Abstract
Background and Objectives
Although Alzheimer disease (AD) and dementia with Lewy bodies (DLBs) represent 2 different pathologies, they have clinical overlap, and there is a significant degree of co-occurrence of their neuropathologic findings. Many studies have examined imaging characteristics in clinically diagnosed patients; however, there is a relative lack of longitudinal studies that have studied patients with pathologic confirmation. We examined whether there were differences in longitudinal patterns of cortical atrophy between patients with both AD and DLB (AD/DLB) vs those with AD alone.
Methods
We collected and analyzed clinical and neuroimaging data from the AD Neuroimaging Initiative (ADNI) database for patients who underwent autopsy. The rates of change in various neuropsychological assessments were not significantly different between patients with AD/DLB and AD, and each group had neuropsychological outcomes consistent with disease progression. For our neuroimaging analysis, we used a linear mixed-effects model to examine whether there were longitudinal differences in cortical rates of atrophy between patients with AD/DLB and AD.
Results
Autopsies and serial neuroimaging were available on 48 patients (24 AD and 24 AD/DLB). Patients with AD alone had significantly higher atrophy rates in the left cuneus, lateral occipital, and parahippocampal regions over time when compared with patients with concomitant DLB, after covarying for interval from imaging to autopsy, sex, and total estimated intracranial volume. Site ID was included as a random effect to account for site differences. For these regions, the rate of decline over time in the AD/DLB group was less steep by a difference of 0.1887, 0.395, and 0.0989, respectively (p = 0.022, 0.006, and 0.006). The lattermost left cuneus volume measurement and Braak Lewy score had a Pearson product-moment correlation of 0.37, p = 0.009, while the lattermost left parahippocampal volume measurement and Braak neurofibrillary tangle score had a Pearson product‐moment correlation of -0.327, p = 0.02.
Discussion
Patients with AD had more significant atrophy in the left cuneus, lateral occipital, and parahippocampal regions when compared with patients with AD/DLB. These regions are known to distinguish DLB and AD pathology cross-sectionally but here are shown to distinguish longitudinal disease progression.
Neurodegenerative diseases are major causes of disability among the aging population, with Alzheimer disease (AD) and dementia with Lewy bodies (DLBs) being the most frequently diagnosed dementia conditions. Clinically, AD and DLB may not only be similar but also have some differentiating features. AD presents with episodic memory loss in addition to broader deficits in cognitive functioning, including the visuospatial, language, and executive function domains.1,2 By contrast, core symptoms of DLB include, in addition to dementia, fluctuating cognition, visual hallucinations, and spontaneous parkinsonism.2 Furthermore, each condition has its own dominant neuropathology: Beta-amyloid plaques, neurofibrillary tangles (NFTs), and medial temporal lobe atrophy are characteristic of AD, whereas alpha-synuclein deposits are characteristic of DLB.2,3 However, the 2 diseases may co-occur neuropathologically. In fact, of the spectrum of neurodegenerative disorders, AD and DLB overlap most frequently.4 Studies show that up to 80%–89% of patients diagnosed with DLB will have some degree of AD pathology present at autopsy.5 It has been shown that AD with concomitant DLB results in poorer outcomes clinically than either disease alone and is associated with a more rapid cognitive decline.6
A reliable method to correctly identify a patient's neuropathology antemortem would be useful because AD and DLB often have overlapping neuropsychiatric profiles. Patients with AD may experience hallucinations and delusions during disease progression, even without the presence of Lewy body (LB) pathology at autopsy.7 Furthermore, the diagnosis of DLB based on the core clinical features alone is estimated to be unreliable; for instance, one study examined postmortem neuropathology in over 3,000 patients to show that the sensitivity of antemortem clinical diagnoses was low, at 32.1% for DLB cases without AD-related pathology and only 12.1% for DLB and AD concomitant pathology, although specificity was over 95% in each of these groups. Interestingly, the same study found that the clinical diagnosis of AD alone had a higher sensitivity (85%) but lower specificity (51.5%).7 This indicates that patients with LB pathology are underdiagnosed, and patients clinically diagnosed with AD alone have elevated instances of alternate or concomitant pathology. Another study found that specialist psychiatrists who examined clinical records & summaries of patients with pathologic confirmation were only able to correctly identify the comorbidity in 23% of mixed AD/DLB cases.8 These studies suggest that current clinical practice is unable to highly discriminate between these diseases antemortem.
