Abstract
Background and Objectives
Migraine has consistently been associated with an increased risk of cardiovascular disease (CVD) events. It remains, however, unclear to what extent cardiovascular risk profiles might be linked with migraine activity status and how these profiles relate to the development of migraine.
Methods
We used data from a cohort study of female health professionals (Women's Health Study, n = 27,539, age ≥45 years at baseline) without a history of CVD or other major diseases and who provided a blood sample at baseline. Framingham risk scores (FRSs) estimating the 10-year risk of coronary heart disease calculated at baseline were used to create vascular risk categories. The presence or development of self-reported migraine was assessed by questionnaires. Women were classified as having no migraine, history of migraine (experienced migraine in the past but did not experience any migraine attacks in the year before enrollment), active migraine at baseline (active), or incident migraine (first report of migraine during follow-up but not at baseline). We used multinomial logistic regression models to calculate ORs for the association between FRS categories and migraine status.
Results
Of the 27,539 participants, a total of 21,927 women did not report migraine, 1,500 women reported a history of migraine, 3,579 had migraine at baseline, and 533 reported migraine for the first time during follow-up. The odds of the probability of having a history of migraine at baseline (vs never migraine) was 76% higher among those with FRS ≥10% compared with FRS ≤1% after adjustment (OR = 1.76, 95% CI 1.39–2.23). In contrast, having FRS ≥10% was associated with reduced odds of having active migraine at baseline (OR = 0.64, 95% CI 0.52–0.80) and with newly reported migraine during follow-up (OR = 0.42, 95% CI 0.22–0.81) when compared with women with FRS category ≤1% and those not reporting migraine. A similar association pattern was observed for FRS categories 5%–9% and 2%–4%.
Discussion
High FRS categories were only observed among women with a history of migraine but not with active migraine at baseline or incident migraine after baseline. Our results suggest that the life course of migraine should be considered when studying associations with the vascular system. Our data further suggest that a relatively healthy vascular system, as assessed by the FRS, is associated with active migraine status or developing migraine in the future.
Migraine is a chronic-intermittent primary headache disorder that affects about one-sixth of the adult population, particularly females, and is not only associated with a substantial burden for the individual migraine patients but for society as a whole.1,2 Several aspects of the pathomechanisms of migraine directly or indirectly involve the vascular system,3 and migraine-specific medications may affect vasomotor tone, either directly by inducing vasoconstriction4 or indirectly by affecting vasodilation.5,6
Migraine has been linked with an increased risk of cardiovascular disease (CVD) in numerous studies7-10 and, as such, was included among the risk factors for CVD in guidelines from the European Society for Cardiology11 and in a recently developed cardiovascular risk score.12 There appears to be a paradoxical interrelationship between migraine, the presence of specific vascular risk factors, and CVD. For instance, the association between prevalent migraine and ischemic stroke is more pronounced among individuals with lower vascular risk,13,14 whereas the risk for myocardial infarction is only apparent among those with higher vascular risk, as measured by the Framingham Risk Score (FRS).13
Whereas some studies reported increased carotid intima-media wall thickness in people with migraine that are suggestive of subclinical atherosclerosis,15-17 other studies did not confirm such findings18 and provided evidence that having active migraine is an indicator of an arterial system that is less affected by atherosclerotic changes.19,20 Moreover, genetic studies have suggested protective associations of migraine with coronary artery disease.21-23 Thus, we hypothesized that vascular risk profile, in terms of traditional atherosclerotic risk factors as captured by the FRS, would be differentially associated with migraine status (having a history of migraine but not active migraine anymore, having active migraine, or first reporting new, incident migraine during follow-up). We evaluated these hypotheses in the Women's Health Study, for which information on the FRS and migraine status were available at baseline, and the included women reported incident migraine during follow-up.
