrial debris after menstruation [21]. Moreover, endometrial NK cells are the most abundant immune cells in the endometrium throughout the menstrual cycle and their concentration increases significantly in the secretory phase [21].
Given the above, endometrial immune cells dysregulation may lead to abnormal uterine bleeding [20]. For example, a recent study has found that heavy menstrual bleeding is associated with increased uterine NK cells in the proliferative and early secretory phase and decreased uterine NK cells in the mid secretory phase when compared to the control group [20]. This suggests that endometrial leukocytes may impact the endometrial vascular development, hence causing abnormal uterine bleeding [20]. So far, ovarian hormone receptors have not been identified on the endometrial leukocytes; however, steroid hormones appear to have indirect impact on the endometrial immune cells that need further research in the future [22, 24]. Thus, we hypothesize that the immune response-mediated change in cytokines and leukocytes milieu in the endometrium and can explain the heavy menstrual cycles after COVID-19 infection.
COVID-19 infection and the menstrual cycle
Menstrual disturbances
The current evidence on the effect of COVID-19 infection on menstrual cycle is scarce. A recent study showed that out of 177 COVID-19 positive patients (with complete menstrual history records), 132 (75%) had no change in the menstrual volume, while 20% had a significant decrease in menstrual volume and only 9% had an increase in the menstrual volume [25]. There was no significant difference between the mildly ill patients and severely ill patients when it came to menstrual volume; however, patients who were severely ill had longer menstrual cycles [25]. These clinical findings were not backed up by hormonal changes [25]. Another study showed that 16% of women reported menstrual disturbances after COVID-19 infection [2]. Multiple studies have shown that there is no significant difference in the average of sex hormones (including follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, testosterone and AMH), between COVID-19 patients and control patients, or between mild and severe patients, or even between the patients with menstrual changes and those with no menstrual changes [19, 25]. This indicates that the menstrual changes due to COVID-19 infection are thought to be transient and with no reported long-term consequences. Immune response mediated stress may be the main cause behind these temporary changes in the menstrual cycle. This is possible due to the interaction between the HPA and the hypothalamo-pituitary gonadal (HPG) axis.
The interaction between the HPA and HPG axes affecting the menstrual cycle
We hypothesize that the interaction between the HPA axis and the HPG axis can be behind the COVID-19 infection and the menstrual cycle irregularities. Stress has an inhibitory effect on reproduction, and these inhibitory effects are likely the result of disruption of gonadotropin secretion after changes in gonadotropin-releasing hormone (GnRH) output.
Glucocorticoids, the end-organ product of HPA axis activation, typically suppress gonadotropin secretion [26], but outcomes are variable, depending on multiple factors. Glucocorticoid-induced inhibition of the HPG axis is modulated by the effects of the ovarian hormones, estrogen and progesterone [26]. In ovariectomized female rats with low physiological estradiol replacement, both acute and chronic treatment with corticosteroids fail to alter LH pulse frequency or amplitude [25–27], whereas chronic corticosteroid treatment suppressed LH pulse frequency in ovariectomized mice with estrogen supplements suggesting that ovarian hormones influence the action of corticosteroids on the HPA axis [26].
Female hormones also influence the secretion of cortisol under physiological conditions. A recent meta-analysis evaluated the peripheral cortisol levels in normal menstruating women; analysis from 35 studies showed that women during the follicular phase have higher cortisol levels than in the luteal phase [28]. Allopregnanolone, which is a progesterone derivative, is a potent allosteric positive modulator of the actions of the inhibitory neurotransmitter GABA at the GABAA receptor. The exacerbated action of GABA at GABAA receptor leads to inhibition of the paraventricular nucleus (PVN) and in return an attenuated HPA axis [28]. On the other hand, estrogen binds to either estrogen receptor beta (ER-β) or estrogen receptor alpha (ER-α), each of which relay a different function [28]. Binding of estrogen to ER-β in the para-ventricular nucleus leads to decreased cortisol levels, while binding of estrogen to ER-α indirectly activates the PVN, and binding of estrogen to the ER-α receptors in peri-PVN region can impair glucocorticoid-mediated negative feedback regulation of the HPA axis, resulting in increased cortisol synthesis [28]. Hence, higher cortisol levels are recorded in the follicular phase than in the luteal phase. In the luteal phase, depending on the extent of ER-β or ER-α expression and activation in or near the PVN, estradiol can either decrease or increase circulating cortisol levels [28].
The extent to which glucocorticoids will influence the menstrual cycle will depend on the timing of the COVID-19 infection during the menstrual cycle. Given our understanding of the effect of estrogen of glucocorticoids, it can be hypothesized that those who are infected in the follicular phase are more likely to potentiate the glucocorticoids effects and lead to more suppression of the GnRH and of the HPG axis. This, however, needs further studies that keep record of the patient menstrual changes in accordance with the timing of infection in the menstrual cycle.
In addition to suppressing the release of GnRH, glucocorticoids suppress the response to GnRH at the pituitary level [26]. In ovariectomized ewes, GnRH pulse amplitude and frequency were not suppressed, most probably due to the lack of estrogen modulation, but LH pulse amplitude was suppressed indicating that corticosteroids also act at lower levels, and not just at the hypothalamus level [26, 29].