The desire to clarify the clinical diagnoses of AD and DLB has prompted many investigations into the distinguishing features and potential biomarkers for each group. A 2017 meta-analysis found that patients with confirmed AD pathology score worse in the memory domain of cognitive examinations and patients with confirmed DLB pathology score worse in the visuospatial domain; however, there were no significant differences identified between the groups for a single cognitive test.9 Performance on neuropsychological examinations may be suggestive of a certain diagnosis but are not alone traditionally definitive; as such, the quest for biomarkers of neuropathology in DLB and AD is of high interest. Many studies have examined the respective neuroimaging patterns associated with each disease. Neuroimaging with MRI has shown that medial temporal lobe atrophy is more characteristic of AD than DLB in pathologically confirmed cases (sensitivity 91% and specificity 94%).3 Relative preservation of the medial temporal lobe is considered to be a supportive biomarker for the clinical diagnosis of DLB.2 In one study, DLB was more associated with subcortical brain atrophy; of note, however, the participants in this study did not have pathologically confirmed diagnoses, and it is therefore possible that comorbid pathology was not accounted for.10 Indeed, few studies have examined the patterns of concurrent AD and DLB, especially given the need of accurate neuropathologic confirmation and the high rate of attrition of those who go on to autopsy. One study used an automated boundary shift integral algorithm and tensor-based morphometry method to analyze whole brain atrophy and regional gray matter loss, respectively, in patients with DLB, AD, and AD/DLB; this study found no difference in the rates of whole brain or gray matter atrophy between patients with DLB and older controls or patients with AD and AD/DLB. The authors suggest that neuronal loss because of alpha-synuclein pathology is therefore subcortical or biochemical rather than structural. However, this study used only 2 serial MRI scans for each patient, on average 2 years apart.11 It is possible that end-stage disease is indistinguishable between patients with AD/DLB and AD as measured by MRI; however, the possibility remains that differences may be identified over a longer period of disease progression, with more frequent assessments and scans.
The aim of this study was to examine the patterns of longitudinal cortical volume loss between patients with pathologically confirmed AD and AD/DLB over a range of up to 8 years. Structural abnormalities in AD vs comorbid AD/DLB may elucidate a neuroanatomic pattern for AD and provide insight into the additional anatomic pathology that is present due to DLB. This insight is critical for a potentially earlier and more accurate diagnosis of comorbid AD/DLB and for examining target engagement in clinical trials which aim to address alpha-synuclein pathology. The null hypothesis tested in this study is that there is no difference in the rates of regional atrophy between patients with AD alone vs patients with comorbid AD/DLB in any cortical brain region.
Methods
Data Collection
Data used in the preparation of this article were obtained from the AD Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. We analyzed neuropathologic data from ADNI. The control population for this study were patients with AD neuropathologic change (ADNC) without LB pathology. The case population for this study included patients with both ADNC and LB pathology. We also collected Braak Lewy, Braak NFT, and Thal amyloid beta (AB) scores, which describe the distribution of Lewy bodies, NFTs, and AB plaques within the brain, respectively, with higher scores corresponding to higher disease burden.12-14 Patients with Lewy bodies localized only to the amygdala were excluded from this study because DLBs and amygdala with Lewy bodies are considered to be distinct alpha-synuclein pathologies.15 We obtained all T1-weighted structural MRI scans (magnetization prepared rapid gradient‐echo or inversion recovery‐spoiled gradient echo) for every subject within the ADNI database (n = 273). We also collected clinical data for these subjects throughout all initial and follow-up visits, including neuropsychological assessments, genetic testing, and CSF analyses. Assessments included the Mini-Mental Status Examination (MMSE), Alzheimer Disease Assessment Scale-Cognitive, Neuropsychiatric Inventory Questionnaire, Clinical Dementia Rating scale (CDR), and Neuropsychological Summary scores from the University of Washington. These summary scores are a composite score based on the patient's answers to questions in multiple assessments; the questions are grouped by the cognitive domain which it aims to evaluate (including memory, executive function, language, and visuospatial domains).
Clinical Data Analysis
For each of the neuropsychological assessments, we calculated the rate of change in scores by using a simple linear regression model of the scores and time from baseline (years). We then compared the average slopes from the linear regression model between the AD/DLB cases and AD controls for each of the assessments. For the NPIQ, we also examined each element individually. Each subject was given a 0 for a particular element if he/she never had a recorded instance during follow-up or a 1 if he/she had. The distribution of 1's for each element was analyzed between groups using a Fisher exact test. In addition to analyzing the rates of change for these assessments, we also examined the scores between groups at each patient's final visit, similarly to Toledo et al., in 2013. We correlated individual neuropsychological assessments with Braak Lewy, Braak NFT, and Thal AB staging, using both the Pearson product-moment and Spearman rank correlation tests.
Imaging Analysis
All structural images scans were obtained through the ADNI's MRI protocol. Of the 48 total subjects, 37 were included in ADNI1 phase, which used 1.5T scanners to obtain T1-weighted and T2-weighted images. The remaining 11 subjects were only included in the ADNI2 phase. Of the 37 subjects included in ADNI1, 16 were also included in the ADNI2 phase. ADNI2 imaging protocol used 3T scanners. We registered each subject's follow-up scan to the initial baseline scan using a robust and consistent algorithm and then created subject-specific templates for longitudinal processing using FreeSurfer software as outlined in previous studies.16-18 Specifically, the longitudinal pipeline in FreeSurfer 6.0 was used for all subjects, and all images were manually inspected for gross topological defects. The software parcellated the cortex into 31 distinct regions for each hemisphere (62 total regions) and produced an output of measurements for each of these regions. We collected all cortical volume measurements from each hemisphere using the DKT Atlas. All subjects included in this study had at least 2 serial T1-weighted MRI scans.
