Abstract
As part of our ongoing effort to develop a standardized competitive enzyme immunoassay for human autoantibodies to oxidized low-density lipoprotein (oxLDL), we have generated a reference human antibody standard and revised some of the conditions of the assay. The preparation of the standard involved purification of human anti-oxLDL antibodies by affinity chromatography using immobilized oxLDL. The total concentration of antibody in this purified human oxLDL antibody was established by adding the concentrations of immunoglobulin G (IgG), IgA, and IgM determined in the standard by radial immunodiffusion. The isolated antibody was used to calibrate a whole human serum standard, which was used to calibrate the assays to detect antibody in serum samples. We also revisited the general conditions for performance of our competitive assay. We determined that 1/20 was the ideal dilution for performing the absorption step, and that 1/20 and 1/40 were optimal dilutions to assay oxLDL antibody in unknown serum samples. We also established that the optimal concentration of oxLDL for absorption of free antibody in serum samples was 200 μg of oxLDL/ml; no significant decrease in the reactivity of samples with immobilized oxLDL was observed when higher concentrations of oxLDL were used for absorption. The minimum detection level of the assay is 0.65 mg/liter. Because serum samples are diluted 1/20 and 1/40 for the assay, the minimal concentration of antibody detectable in serum is 20-fold higher, i.e., 13 mg/liter. The intraassay coefficient of variation calculated from seven determinations of three samples containing antibody concentrations of 240, 340, and 920 mg/liter ranged from 8 to 6.1%. The interassay coefficients of variation for sera with antibody levels of 100 to 594 mg/liter varied from 9.2 to 7.0%, and for isolated antibodies with concentrations of 52 to 111 mg/liter, the coefficients varied from 5.8 to 3.9%.
The role of autoantibodies against oxidatively modified low-density lipoproteins (oxLDL) in the pathogenesis of atherosclerosis is, presently, the object of intense investigation. Experiments conducted in vitro have shown that LDL may be oxidized by several types of cells, including endothelial cells, smooth muscle cells, and macrophages (2, 11, 13, 17). oxLDL has been found in atheromatous lesions (5, 12, 22), and LDL extracted from atherosclerotic lesions exhibits nearly all of the physicochemical and immunological properties of copper-oxidized LDL (21). Antibodies against oxLDL (anti-oxLDL) have been demonstrated in human serum (15, 19) and in atherosclerotic lesions of rabbits and humans (6, 20, 21). Such antibodies recognize epitopes expressed in atherosclerotic lesions of rabbits and humans but not in normal arteries (1, 5, 12). However, the pathogenic significance of anti-oxLDL antibodies remains uncertain due to the discrepant results published by several groups of investigators who found either a significant correlation between circulating anti-oxLDL antibody levels and manifestations of atherosclerosis or no correlation at all (4, 15, 18, 19).
The methods used for the measurement of circulating anti-oxLDL antibodies include radioimmunoassays (15) and enzymeimmunoassays (4, 14, 18, 19). Most of the assays are based on a comparison of the reactivities of a sample with immobilized oxLDL and with immobilized native LDL, and the results are expressed either as a difference or a ratio that reflects the increased binding to oxLDL (4, 14, 15, 18). This methodology can underestimate the antibody levels if the anti-oxLDL antibodies cross-react with native LDL (10) or may result in falsely elevated values due to the lack of correction for charge-dependent nonspecific interactions that are likely to be quite different between native LDL and oxLDL, which, as it is well known, has an increased negative charge. Furthermore, the expression of data in arbitrary units, whether they are ratios or differences in optical density (OD), represents a significant obstacle in the comparison of data obtained by different groups.
To solve these problems, it seemed essential to devise assays that were not only of satisfactory specificity and reproducibility but also adequately standardized. Issues of specificity and reproducibility were approached by our group several years ago, with the development of a competitive assay, based on the measurement of binding values before and after absorption with oxLDL, which we have routinely used in our laboratory (19). The development of calibrator standards with known antibody concentrations, allowing expression of antibody concentration in standard mass units rather than in arbitrary units, appears to be an important step towards standardization. Originally, we used immunoglobulin G (IgG) isolated from a rabbit hyperimmune anti-human LDL antiserum to standardize the assay (19), but this approach had the important drawback that only an undetermined proportion of this IgG reacted with oxLDL. In addition, two different conjugates had to be used in each plate: an anti-rabbit IgG for the calibration curve and anti-human IgG for the human samples. This report describes the standardization of our oxLDL antibody assay for whole human serum by using a human serum standard to calibrate the assay, as well as other modifications in the assay conditions that appear to improve the reproducibility of the assay.