Intermediates in the HPA axis are also involved in the suppression of HPG axis in conditions of acute stress like COVID-19 infection. Chemically stimulating CRH neurons in the PVN resulted in suppression of LH pulse frequency in ovariectomized mice [30]. The effects of this chemical stimulation are not only due to glucocorticoid synthesis because intravenous injection of CRH in adrenalectomized monkeys, which are unable to produce adrenal glucocorticoids, was still able to inhibit LH pulse frequency and amplitude. [31]. This indicates that CRH itself can act on the reproductive system independent of glucocorticoids. CRH acts centrally to modulate the pituitary function. Treatment with nonspecific CRHR antagonists prevents suppression of LH pulse frequency caused by interleukin-1α (IL-1α) in ovariectomized monkeys [32], confirming that CRH directly affects LH levels and that the activation of CHRH2 or CHRH1 can alter secretion of LH independent of the HPA axis [26].
We hypothesize that COVID-19 infection generates a cytokine storm that up alters the HPA axis on every level, increasing CRH, ACTH, and glucocorticoids. CRH and glucocorticoids are especially involved in suppressing the HPG axis at different levels, leading to overall a decrease in the LH pulse amplitude or frequency, resulting in anovulatory cycles and menstrual disturbances. These effects are suspected to subside when the cytokine storm resolves and when the intermediates and final product of HPA axis (glucocorticoids), go back to physiological concentrations (Figure 1). Heavy menstrual bleeding post COVID-19 infection could also be explained by the cytokine storm altering the leukocyte milieu of the endometrium.
Figure 1.
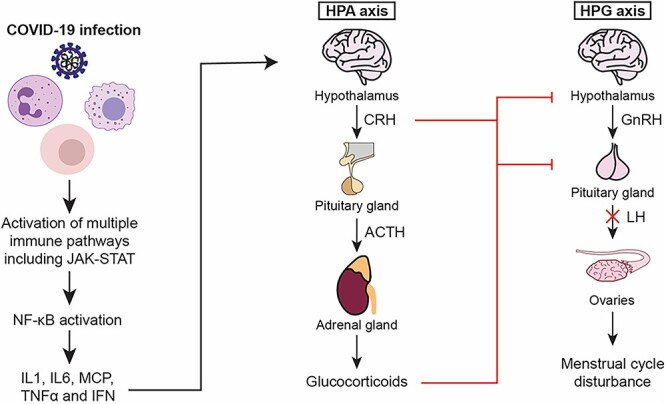
The effect of the cytokine storm on the menstrual cycle. The increased levels of cytokines, specifically cytokine IL-1, act at different levels of the HPA axis activating the hypothalamus, pituitary gland, and adrenal gland to increase the production of glucocorticoids. Glucocorticoids, modulated by progesterone and estrogen, inhibit the secretion of GnRH by the hypothalamus altering secretion of LH by the pituitary gland, thus delaying or inhibiting ovulation.
COVID-19 vaccine and the menstrual cycle
Although a major concern for women on reproductive age, very few studies have since assessed the relationship between COVID-19 vaccination and menstrual disturbances. Currently, the menstrual disturbances associated with COVID-19 vaccination appear to be temporary and resolve within two cycles [33, 34]. Among the most reported menstrual disturbances are heavier bleeding, prolonged bleeding, and/or shorter inter-menstrual intervals [35]. A Norwegian study collected information from 5688 women aged between 18 and 30 years using a mobile phone application found a significant increase in menstrual disturbances after COVID-19 vaccination [35]. The prevalence of heavy menstrual bleeding almost doubled after COVID-19 vaccination when compared to pre-vaccination rates. The prevalence of menstrual disturbances increased from 7.6% to 13.6% after the first dose and from 8.2% to 15.3% after the second COVID-19 vaccine dose [35]. Similarly, another US-based study included 3959 women between the age of 18 and 45 years and assessed the prevalence of menstrual disturbances using Natural Cycles mobile application [34]. In this study, vaccination increased the length of the menstrual cycle by 0.64 and 0.79 days after the first and second doses, respectively [34]. Interestingly, more substantial changes were observed in those who received two COVID-19 vaccine doses within the same menstrual cycle, with 2 days increase in the menstrual cycle length [34]. Women who experienced menstrual disturbances after the first dose were at higher risk of developing more severe symptoms after the second dose. However, the prevalence of menstrual disturbances after the second dose in those who did not experience any after the first dose was almost equal to before vaccination [35]. The above findings, especially the transience of the symptoms and the more robust symptoms when both doses are administered close in time, support our theory that the menstrual changes may be due to the immunologic reaction and transient cytokine storm against COVID-19, and will resolve after the cytokine storm and its effect on the HPG axis subsides. More prospective studies are needed to assess the link between COVID-19 vaccine on menstrual health and to confirm the effect of two vaccinations within one menstrual cycle, which can potentially change the timing of vaccination for women of reproductive age. Moreover, more studies are needed to compare the effects of the different types of COVID-19 vaccines on the menstrual cycle. Four types of COVID-19 vaccines are available until now: the messenger RNA (m-RNA) vaccines (e.g., Pfizer-BioNTech and Moderna), the vector vaccines (Janssen/Johnson & Johnson and AstraZeneca), the protein subunit vaccine (Novavax), and the inactivated virus vaccine (e.g., Sinovac) [36]. Due to the few studies available in the literature that assesses the effect of the COVID-19 vaccine on the menstrual cycle, not enough data are available to compare the different types of vaccines. However, menstrual disturbances were observed in all vaccine types, and increased women’s age, smoking, second vaccine dose, and history of pregnancy were predictors of menstrual disturbances after COVID-19 vaccination [37].