Methods
Study Population
The Women's Health Study started as a randomized controlled trial that aimed to test the benefits of low-dose aspirin and vitamin E in the primary prevention of CVD and cancer. The detailed design and main findings of this study have been previously published.24,25 Briefly, US female health professionals (n = 39,876, age ≥45 years) without a history of CVD, cancer, or any other major disease were enrolled from 1992 until 1995. After the trial concluded in 2004, the study continued as an observational cohort. Baseline information on vascular risk factors and lifestyle variables was self-reported and collected by a mailed questionnaire. Twice in the first year and yearly thereafter, participants were sent follow-up questionnaires to gather additional information about study outcomes, participant characteristics, medical history, and health habits. To date, follow-up is 98% complete. Written informed consent was obtained from all participants, and the Women's Health Study was approved by the Institutional Review Board at Brigham and Women's Hospital, Boston, MA (clinicaltrials.gov identifier: NCT00000479).
Before randomization, blood samples from 28,345 participating women were collected in tubes containing EDTA and stored in vapor-phase liquid nitrogen (−170°C). Samples were analyzed for lipids and inflammatory biomarkers. Total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were assayed with reagents from Roche Diagnostics (Basel, Switzerland) and Genzyme (Cambridge, MA). Viable cholesterol measurements, necessary to calculate the FRS, were available for 27,939 of the stored blood samples.
Inclusion and Exclusion Criteria
Of the 27,939 women with available cholesterol measurements, we excluded 79 with missing migraine status information at baseline and 321 missing other information needed to calculate the FRS, resulting in a study population of 27,539 women for the present investigation.
Framingham Risk Score
To evaluate the exposure variable, vascular risk score status at baseline, we used the FRS, which is a sex-stratified assessment tool used to predict the 10-year risk of coronary heart disease.26 This score assigns points based on age, total cholesterol, high-density lipoprotein cholesterol, smoking, and systolic blood pressure (stratified by antihypertensive treatment). Similar to previously published work from the Women's Health Study, we created 4 groups based on 10-year predicted risk of coronary heart disease as a proxy for vascular risk according to the summed points of the individual components of the risk score as follows: ≤1% (corresponding to ≤12 points), 2%–4% (13–16 points), 5%–9% (17–19 points), and ≥10% (≥20 points).13
Migraine Assessment
On the baseline questionnaire, participating women were asked 2 questions relevant for the classification of migraine: (1) “Have you ever had migraine headaches?” and (2) “In the past year, have you had migraine headaches?” Subsequently, the participants were categorized as having no migraine, history of migraine (women who self-reported having migraine in the past but reported having no migraine in the year before study enrollment), and active migraine at baseline (women who self-reported migraine in the year before study enrollment). On each of the follow-up questionnaires, the participants were asked: “Have you had newly diagnosed migraine headaches since you last returned a questionnaire?” From these follow-up responses, we categorized participants as having incident migraine when the women did not report any migraine on the baseline questionnaire and only reported migraine during the follow-up. Because an individual's FRS status may change over the period of several years, we only included reports of incident migraine from the first 5 annual follow-up questionnaires in this analysis. Previously, we found good agreement between self-reported migraine at baseline in the Women's Health Study and self-reported symptoms of the International Classification of Headache Disorders II criteria.27
Statistical Analysis
We report the computed means and SDs of continuous variables and the frequencies and percentages of categorical baseline characteristics according to the participants' FRS categories. To quantify the associations between the FRS categories (≤1%, 2%–4%, 5%–9%, and ≥10%) and migraine status (no migraine, history of migraine, active migraine at baseline, and incident migraine), we used multinomial logistic regression models to estimate ORs and corresponding 95% CIs. Multinomial logistic regression is an extension of binary logistic regression, in which the dependent variable (migraine status) is allowed to have more than 2 categories. The status of never reporting migraine was used as the reference category for the outcome. We report the odds of the probability of the outcome among women belonging to each of the higher FRS categories relative to the group in the lowest FRS category (≤1%) computed from the baseline measurements.