MATERIALS AND METHODS
Lipoprotein isolation, modification, and characterization.
Blood for lipoprotein isolation was collected from healthy volunteers after 12 h of fasting into a 0.4-mmol/liter concentration of EDTA. Plasma from three to four healthy volunteers was used for separation of LDL by preparative ultracentrifugation at 50,000 rpm for 17 h on a Beckman L-80 ultracentrifuge after density adjustment with potassium bromide (1.019 < density < 1.063 g/ml) using a type 70 Ti rotor (8). Isolated LDL was washed by ultracentrifugation, dialyzed against a 0.15-mol/liter sodium chloride solution containing 300 μmol of EDTA per liter, pH 8.0, passed through an Acrodisc filter (0.22 μm pore size) in order to sterilize and remove aggregates, and stored under nitrogen in the dark at 4°C.
Oxidation of LDL was performed in accordance with the protocol described by Steinbrecher (16). To remove residual KBr and EDTA, the freshly isolated LDL was passed through a PD-10 column (Pharmacia Biotech, Uppsala, Sweden). Phosphate-buffered saline (PBS), pH 7.4, was oxygenated at 2 liters/min for 10 min, and copper chloride was added to a final concentration of 10 μmol/liter for every 300 μg of LDL. The LDL final concentration was adjusted not to exceed 1.5 mg/ml, and the highest concentration of CuCl2 used was 40 μmol/liter when the concentration of oxLDL was between 1.2 and 1.5 mg/ml. The oxidation reaction was carried out at 37°C, and to monitor the degree of oxidation, an aliquot (100 μg) of LDL, prepared as described above, was diluted in 2 ml of PBS and continuously monitored at 37°C on a luminescence spectrophotometer (SLM-Aminco Series 2; Spectronic Instruments, Rochester, N.Y.) using a wavelength of 360 nm for excitation and a wavelength of 430 nm to measure fluorescence emission (3). The fluorescence profile shows a steady increase during the first 8 to 12 h, after which a plateau is reached, and the fluorescence values remain stable for up to 6 days after the plateau is reached (8a). The results of oxLDL antibody assays using oxLDL preparations obtained 5 to 48 h after the fluorescence levels reached their peak do not differ significantly. To avoid unnecessary degradation, we decided to stop the oxidation reaction 4 to 6 h after the fluorescence reached its peak by adding EDTA and butyl-hydroxytoluene (BHT) to final concentrations of 300 and 200 μmol/liter, respectively. LDL preparations obtained in this manner have yielded reproducible results in our competitive enzyme immunoassay. Copper and BHT were removed from the oxLDL preparations after termination of the oxidation reaction by overnight dialysis against 4 liters of a 0.15-mol/liter concentration of sodium chloride containing 300 μmol of EDTA (pH 8.0) per liter. After dialysis, the oxLDL preparations were filtered through a 0.22-μm-pore-size filter to sterilize and remove aggregates and stored at 4°C. The final protein concentration in each preparation was determined by a Lowry assay (7).
Isolation of anti-oxLDL antibodies.
Anti-oxLDL antibodies were isolated by using an affinity chromatography protocol previously described by Mironova et al. (10). In summary, freshly isolated LDL was dialyzed against a coupling buffer containing 0.1 mol of NaHCO3 per liter and 0.5 mol of NaCl (pH 8.3) per liter and incubated overnight on a rocker at 4°C with CNBr-activated Sepharose 4B (Pharmacia Biotech) prepared in accordance with the manufacturer’s instructions and equilibrated with the same coupling buffer. At the end of this incubation, free binding sites were blocked with a solution containing 0.2 mol of glycine (pH 8.0) per liter, and after extensive washing and degassing, the gel suspension was transferred to a chromatography column and washed thoroughly with PBS, pH 7.4. The column was then equilibrated with PBS containing 10 μmol of CuCl2 per liter, and oxidation was allowed to proceed for 18 h at 37°C. The protein concentration is estimated to correspond to about 300 to 400 μg/ml of gel, based on the coupling efficiency data published by Pharmacia Fine Chemicals. Therefore, the conditions of immobilized LDL oxidation were similar to those used for LDL in solution. The oxidation reaction was stopped by washing the column extensively with PBS containing 300 μmol of EDTA per liter and 200 μmol of BHT per liter followed by washes with NaHCO3 buffer and acetate buffer. Finally, the column was equilibrated with 0.01 mol of NaHCO3 buffer (pH 8.3) per liter.