COVID-19 and the placenta
The effect of COVID-19 infection on the placenta is still not well understood. For COVID-19 to infect and replicate in target cells, the spike protein should be able to bind to its ACE-2 receptor on the cell surface. S protein is then primed by cellular transmembrane serine protease 2 (TMPRSS2) that allows the fusion of the virus with host cellular membranes [38]. The co-expression of ACE-2 and TMPRSS2 in a cell makes it more susceptible to COVID-19 infection. It was shown that developing embryos co-express ACE-2 and TMPRSS2 due to co-localization of these two genes, which suggests the possibility of fetal infection by COVID-19 [38]. On the other hand, it has been found that both placental cytotrophoblasts and syncytiotrophoblasts express ACE-2 starting 7 weeks of gestation [39], but only few cells in the placenta co-express ACE-2 and TMPRSS2, suggesting that COVID-19 infection of the placenta is low or that the virus uses other host proteins to infect placental cells [40].
Multiple studies on placentas derived from pregnant women who were infected with COVID-19 failed to detect COVID-19 infection in the placenta most of the time [41, 42]. This nonappearance of placental infection can be explained by the lack of the co-expression of all the factors needed for proper infection and replication. Given the above, the general understanding leans toward a scarce chance of COVID-19 infecting the placenta, thus decreasing the probability of vertical transmission as well. Theoretically, vertical transmission can still occur. The cytokine storm in COVID-19 infection might alter the maternal–fetal interface and make it more permeable to pathogens, which leads to fetal COVID-19 infection [43]. A recent systemic review of the available literature found that vertical transmission of COVID-19 is possible, but it is a rare incidence [44]. While 1.8% of the fetuses born to COVID-19 positive mothers tested positive for COVID-19 infection, less than 0.001% had confirmed maternal–fetal transmission of COVID-19 infection when combining data on the timing of exposure to COVID-19 [44]. The decidua plays an important role in protecting the fetus against viral infections, and increased levels of NK cells and macrophages were detected in the decidua of COVID-19 positive pregnant mothers, suggesting that the decidual immune reaction may be protect against the maternal-fetal transmission of COVID-19 [45]. Another possible protective mechanism against COVID-19 infection of the placenta is the expression of IFN-induced transmembrane (IFITM) antiviral transcripts. IFITM are proteins that are expressed in epithelial cells including placental cells and that are known to restrict the replication of many viruses including influenza, SARS-CoV-1, flavivirus, and many other enveloped viruses [46]. IFITM3 was recently found to inhibit the replication of COVID-19 as well [46]. IFITM3 can also inhibit the membrane fusion of enveloped viruses, which is a unique feature that is not observed in other IFN-inducible proteins, but IFITM activity may be attenuated by the expression of TMPRSS2 [46]. Interestingly, the levels of TMPRSS were not significantly elevated in patients with severe COVID-19 infection when compared to mild/moderate COVID-19 infection [46]. However, the placental expression of IFITM1 and IFITM3 were found to be higher in placental cells of patients with severe COVID-19 infection indicating that these proteins may play a role in protecting the placenta from COVID-19 infection in severe COVID-19 infection [46].
On the other hand, ex vivo studies of the placenta recently showed that COVID-19 can infect and propagate in the human placental cells [47]. Infecting precision–cut slices of placenta with COVID-19 leads to an increase of viral release from these cells indicating the ability of COVID-19 to propagate and replicate in the placenta with possible propagation to surrounding tissues [47]. Nevertheless, nonspecific placental histopathological changes have been detected in pregnant patients with COVID-19 infection. These changes might be explained by the cytokine storm in adult patients infected with COVID-19 infection or by placental ischemia that results from maternal hypoxia [48]. Acute chorioamnionitis was relatively common and detected in 26% of the placentas of COVID-19 patients [48]. Moreover, chronic histiocytic intervillositis and massive fibrin deposition detection rates were comparable to non-COVID-19 placentas indicating that these are not specific findings to COVID-19 infection [48]. The most likely mechanism is that the disease severity in COVID-19 patients (thus the hypoxia and extent of the cytokine storm) is associated with the ischemic placental pathologies and placental insufficiency [49]. Given all the above, a recent article was published with a standardized definition and classification of placental infection by COVID-19 to make it easier to compare results across studies and resolve the controversy around placental infection by COVID-19 [50]. According to their recommendations, placental infection can be reported as either definite (documentation and localization), probable (documentation of viral proteins or RNA without evidence of replication), possible (detection of viral particles), unlikely (no detection), and not tested [50].
COVID-19 and pregnancy outcomes
While ACE2 is expressed in the uterus, the accumulating evidence suggests that the COVID-19 virus does not cross the placenta, and it is not vertically transmitted [51]. Nevertheless, COVID-19 infections have been associated with multiple pregnancy adverse outcomes [51]. A recent meta-analysis concluded that COVID-19 infection was associated with preterm birth, stillbirth, and lower birth weight but not with increased cesarean delivery [3]. Another meta-analysis found that COVID-19 infection in pregnancy has been associated with increased risk of preterm delivery, post-partum hemorrhage, and low birth weight [52]. In this paper, COVID-19 infection was also associated with increased risk of cesarean sections. Many of these adverse events can be explained by the increased inflammatory state during COVID-19 infections.
Inflammation and pregnancy
The inflammatory state alternates throughout pregnancy to serve both the mother and the fetus [53] (Figure 2). Embryo implantation elicits an inflammatory reaction in the endometrium, where upregulation of multiple cytokines including IL-1, IL-6, IL-8, and TNF-αis evident [53]. The implantation process in pregnancy requires a strong inflammatory response. The blastocyst implants by breaking through the endometrial lining. Implantation is followed by invading of the endometrial tissue to pave the way for the trophoblast to replace the endometrium. This allows the establishment of the placental–fetal blood supply [53]. All these stages lead to an active inflammatory stage to repair the uterine epithelium and to remove any cellular debris [53]. However, postimplantation, decidual stromal cells dampen the immune response in the endometrium for the pregnancy to proceed [54]. For example, decidual stromal cells silence cytokines like CXCL9 and CXCL10, which are responsible for recruiting cytotoxic T cells [54]. The dampened immune reaction is an important step for the maintenance of pregnancy because the semi-allogenic fetus needs to be tolerated by the mother [55].
