Abstract
Introduction:
Parkinson’s Disease (PD) is a neurodegenerative disorder caused by disruption of dopamine-producing cells. PD is associated with motor symptoms and non-motor symptoms including depression and cognitive impairment. Past research suggests an association between depression and cognitive impairment in PD. Physical activity may have a therapeutic effect on both depression and cognitive impairment. The present study investigates if physical activity mediates the association between depressive symptoms and cognition in a longitudinal sample of individuals with PD.
Methods:
Participants include individuals newly diagnosed with PD (N = 487) enrolled in the Parkinson’s Progression Marker Initiative (PPMI). Participants completed an array of neuropsychological tests over the course of five years, as well as questionnaires of depression and physical activity. Between-person and within-person effects of depression and cognition mediated through physical activity were analyzed using structural equation modeling.
Results:
A significant direct effect demonstrated depression was associated with worse global cognitive functioning. Furthermore, there was a significant indirect within-person effect, indicating that physical activity fully mediated the association between depression and cognition. Individuals who became more depressed over time became less physically active and subsequently experienced cognitive decline over the five-year period.
Discussion:
Findings have implications for prognostic detection and/or the role of physical activity interventions to buffer effects of depression on cognitive impairment among individuals diagnosed with PD. Physical interventions may potentially be implemented among depressed persons to preserve cognitive functioning. Worsened depression early during PD may be a risk factor for inactivity and cognitive diminishment.
Introduction
Parkinson’s disease (PD) is the second most widespread neurodegenerative disorder and affects 1% of individuals over the age of 60 (Tysnes & Storstein, 2017). PD is characterized by both motor (e.g. tremor, rigidity, akinesia, postural instability) and nonmotor symptoms. Common non-motor symptoms include changes in cognition, mood disorders, gastrointestinal symptoms, fatigue, loss of sense of smell, and sleep disorders.
Cognitive decline is a commonly reported symptom of PD. There is debate about the heterogeneity of cognitive symptoms. In general, executive functioning, attention, working memory, and visuospatial functioning difficulties are commonly affected in PD (Litvan et al., 2012). According to Muslimovic et al. (2005), 25% of individuals newly diagnosed with PD experience cognitive impairment on objective neuropsychological assessments.
Along with cognitive impairment, depression is commonly experienced among individuals with PD. Depression is associated with accelerated cognitive decline and increased risk of dementia among elderly individuals (Jorm, 2000; Sachs-Ericsson et al., 2005; Rapp et al., 2011). Among PD individuals, persistent symptoms of mood disorders are associated with poorer immediate and delayed memory recall (Jones et al., 2019; Szymkowicz et al., 2018), executive functioning (Hurtado-González et al., 2018), and semantic verbal fluency tasks and task change (Hurtado-González et al., 2018). PD individuals with major depression experienced greater cognitive decline and progressed through stages of PD severity more rapidly than those with minor or no depression (Starkstein et al., 1992). A past paper by our group utilizing data from the Parkinson Progression Marker Initiative (PPMI) cohort, examined the bidirectional association between cognition and multiple mood/affective symptoms in PD (depression, anxiety, and apathy; Jones et al., 2019). Results suggested that severity of depressive symptoms, but not anxiety or apathy, is associated with longitudinal cognitive decline. Furthermore, we found that depressive symptoms predicted cognitive functioning in the subsequent year, but that cognitive functioning did not predict future changes in depression, providing evidence of a uni-directional relationship between cognition and depression.
In recent years, the therapeutic effect of physical activities on cognitive decline and depression among individuals with PD has been a topic of interest (Lauze et al., 2016). The contribution of physical activity to clinical outcomes may be especially relevant for individuals with PD. Individuals with PD tend to be physically inactive and this is likely due to both motor and non-motor complications (van Nimwegen et. al., 2011). Chastin et al. (2010) found that while there was no difference in total amount of sedentary time over a 7-day period, individuals with PD had longer bouts of sedentary behavior than healthy, age-matched controls. Randomized controlled trials/exercise interventions show that physical activity is associated with improvements in executive functioning (Altmann et al., 2016; Silveira et al., 2018), auditory memory and attention (Fiorelli et al., 2019), and language and episodic memory (Altmann et al., 2016) among individuals diagnosed with PD. Additionally, research utilizing self-report measures of participation in physical activities have shown that physical activity is associated with decreased depression and better cognitive performance among individuals diagnosed with PD (Amara et al., 2019). Specifically, participating in physical activity is negatively correlated with depressive symptoms even when controlling for social support, demographic variables, and physical health among middle-aged adults (Lu, 2011). Additionally, Bak (2009) found that physical activity was negatively correlated with depression and positively correlated with perceived health and life satisfaction among healthy older adults. Physical activity also plays a role in minimizing depressive and anxiety symptoms in subclinical populations (Rebar et al., 2015). Overall, the literature provides reasonable support for direct associations between 1) physical activity and cognition, and 2) physical activity and depression. However, we are unaware of research examining both physical activity and depression and their temporal associations with cognitive function in individuals with PD.