To isolate anti-oxLDL antibodies from serum samples, 1 ml of serum was diluted with 4 ml of the bicarbonate buffer used to equilibrate the affinity chromatography column, and it was allowed to diffuse into the column. After the column loaded with diluted serum was incubated overnight at 4°C, unbound proteins were washed off with the equilibrating buffer. The bound antibodies were eluted with a 0.1-mol/liter concentration of NaHCO3 (pH 8.3) buffer containing 0.5 mol of NaCl per liter, and after the OD returned to baseline, the column was eluted a second time with 0.2 mol of glycine-HCl buffer (pH 2.3) per liter. All of the bound antibody was eluted with 0.1 mol of NaHCO3 (pH 8.3) per liter. As shown in Table 1, the antibody purified in this manner was predominantly of the IgG isotype, but IgA and IgM antibodies were also isolated. No contaminating proteins were detected in the purified antibody preparations by immunoelectrophoresis. The purified antibodies were aliquoted and stored at −20°C without addition of preservatives until use. Under these conditions, the purified antibody remained stable for at least 6 months, as demonstrated by its reproducible reactivity with immobilized oxLDL.
TABLE 1.
General characteristics of the samples used in the study
| Sample | Aba concn (mg/liter) | % Isotypeb
|
Kd (mol/liter)c | ||
|---|---|---|---|---|---|
| IgG | IgA | IgM | |||
| Whole human serum | |||||
| Sample A | 100 | ||||
| Sample B | 177 | ||||
| Sample C | 287 | ||||
| Sample D | 594 | ||||
| Purified Ab from: | |||||
| Serum sample A | 111d | 88 | 4.5 | 7.5 | 1.9 × 10−8 |
| Serum sample B | 82d | 82 | 12 | 6 | 7.7 × 10−8 |
| Serum sample C | 52d | 76 | 10.5 | 13.5 | 2.0 × 10−8 |
| Purified Ab standard | 117d | 68.5 | 11 | 24 | 6.6 × 10−9 |
| Purified rabbit Ab | 84.0 | 1.4 × 10−11 | |||
Ab, antibody.
In isolated antibody preparations; derived from the sum of the three isotype concentrations as determined by radial immunodiffusion.
High-affinity component.
On the pool of antibody-containing fractions eluted from the immobilized oxLDL column; values not corrected for dilution during isolation.
Standards and quality control sera.
Purified human oxLDL antibody, prepared as described above, was used to calibrate a whole human serum sample, which was subsequently used as the standard for all assays measuring serum oxLDL antibody levels. Since the assay, as described in this manuscript, will detect antibodies of all isotypes, and since the oxLDL antibodies purified by affinity chromatography can be distributed among the three major immunoglobulin isotypes (Table 1), we used the total antibody concentration in the purified human antibody preparation, obtained by adding the concentrations of IgG, IgA, and IgM determined by radial immunodiffusion (medium-level radioimmunodiffusion kits; The Binding Site), as our reference value.
As quality controls for the assay, we used three serum samples with different levels of oxLDL antibodies (Table 1). The isotype distribution and the dissociation constant values (Kd) for our human antibody standard and for the oxLDL antibodies isolated from three of the quality control serum samples, determined as previously described (10), are also shown in Table 1. Table 1 also includes, for reference purposes, the Kd value for a rabbit anti-human LDL antibody isolated by affinity chromatography using the same column and general procedure outlined for isolation of human oxLDL antibodies. Standards and quality controls were aliquoted and stored at −20°C without additives and have remained stable for at least 6 months (their reactivity in the oxLDL antibody enzyme immunoassay has remained basically unchanged).
Enzyme immunoassay.