Statistical Analysis
We analyzed demographic and clinical data with two-tailed t tests, Fisher exact tests, Pearson product-moment correlation tests, and Spearman rank correlation tests as appropriate for the data type. For the imaging data, we used a linear mixed-effects (LMEs) model. LME modeling has previously been shown to be an effective method for capturing group differences in hippocampal volume and entorhinal cortex atrophy as measured by longitudinal structural MRI between healthy controls, patients with MCI, and patients with AD.19 There are several benefits in using an LME model for longitudinal data including minimization of between-subject variability and imperviousness to irregular timing and subject drop-out.19 We chose fixed effects to be group (either AD or AD/DLB), time since date of death (DOD; days), group × time since DOD, sex (male or female), and estimated intracranial volume (eTIV) (mm3). We chose to use time since DOD instead of time since baseline as patients entered this study at different points during disease progression; time since DOD was a more precise variable for indicating a temporal relationship with severe disease. Each patient's unique ID was used as a random effect because multiple scans from the same patient are nonindependent samples. In addition, RID was nested within a second random effect of site ID, to account for differences between sites, such as scanner strength. Our model was programed using R and was formulated as follows for each region:
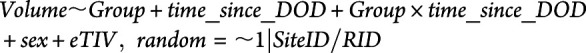 |
Output from the model included a coefficient for each term, in addition to a p value (62 total), and the group × time_since_DOD term; this coefficient indicates the difference in slope between patient groups over time. We applied FDR multiple comparison analysis on the 62 p values and established significance as p < 0.05 after this analysis.
Data Availability
All data used were gathered through ADNI. Python and R codes used for statistical analysis and display are available on request.
Standard Protocol Approvals, Registrations, and Patient Consents
All data used were gathered through ADNI, which approved this manuscript, which provides deidentified patient records to qualifying researchers. Each site that participated in this study individually obtained informed consent and received IRB approval from each respective site. Our secondary analysis of this was found to be exempt by Columbia's IRB (Protocol Number IRB-AAAS6975). No experiments were performed on live vertebrates.
Results
Clinical Data
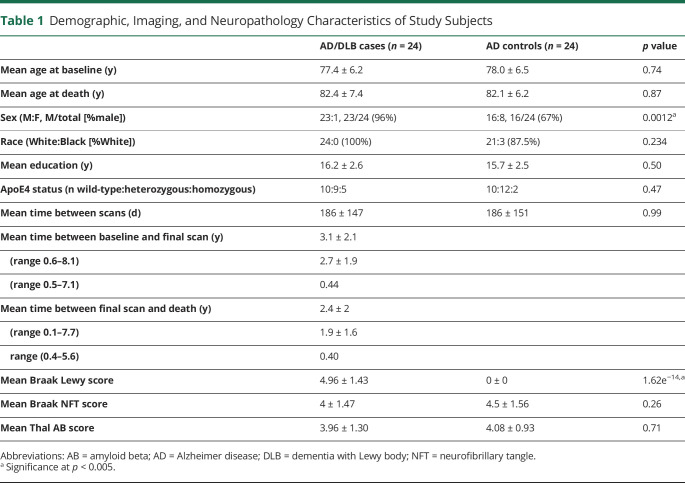
Of the 64 patients in the ADNI database with recorded neuropathology, 24 patients were identified as having LB pathology in addition to ADNC; these patients comprised the AD/DLB group. There were 0 patients who had LB pathology without ADNC. Another 24 patients had recorded ADNC without LB pathology and served as the control population or AD group. Therefore, a total of 48 subjects with confirmed neuropathology were included in this study. Clinical diagnoses (normal, MCI, or dementia) at baseline visit are presented in eTable 1 (links.lww.com/WNL/C356) and are not significantly different between groups (p = 0.1534). The demographic information for all subjects is presented in Table 1. The mean age at baseline (77.4 and 78 years for AD/DLB and AD groups) and the mean years of education (16.2 and 15.7 years for AD/DLB and AD groups) were not significantly different between groups as measured by the t test (p = 0.74 and 0.5, respectively). Although both groups were predominantly male, there was a significantly higher proportion of men in the AD/DLB group (96%) compared with the AD group (67%) as measured by the Fisher exact test (p = 0.0012). This is consistent with previous literature which has shown that the incidence of DLB is higher in men than in women.20 Proportions of race (100% and 87.5% White in AD/DLB and AD groups) and ApoE4 status (79.2% and 91.2% with at least 1 copy of ApoE4 allele in AD/DLB and AD groups) were not statistically different between groups as measured by the Fisher exact test (p = 0.234 and 0.47, respectively). The aforementioned regions remained significant when including both APOE4 status (eAnalysis 1, links.lww.com/WNL/C356) and final CDR Sum of Boxes (eAnalysis 2, links.lww.com/WNL/C356) as fixed effects.