Past research provides evidence for physical inactivity, to be a behavioral pathway linking anxiety to cognitive impairment in PD or linking depression to cognitive impairment in healthy older adults (Jones et al., 2021a; Sharifian et al., 2021). However, examining the associations between depression, physical activity and cognition in PD is particularly relevant because: 1) past research suggests that depression may be associated with future cognitive impairment above and beyond other mood symptoms (Jones et al., 2019), and 2) individuals with PD experience a variety of unique neurobiological (e.g. dopamine depletion and disruption of frontal-subcortical pathways important for both cognition and emotion) and psychosocial changes (e.g. embarrassment from motor symptoms, physical disability) that raise concerns about the extent that findings from healthy older adults generalize to the PD population. The current study expands upon our past finding of a directional association between depression and cognitive functioning by examining physical activity as a potential behavioral pathway linking the two constructs (Jones et al., 2019). Although past studies provide initial evidence of direct effects between these three constructs, we are unaware of any study examining the direct and indirect effects of depression and physical activity on cognition in individuals with PD.
Methods
Participants
Data was obtained via the Parkinson’s Progression Markers Initiative (PPMI), a longitudinal multisite observational study of the progression of PD. Data from 10 national or international sites from were utilized in the current sample (see https://www.ppmiinfo.org/about-ppmi/ppmi-clinical-sites for a complete list of sites). The sample has 100% (Jones et al., 2021a; Jones et al., 2021b; Jones et al., 2021c), 88% (Jones et al., 2020a; Jones et al., 2020b) and 64% (Jones et al., 2019) overlap with our past work. For additional details please see Marek et al. (2011) and https://www.ppmi-info.org/. The institutional review board approved the study at each site, and all participants provided consent prior to participation. The current study consisted of 487 individuals newly diagnosed with PD (less than 1 year of clinical diagnosis). Data from each participant was collected over the course of 5 years (baseline, 1st, 2nd, 3rd, 4th, 5th). Inclusion/exclusion criteria for the parent study (PPMI) have been previously published (Marek, et al., 2011). Briefly, inclusion criteria for PD patients included diagnosis of PD based of presence of resting tremor, bradykinesia, or rigidity within two years, and being untreated for PD. For the current analyses, participants were included if they completed at least one clinical visit where neuropsychological tests and questionnaires of depression and physical activity were completed.
Measures
Depression.
Depressive symptoms were measured with the Geriatric Depression Scale-Short Form (GDS; Greenberg, 2012), a self-report measure of depression in older adults. Research has shown the GDS to be a valid measure of depression in individuals with PD (Schrag et al., 2007). The GDS was administered at each annual follow-up. Higher scores indicate more depressive symptoms.
Physical Activity.
The Physical Activity Scale for the Elderly (PASE; Washburn et al., 1993) is a brief self-report measure that assesses physical activity and consists of two domains: leisure activities and household activities. Intensity, frequency, and duration of activities over a one-week period are measured, with higher scores representing higher levels of activity. The PASE was found to be a valid measure for use in studies on physical activity in older populations (Washburn et al., 1993). The PASE was not consistently administered in the study until year two, therefore only years 2–5 were used to model the within- and between-person effects.
Cognition.
Participants were given a battery of neuropsychological tests annually. The Hopkins Verbal Learning Test- Revised (HVLT-R; Brandt & Benedict, 2001) assessed learning (sum of trials 1–3) and delayed free recall/memory. PPMI uses HVLT-R versions 1–5 in sequential order (version 1 administered at baseline, version 2 at the first annual follow-up, etc.). The Judgement of Line Orientation Test (JLO; Benton et al., 1983) assessed visuospatial functioning. The odd and even trials were administered at alternating years (odd trials administered as baseline, year 2 and 4; even trials administered at years 1, 3 and 5). The Letter-Number Sequencing task (LNS; Wechsler, 2008) assessed attention/working memory. Language was assessed with the Animal Fluency test (Gladsjo, et al., 1999). The Symbol Digit Modalities Test (SDMT; Smith, 1982) assessed processing speed. Version 1 and 2 were administered in alternating years. Lastly, to reduce the number of analyses, a composite score of global cognition was created by averaging the demographically corrected z-scores for each cognitive score. The majority of tests were normed based on age only (HVLT-R immediate and delayed memory, letter number sequencing, SDMT), with the exception of JLO (age & education) and Animal Fluency (age, education and ethnicity).
Severity of Motor Symptoms.
The Unified Parkinson’s Disease Rating Scale – Part III (UPDRS) assessed motor symptoms severity (Goetz et al., 2008). Higher scores indicate greater severity of motor symptoms (e.g., speech, facial expression, rigidity, hand movements, tremors, gait, postural stability, body bradykinesia).