The concentration of oxLDL antibody was determined in all samples (whole serum samples and isolated antibodies) by the competitive enzyme immunoassay previously described by us (19). The assay was conducted on flat-bottom Immulon type 1 enzyme immunoassay plates purchased from Dynatech (Chantilly, Va.). The plates were coated immediately prior to use by overnight incubation at 4°C with 100 μl of oxLDL (7.5 mg/liter in a 1-mol/liter concentration of carbonate-bicarbonate buffer [pH 9.6]). Serum calibrators, controls, and unknown samples were diluted 1/10 in PBS containing 1% bovine serum albumin (PBS-BSA) and were then separated into two 200-μl aliquots. One of the aliquots was absorbed with an equal volume of oxLDL (400 μg/ml in PBS-BSA), with the same batch of oxLDL used to coat the plates in the absorption step. The other aliquot (unabsorbed) was mixed with an equal volume of PBS-BSA. The final dilution of these aliquots was 1/20. All samples (absorbed and unabsorbed) were incubated overnight at 4°C on a rocker. After incubation, the plates were washed with 0.05% Tween 20 (Sigma Chemical Co.) in PBS, pH 7.8, to remove unbound oxLDL, blocked with 5% BSA in PBS (1 h at room temperature), and washed four more times. Neither the coating buffer nor any of the remaining buffers used to perform the assay contained antioxidants because a comparison of assays performed with and without addition of antioxidants (EDTA at 0.27 mmol/liter and BHT at 20 μmol/liter) to the buffers showed no difference in the final results. The absorbed and unabsorbed aliquots were spun at 9,000 × g in an Eppendorf centrifuge (model 5413) for 30 min. The supernatants of the centrifuged samples, unabsorbed and absorbed, were divided into two portions, one to be tested as it was absorbed (1:20 dilution) and the other one to be tested after being diluted 1:2 (final dilution, 1:40). One-hundred-microliter volumes of both dilutions were transferred to the wells of the oxLDL-coated plates. After overnight incubation at 4°C and extensive washing, peroxidase-conjugated rabbit anti-human IgG (IgG fraction), reacting with both IgG heavy chains and light chains (Cappel Organon Teknika, Durham, N.C.), was added to the wells. After incubation for 1 h at 4°C the unbound antibody was washed off and the plates were incubated for 10 min in the dark with the substrate (0.5 mmol of 2,2′-azino-di-[3-ethylbenzthiazoline-6-sulfonate] [ABTS; Sigma Chemical Co., St. Louis, Mo.] per liter and 3% [vol/vol] hydrogen peroxide in a 45-mmol/liter concentration of citric acid buffer [pH 4.0]). The reaction was stopped with 0.1 mol of citric acid (pH 2.1) per liter and the absorbance was measured at 414 nm in a VMax enzyme-linked immunosorbent assay reader (Molecular Devices, Sunnyvale, Calif.).
The OD values used to calculate antibody concentrations in both standards and unknown samples were obtained by subtracting the OD values of the oxLDL-absorbed samples from those of the unabsorbed samples. Five serial dilutions of the whole human serum standard were used to calibrate the assay. The best fit for the calibration curve was obtained with a third-degree polynomial function. The concentration of the antibody in unknown samples represents the average of two determinations (1/20 and 1/40 dilutions) after correction by the dilution factor.
RESULTS
Characterization of the calibration standard.
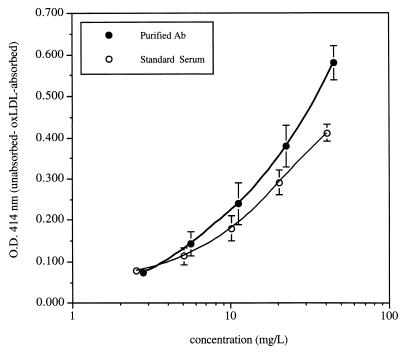
The dose-response curves obtained with purified human oxLDL antibody and the calibrated whole human serum standard are reproduced in Fig. 1. Each point represents the mean of results obtained in four (human antibody standard) to six (purified anti-oxLDL) different assays run in duplicate.
FIG. 1.
Composite concentration versus OD interpolation plots for oxLDL standards used for calibration of our oxLDL antibody assay. The two plots correspond to data generated with isolated human antibodies and whole human serum (plot of the means of six successive determinations of isolated human anti-oxLDL antibodies [•] and plot of the means of four successive determinations of the whole human serum standard [○]. The concentrations of antibody in each standard were determined as detailed in the text. Each value corresponds to the mean of six assays ± 1 standard deviation for the isolated antibody and of four assays ± 1 standard deviation for the serum standard. Each point in the figure corresponds to the difference in OD414 readings between unadsorbed and oxLDL-adsorbed samples.
The mean concentration of the purified human antibody, calculated by adding the concentrations of IgG, IgA, and IgM in the purified reference antibody preparation determined in triplicate by radial immunodiffusion, was 117 mg/liter. The antibody concentration on the lowest dilution (1/20) of the whole human serum standard used for calibration purposes, determined against the calibration curve constructed with purified human oxLDL antibody, was 40.8 ± 5.38 mg/liter (mean ± standard deviation of three different assays, each one of which included three dilutions of the serum standard run in duplicate). By running seven series of dilutions of the serum standard, we found that the lowest concentration of antioxLDL antibodies that we could determine with satisfactory accuracy was 0.65 mg/liter, which translates to a minimal concentration of 13 mg/liter on serum samples (which are diluted 1/20 and 1/40 for the assay) and 1.3 mg/liter on purified antibody preparations (which only need to be diluted 1/2 for the absorption step).