Figure 2.
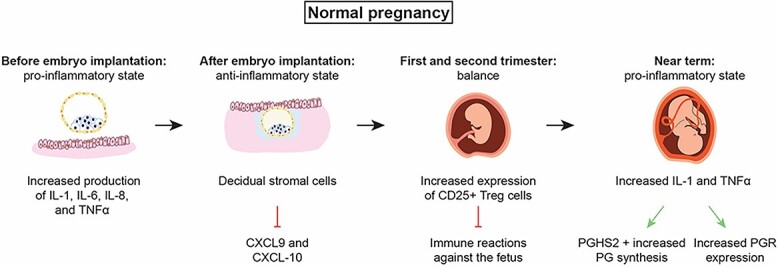
The inflammatory states in normal pregnancy. For the embryo to implant, a pro-inflammatory milieu is achieved by increased expression of inflammatory cytokines. After that, decidualization takes place, and decidual stromal cells inhibit multiple proinflammatory cytokines including CXCL9 and CXCL10, which usually recruit cytotoxic T-cells and protect against the semi-allogenic fetus. By the end of pregnancy, cytokines expression increases again to stimulate uterine activation proteins including prostaglandin receptors, oxytocin and its receptor, connexin43, and iNOS (not shown in the diagram).
One mechanism in which trophoblasts evade recognition by the maternal immune system is the downregulation of the polymorphic HLA-A, HLA- B, and HLA class II molecules on their cell surface [55]. However, extravillous trophoblasts express HLA-C, HLA-E, and HLA-G molecules. Of these molecules, only HLA-C is polymorphic and is thus the primary candidate to attract an antigen-specific response by CD8+ T cells [56]. This can elicit an Major histocompatibility complex/human leucocyte antigen-C specific response that is controlled by the induction CD4+ CD25+ T-regs [56]. The expansion of T-reg cells during pregnancy is an important protective mechanism to prevent a detrimental immune reaction against the fetus [55]. Injecting abortion prone mice with CD25+ T-regs from wild-type pregnant mice led to a significantly increased litter size [57]. Alternatively, depleting CD25+ T-regs during the implantation period of mice caused high fetal resorption [58]. Moreover, a recent systematic review of 17 studies on human pregnancy showed that the number and functionality of T-regs are diminished in women experiencing recurrent pregnancy loss [59]. Similarly, there is decreased T-reg numbers in both the periphery and the decidua and impairment in the signaling of peripheral blood T-regs in women with pre-eclampsia, a serious pregnancy complication [55].
Toward the end of the pregnancy, a proinflammatory state develops again. One of the most important mediators of parturition is prostaglandins (PGs) [60]. Prostaglandins are formed when arachidonic acid is released from the plasma membrane by phospholipases and metabolized by the actions of prostaglandin H synthase (PGHS) [60]. Two isoforms of the PGHS enzyme exist: PGHS-1 that is expressed in many tissues and is responsible for constitutive PG production and PGHS-2 that is an inducible form of the enzyme. The increase in PG production prior to parturition is largely due to increased PGHS-2 [61]. Several cytokines, including IL-1 beta and TNF-α, regulate and stimulate PGHS-2 expression and prostaglandin synthesis [62, 63]. PGHS-2 is one of the many other proteins that are called uterine activation proteins (UAPs), which mediate parturition. Proinflammatory cytokines also stimulate prostaglandin receptor expression, along with other UAPs such as oxytocin and its receptor (OT/OTR), connexin-43 (CX43), inducible nitric oxide synthase (iNOS), and prostaglandin receptor (PTGFR) [61, 64].
Preterm delivery in COVID-19
Preterm delivery has been documented as one of the adverse events of pregnancy in COVID-19 infected pregnant patients [3], and 83% of preterm deliveries were medically indicated for conditions such as preeclampsia, HELLP syndrome, or eclampsia [65]. However, even in the absence of any medical complications, the gestational age was reported to be 0.6 weeks shorter at time of delivery in all women with a COVID-19 diagnosis and 0.8 weeks shorter in symptomatic women with a COVID-19 diagnosis than in women without a COVID-19 diagnosis [65]. As explained above, the pregnancy is highly regulated by alternating pro-inflammatory and anti-inflammatory states. The cytokine storm in COVID-19 infections can create a pro-inflammatory state with increased corticosteroids production, which may have a role in labor initiation. In humans, glucocorticoids induce expression of aromatase and CRH in the placenta and induce expression of prostaglandin synthetic enzymes but downregulate expression of 15-hydroxyprostaglandin dehydrogenase (PGDH), an enzyme that is involved in the catabolism of PGs. All of which drives the pro-parturition processes [66] (Figure 3). This pathway has not been studied in viral infections, but we hypothesize that it can possibly be involved in preterm labor in those infected with COVID-19, but more research needs to be done to elucidate this hypothesis.
Figure 3.