Data and Data Analysis
Structural equation modeling (SEM) was conducted in SPSS AMOS (Preacher et al., 2011). The direct and indirect effects of depression on cognition through physical activity were examined. Missing data was estimated with maximum likelihood estimates. We utilized 2000 iterations of bootstrapped samples to calculate the standard error of indirect effects (Gunzler et al., 2013). Within-person and between-person effects were separately modeled for depression, cognition, and the two physical activity domains (household activities and leisure activities; Figure 1). The within-person effects examined whether longitudinal changes in physical activity mediate the longitudinal relationship between depression and cognition. The within-person effects were modeled based on linear growth (Supplemental Figure 1; Preacher et al., 2011). The between-person effects examined if the average level of physical activity over the study period mediated the association between depression and cognitive functioning. Motor severity, age at baseline, and gender were also included as predictors of global cognition in statistical models.
Figure 1.
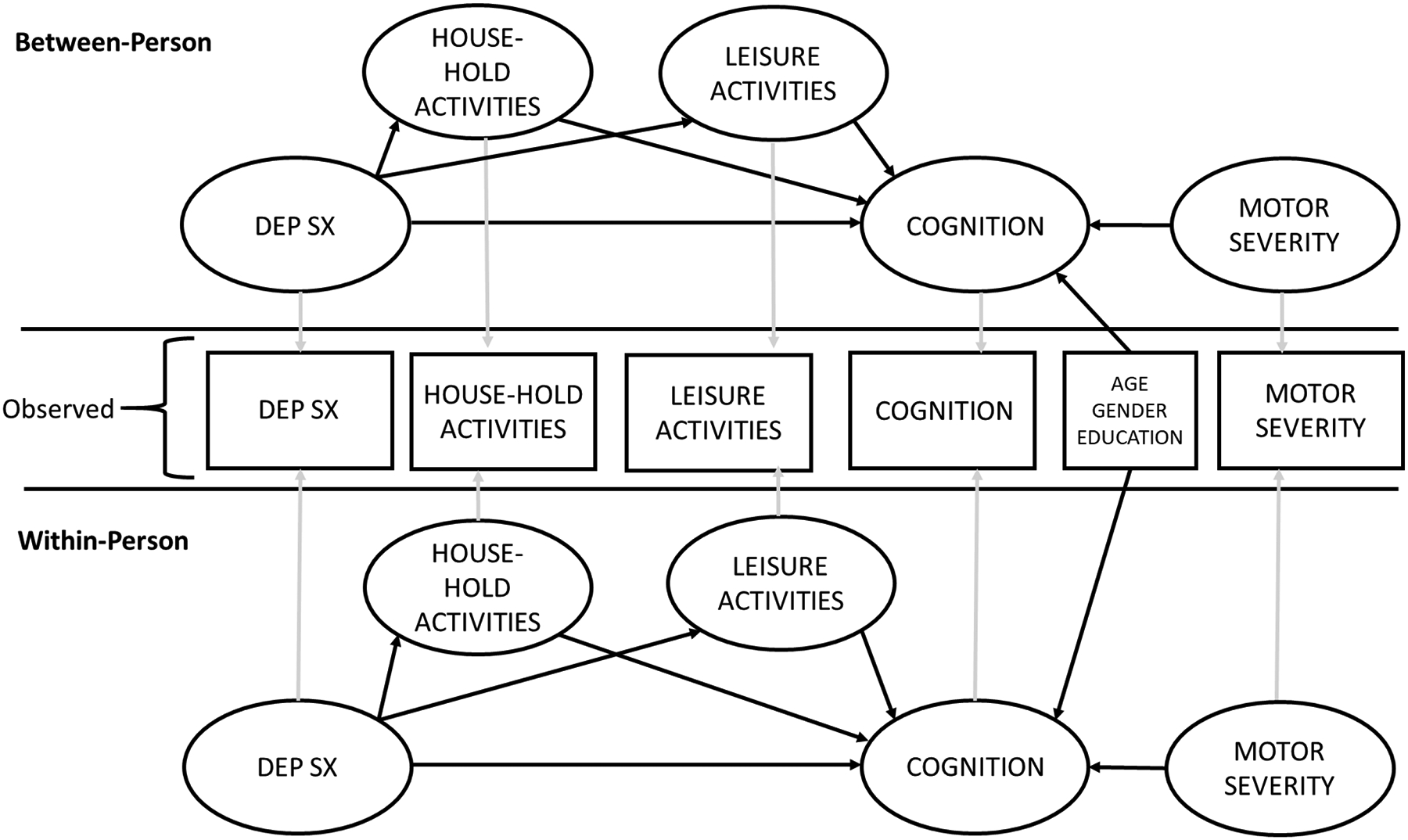
Depiction of Model.