Table 1.
Demographic, Imaging, and Neuropathology Characteristics of Study Subjects
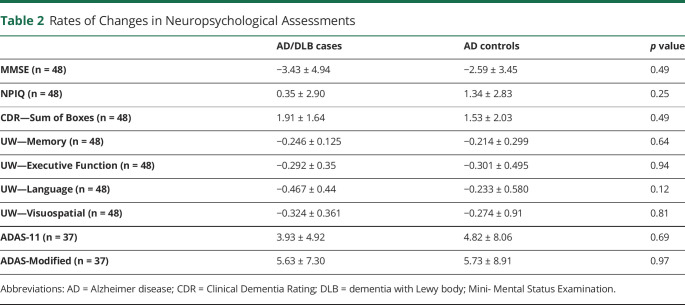
We used simple linear regression to examine the rate of change in several neuropsychological assessments over time and compared these rates between patients with AD/DLB and AD (Table 2). As expected, overall, the average score for each group declined over time for tests that measured cognitive ability (MMSE and UW Neuropsychological Examination) and increased over time for tests that measured symptom severity (CDR Sum of Boxes, NPIQ, ADAS). Specifically, the average slope for MMSE was −3.43 for the AD/DLB group and −2.59 for the AD group (p = 0.49). For UW Memory, Executive Function, Language, and Visuospatial scores, the average slopes for the AD/DLB group were −0.246, −0.292, −0.467, and −0.234, while for the AD group, the average slopes were −0.214, −0.301, −0.233, and −0.274, respectively (p value = 0.64, 0.94, 0.12, and 0.81, respectively). The average slope for NPIQ was 0.35 and 1.34 for the AD/DLB and AD groups, respectively (p value = 0.25). For CDR Sum of Boxes score, the average slopes were 1.91 for patients with AD/DLB and 1.53 for patients with AD (p value = 0.49). The slopes of CDR Global score could not be accurately compared between groups using linear regression because the change in score was so small over such a long follow-up period that many subjects had slopes near 0. For ADAS scores, the average slope for ADAS-11 and ADAS-modified was 3.93 and 5.63 for the AD/DLB group and 4.82 and 5.73 for the AD group (p = 0.69 and 0.97, respectively). Notably, only 37 of the 48 patients included had ADAS scores. There were no significant differences in the rates of change in any of neuropsychological examination scores between patients with AD/DLB and AD as measured by the t test and as presented in Table 2. Ranges for the final score on these neuropsychological assessments are presented in eFigure 1 (links.lww.com/WNL/C356).
Table 2.
Rates of Changes in Neuropsychological Assessments
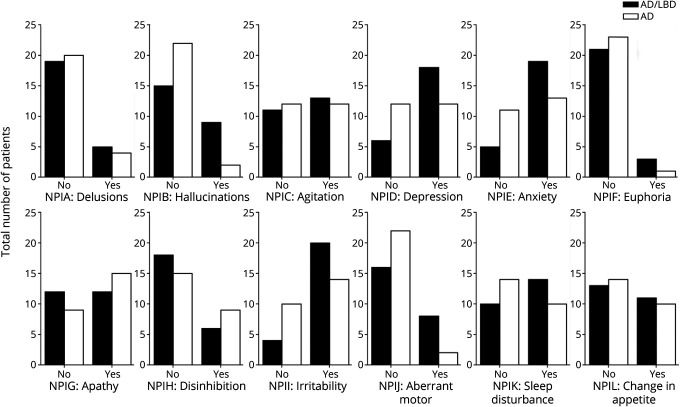
We investigated what symptoms patients experienced throughout the course of illness, as measured by the NPIQ (Figure 1). Compared with patients with AD, there were a higher proportion of patients with AD/DLB who had ever experienced delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, irritability/lability, aberrant motor behavior, and sleep disturbances, as measured by the NPIQ. Data are presented in eTable 2 (links.lww.com/WNL/C356). The only statistically significant difference was in the NPIB element, which measured whether the patient had experienced hallucinations. A significantly higher proportion of patients with AD/DLB (37.5%) experienced hallucinations throughout the course of their illness than patients with AD (8.3%, p value of Fisher exact test = 0.036). This is unsurprising because the presence of hallucinations is a core clinical feature of DLB. Compared with patients with AD/DLB, there were a higher proportion of patients with AD who had ever experienced apathy/indifference and disinhibition. However, these results were not statistically significant. Sensitivities and specificities of hallucinations and relevant rates of change are presented in eTable 3 (links.lww.com/WNL/C356).
Figure 1. Differences in the Recorded Presence of NPIQ Elements Between Groups.
Histogram showing a total number of patients in AD/DLB and AD groups who had ever experienced the symptom targeted by each particular NPIQ element. AD = Alzheimer disease; DLB = dementia with Lewy body.