The slopes of the concentration versus OD plots of purified antibody and serum antibody are slightly different, probably reflecting the selection of antibodies of relatively higher affinity in the purification process. Thus, the preparation of a whole human serum standard has the advantage not only of making frequent antibody purification unnecessary but also of providing a more accurate calibration curve for the assay of circulating antibodies.
Precision parameters.
The intraassay coefficient of variation (CV) calculated from seven determinations of three samples containing antibody concentrations of 240, 340, and 920 mg/liter ranged from 8 to 6.1%. The interassay CVs for serum samples with antibody levels of 100 to 594 mg/liter varied from 9.2 to 7.0%, and for isolated antibodies with concentrations of 52 to 111 mg/liter, the CVs varied from 5.8 to 3.9%. Three to five runs were performed to calculate the interassay CVs (Table 2).
TABLE 2.
Interassay variation of the standards and quality control samples
| Purified antibody or serum sample | na | Anti-oxLDL antibodies (mg/liter)
|
CV (%) | |
|---|---|---|---|---|
| Mean | SD | |||
| Purified antibodies | ||||
| B | 3 | 111 | 4.2 | 3.9 |
| C | 3 | 82 | 4.1 | 4.9 |
| D | 3 | 52 | 3.0 | 5.8 |
| Serum samples | ||||
| A | 4 | 100 | 7.4 | 7.4 |
| B | 3 | 177 | 16.3 | 9.2 |
| C | 5 | 287 | 21.2 | 7.4 |
| D | 5 | 594 | 41.83 | 7.0 |
n, number of determinations.
Determination of the optimal conditions for the assay of oxLDL antibodies.
In a competitive immunoassay such as the one that we developed, three major variables need to be considered: the degree of LDL oxidation and its consistency from batch to batch, the concentration of antigen used in the absorption step, and the dilution of the unknown samples in order to fall in the linear part of the calibration curve.
The degree of LDL oxidation can be monitored by several different parameters, such as formation of thiobarbituric acid reactive substances (7), formation of conjugated dienes (7), and generation of fluorescent compounds during oxidation (3). We have used the last parameter to ensure that the degree of oxidation was similar in the different batches of oxLDL used in our experiments, thus ensuring optimal and reproducible reactivity with oxLDL antibodies. Such optimal reactivity of anti-oxLDL antibodies was observed with LDL preparations that had been stopped from further oxidation 4 to 6 h after fluorescence levels reached their maximum level.
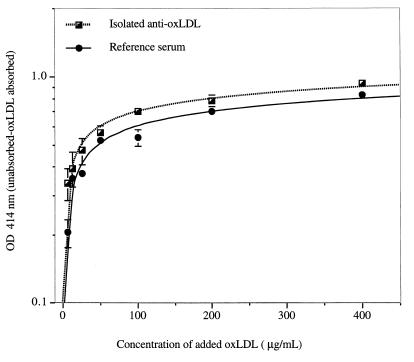
To determine the concentration of oxLDL that led to maximal absorption of oxLDL antibody, we absorbed both our reference whole serum (at a final dilution of 1/20) and our reference isolated antibody (at a final dilution of 1/2) with various concentrations of oxLDL (12.5 to 800 μg of oxLDL per ml). As shown in Fig. 2, we observed a direct correlation between the concentration of oxLDL used for absorption and the difference in OD values between absorbed and unabsorbed samples. Figure 2 also shows that the degree of absorption starts to reach a plateau at an oxLDL concentration of 100 μg/ml, and there is no evidence for significant increases in absorption with concentrations higher than 200 μg/ml. The percentage reduction of OD after absorption with 200 μg of oxLDL for serum samples containing a high titer of antioxLDL antibodies was similar for dilutions ranging from 1/10 to 1/80 (results not shown). In accordance with these results, we have chosen 200 μg/ml as the concentration of oxLDL used for absorption.
FIG. 2.
Concentration of oxLDL required for maximal absorption of free anti-oxLDL antibodies. Each point in the figure corresponds to the difference in OD readings at 414 nm between unabsorbed and absorbed aliquots of our reference purified antibody and serum D (see Table 1). Identical dilutions of the antibody preparations (1/2 for isolated antibody, 1/20 for serum) were absorbed with increasing concentrations of oxLDL, as shown in the figure. In the unabsorbed aliquots, oxLDL was replaced by PBS. Each value corresponds to the mean duplicate values ± 1 standard deviation.