We present results from both crude and multivariable-adjusted models. In the multivariable model, we adjusted for baseline variables including body mass index (weight in kg divided by height in meters squared, continuous), diabetes (yes/no), alcohol consumption (rarely/never, 1–3 drinks per month, 1–6 drinks per week, or ≥1 drink per day), vigorous physical activity (rarely/never, <1/wk, 1–3 times per week, or ≥4 times per week), ever use of oral contraceptives (yes/no), use of postmenopausal hormones (never, past, or current), and family history of premature myocardial infarction (younger than 60 years) (yes/no). Less than 1.7% of the study population had missing information for these covariates. Those missing information on the covariates were assigned to the most common category or to the mean value for the cohort in the case of body mass index. We did not adjust for age, total cholesterol, high-density lipoprotein cholesterol, smoking, systolic blood pressure, and antihypertensive medication use because these factors are used to compute the FRS and, as such, are components of our operationalization of vascular risk status.
In 2 sensitivity analyses, we further evaluated the association between the FRS categories and newly reported migraine during follow-up using a Cox proportional hazards regression model, adjusting for the same set of covariatesas in the multivariable multinomial model. We also additionally adjusted the multinomial logistic regression model for age in categories(<55 years, 55 to <65 years, 65 to <75 years, and ≥75 years) to account for the strong influence of age in determining the FRS categories.
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). For the Figure, we used the ggplot2 package in R/R Studio (RStudio 2022.02.1 Build 461, R version 4.1.3).
Data Availability
The data analyzed in this study are not publicly available because of restricted access regulated by original participant consent; however, further information about the data set is available from the corresponding author upon reasonable request.
Results
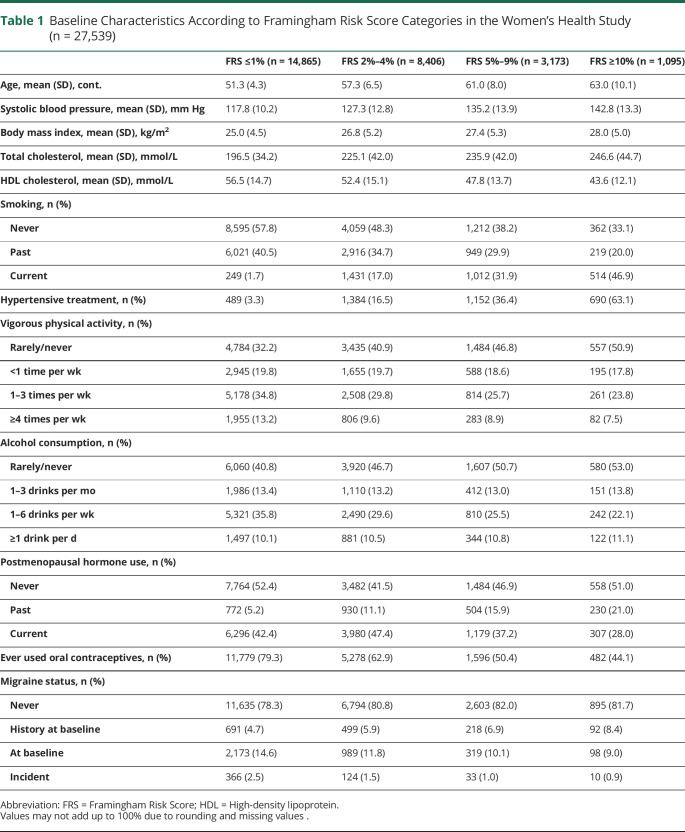
Of the 27,539 participants in our study, 14,865 had an FRS category ≤1%, 8,406 of 2%–4%, 3,173 of 5%–9%, and 1,095 of ≥10% (Table 1). As expected, age, mean systolic blood pressure, body mass index, cholesterol values, and current smoking increased across the FRS categories, whereas reports of ever using oral contraceptives decreased (Table 1). A total of 1,500 (5.5%) women reported a history of migraine, 3,579 (13.0%) reported having active migraine at baseline, and 533 (1.9%) newly reported migraine during the first 5 years of follow-up. Across all FRS categories, compared with women without migraine and having an FRS category of ≤1%, women with a history of migraine were more likely to be in the categories of the FRS category of 2%–4%, 5%–9%, and ≥10% (Figure, Table 2).
Table 1.