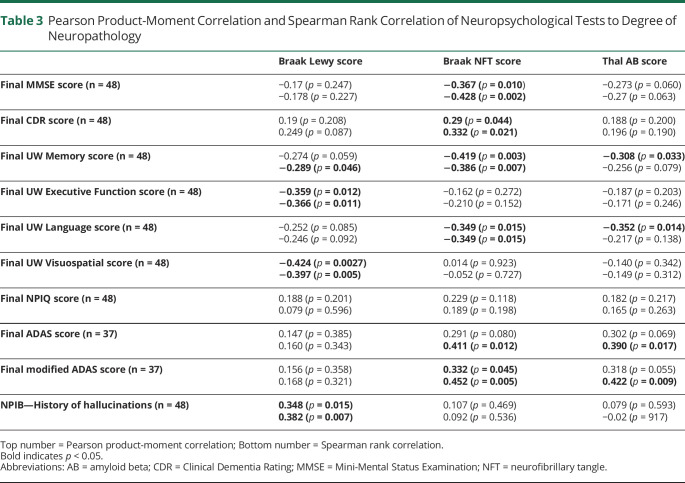
The results from Spearman rank correlation between neuropathology score and the most proximal neuropsychological examination score to the patient's DOD are presented in Table 3. The Braak Lewy score was negatively correlated with the final UW executive function and visuospatial scores (rho = −0.366 and −0.397, respectively), indicating that lower scores in these examinations were associated with a higher burden of Lewy bodies (p value = 0.011 and 0.005, respectively). In addition, whether a patient had ever experienced hallucinations as measured by the NPIB element was positively correlated with the Braak Lewy score (rho = 0.382, p = 0.007); however, the total NPIQ score was not (rho = 0.079, p = 0.596). The Braak NFT score was negatively correlated with the final MMSE (rho= -0.428, p = 0.002), CDR (rho = 0.332, p = 0.021), ADAS (rho= 0.452, p = 0.005), UW Memory (rho = −0.386, p = 0.007), and UW Language scores (rho = −0.349, p = 0.015). Thal AB was also significantly correlated with the final UW Memory score with Pearson rank correlation (rho = −0.308, p = 0.033) and final UW Language score (rho = −0.352, p = 0.014) and ADAS (rho = 0.390, p = 0.017) and modified ADAS scores with Spearman rank correlation (rho = 0.422, p = 0.009). Of note, only 37 of 48 patients had ADAS scores available.
Table 3.
Pearson Product-Moment Correlation and Spearman Rank Correlation of Neuropsychological Tests to Degree of Neuropathology
Imaging Data
There were 148 total structural T1-weighted MRI scans in the AD/DLB group (median 5.5/patient) and 125 total structural T1-weighted MRI scans in the AD group (median 6/patient). The number of scans per patient was not significantly different between groups as measured by a two-tailed t test of equal variance (p value = 0.128). In addition, AD/DLB cases and AD controls had similar times in between scans (Table 1), from baseline scan to the final scan (2.7 and 3.1 years for AD and AD/DLB, respectively), and from the final scan to the DOD (1.9 and 2.4 years for AD and AD/DLB, respectively). This indicates that the tracking of disease progression was comparable between the 2 groups. As expected, only AD/DLB cases had a Braak Lewy score above 0; therefore, there was a significant difference in Braak Lewy scores between groups (p = 1.62e−14). However, the mean Braak NFT (4.5 for patients with AD and 4 for patients with AD/DLB) and Thal AB (4.08 for patients with AD and 3.96 for patients with AD/DLB) scores were similar between groups as measured by a two-tailed t test (p value = 0.26 and 0.51, respectively), suggesting a similar severity of AD neuropathology between AD/DLB cases and AD controls. Our imaging data are graphically presented in eFigure 2 (links.lww.com/WNL/C356).
The LME identified 7 regions as being significantly different between groups: the left caudal anterior cingulate (p = 0.0315), left cuneus (p = 0.0011), left lateral occipital (p = 0.0002), left lateral orbitofrontal (p = 0.0035), left lingual (p = 0.0103), left parahippocampal (p = 0.0001), and right cuneus regions (p = 0.0135). After adjusting for multiple comparisons using false discovery rate correction, only the left cuneus, left lateral occipital, and left parahippocampal regions remained significant (p = 0.023, 0.006, and 0.006, respectively). In these regions, the coefficients corresponding to the time variable, which indicate the rate of change for the AD group, were −0.211, −0.68, and −0.155; as expected, volumes in these regions decrease over time. The coefficients of the interaction term group*time were all positive in these regions (0.1884, 0.395, and 0.0989, respectively). This indicates that, on average, patients with AD/DLB had a lower rate of change in these regions over time. The rate of change for patients with AD/DLB when covarying for sex and eTIV can be calculated by summing the coefficient of the time variable and the coefficient of the interaction term (group × time). Therefore, the rates of change in patients with AD/DLB in the left cuneus, left lateral occipital, and left parahippocampal regions when covarying for sex and eTIV were −0.0226, −0.285, and −0.0561, respectively.