Results
Demographic and clinical characteristics at baseline are reported in Table 1.
Table 1.
Baseline Characteristics (n = 487).
| Mean | SD | Range | |
|---|---|---|---|
| Age | 61.07 | 9.7 | 33 – 84 |
| Education | 15.48 | 3.1 | 5 – 26 |
| % Male | 65.1% | -- | -- |
| % Caucasian | 92.8% | -- | -- |
| % African American/Black | 1.4% | -- | -- |
| % Asian | 1.9% | -- | -- |
| % Other | 3.9% | -- | -- |
| UPDRS-III Motor | 20.03 | 9.2 | 2 – 51 |
| GDS | 2.46 | 2.6 | 0 – 14 |
| Global Cognition (Z score) | 0.01 | 0.6 | −1.8 – 1.4 |
| HVLT-II Trials 1–3 (Z score) | −0.36 | 0.9 | −2.7 – 3 |
| HVLT-II Delayed Recall (Z score) | −0.30 | 1.9 | −2.9 – 3 |
| Judgement of Line Orientation (Z score) | 0.68 | 1.0 | −2.3 – 3 |
| Letter Number Sequencing (Z score) | 0.45 | 0.7 | −2.7 – 3 |
| Animal Fluency (Z score) | 0.038 | 1.0 | −3.5 – 3.3 |
| Symbol Digit Modalities Test (Z score) | −0.48 | 0.9 | −3 – 2.25 |
| PASE Household Activities | 256.55 | 43.7 | 171 – 342 |
| PASE Leisure Activities | 114.98 | 77.1 | 0 – 351 |
Note. UPDRS = Unified Parkinson’s Disease Rating Scale- part III; GDS = Geriatric Depression Scale; PASE = Physical Activity Scale for the Elderly.
Direct effect model: Depression predicts cognition
SEM assessed the direct effect of depression on global cognition. Model fit was adequate (χ2 = 428.66, p < 0.001; RMSEA = 0.048; CFI = 0.946; NFI = 0.904). The analyses revealed a significant direct within-person effect (Table 2); individuals who became more depressed over the course of 5 years experienced greater longitudinal declines in global cognition. Additionally, there was a significant direct between-person effect. Individuals who on average reported more depressed symptoms across the 5 years also demonstrated worse cognitive performance on average over the study period. Worse cognitive performance was also significantly associated with older age and less severe motor symptoms.
Table 2.
Direct Effects Standardized Estimates and Significance
| Variable | Standardized Estimate | S.E. | p |
|---|---|---|---|
| Within-Person Effects | |||
| Depression -- > Global Cognition | −0.308 | 0.092 | <0.001** |
| Age -- > Global Cognition | −0.031 | 0.009 | <0.001** |
| Education -- > Global Cognition | −0.007 | 0.010 | 0.492 |
| Gender -- > Global Cognition | −0.004 | 0.009 | 0.617 |
| Motor Severity -- > Global Cognition | −0.143 | 0.070 | 0.041* |
| Between-Person Effects | |||
| Depression -- > Global Cognition | −0.204 | 0.047 | <0.001** |
| Age -- > Global Cognition | −0.023 | 0.036 | 0.525 |
| Education -- > Global Cognition | 0.109 | 0.040 | 0.007* |
| Gender -- > Global Cognition | −0.070 | 0.035 | 0.043* |
| Motor Severity --> Global Cognition | −0.311 | 0.063 | <0.001** |
S.E. = Standard Error
< 0.05
< 0.001
Mediation Model
An additional SEM assessed the mediating effect of physical activity on the relationship between depression and global cognition. Model fit was adequate (χ2 = 821.63, p < 0.001; RMSEA = 0.047; CFI = 0.955; NFI = 0.918).
The model revealed a significant within-person indirect effect (Table 3); individuals who became more depressed over the 5-year period became less physically active in household activities and experienced declines in global cognition. The direct effect between depression and global cognition was not significant in this model, indicating the relationship between depression and global cognition was fully mediated by household physical activities. Worse within-person cognitive performance was also significantly associated with younger age and more severe motor symptoms.
Table 3.