Baseline Characteristics According to Framingham Risk Score Categories in the Women's Health Study (n = 27,539)
Figure. Adjusted ORs and 95% CIs of the Association Between Framingham Risk Score Categories and Migraine Groups.
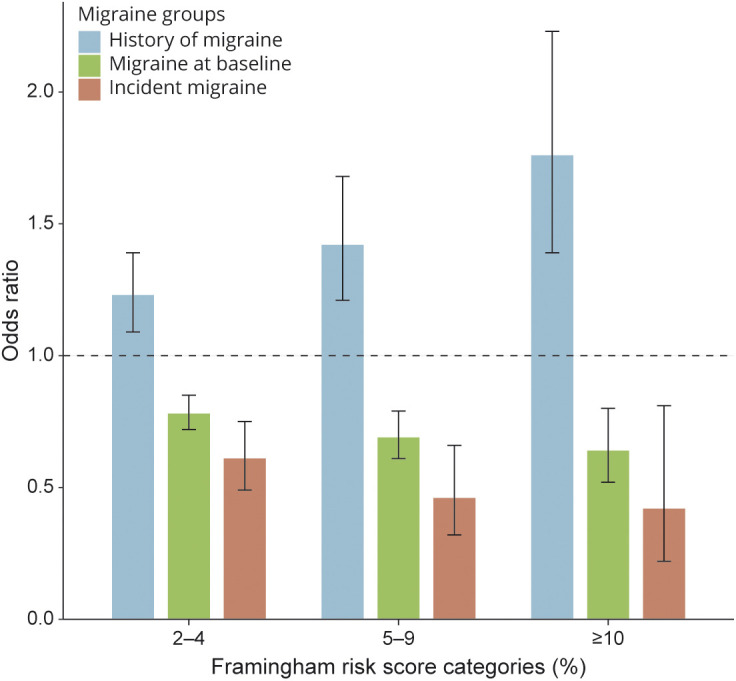
ORs are adjusted for body mass index, diabetes, alcohol consumption, physical activity, oral contraceptive use, postmenopausal hormone use, and family history of premature myocardial infarction <age 60 years. Reference groups are Framingham Risk Score category ≤1% and never reporting migraine (not shown in the Figure).
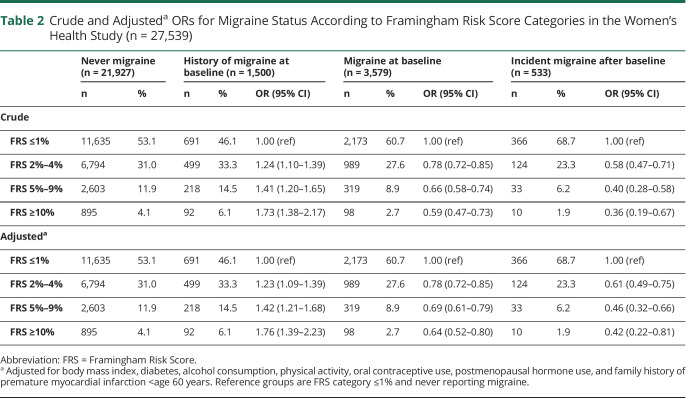
Table 2.
Crude and Adjusteda ORs for Migraine Status According to Framingham Risk Score Categories in the Women's Health Study (n = 27,539)
The odds of the probability of having a history of migraine at baseline (vs never migraine) were 76% higher among those with FRS ≥10% compared with FRS ≤1% after adjustment in the multinomial logistic regression model (OR = 1.76, 95% CI 1.39–2.23) (Figure and Table 2). In contrast, having FRS ≥10% was inversely associated with (active) migraine at baseline after adjustment (OR = 0.64, 95% CI 0.52–0.80) and with newly reported migraine during follow-up (OR = 0.42, 95% CI 0.22–0.81) when compared with women with FRS category ≤1% and those not reporting migraine (Figure and Table 2). Compared with women with FRS category ≤1% and never reporting migraine, women who reported incident migraine had adjusted OR (95% CI) of 0.61 (0.49–0.75) for FRS category 2%–4%, 0.46 (0.32–0.66) for FRS category 5%–9%, and 0.42 (0.22–0.81) for FRS category ≥10%.