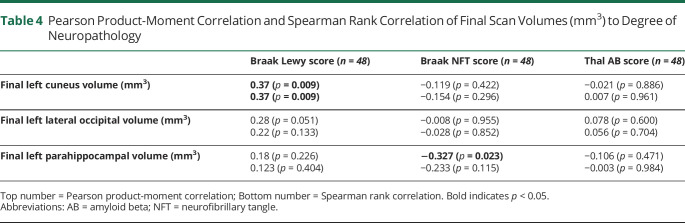
The results of Spearman rank correlation between the neuropathology score and the most proximal cortical volume measurement to the patient's DOD are presented in Table 4. Only the final left cuneus volume was correlated with the Braak Lewy score, with a positive coefficient of 0.37, indicating that higher final left cuneus volumes were associated with a higher Braak Lewy score (p = 0.009). The final left parahippocampal volume had a significant negative correlation with a Braak NFT score as measured by Pearson correlation (rho = −0.327, p value = 0.023) but not Spearman (rho = −0.233, p value = 0.115). Both coefficients were negative, indicating that lower final left parahippocampal volumes were associated with a higher Braak NFT score. None of the final volumes were correlated with Thal AB score.
Table 4.
Pearson Product-Moment Correlation and Spearman Rank Correlation of Final Scan Volumes (mm3) to Degree of Neuropathology
Discussion
Our study provides insight into the neuropsychological and imaging patterns in longitudinal data of patients with AD/DLB compared with patients with AD alone. Final MMSE, CDR, UW Memory, UW Language, and ADAS (modified) scores all correlated with Braak NFT scores, which is consistent with previous studies that have shown cognitive performance to be reflective of AD pathology severity.21 In addition, UW executive function, UW visuospatial scores, and history of hallucinations were correlated with Braak Lewy staging, which is also consistent with the current neuropsychiatric profile of patients with DLB.2,22
For neuroimaging, our results suggest that, over the course of disease progression, patients with mixed AD/DLB have relative structural preservation of the left cuneus, lateral occipital, and parahippocampal regions when compared with patients with AD alone. The asymmetry of findings in the left hemisphere may be reflective of previous studies which have found that AD pathology is often more pronounced on this side, although there is some evidence of trending results on the right, although they fail to survive corrections for multiple testing.23 Measurements of the left cuneus from the MRI scan most proximal to death had a positive correlation with Braak Lewy staging, suggesting that the distribution of Lewy bodies in the cortex (highest Braak score) is correlated with more significant preservation in this region. However, this was not true for the other regions found to be significant from the model. Only the left parahippocampal region from the MRI scan most proximal to death had a negative correlation with Braak NFT staging, indicating that a lower final volume in the parahippocampal region correlated with higher Braak NFT scores. This is consistent with previous findings that the parahippocampal region is susceptible to atrophy in Alzheimer disease.3,11,24
There has been much research indicating that LB pathology does not necessarily contribute to volumetric atrophy alone, especially to a similar degree as that seen in Alzheimer disease pathology. Previous neuropathologically confirmed studies have shown that cortical volumetric loss in patients with LB pathology is less pronounced than in patients with AD alone as measured by structural MRI.11,25 Cross-sectional studies of a single MRI scan per patient have examined gray matter atrophy patterns in patients with clinical diagnoses of probable DLB and AD.26-28 Patients with DLB were noted to have less substantial gray matter atrophy than in patients with AD, particularly in the orbitofrontal and temporal regions.26,27 Other studies with clinically diagnosed patients with AD and DLB showed that patients with AD had higher rates of global atrophy and temporal thinning on MRI at 1 year of follow-up and that patients with DLB had rates closer to controls.29,30 Our findings extend these previous studies to suggest that increasing atrophy is not necessarily characteristic of LB pathology in the presence of concurrent AD.