Mediation Model
| Variable | Standardized Estimate | S.E. | p |
|---|---|---|---|
| Within-Person Effects | |||
| Depression -- > Global Cognition | 0.302 | 1.567 | 0.847 |
| Depression -- > Household Activities | −0.524 | 0.090 | <0.001** |
| Depression -- > Leisure Activities | −0.156 | 0.077 | 0.043 |
| Household Activities -- > Global Cognition | 1.198 | 2.928 | 0.682 |
| Leisure Activities -- > Global Cognition | Unable to Estimate* | ||
| Age -- > Global Cognition | −0.028 | 0.007 | <0.001** |
| Education -- > Global Cognition | −0.005 | 0.007 | 0.475 |
| Gender -- > Global Cognition | −0.006 | 0.006 | 0.304 |
| Motor Severity -- > Global Cognition | −0.142 | 0.060 | 0.018 |
| Indirect Effect: Depression -- > Global Cognition | −0.628 | 0.742 | 0.017* |
| Between-Person Effects | |||
| Depression -- > Global Cognition | −0.227 | 0.046 | <0.001** |
| Depression -- > Household Activities | −0.162 | 0.045 | <0.001** |
| Depression -- > Leisure Activities | −0.264 | 0.043 | <0.001** |
| Household Activities -- > Global Cognition | −0.003 | 0.056 | 0.951 |
| Leisure Activities -- > Global Cognition | −0.085 | 0.055 | 0.124 |
| Age -- > Global Cognition | −0.031 | 0.035 | 0.371 |
| Education -- > Global Cognition | 0.111 | 0.039 | 0.004* |
| Gender -- > Global Cognition | −0.066 | 0.034 | 0.050* |
| Motor Severity -- > Global Cognition | −0.299 | 0.059 | <0.001** |
| Indirect Effect: Depression --> Global Cognition | 0.022 | 0.018 | 0.223 |
S.E. = Standard Error; *Unable to estimate due to convergence/minimal within-person variability in leisure activities.
< 0.05
< 0.001
No significant between-person indirect effect was revealed, indicating that mediation did not occur. However, there was a significant direct effect between depression and global cognition, meaning that individuals who were more depressed on average declined more cognitively on average. Furthermore, more severe depression on average was associated with less participation in household and leisure activities. Performance in global cognition on average was not associated with either household or leisure activities. Worse within-person cognitive performance was also significantly associated with fewer years of education, male gender and more severe motor symptoms.
Discussion
Findings expand upon our 2019 finding of depression predicting cognitive decline by showing that longitudinal changes in physical activity mediate this association among individuals diagnosed with PD. Specifically, individuals who experienced a worsening of depression over time were less likely to be active in household physical activities, which was indirectly associated with declines in global cognition.
Past studies have also examined the direct association between physical activity and depression/cognition. For instance, Amara et al. (2019) found that physical activity early in PD is associated with less severe depression. Additionally, individuals who are more physically active experienced less severe cognitive decline than compared to their inactive counterparts, suggesting that physical activity preserves cognition in early PD (Mantri et al., 2019). Furthermore, Altmann et al. (2016) conducted a longitudinal study comparing individuals diagnosed with PD randomized to an aerobic exercise groups and a stretch-balance control group. Individuals in the aerobic exercise group performed better on executive functioning and language tasks than individuals in the control group. Silveira et al. (2018) found similar results, with aerobic exercise halting declines in executive functioning over a 12-week period. Our findings, in conjunction with the previous literature, suggests that increasing physical activity may be an effective strategy for mitigating cognitive losses. Certainly, there are benefits to physical activity for individuals with PD. Past studies support a direct relationship between physical activity and mood, or physical activity and cognition, but our study expands upon the literature by integrating the separate direct effects.
The clinical implications of the study suggest that early implementation of physical activity interventions could potentially help prevent negative clinical outcomes associated with physical inactivity. Specifically, physical interventions could be valuable for PD patients with comorbid depression, who are at increased risk for cognitive decline. Furthermore, clinicians may consider assessing for sedentary behaviors or changes/decreases in physical activities in their clinical interviews. Findings suggest the combination of depression and decreased participation in physical activities should raise concerns that patients may be at risk for future cognitive decline. The importance of maintaining physical activity may be more relevant/important for PD patients, relative to non-PD older adults. Van Nimwegen et al. (2011) reported that PD patients become more physically inactive as the disease progresses. Moreover, individuals with PD are 24% less active compared to their healthy counterparts (van Nimwegen et al., 2011). PD patients with comorbid psychiatric complications, may be at elevated risk for physical inactivity and subsequent complications. Indeed, other psychiatric complications such as anxiety and apathy are common in PD and associated with physical inactivity, cognitive impairment, and quality of life (Jones et al., 2021; Jones et al., 2015).