In a sensitivity analysis using Cox proportional hazards models to estimate the association between FRS categories and incident migraine accounting for the time to the event, we found very similar estimates as those obtained from the multinomial logistic regression models. Compared with women with FRS category of ≤1% and after adjustment for all covariates as in the multivariable multinomial model, the hazard ratios (95% CI) for incident migraine were 0.62 (0.50–0.77) for FRS category 2%–4%, 0.47 (0.33–0.68) for FRS category 5%–9%, and 0.43 (0.23–0.82) for FRS category ≥10%.
In a further sensitivity analysis, we additionally adjusted the multivariable multinomial logistic regression model for age categories. The estimates were largely comparable when additionally adjusting for age, which is one of the variables used to calculate the FRS (eTable, links.lww.com/WNL/C240). The ORs for women with migraine at baseline and women with incident migraine were attenuated after age category adjustment (eTable, links.lww.com/WNL/C240).
Discussion
In this cohort of apparently healthy women aged ≥45 years at inclusion, those having estimated 10-year risks of coronary heart disease at baseline according to the FRS of 2%–4%, 5%–9%, and ≥10% were less likely to report (active) migraine at baseline or newly report migraine during the 5-year follow-up period compared with those in the FRS category of ≤1% and with those never reporting migraine during the study period. In contrast, women in the highest 3 FRS categories were more likely to report having a history of migraine before baseline.
Prior studies on the relationship between vascular risk, as operationalized by the FRS or by one of its vascular risk factor components, and migraine status have shown conflicting results. Although some studies reported increased FRS among those with migraine,28,29 another study reported a decreased risk in the hypertension component of the FRS among those with migraine compared with those without migraine.30 Yet other studies reported increased risk of developing hypertension among women with migraine.31,32 Comparing prior studies to the current analysis may be challenging due to differences in the target populations, including differences in sex of the included individuals (our study only included women), data ascertainment, and different definitions of migraine status.
Although we cannot rule out that the observed associations between the FRS categories and migraine are mainly driven by the influence of age on migraine activity status, we discuss 2 potential explanations for the observed associations. First, in line with the classical vascular hypothesis of migraine,33 it could be argued that among women with higher vascular risk according to the FRS at baseline who also had a history of migraine, vessel stiffness likely occurred,34 which in turn prohibited vasodilation of the dural arteries and subsequent headache development during a migraine attack. We acknowledge that such a hypothesis is not in line with the current view of migraine being a neurovascular,35 rather than a purely vascular disorder.36 However, the relevance of this first potential explanation is that, from a cardiovascular risk perspective, patients with a history of migraine, in whom active migraine complaints have ceased, may represent a subgroup of people with migraine who are actually at increased cardiovascular risk. Thus, a cardiovascular follow-up of such patients, similar to, e.g., in patients with prior preeclampsia,37 could be warranted.