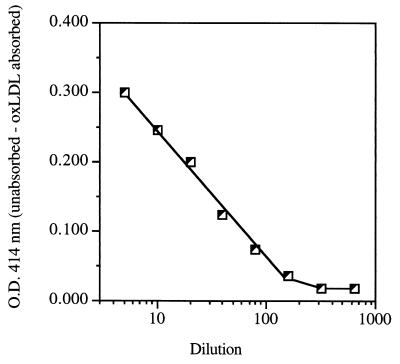
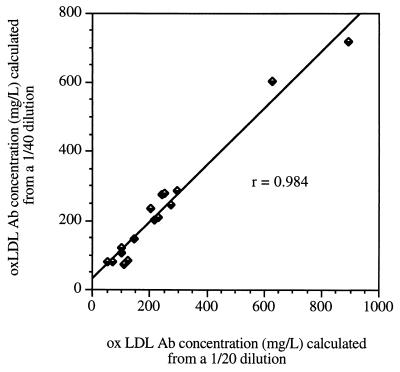
To determine the optimal sample dilution to adequately measure oxLDL antibody concentrations in whole serum, we prepared several dilutions of our reference serum, ranging from 1/5 to 1/800, to determine which dilutions would fall in the linear part of the calibration curve (Fig. 3). Dilutions near the middle range of the calibration curve are preferred to avoid errors due to either antigen excess or excessively low antigen concentrations. Therefore, 1/20 and 1/40 appeared to be optimal. We adopted 1/20 as the dilution to be absorbed, and each sample is assayed at 1/20 and 1/40 dilutions. The correlation between the values obtained with these two dilutions after correction by the dilution factor is excellent (Fig. 4). Linear regression analysis showed a highly significant (P < 0.0001) correlation between the two sets of values (r = 0.984).
FIG. 3.
Effect of dilution on the measurement of OD differences between unabsorbed and oxLDL-absorbed samples of serum sample D (see Table 1). The initial absorbed and unabsorbed aliquots were prepared at a 1/5 dilution, and a series of dilutions was prepared from those aliquots. Each point in the graph corresponds to the difference in OD between identical dilutions of the unabsorbed and absorbed aliquots run in duplicate.
FIG. 4.
Degree of agreement between oxLDL antibody concentrations calculated from 1/20 and 1/40 dilutions of the unknown samples. The plotted data correspond to a single run in which all samples were tested at both dilutions. Values entered in the plot were corrected by multiplication by the respective dilution factors.
DISCUSSION
There is a recognized need to establish standardized assays for anti-oxLDL antibodies that would allow different groups to generate comparable data. Several issues need to be addressed in this effort, including choice of the assay procedure, choice of a suitable antigen, and development of calibrators that would allow the expression of data in standard units of antibody concentration.
We report in this publication the results of our efforts leading to the establishment of calibration standards for oxLDL antibody assays, which, to our knowledge, had not been previously established. This was possible because of the development of a reliable affinity chromatography method for the isolation of oxLDL antibodies (10) that allowed us to isolate antibodies from human sera. The isolated oxLDL antibodies always include molecules of the three major isotypes (IgG, IgA, and IgM), and since our assay is designed to measure total antibody concentrations, the total antibody concentration in the isolated antibody preparation was established by adding the concentrations of IgG, IgA, and IgM, determined by radial immunodiffusion, and this concentration was used as the starting point to calibrate a whole serum sample.
The advantages of establishing a whole serum standard are manifold. On the one hand, it is easier to calibrate a serum sample from which a large number of aliquots can be prepared and properly stored, avoiding the problems inherent to repeated and frequent antibody isolations that would be a major drawback if the standard was prepared with purified antibody. In addition, Fig. 1 shows that the concentration versus OD curves obtained with the antibody standard and the serum standard have different slopes. The most likely cause for this difference in the calibration curves is the selection of an antibody population of higher affinity during purification by affinity chromatography. Those antibodies with lower affinity are likely to have been washed off the column, while the population with a relatively higher affinity would have remained bound to the column. For practical purposes, using a purified antibody standard would add an element of imprecision to the assay.