This study did not directly examine potential neurobiological mechanisms. Future studies could consider several biologic mechanisms that potentially underlie the associations between physical activity, depression and global cognition. The mediating effect of physical activity on the relationship between depression and cognition may also be explained by adult neurogenesis. Within animal studies, exercise has been shown to alleviate symptoms of depression due to hippocampal neurogenesis (Ernst et al., 2006). Exercise allows for the synthesis of new neurons within the hippocampus, thus leading to decreases in depressive symptoms (Ernst et al., 2006). The relationship between hippocampal neurogenesis and physical activity has also been associated with reduced cognitive impairment within chemotherapy treated rats (Winocur et al., 2014). Besides neurogenesis, Apolipoprotein E type 4 (APOE-E4) is a risk factor for dementia and cognitive decline seen in PD and Alzheimer’s Disease. Exercise has been hypothesized to reduce mood and cognitive symptoms among APOE-E4 carriers (Fenesi er al., 2017). Ku et al. (2017) measured differences in depressive symptoms among APOE-E4 carriers and non-carriers, and high/low physically active individuals. Differences were observed between high active APOE-E4 non-carriers and low-active APOE-e4 carriers; however, the benefits of exercise were only observed among APOE-E4 non-carriers. Regarding cognition, greater semantic memory activation was observed among highly active APOE-E4 carriers (Smith et al., 2011). In an animal study, cognitive performance improved after six weeks of wheel running among APOE-E4 mice (Nichol et al., 2009). A recent PPMI study found that APOE genotype and physical activity interacted in predicting cognitive impairment. Specifically, higher physical activity was associated with slower APOE-E4 related cognitive decline (Kim et al., 2021). Studies commonly suggest the association between depression and cognition may reflect shared neural circuitry, such as disruption of the frontostriatal circuitry (Levin & Katzen, 1995; Zgaljardic et al., 2003). Indeed, depressive symptoms are associated with altered grey matter volume and white matter integrity in frontal-striatal regions among individual with PD (Li et al., 2020). Indeed, neuroimaging studies provide support that exercise may increase dopamine release and retain functional activation in striatal regions (Sacheli et al., 2018; Shih et al., 2019).
The current study is not without limitations. Physical activity was measured with a subjective self-report questionnaire. An objective measurement of physical activity may be used to strengthen future studies. Furthermore, the current study utilized a global metric of physical activity that assess frequency and intensity of activities. Future studies may benefit from examining individual dimensions of physical activity (e.g., intensity, complexity, frequency, and modality). Caution should be taken when interpreting causation among constructs. It is plausible that alternative directionalities may exist (physical inactivity may cause future depression, or cognitive impairment may cause future physical inactivity). Although indirect models can provide insight regarding potential mediators/behavioral mechanisms of associations, future studies utilizing an experimental approach can best determine directionality and causation. The severity of Parkinsonian motor symptoms may impact one’s ability to engage in physical activity. The present study consisted of individuals newly diagnosed with PD, and participants are generally cognitively intact; therefore, findings may have limited generalizability to PD samples with more severe motor symptoms and cognitive impairments. Similarly, participants predominantly consisted of Caucasians, which may limit generalizability.
Ultimately, results suggest that physical activity may be a mediator between depression and cognitive functioning among individuals diagnosed with PD. Greater depression is associated with inactivity in household activities, which is indirectly linked to cognitive diminishment. Behavioral interventions promoting increased participation in physical activity could have implications for preserving cognition and mental health.
Supplementary Material
Key Points.
The present study investigated whether physical activity mediates the relationship between depression and cognition among individuals with Parkinson’s Disease (PD). Results revealed a significant within-person indirect effect; individuals who became more depressed over the course of 5 years became less physically active and experienced cognitive diminishment over time. Findings suggest that depression early following diagnosis may be a risk factor for cognitive decline. Future research should examine individual dimensions of physical activity (e.g., intensity, complexity, frequency, and modality) and its effects on cognition in PD.
Acknowledgements/Study Funding:
Jacob Jones was support by NIH SC3NS124906
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research funding partners 4D Pharma, Abbvie, Acurex Therapeutics, Allergan, Amathus Therapeutics, ASAP, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, Dacapo Brain Science, Denali, The Edmond J. Safra Foundaiton, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.