As a second hypothesis, the FRS may not be a good proxy for underlying vascular health among people with migraine, as it may not cover all vascular aspects in patients with migraine. In the Women's Health Study, a history of migraine and active migraine at baseline was found to be associated with an increased likelihood of reporting a family history of myocardial infarction before age 60.13 Family history of myocardial infarction is known to be related to subclinical atherosclerosis38 and, similar to the FRS, is related to an increased cardiovascular risk in the general population.39 This may suggest that in patients with migraine, a family history of myocardial infarction is mediated by mechanisms different from classical vascular risk factors. Such a distinct mechanism for the risk of myocardial infarction in people with migraine, not depending on the FRS, might play an even stronger role in ischemic stroke risk. In the Women's Health Study, the risk for ischemic stroke was found to be strongest among women with a low FRS.13 This seems to be in line with findings showing no increased atherosclerosis in large vessels in patients with migraine with acute ischemic stroke compared with patients with stroke without migraine.40
One potential nontraditional vascular mechanism that could be responsible for increased cardiovascular risk, which is not represented by the FRS, is microvascular function. Indeed, atherosclerotic processes, as predicted by the FRS, are less abundant in microarteries.41 Taken together, we cannot exclude that 2 separate mechanisms might affect people with migraine simultaneously, as the microcirculation may also show diminished endothelial vasodilator function due to atherosclerosis in the larger, more proximal arteries.42
Migraine is considered to be a neurovascular disorder, where factors such as nitric oxide and calcitonin gene-related peptide (CGRP), which are produced by trigeminal afferents and act on blood vessels, play a prominent role.35 Furthermore, the exposure of the trigeminovascular system to exogenously administered CGRP or nitric oxide (not of neuronal origin) is known to provoke migraine-like attacks.43,44 Consideration should be given to the fact that besides the trigeminal afferents, the blood vessels could act as a further source of nitric oxide.3 Because atherosclerosis, which is related to a high FRS,45 decreases endothelial nitric oxide production,46 such a decreased nitric oxide production might well be underlying the decreased prevalence of migraine in women with a high FRS. Alternatively, activation of cation channel subfamily V member 1 channels, resulting in CGRP release, has been suggested to exhibit a protective effect against atherosclerosis.47 Although it is questionable whether such an effect may be relevant to systemic factors that are implicated in the FRS, such a mechanism provides an alternative hypothesis to explain our observations.
Pharmacologic treatment with antihypertensives could have affected the FRS, and we cannot exclude that these medications were used as a prophylactic treatment for migraine. In addition, as described above, the age distribution was not identical between the 4 groups in our study. However, when additionally controlling for age group in our multinomial model (an approach that might be debated because age is also reflected in the FRS), the observed result of lower odds of the probability of having incident migraine among women with high FRS scores persisted.
The results of this study suggest that traditional measures of vascular risk such as the FRS are linked with migraine activity status, but migraine itself may also capture unique pathways of cardiovascular risk. Therefore, future studies developing cardiovascular risk prediction algorithms should consider including migraine as a candidate variable, as was done in the QRISK 3 score.12 This concept is further supported by recent work demonstrating that migraine is associated with a similar or even higher absolute risk of CVD events as other commonly used vascular risk factors.10
Strengths of this study include the large number of participants, the prospective data collection, and lower risk for misclassification of health-related information among this cohort of health care professionals. Some potential limitations to this study should be noted when interpreting our results. New reports of migraine during follow-up by women who did not report migraine nor a history of migraine at baseline may represent true incident migraine onset, which becomes less likely with increasing age, or may instead represent reoccurrence of prior migraine attacks that were not reported as such. Regardless, we consider such reports to be representative of the biological ability to continue to have active migraine. Furthermore, we did not ask about aura status in the follow-up questionnaires, so we could not include data on migraine aura status in our analysis, and our results thus reflect a mixture of migraine with and without aura subtypes. Earlier findings in the Women's Health Study showed no substantial difference between the FRS categories and migraine with or migraine without aura at baseline.13 In our primary analysis, we did not account for the time to incident migraine during the follow-up period. However, in a sensitivity analysis using a Cox proportional hazards model, the results were similar. As the Women's Health Study only included women, our data cannot be extrapolated to men and therefore cannot provide insights into possible relationships or mechanisms in men, similar to many other studies probing the relationship between migraine and CVD.7,10,48,49 Readers should cautiously interpret our findings, as we relate information about the FRS calculated at the time of inclusion into the study with information from the past, present, and future (report of prior, current, and newly incident migraine). More specifically, our findings should be interpreted as predictive and not causal, as we mix prevalent with incident migraine activity statuses.50 Future studies, ideally with multiple measurement time points, are warranted to evaluate the causal interrelationships of migraine activity status with the vascular system.
High FRS categories were only observed among women with a history of migraine but not with (active) migraine at baseline or incident migraine. Our results suggest that a relatively healthy vascular system, as assessed by the FRS, is associated with migraine activity status or developing migraine in the future. The biological mechanisms underlying these observations, as well as how our observations might be related to specific CVD end points, remain to be studied. Further work should investigate whether documented changes in migraine activity status might be linked to vascular risk (or vice versa).