To further increase the precision of the assay, it is important to avoid confounding factors and to standardize as many of the assay steps as possible. Our decision to develop a competitive assay which allows the calculation of specific binding, i.e., the difference in binding between unabsorbed and oxLDL-absorbed aliquots, rather than a comparative assay based on the different reactivities of sera with oxidized and native LDL, was based on the desire to avoid the interference of two sources of nonspecific binding (“charged” oxLDL and native LDL). Most of the oxLDL antibody immunoassays compare the reactivity of a given sample with oxidized LDL and that of a sample with native LDL. The values for oxLDL antibodies obtained in these assays are expressed in arbitrary units, either as the ratios of the OD value recorded with oxLDL to that with native LDL or as the differences between these two OD values (4, 14, 15, 18). The problem with this approach is that oxidized LDL has a much stronger negative charge than native LDL. Therefore, the measurement of the reactivity with native LDL as an index of nonspecific binding ignores the fact that the nonspecific binding levels of IgG molecules (which at pH 7.4 have a charge between neutral and weakly positive) to oxidized and native LDL are significantly different and constitutes an uncontrolled variable factor which interferes with the final calculation of antibody concentrations.
There is general agreement about the use of copper-oxidized LDL as an antigen in oxLDL antibody assays because, according to observations of Ylä-Herttuala et al., copper-oxidized LDL carries nearly the same properties as the oxLDL found in human atheromatous lesions (21). It must be noted, however, that we and others (15) have experienced a considerable degree of variability when testing for anti-oxLDL antibodies using different batches of oxLDL, even when they are prepared under the same conditions and from the same donor pool. This seems to be related to differences in oxidation susceptibility between different batches of LDL, which results in difficulties when attempts are made to reproduce the same degree of oxidation at different times under the same apparent experimental conditions. Thus, it is likely that the epitopes exposed by different batches of oxLDL may be quite variable, unless the degree of oxidation is properly controlled.
In our laboratory, copper-oxidized LDL has allowed us to obtain data with the highest reproducibility, providing that the degree of oxidation of LDL is properly standardized to ensure that different batches have similar degrees of reactivity with the corresponding antibodies. This reproducibility has been achieved with oxLDL preparations collected 3 to 5 h after the formation of fluorescent LDL protein compounds reaches its peak. Under those conditions, regardless of the time required for the fluorescence values to reach their peak, each batch of LDL used in our experiments is at the same stage of oxidation and the reactivity with reference antibodies remained reproducible.
In addition, we decided it was also important to establish the optimal concentrations of oxLDL required for the absorption step as well as the optimal sample dilution for performance of the assay. The parameters now recommended are slightly different from those included in the original description of the assay, increasing the concentration of oxLDL used for absorption to 200 μg/ml and increasing the serum dilution used in the absorption step from 1/10 to 1/20. These changes were adopted to ensure a more complete blocking of free antibody sites and to ensure that the OD values obtained with most unknown samples fall in the optimal range of the calibration curve. The antibody concentrations calculated from the two dilutions correlated extremely well (Fig. 4). Testing two different dilutions has an advantage over duplicates of a single dilution because in the occasional serum sample in which the antibody concentrations may be excessive, the two dilutions will give dissimilar results (the concentration calculated from the 1/40 dilution exceeding the concentration calculated from the 1/20 dilution), and the lack of concordance between the two dilutions will alert us to the problem. Such samples are then tested at higher dilutions, seeking those that give concordant results.
The final assessment of the performance of our assay was to determine the intra- and interassay CVs both for the measurement of isolated anti-oxLDL antibodies and serum samples. The CVs corresponding to the intraassay variability varied from 8 to 6.1%, and the CVs corresponding to the interassay variability ranged from 5.8 to 3.9% with purified antibodies and from 9.2 to 7.0% with serum samples (Table 2). Comparing the CVs for purified antibodies and serum samples at similar antibody concentrations, it is obvious that the reproducibility is better with purified antibodies, which is usually the case in all enzyme immunoassays. Still, an interassay CV of 9.2% at the lower level of detection is an excellent index of the stability and precision of the method, particularly when we take into consideration that the analyte is an antibody of moderate to low affinity.
In conclusion, we have developed a human antibody standard for the assay of oxLDL antibodies and have optimized the competitive enzyme immunoassay for oxLDL antibodies that was previously described (19). These new developments will allow a more precise and reproducible determination of the levels of circulating anti-oxLDL antibodies. As the assays for oxLDL antibodies become more accurate and reproducible, we expect that the discrepancies found in the literature on the correlation between circulating anti-oxLDL antibody levels and manifestations of atherosclerosis (4, 15, 18, 19) will be finally resolved.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HL-55782, by the Research Service of the Department of Veterans Affairs, and by grants from the Academy of Finland, Pohjois-Karjala Cultural Foundation, Aarne Koskelo Foundation, and Jalmari and Rauha Ahokas Foundation. Sinikka Koskinen is a postdoctoral fellow of the Juvenile Diabetes Foundation International.
We thank Charlyne Chassereau for her skilled technical assistance and Alva Mullins for editorial assistance.