References
- Amara AW, Chahine L, Seedorff N, Caspell-Garcia CJ, Coffey C, Simuni T, & the Parkinson’s Marker’s Initiative (2019). Self-reported physical activity levels and clinical progression in early Parkinson’s disease. Parkinsonism and Related Disorders, 61, 118–125. 10.1016/j.parkreldis.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Altmann LJ, Stegemöller E, Hazamy AA, Wilson JP, Bowers D, Okun MS, & Hass CJ (2016). Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: Results of a controlled study. Journal of the International Neuropsychological Society, 22(9), 878–889 [DOI] [PubMed] [Google Scholar]
- Bak HK (2009). A study on leisure activities, leisure life satisfaction, perceived health status and depression in the elderly. The Korean Journal of Rehabilitation Nursing, 12(2), 112–119. [Google Scholar]
- Benton AL, Hamsher KD, Varney NR, & Spreen O (1983). Judgment of line orientation. New York: Oxford University Press. [Google Scholar]
- Brandt J, & Benedict RH (2001). Hopkins verbal learning test--revised: professional manual. Psychological Assessment Resources. [Google Scholar]
- Chastin SF, Baker K, Jones D, Burn D, Granat MH, & Rochester L (2010). The pattern of habitual sedentary behavior is different in advanced Parkinson’s disease. Movement Disorders, 25(13), 2114–2120. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JPJ, Lam RW, & Christie BR (2006). Antidepressant effects of exercise: Evidence for an adult-neurogenesis hypothesis? Journal of Psychiatry & Neuroscience, 31(2), 84–92. [PMC free article] [PubMed] [Google Scholar]
- Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, & Heisz JJ (2017). Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: A population-based study. Journal of Alzheimer’s Disease, 56(1), 297–303. https://doi-org.libproxy.lib.csusb.edu/10.3233/JAD-160424 [DOI] [PubMed] [Google Scholar]
- Fiorelli CM, Ciolac EG, Simieli L, Silva FA, Fernandes B, Christofoletti G, & Barbieri FA (2019). Differential acute effect of high-intensity interval or continuous moderate exercise on cognition in individuals with Parkinson’s Disease. Journal of Physical Activity & Health, 16i(2), 157–164. https://doiorg.libproxy.lib.csusb.edu/10.1123/jpah.2018-0189 [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, & Heaton RK (1999). Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment, 6(2), 147–178. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐ Martin P, … & Dubois B (2008). Movement Disorder Society‐ sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐ UPDRS): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society, 23(15), 2129–2170. [DOI] [PubMed] [Google Scholar]
- Greenberg SA (2012). The geriatric depression scale (GDS). Best Practices in Nursing Care to Older Adults, 4(1), 1–2. [Google Scholar]
- Gunzler D, Chen T, Wu P, & Zhang H (2013). Introduction to mediation analysis with structural equation modeling. Shanghai archives of psychiatry, 25(6), 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-González CA, De la Cruz-Cifuentes O, Triviño O, Rinco A Seminec D, De la Cruz-Cifuentes G, & Olayo B (2018). Influence of depression on the executive functioning of patients with advanced idiopathic Parkinson’s disease without -dementia. Biomedical Research, 29(7), 1481–1483. [Google Scholar]
- Jones JD, Kurniadi NE, Kuhn TP, Szymkowicz SM, Bunch J, & Rahmani E (2019). Depressive symptoms precede cognitive impairment in de novo Parkinson’s disease patients: Analysis of the PPMI cohort. Neuropsychology. 10.1037/neu0000583. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Mangal P, Lafo J, Okun MS, & Bowers D (2016). Mood differences among Parkinson’s disease patients with mild cognitive impairment. Journal of Neuropsychiatry and Clinical Neurosciences, 28, 211–216. 10.1176/appi.neuropsych.15090221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Burroughs M, Apodaca M, & Bunch J (2020a). Greater intraindividual variability in neuropsychological performance predicts cognitive impairment in de novo Parkinson’s disease. Neuropsychology, 34(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Rahmani E, Garcia E, & Jacobs JP (2020b). Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Parkinsonism & related disorders, 72, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Timblin H, Rahmani E, Garrett S, Bunch J, Beaver H, & Hill CR (2021a). Physical Activity as a Mediator of Anxiety and Cognitive Functioning in Parkinson’s Disease. Journal of Mental Health and Physical Activity, 20, 1–6. 10.1016/j.mhpa.2021.100382 [DOI] [Google Scholar]
- Jones JD, Dominguez B, Bunch J, Uribe C, Valenzuela Y, & Jacobs JP (2021). A bidirectional relationship between anxiety, depression and gastrointestinal symptoms in Parkinson’s disease. Clinical parkinsonism & related disorders, 5, 100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Uribe C, Bunch J, & Thomas KR (2021c). Beyond PD-MCI: objectively defined subtle cognitive decline predicts future cognitive and functional changes. Journal of neurology, 268(1), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF (2000). Is depression a risk factor for dementia or cognitive decline? Gerontology, 46(4), 219–227. [DOI] [PubMed] [Google Scholar]
- Kim R, Park S, Yoo D, Jun JS, & Jeon B (2021). Association of Physical Activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology, 96(19), e2429–e2437. [DOI] [PubMed] [Google Scholar]
- Ku P-W, Steptoe A, & Chen L-J (2017). Prospective associations of exercise and depressive symptoms in older adults: The role of apolipoprotein E4. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 26(7), 1799–1808. https://doi-org.libproxy.lib.csusb.edu/10.1007/s11136-017-1537-1 [DOI] [PubMed] [Google Scholar]
- Lauze M, Daneault JF, & Duval C (2016). The effects of physical activity in Parkinson’s disease: A review. Journal of Parkinson’s Disease, 6, 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, & Katzen HL (1995). Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. In Weiner WJ & Lang AE (Eds), Behavioral neurology of movement disorders. (pp. 85–95). Raven Press. [PubMed] [Google Scholar]