Glossary
- CVD
cardiovascular disease
- FRS
Framingham Risk Score
- CGRP
calcitonin gene-related peptide
Appendix. Authors
Footnotes
Editorial, page 681
Study Funding
The Women's Health Study is funded by the NIH grants: CA047988, HL043851, HL080467, HL099355, and UM1 CA182913. PMR is funded by K01 HL128791. A. Maassen van den Brink and T. Kurth received funding from the Berlin Institute of Health's Excellence award for Sex and Gender Aspects in Health Research (granted to A. Maasen van den Brink).
Disclosure
P. Rist reports having received research grants from Biogen and honoraria from the American Heart Association for editorial work. J. Rohmann reports receiving a grant from Novartis Pharma for a self-initiated research project focused on quantifying migraine activity remission rates on the population level. A. Maassen van den Brink reports having received research grants and/or consultation fees from Allergan/AbbVie, Amgen/Novartis, Lilly/CoLucid, Teva, and ATI and is funded by the Dutch Research Council (Vici grant 09150181910040). T. Kurth reports having received research grants from the German Joint Committee and the German Federal Ministry of Health as well as honoraria from Eli Lilly & Company, Teva Pharmaceuticals, TotalEnergies S.E., and from BMJ, and Frontiers. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Primers. 2022;8(1):2. [DOI] [PubMed] [Google Scholar]
- 2.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21-34. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs B, Dussor G. Neurovascular contributions to migraine: moving beyond vasodilation. Neuroscience. 2016;338:130-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, et al. Characterisation of vasodilatory responses in the presence of the CGRP receptor antibody erenumab in human isolated arteries. Cephalalgia. 2019;39(14):1735-1744. [DOI] [PubMed] [Google Scholar]
- 6.de Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020;211:107528. [DOI] [PubMed] [Google Scholar]
- 7.Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adelborg K, Szépligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8(3):e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurth T, Rist PM, Ridker PM, Kotler G, Bubes V, Buring JE. Association of migraine with aura and other risk factors with incident cardiovascular disease in women. JAMA Am Med Assoc. 2020;323(22):2281-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visseren FLJ, Mach F, Smulders YM, et al. , ESC National Cardiac Societies, ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337. [DOI] [PubMed] [Google Scholar]
- 12.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38(9):2438-2445. [DOI] [PubMed] [Google Scholar]
- 15.Poyrazoglu HG, Vurdem UE, Arslan A, Uytun S. Evaluation of carotid intima-media thickness in children with migraine: a marker of subclinical atherosclerosis. Neurol Sci. 2016;37(10):1663-1669. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz Avci A, Akkucuk MH, Torun E, Arikan S, Can U, Tekindal MA. Migraine and subclinical atherosclerosis: endothelial dysfunction biomarkers and carotid intima-media thickness: a case-control study. Neurol Sci. 2019;40(4):703-711. [DOI] [PubMed] [Google Scholar]
- 17.Magalhães JE, Barros IMLde, Pedrosa RP, Sampaio Rocha-Filho PA. Migraine and markers of carotid atherosclerosis in middle-aged women: a cross-sectional study. Headache. 2019;59(1):77-85. [DOI] [PubMed] [Google Scholar]
- 18.Stam AH, Weller CM, Janssens ACJ, et al. Migraine is not associated with enhanced atherosclerosis. Cephalalgia. 2013;33(4):228-235. [DOI] [PubMed] [Google Scholar]
- 19.Wen K-X, Ikram MA, Franco OH, et al. Association of migraine with calcification in major vessel beds: the Rotterdam Study. Cephalalgia. 2019;39(8):1041-1048. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed B, Bairey Merz CN, McClure C, et al. , WISE Study Group. Migraines, angiographic coronary artery disease and cardiovascular outcomes in women. Am J Med. 2006;119(8):670-675. [DOI] [PubMed] [Google Scholar]