Several studies have examined occipital lobe atrophy and hypometabolism associated with Lewy bodies, given the prominence of visual symptoms in DLB. It has been shown that occipital lobe atrophy as measured on structural MRI is similar between patients with DLB and AD, with few studies (i.e., Toledo et al.) using patients with pathologic confirmation.22,28,31,32 In addition, one study found that cortical thickness in occipital regions was actually more affected in patients with AD than in patients with DLB, in addition to the midanterior temporal, subgenual cingulate cortex, and parahippocampal regions; this study used 97 clinically diagnosed patients, 7 of whom had pathologic confirmation at death.24 Our results showed that the left lateral occipital region and left cuneus had steeper rates of atrophy in the AD group compared with the AD/DLB group when covarying for sex, time since death, and eTIV. This finding supports the hypothesis that hallucinations associated with DLB are not explained by structural atrophy within the occipital lobe.28 However, functional change within the occipital lobe, specifically hypometabolism as measured by SPECT/PET, is considered a supportive biomarker for the clinical diagnosis of DLB.2,33
In particular, the phenomenon of more intense hypometabolism in the precuneus and cuneus regions relative to the posterior cingulate cortex, known as the cingulate island sign, is also a supportive biomarker of DLB diagnosis.2 Hypometabolism within the precuneus and cuneus has been shown to correlate with primary DLB symptoms, such as parkinsonism and global cognitive function.34 Because our results show that patients with AD alone have more precipitous atrophy in the occipital region, particularly in the left cuneus, it may be plausible that concomitant LB pathology and resulting dysfunction in metabolic activity are also in some way related to the relatively diminished atrophy that would have otherwise followed the pattern seen in traditional AD pathology. Previous research investigating the neuropathologic mechanisms of Lewy bodies has suggested that synaptic dysfunction precedes neuronal volume loss, and it may be the case that this dysfunction, not atrophy, drives DLB symptomatology.32,35,36
There are a few limitations to this study. First, we were unable to include subjects without any AD or AD/DLB pathology, given that all autopsy patients included in the ADNI database had some degree of neuropathology. Second, it is impossible to determine when this pathology appeared during disease progression, given that it is only discovered during autopsy. We are therefore unable to establish a causal relationship between the types of neuropathology and structural MRI patterns. Future studies focused more on the location and quantity of pathologies in these groups may help further elucidate causal factors driving these processes. In addition, as with any multisite study, there are differences in scanners which maybe contribute to a source of variance. A further limitation is that our study does not include information regarding the synaptic density in the dopaminergic system, which has been shown to be an important pathologic factor in LB pathologies.2 Indeed, using neuroimaging of different types would better characterize LB disease progression; future studies may also examine molecular PET scans because a recent study indicated that lower Pittsburgh compound B uptake can accurately distinguish cases of DLB from AD or mixed cases.37 In addition, future studies using our methods in a larger and more diverse patient population may help increase the generalizability of our results, and the results from such a data set could potentially be used to develop a risk calculator to help identify possible comorbid disease state antemortem. This would be an exciting and clinically applicable tool.
When compared with patients with AD neuropathology alone, patients with comorbid AD/DLB neuropathology at autopsy have less significant rates of cortical atrophy in the left cuneus, left lateral occipital, and left parahippocampal regions, when controlling for time since death, sex, and eTIV. Of these regions, the left cuneus was the most distinctive between groups because final cuneus volume was positively correlated with the Braak Lewy score, indicating that those with more extensive distribution of LB pathology had larger final volumes. Our findings corroborate existing literature that LB and alpha-synuclein pathology are more likely to induce functional deficits rather than structural atrophy and further suggests that patients with AD alone are more likely to have steeper rates of cortical atrophy than patients with comorbid AD/DLB.
Acknowledgment
We would like to acknowledge statistician Jimmy Duong for his help in this study.
Glossary
- AB
amyloid beta
- AD
Alzheimer disease
- ADNI
AD Neuroimaging Initiative
- ADNC
AD neuropathologic change
- DLB
dementia with Lewy body
- eTIV
estimated intracranial volume
- NFT
neurofibrillary tangle
- LB
Lewy body
- LME
linear mixed effect
- MMSE
Mini-Mental Status Examination
Appendix 1. Authors
Appendix 2. Coinvestigators

Study Funding
This work was funded by Columbia University's Vagelos College of Physicians & Surgeons through the Dean's Research Fellowship, an award provided to current medical students. This study was supported by NIH (5P30AG066462). Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Disclosure
F.A. Provenzano was a consultant for Imij Technologies and has several granted patents and applications in neuroimaging unrelated to this study. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Förstl H, Kurz A. Clinical features of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288-290. doi: 10.1007/s004060050101 [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. BRAIN A J Neurol. 2009;(132):195-203. doi: 10.1093/brain/awn298 [DOI] [PubMed] [Google Scholar]