- Li Y, Huang P, Guo T, Guan X, Gao T, Sheng W, Zhou C, Wu J, Song Z, Xuan M, Gu Q, Xu X, Yang Y, & Zhang M (2020). Brain structural correlates of depressive symptoms in Parkinson’s disease patients at different stage. Psychiatry Research: Neuroimaging, 296, 1–7. 10.1016/j.pscychresns.2019.111029 [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, … & Aarsland D (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement disorders, 27(3), 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L (2011). Leisure and depression in midlife: A Taiwanese national survey of middle-aged adults. Journal of health psychology, 16(1), 137–147. [DOI] [PubMed] [Google Scholar]
- Mantri S, Tropea T, & Morley J (2019, April). Physical Activity and Rates of Cognitive Decline in Early Parkinson Disease. NEUROLOGY (Vol. 92, No. 15). [Google Scholar]
- Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, … & Poewe W (2011). The parkinson progression marker initiative (PPMI). Progress in neurobiology, 95(4), 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimović D, Post B, Speelman JD, & Schmand B (2005). Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology, 65(8), 1239–1245. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, & Cotman CW (2009). Exercise improves cognition and hippocampal plasticity in APOEε4 mice. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 5(4), 287–294. https://doiorg.libproxy.lib.csusb.edu/10.1016/j.jalz.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky-Turk E, Moore RC, Filoteo JV, Litvan I, Song DD, Lessig SL, & Schiehser DM (2017). Neuropsychiatric predictors of cognitive decline in Parkinson disease: A longitudinal study. American Journal of Geriatric Psychiatry, 25, 279–289. 10.1016/j.jagp.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Zhang Z, & Zyphur MJ (2011). Alternative methods for assessing mediation in multilevel data: The advantages of multilevel SEM. Structural Equation Modeling, 18(2), 161–182. [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Wysocki M, Guerrero-Berroa E, Grossman HT, Heinz A, & Haroutunian V (2011). Cognitive decline in patients with dementia as a function of depression. The American Journal of Geriatric Psychiatry, 19(4), 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C (2015). A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adults populations. Health Psychology Review, 9(3), 366–378. [DOI] [PubMed] [Google Scholar]
- Sacheli MA, Murray DK, Vafai N, Cherkasova MV, Dinelle K, Shahinfard E, … & Jon Stoessl A (2018). Habitual exercisers versus sedentary subjects with Parkinson’s Disease: Multimodal PET and fMRI study. Movement Disorders, 33(12), 1945–1950. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Joiner T, Plant EA, & Blazer DG (2005). The influence of depression on cognitive decline in community-dwelling elderly persons. The American journal of geriatric psychiatry, 13(5), 402–408. [DOI] [PubMed] [Google Scholar]
- Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, … & Stebbins GT (2007). Depression rating scales in Parkinson’s disease: critique and recommendations. Movement disorders, 22(8), 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian N, Gu Y, Manly JJ, Schupf N, Mayeux R, Brickman AM, & Zahodne LB (2020). Linking depressive symptoms and cognitive functioning: The mediating role of leisure activity. Neuropsychology, 34(1), 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CH, Moore K, Browner N, Sklerov M, & Dayan E (2019). Physical activity mediates the association between striatal dopamine transporter availability and cognition in Parkinson’s disease. Parkinsonism & related disorders, 62, 68–72. [DOI] [PubMed] [Google Scholar]
- Silveira CRA, Roy EA, Intzandt BN, & Almeida QR (2018). Aerobic exercise is more than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain and Cognition, 122, 1–8. http://dx.doi.org.libproxy.lib.csusb.edu/10.1016/j.bandc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Smith A (1982). Symbol digit modalities test (SDMT) manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, Butts AM, Hantke NC, Lancaster MA, & Rao SM, (2011). Interactive effects of physical activity and APOE-E4 on BOLD semantic memory activation in healthy elders. NeuroImage, 54, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, & Robinson RG (1992). A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 55(5), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowicz SM, Dotson VM, Jones JD, Okun MS, & Bowers D (2018). Symptom dimensions of depression and apathy and their relationship with cognition in Parkinson’s disease. Journal of the Inter- national Neuropsychological Society, 24, 269–282. 10.1017/S1355617717001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes OB, & Storstein A (2017). Epidemiology of Parkinson’s disease. Journal of Neural Transmission, 124(8), 901–905. [DOI] [PubMed] [Google Scholar]
- van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, Overeem S, Deeg DJ, Borm GF, … & Munneke M (2011). Physical inactivity in Parkinson’s disease. Journal of neurology, 258(12), 2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, & Janney CA (1993). The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology, 46(2), 153–162. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson, 22, 498. [Google Scholar]
- Winocur G, Wojtowicz JM, Huang J, & Tannock IF (2014). Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology, 231(11), 2311–2320. https://doiorg.libproxy.lib.csusb.edu/10.1007/s00213-013-3394-0 [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, & Mattis P (2003). A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cognitive and behavioral neurology, 16(4), 193–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