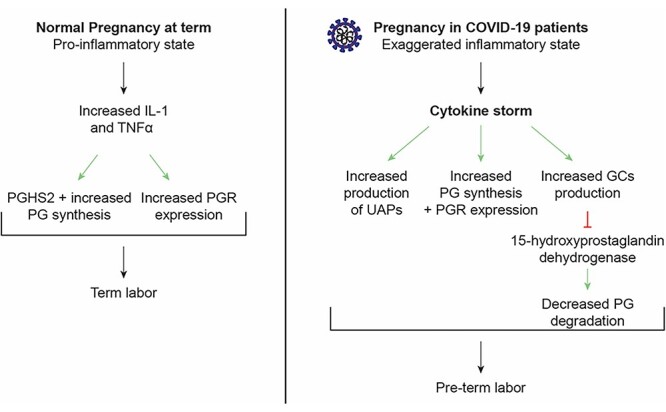
Comparison between normal proinflammatory state in normal pregnancy in healthy female patients and exaggerated inflammatory state in COVID-19 infected patients.
Miscarriage
According to the American College of Obstetrics and Gynecology (ACOG), miscarriage is the unintentional pregnancy loss before 22 weeks. The overall miscarriage rate before 20 weeks gestation ranges from 10% to 26% [67]. In a recent meta-analysis, the pooled proportion of miscarriage in pregnant women with COVID-19 was 15.3% and 23.1% using fixed and random effects models, respectively [67]. Thus, the miscarriage rate of COVID-19 cases seems to be in the range of the normal pregnant population. However, more research needs to be done on larger samples that are more homogeneous. Miscarriage and pregnancy loss in COVID-19 infection can be due to systemic inflammation that interfere with trophoblast invasion [67]. Moreover, the cytokine storm (IL-6, IL-8, TNF-α) would result in an unbalanced Th1/Th2 response and can induce a hypercoagulable state that is detrimental to fetal development [67–69]. Another mechanism for miscarriage is the increased Th17/Treg ratio [69–71]. Treg and Th17 cells are part of the complex machinery that constitutes the immune system. The differentiation of Th17 and Treg cells from naïve CD4+ T cells is mediated by TGF-β [70]. However, in the presence of IL-6 or IL-21 (together with TGF-β), naïve CD4+ T cells differentiate into Th17 cells [72]. During healthy pregnancy, the Treg/Th17 ratio shifts in favor of Treg cells [70]. Severe COVID-19 infection might shift the Treg/Th17 ration towards increase in the Th17 cells, which result in uncontrolled systemic inflammation that might contribute to pregnancy outcomes such as pregnancy loss [71] (Figure 4).
Figure 4.
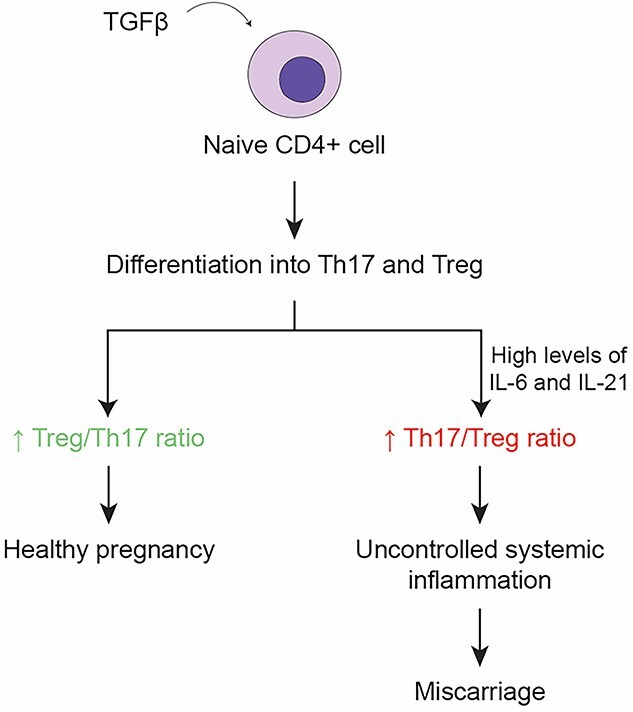
The consequences of the shift in Treg/Th17 ratio on pregnancy. The cytokine storm in COVID-19 infections can shift the Th17/Treg ratio towards increased levels of Th17 which may result in a miscarriage.
COVID-19 vaccine and pregnancy outcomes
Given the adverse events associated with COVID-19 infection, the center of disease control (CDC) and the ACOG strongly recommend that pregnant and lactating women be vaccinated against COVID-19. Recent papers have shown that COVID-19 vaccine is effective and safe in pregnancy [73–75] and that pregnant vaccinated women are 50% less likely to be infected with COVID-19 and 50% less likely to be hospitalized due to COVID-19 infection [75]. Moreover, multiple registry studies, cohort studies, and case control studies have shown that COVID-19 vaccination was not associated with increased risk of miscarriage, preterm labor, hypertensive disease of pregnancy, small gestational age, stillbirth, or cesarean delivery [74, 76]. Systematic reviews of available studies confirm the above findings, and show a 15% decrease in the odds of stillbirth in the vaccinated group [73]. However, COVID-19 vaccine may be associated with nonserious obstetrics symptoms like uterine tension, uterine pain, and uterine contraction [77]. Importantly, COVID-19 vaccine was not associated with neonatal adverse events, including low Appearance, Pulse, Grimace, Activity and Respiration (APGAR) scores, severe academia, respiratory distress in the newborn, meconium aspiration, and the need for mechanical ventilation or neonatal intensive care unit (NICU) hospitalization [75].