- 21.Winsvold BS, Nelson CP, Malik R, et al. , CARDIoGRAM Consortium and the International, Headache Genetics Consortium. Genetic analysis for a shared biological basis between migraine and coronary artery disease. Neurol Genet. 2015;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winsvold BS, Bettella F, Witoelar A, et al. , International Headache Genetics Consortium. Shared genetic risk between migraine and coronary artery disease: a genome-wide analysis of common variants. PLoS One. 2017;12(9):e0185663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daghlas I, Guo Y, Chasman DI. Effect of genetic liability to migraine on coronary artery disease and atrial fibrillation: a Mendelian randomization study. Eur J Neurol. 2020;27(3):550-556. [DOI] [PubMed] [Google Scholar]
- 24.Lee I-M, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56-65. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. [DOI] [PubMed] [Google Scholar]
- 26.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486-2497. [DOI] [PubMed] [Google Scholar]
- 27.Schürks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women's Health Study. Cephalalgia. 2009;29(10):1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bank G, Kapus K, Meszaros J, et al. Framingham risk stratification of middle-aged migraineurs. Behav Neurol. 2020;2020:7351214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winsvold BS, Hagen K, Aamodt AH, Stovner LJ, Holmen J, Zwart J-A. Headache, migraine and cardiovascular risk factors: the HUNT study. Eur J Neurol. 2011;18(3):504-511. [DOI] [PubMed] [Google Scholar]
- 30.Benseñor IM, Goulart AC, Lotufo PA, Menezes PR, Scazufca M. Cardiovascular risk factors associated with migraine among the elderly with a low income: the Sao Paulo Ageing & Health Study (SPAH). Cephalalgia. 2011;31(3):331-337. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald CJ, El Fatouhi D, Madika A-L, et al. Association of migraine with incident hypertension after menopause: a longitudinal cohort study. Neurology. 2021;97(1):e34-e41. [DOI] [PubMed] [Google Scholar]
- 32.Rist PM, Winter AC, Buring JE, Sesso HD, Kurth T. Migraine and the risk of incident hypertension among women. Cephalalgia. 2018;38(12):1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham JR, Wolff HG. Mechanism of migraine headache and action of ergotamine tartrate. Arch Neurpsych Am Med Assoc. 1938;39:737-763. [Google Scholar]
- 34.Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc. 2018;7(2):e007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edvinsson L, Villalón CM, MaassenVanDenBrink A. Basic mechanisms of migraine and its acute treatment. Pharmacol Ther. 2012;136(3):319-333. [DOI] [PubMed] [Google Scholar]
- 36.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105(16):1273-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheuner MT, Setodji CM, Pankow JS, Blumenthal RS, Keeler E. Relation of familial patterns of coronary heart disease, stroke, and diabetes to subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. Genet Med. 2008;10(12):879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivapalaratnam S, Boekholdt SM, Trip MD, et al. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96(24):1985-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Os HJA, Mulder IA, Broersen A, et al. , DUST Investigators. Migraine and cerebrovascular atherosclerosis in patients with ischemic stroke. Stroke. 2017;48(7):1973-1975. [DOI] [PubMed] [Google Scholar]
- 41.Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis. 2007;50(2):112-125. [DOI] [PubMed] [Google Scholar]
- 42.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res. 1992;70(3):465-476. [DOI] [PubMed] [Google Scholar]
- 43.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54-61. [DOI] [PubMed] [Google Scholar]
- 44.Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38(1):17-24. [DOI] [PubMed] [Google Scholar]
- 45.Karim R, Hodis HN, Detrano R, Liu C-R, Liu C-H, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol. 2008;102(7):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713-735. [DOI] [PubMed] [Google Scholar]
- 47.Randhawa PK, Jaggi AS. TRPV1 channels in cardiovascular system: a double edged sword? Int J Cardiol. 2017;228:103-113. [DOI] [PubMed] [Google Scholar]
- 48.Scher AI, Terwindt GM, Picavet HSJ, Verschuren WMM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64(4):614-620. [DOI] [PubMed] [Google Scholar]
- 49.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia. 2011;31(14):1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study are not publicly available because of restricted access regulated by original participant consent; however, further information about the data set is available from the corresponding author upon reasonable request.