REFERENCES
- 1.Boyd H C, Gown A M, Wolfbauer G, Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989;135:815–825. [PMC free article] [PubMed] [Google Scholar]
- 2.Cathcart M K, Morel D W, Chisolm G M. Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985;38:341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- 3.Cominacini L, Garbin U, Davoli A, Micciolo R, Bosello O, Gaviraghi G, Scuro L A, Pastorino A M. A simple test for predisposition to LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J Lipid Res. 1991;32:349–358. [PubMed] [Google Scholar]
- 4.Craig W Y, Davis A E, Poulin S E. Effects of incubation conditions on ELISA for autoantibodies against oxidized low-density lipoprotein. Clin Chem. 1996;42:1709–1711. [PubMed] [Google Scholar]
- 5.Haberland M E, Fong D, Cheng L. Malonyldialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988;241:215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- 6.Hoff H, O’Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb. 1991;11:1209–1222. doi: 10.1161/01.atv.11.5.1209. [DOI] [PubMed] [Google Scholar]
- 7.Kim R S, LaBella F S. Comparison of analytical methods for monitoring auto-oxidation profiles of authentic lipids. J Lipid Res. 1987;28:1110–1117. [PubMed] [Google Scholar]
- 8.Lopes-Virella M F, Sherer G K, Lees A M, Wohltmann H, Mayfield R, Sagel J, LeRoy E C, Colwell J A. Surface binding, internalization, and degradation by cultured human fibroblasts of LDL isolated from type 1 (insulin-dependent) diabetic patients: changes with metabolic control. Diabetologia. 1982;22:430–436. doi: 10.1007/BF00282585. [DOI] [PubMed] [Google Scholar]
- 8a.Lopes-Virella, M. F., et al. Unpublished data.
- 9.Lowry O H, Rosebrough N J, Farr A L, Randall A J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.Mironova M, Virella G, Lopes-Virella M F. Isolation and characterization of human antioxidized LDL antibodies. Arterioscler Thromb Vasc Biol. 1996;16:222–229. doi: 10.1161/01.atv.16.2.222. [DOI] [PubMed] [Google Scholar]
- 11.Morel D W, DiCorleto P E, Chisolm G M. Endothelial and smooth muscle cells alter low-density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984;4:357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- 12.Palinski W, Rosenfeld M E, Ylä-Herttuala S, Gurtner G C, Socher S S, Butler S W, Parthasarathy S, Carew T E, Steinbergand D, Witztum J L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parthasarathy S, Printz D J, Boyd D, Joy L, Steinberg D. Macrophage oxidation of low-density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986;6:505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- 14.Raitakari, O. T., O.-P. Pitkänen, T. Lehtimäki, S. Lahdenperä, I. Hidehiro, S. Ylä-Herttuala, J. Luoma, K. Mattila, T. Nikkari, M. R. Taskinen, J. S. Viikari, and J. Knuuti. In vivo low density lipoprotein oxidation relates to coronary reactivity in young men. J. Am. Coll. Cardiol. 30:97–102. [DOI] [PubMed]
- 15.Salonen J T, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum J L. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrecher U P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–3608. [PubMed] [Google Scholar]
- 17.Steinbrecher U P, Parthasaranthy S, Leake D S, Witzum J L, Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci USA. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uusitupa M I J, Niskanen L, Luoma J, Vilja P, Rauramaa R, Ylä-Herttuala S. Autoantibodies against oxidized LDL do not predict atherosclerosis vascular disease in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:1236–1242. doi: 10.1161/01.atv.16.10.1236. [DOI] [PubMed] [Google Scholar]
- 19.Virella G, Virella I, Leman R B, Pryor M B, Lopes-Virella M F. Anti-oxidized low-density lipoprotein antibodies in patients with coronary heart disease and normal healthy volunteers. Int J Clin Lab Res. 1993;23:95–101. doi: 10.1007/BF02592290. [DOI] [PubMed] [Google Scholar]
- 20.Ylä-Herttuala S, Butler S, Picard S, Palinski W, Steinberg D, Witztum J L. Rabbit and human atherosclerotic lesions contain IgG that recognizes MDA-LDL and copper-oxidized LDL. Atherosclerosis. 1991;11:1426a. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Ylä-Herttuala S, Palinski W, Rosenfeld M E, Parthasarathy S, Carew T E, Butler S, Witztum J L, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ylä-Herttuala, S., W. Palinski, M. E. Rosenfeld, D. Steinberg, and J. L. Witztum. 1990. Lipoproteins in normal and atherosclerotic aorta. Eur. Heart J. 11(Suppl. E):88–99. [DOI] [PubMed]