COVID-19 and fertility
When discussing fertility and COVID-19, both male and female factors should be considered. In females, the immune response against COVID-19 infection appears to have no effects on fertility of infected patients [25]. Indicators of fertility like serum levels of FSH, AMH, and antral follicular count are very similar when comparing healthy childbearing aged women with their COVID-19 infected cohort [78]. Moreover, ovarian reserve was unaltered after mild COVID-19 infection [79]. On the other hand, COVID-19 infection seems to affect the male reproductive system. Multiple cases of orchitis were documented during acute COVID-19 infection at a rate of 22% [80]. A recent study also showed that male partner infection with COVID-19 is associated with decreased fecundability if the infection is within 60 days, indicating that COVID-19 infection can be associated with short term, but not long term, reduction of fertility in men [81]. ACE-2 is mainly expressed in the Sertoli and Leydig cells, and TMPSS is mainly expressed in spermatogonia, so despite the lack of co-expression of ACE-2 and TMPSS in the testicles, infection of testicles is possible, suggesting that there may be another mechanism of entry [80]. Moreover, endothelial dysfunction from COVID-19 infection seems to affect the cavernosal endothelium that leads to male sexual dysfunction, such as erectile dysfunction, lasting for up to 7 months after infection [80]. From an immunological point of view, the male reproductive system was shown to stay in a proinflammatory state after COVID-19 infection evidenced by the presence of inflammatory cytokines in the semen analysis of men recovering from their infection [82]. The exaggerated cytokine storm in men (especially IL-6 and IFN-α) can be involved in the damage of the blood-testes barrier, leading to testicular infection with COVID-19 especially in moderate/severe cases where the increased viral load in the blood facilitate dissemination [82]. Moreover, the increased body temperature mediated by cytokines might alter spermatogenesis [83]. Finally, the cytokine storm leads to increased production of reactive oxygen species (ROS) that increase the oxidative stress in the testes thus affecting spermatogenesis, motility, and fertilization capacity of mature sperms [83].
Conclusion
Some of the severe COVID-19 infection creates a cytokine storm that might influence the HPG axis in female patients, leading to short-term menstrual disturbances. The cytokine storm also alters the proinflammatory and anti-inflammatory equilibrium in pregnancy leading to pre-term deliveries and possible miscarriages. Placental infection is still less understood and requires further research, but the inflammatory state in COVID-19 infection does not seem to be the culprit behind placental infection if it took place. Moreover, the cytokine storm can damage the blood-testicles barrier and can produce ROS species that might adversely affect the fertility of male infected patients. Given the above, more research needs to be done to identify if there will be clinically significant difference in symptoms (whether systemic or menstrual) or duration of symptoms between females who got infected with COVID-19 in their follicular phase and those who got infected in their luteal phase of the menstrual cycle, given the different expression levels of female hormones in these two phases. Finally, given the interaction between the immune system and the ovarian hormones, more research needs to be conducted to assess whether the immune response and the acquired immunity due to COVID-19 vaccine differ according to whether the COVID-19 infection or vaccine is given in the luteal or follicular phase.
Authors’ contributions
MS and MES contributed to the writing, literature review search, and the design of the manuscript. MAB and GD contributed to the design of the manuscript, analysis of the content and critical discussion of the manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists
Contributor Information
Mariam Saadedine, Department of Anatomy, Cell Biology and Physiological Sciences, American University of Beirut, Beirut, Lebanon.
Malak El Sabeh, Department of Gynecology and Obstetrics, Johns Hopkins University, Baltimore, Maryland, USA.
Mostafa A Borahay, Department of Gynecology and Obstetrics, Johns Hopkins University, Baltimore, Maryland, USA.
Georges Daoud, Department of Anatomy, Cell Biology and Physiological Sciences, American University of Beirut, Beirut, Lebanon.
References
- 1. Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online 2020; 22:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan SM, Shilen A, Heslin KM, Ishimwe P, Allen AM, Jacobs ET, Farland LV. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol 2022; 226:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Cmaj 2021; 193:E540–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev 2012; 11:A486–A492. [DOI] [PubMed] [Google Scholar]
- 5. Ursin RL, Klein SL. Sex differences in respiratory viral pathogenesis and treatments. Annu Rev Virol 2021; 8:393–414. [DOI] [PubMed] [Google Scholar]
- 6. Lotfi R, Kalmarzi RN, Roghani SA. A review on the immune responses against novel emerging coronavirus (SARS-CoV-2). Immunol Res 2021; 69:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Vafaeinezhad A, Atashzar MR, Baharlou R. The immune responses against coronavirus infections: friend or foe? Int Arch Allergy Immunol 2021; 182:863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jara LJ, López-Zamora B, Ordoñez-González I, Galaviz-Sánchez MF, Gutierrez-Melgarejo CI, Saavedra M, Vera-Lastra O, Cruz-Domínguez MP, Medina G. The immune-neuroendocrine system in COVID-19, advanced age and rheumatic diseases. Autoimmun Rev 2021; 20:102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, Capela C, Pedrosa J, Castro AG, Silvestre R. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol 2021; 12:613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol 2005; 18:41–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R, Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022; 19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol 2010; 35:109–125. [PMC free article] [PubMed] [Google Scholar]
- 14. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–638. [DOI] [PubMed] [Google Scholar]