- 4.Matej R, Tesar A, Rusina R. Alzheimer's disease and other neurodegenerative dementias in comorbidity: a clinical and neuropathological overview. Clin Biochem. 2019;73:26-31. doi: 10.1016/J.CLINBIOCHEM.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 5.Dugger BN, Adler CH, Shill HA, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014;20(5):525-529. doi: 10.1016/J.PARKRELDIS.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc F, Mahmoudi R, Jonveaux T, et al. Long-term cognitive outcome of Alzheimer's disease and dementia with Lewy bodies: dual disease is worse. Alzheimer’s Res Ther. 2017;9(1). doi: 10.1186/S13195-017-0272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2009 2573. 2010;257(3):359-366. doi: 10.1007/S00415-009-5324-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas AJ, Mahin-Babaei F, Saidi M, et al. Improving the identification of dementia with Lewy bodies in the context of an Alzheimer’s-type dementia. Alzheimer’s Res Ther. 2018;10(1). doi: 10.1186/S13195-018-0356-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurnani AS, Gavett BE. The differential effects of Alzheimer's disease and Lewy body pathology on cognitive performance: a meta-analysis. Neuropsychol Rev. 2016. ;27(1):1-17. doi: 10.1007/S11065-016-9334-0 [DOI] [PubMed] [Google Scholar]
- 10.Watson R, Colloby SJ, Blamire AM, O'Brien JT. Subcortical volume changes in dementia with Lewy bodies and Alzheimer's disease. A comparison with healthy aging. Int Psychogeriatrics. 2016;28(4):529-536. doi: 10.1017/S1041610215001805 [DOI] [PubMed] [Google Scholar]
- 11.Nedelska Z, Ferman TJ, Boeve BF, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging. 2015;36(1):452-461. doi: 10.1016/j.neurobiolaging.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197-211. doi: 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 14.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791 LP–1800. doi: 10.1212/WNL.58.12.1791 [DOI] [PubMed] [Google Scholar]
- 15.Uchikado H, Lin W-L, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of α-synucleinopathy. J Neuropathol Exp Neurol. 2006;65(7):685. doi: 10.1097/01.JNEN.0000225908.90052.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53(4):1181-1196. doi: 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19-21. doi: 10.1016/j.neuroimage.2011.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernal-Rusiel JL, Greve DN, Reuter M, Fischl B, Sabuncu MR. Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage. 2013;0:249. doi: 10.1016/J.NEUROIMAGE.2012.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396-1402. doi: 10.1001/jamaneurol.2013.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenberg AJ, Suemoto CK, de Paula França Resende E, et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer's disease. J Alzheimer’s Dis. 2018;66(1):139-115-126. doi: 10.3233/JAD-180688. Neuropathologic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo JB, Cairns NJ, Da X, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013. ;1(65):1-13. doi: 10.1186/2051-5960-1-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan UA, Liu L, Provenzano F, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci. 2014;17(2):304-311. doi: 10.1038/nn.3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebedev AV, Westman E, Beyer MK, et al. Multivariate classification of patients with Alzheimer's and dementia with Lewy bodies using high-dimensional cortical thickness measurements: an MRI surface-based morphometric study. J Neurol. 2013;260(4):1104-1115. doi: 10.1007/s00415-012-6768-z [DOI] [PubMed] [Google Scholar]
- 25.Harper L, Bouwman F, Burton EJ, et al. Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry. 2017;88(11):908-916. doi: 10.1136/jnnp-2016-314978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballmaier M, O’brien JT, Burton EJ, et al. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004;23:325-335. doi: 10.1016/j.neuroimage.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 27.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130(3):708-719. doi: 10.1093/brain/awl388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlach M, Stadler K, Aichner F, Ransmayr G. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI [4]. Neurology. 2002;59(9):1476. doi: 10.1212/WNL.59.9.1476 [DOI] [PubMed] [Google Scholar]
- 29.Mak E, Su L, Williams GB, et al. Progressive cortical thinning and subcortical atrophy in dementia with Lewy bodies and Alzheimer's disease. Neurobiol Aging. 2015;36:1743-1750. doi: 10.1016/j.neurobiolaging.2014.12.038 [DOI] [PubMed] [Google Scholar]
- 30.Mak E, Su L, Williams GB, et al. Longitudinal assessment of global and regional atrophy rates in Alzheimer's disease and dementia with Lewy bodies. Neuroimage (Amst). 2015;7:456-462. doi: 10.1016/j.nicl.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middelkoop HAM, Van Der Flier WM, Burton EJ, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57(11):2117-2120. doi: 10.1212/WNL.57.11.2117 [DOI] [PubMed] [Google Scholar]
- 32.Watson R, Colloby SJ, Blamire AM, O’brien JT. Assessment of regional gray matter loss in dementia with Lewy bodies: a surface-based MRI analysis. Am J Geriatr Psychiatry. 2015;23:1. doi: 10.1016/j.jagp.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Imamura T, Ishii K, Sasaki M, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease: a comparative study using positron emission tomography. Neurosci Lett. 1997;235:49-52. doi: 10.1016/S0304-3940(97)00713-1 [DOI] [PubMed] [Google Scholar]
- 34.Graff-Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology. 2014;83:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer ML, Schulz-Schaeffer WJ. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27(6):1405-1410. doi: 10.1523/JNEUROSCI.4564-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volles MJ, Peter T. Lansbury J. Zeroing in on the pathogenic form of α-synuclein and its mechanism of neurotoxicity in Parkinson's disease†. Biochemistry. 2003;42(26):7871-7878. doi: 10.1021/BI030086J [DOI] [PubMed] [Google Scholar]
- 37.Kantarci K, Lowe VJ, Chen Q, et al. β-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology. 2020;94(3):e282-e291. doi: 10.1212/WNL.0000000000008818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used were gathered through ADNI. Python and R codes used for statistical analysis and display are available on request.