- 15. Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C et al. (eds.). Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000–2022, MDText.com, Inc.; 2000. [Google Scholar]
- 16. Zhang Y, Zhang Y, Gu W, Sun B. Th1/Th2 cell differentiation and molecular signals. In: Sun B (ed.), T Helper Cell Differentiation and Their Function. Dordrecht: Springer Netherlands; 2014: 15–44. [Google Scholar]
- 17. Calderone A, Menichetti F, Santini F, Colangelo L, Lucenteforte E, Calderone V. Selective estrogen receptor modulators in COVID-19: a possible therapeutic option? Front Pharmacol 2020; 11:1085–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen NT, Chinn J, De Ferrante M, Kirby KA, Hohmann SF, Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS One 2021; 16:e0254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding T, Wang T, Zhang J, Cui P, Chen Z, Zhou S, Yuan S, Ma W, Zhang M, Rong Y, Chang J, Miao X et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in wuhan, China: an observational study. Front Med 2021; 8:635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biswas Shivhare S, Bulmer JN, Innes BA, Hapangama DK, Lash GE. Menstrual cycle distribution of uterine natural killer cells is altered in heavy menstrual bleeding. J Reprod Immunol 2015; 112:88–94. [DOI] [PubMed] [Google Scholar]
- 21. Radović Janošević D, Trandafilović M, Krtinić D, Čolović H, Milošević Stevanović J, Pop-Trajković Dinić S. Endometrial immunocompetent cells in proliferative and secretory phase of normal menstrual cycle. Folia Morphol 2020; 79:296–302. [DOI] [PubMed] [Google Scholar]
- 22. Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update 2000; 6:16–27. [DOI] [PubMed] [Google Scholar]
- 23. Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 2004; 89:6155–6167. [DOI] [PubMed] [Google Scholar]
- 24. Menning A, Walter A, Rudolph M, Gashaw I, Fritzemeier KH, Roese L. Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model. PLoS One 2012; 7:e41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, Lee S, Wang C, Li H, Cheng L, Ai J. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online 2021; 42:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phumsatitpong C, Wagenmaker ER, Moenter SM. Neuroendocrine interactions of the stress and reproductive axes. Front Neuroendocrinol 2021; 63:100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O'Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol 2009; 21:20–29. [DOI] [PubMed] [Google Scholar]
- 28. Hamidovic A, Karapetyan K, Serdarevic F, Choi SH, Eisenlohr-Moul T, Pinna G. Higher circulating cortisol in the follicular vs. luteal phase of the menstrual cycle: a meta-analysis. Front Endocrinol 2020; 11:11:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, Karsch FJ. Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 2002; 143:3748–3758. [DOI] [PubMed] [Google Scholar]
- 30. Yip SH, Liu X, Hessler S, Cheong I, Porteous R, Herbison AE. Indirect suppression of pulsatile LH secretion by CRH neurons in the female mouse. Endocrinology 2021; 162. [DOI] [PubMed] [Google Scholar]
- 31. Xiao E, Luckhaus J, Niemann W, Ferin M. Acute inhibition of gonadotropin secretion by corticotropin-releasing hormone in the primate: are the adrenal glands involved? Endocrinology 1989; 124:1632–1637. [DOI] [PubMed] [Google Scholar]
- 32. Feng YJ, Shalts E, Xia LN, Rivier J, Rivier C, Vale W, Ferin M. An inhibitory effects of interleukin-1a on basal gonadotropin release in the ovariectomized rhesus monkey: reversal by a corticotropin-releasing factor antagonist. Endocrinology 1991; 128:2077–2082. [DOI] [PubMed] [Google Scholar]
- 33. Male V. Menstrual changes after covid-19 vaccination. BMJ 2021; 374:n2211. [DOI] [PubMed] [Google Scholar]
- 34. Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, Pearson JT, Darney BG. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination cohort. Obstet Gynecol 2022; 139:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trogstad L. Increased occurrence of menstrual disturbances in 18- to 30-year-old women after COVID-19 vaccination. 2021. Available at SSRN: https://ssrn.com/abstract=3998180 or 10.2139/ssrn.3998180. [DOI]
- 36. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022; 28:202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nazir M, Asghar S, Rathore MA, Shahzad A, Shahid A, Khan AA, Malik A, Fakhar T, Kausar H, Malik J. Menstrual abnormalities after COVID-19 vaccines: a systematic review. Vacunas 2022; 23:S77–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weatherbee BAT, Glover DM, Zernicka-Goetz M. Expression of SARS-CoV-2 receptorACE2and the proteaseTMPRSS2suggests susceptibility of the human embryo in the first trimester. Open Biol 2020; 10:200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rad HS, Röhl J, Stylianou N, Allenby MC, Bazaz SR, Warkiani ME, Guimaraes FSF, Clifton VL, Kulasinghe A. The effects of COVID-19 on the placenta during pregnancy. Front Immunol 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Constantino FB, Cury SS, Nogueira CR, Carvalho RF, Justulin LA. Prediction of non-canonical routes for SARS-CoV-2 infection in human placenta cells. Front Mol Biosci 2021; 8:614728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang P, Salafia C, Heyman T, Salafia C, Lederman S, Dygulska B. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am J Obstet Gynecol MFM 2020; 2:100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer JA, Roman AS, Limaye M, Grossman TB, Flaifel A, Vaz MJ, Thomas KM, Penfield CA. Association of SARS-CoV-2 placental histopathology findings with maternal-fetal comorbidities and severity of COVID-19 hypoxia. J Matern Fetal Neonatal Med 2021; 1–7. Advance online publication. 10.1080/14767058.2021.1977791. [DOI] [PubMed] [Google Scholar]
- 43. Wong YP, Khong TY, Tan GC. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel, Switzerland) 2021; 11:94. 10.3390/diagnostics11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allotey J, Chatterjee S, Kew T, Gaetano A, Stallings E, Fernandez-Garcia S, Yap M, Sheikh J, Lawson H, Coomar D, Dixit A, Zhou D et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ 2022; 376:e067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Juttukonda LJ, Wachman EM, Boateng J, Jain M, Benarroch Y, Taglauer ES. Decidual immune response following COVID-19 during pregnancy varies by timing of maternal SARS-CoV-2 infection. J Reprod Immunol 2022; 151:103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mourad M, Jacob T, Sadovsky E, Bejerano S, Simone GS, Bagalkot TR, Zucker J, Yin MT, Chang JY, Liu L, Debelenko L, Shawber CJ et al. Placental response to maternal SARS-CoV-2 infection. 2021; 11:14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fahmi A, Brügger M, Démoulins T, Zumkehr B, Oliveira Esteves BI, Bracher L, Wotzkow C, Blank F, Thiel V, Baud D, Alves MP. SARS-CoV-2 can infect and propagate in human placenta explants. Cell Rep Med 2021; 2:100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suhren JT, Meinardus A, Hussein K, Schaumann N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 2021; 117:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Celik E, Vatansever C, Ozcan G, Kapucuoglu N, Alatas C, Besli Y, Palaoglu E, Gursoy T, Manici M, Turgal M, Dogan O, Cekic SG et al. Placental deficiency during maternal SARS-CoV-2 infection. Placenta 2021; 117:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roberts DJ, Edlow AG, Romero RJ, Coyne CB, Ting DT, Hornick JL, Zaki SR, Das Adhikari U, Serghides L, Gaw SL, Metz TD. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 Placental Infection Workshop. Am J Obstet Gynecol 2021; 225:593.e591–593.e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol 2022; 226:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol 2021; 31:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011; 1221:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018; 49:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krop J, Heidt S, Claas FHJ, Eikmans M. Regulatory T cells in pregnancy: it is not all about FoxP3. Front Immunol 2020; 11:1182–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol 2013; 69:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 2005; 166:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 2011; 10:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Keller CC, Eikmans M, van der Hoorn MP, Lashley L. Recurrent miscarriages and the association with regulatory T cells: a systematic review. J Reprod Immunol 2020; 139:103105. [DOI] [PubMed] [Google Scholar]
- 60. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011; 31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79:50–57. [DOI] [PubMed] [Google Scholar]
- 62. Molnár M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol 1993; 169:825–829. [DOI] [PubMed] [Google Scholar]
- 63. Kniss DA, Zimmerman PD, Garver CL, Fertel RH. Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol 1997; 177:559–567. [DOI] [PubMed] [Google Scholar]
- 64. Olson DM. The promise of prostaglandins: have they fulfilled their potential as therapeutic targets for the delay of preterm birth? J Soc Gynecol Investig 2005; 12:466–478. [DOI] [PubMed] [Google Scholar]
- 65. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, do Vale MS, Cardona-Perez JA, Maiz N, Cetin I et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection. Pediatrics 2021; 175:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li XQ, Zhu P, Myatt L, Sun K. Roles of glucocorticoids in human parturition: a controversial fact? Placenta 2014; 35:291–296. [DOI] [PubMed] [Google Scholar]
- 67. Cavalcante MB, de Melo Bezerra Cavalcante CT, ANM C, Sarno M, Barini R, Kwak-Kim J. COVID-19 and miscarriage: from immunopathological mechanisms to actual clinical evidence. J Reprod Immunol 2021; 148:103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rad F, Dabbagh A, Dorgalaleh A, Biswas A. The relationship between inflammatory cytokines and coagulopathy in patients with COVID-19. J Clin Med 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol 2020; 11:2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016; 148:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. 2020; 84:e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saifi B, Rezaee SA, Tajik N, Ahmadpour ME, Ashrafi M, Vakili R, SoleimaniAsl S, Aflatoonian R, Mehdizadeh M. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod Biomed Online 2014; 29:481–489. [DOI] [PubMed] [Google Scholar]
- 73. Prasad S, Kalafat E, Blakeway H, Townsend R, O’Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P, Magee LA et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 2022; 13:2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Male V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat Rev Immunol 2022; 22:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: a systematic review and meta-analysis. Vaccines 2022; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peretz-Machluf R, Hirsh-Yechezkel G, Zaslavsky-Paltiel I, Farhi A, Avisar N, Lerner-Geva L, Meyer R, Tsur A, Yinon Y. Obstetric and neonatal outcomes following COVID-19 vaccination in pregnancy. J Clin Med 2022; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Komine-Aizawa S, Haruyama Y, Deguchi M, Hayakawa S, Kawana K, Kobashi G, Miyagi E, Yamada H, Sugiyama T. The vaccination status and adverse effects of COVID-19 vaccine among pregnant women in Japan in 2021. J Obstet Gynaecol Res 2022; 48:1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang M, Yang Q, Ren X, Hu J, Li Z, Long R, Xi Q, Zhu L, Jin L. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: a retrospective cohort study. EClinicalMedicine 2021; 38:101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kolanska K, Hours A, Jonquière L, Mathieu d'Argent E, Dabi Y, Dupont C, Touboul C, Antoine JM, Chabbert-Buffet N, Daraï E. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod Biomed Online 2021; 43:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nassau DE, Best JC, Kresch E, Gonzalez DC, Khodamoradi K, Ramasamy R. Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int 2022; 129:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wesselink AK, Hatch EE, Rothman KJ, Wang TR, Willis MD, Yland J, Crowe HM, Geller RJ, Willis SK, Perkins RB, Regan AK, Levinson J et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol 2022; 191:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morselli S, Sebastianelli A, Liaci A, Zaccaro C. Male reproductive system inflammation after healing from coronavirus disease 2019, vol. 10. 2022: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 83. Rajak P, Roy S, Dutta M, Podder S, Sarkar S, Ganguly A, Mandi M, Khatun S. Understanding the cross-talk between mediators of infertility and COVID-19. Reprod Biol 2021; 21:100559. [DOI] [PMC free article] [PubMed] [Google Scholar]


