Abstract
COVID-19 global pandemic, originated from Wuhan, resulted in a massive increase in the output of polypropylene (PP)-based personal protective equipment (PPE) for healthcare workers. The continuous demand of PPE across the world caused the PP based plastic wastes accumulation. Some alternative approaches that have been practiced apart from collecting the plastic waste in the landfills are incineration approach and open burning. However, there were many drawbacks of these practices, which promote the release of chemical additives and greenhouse gases into the environment. Therefore, a proper approach in treating the plastic wastes, which introduces conversion of plastic wastes into renewable energy is paramount. Along the way of extensive research and studies, the recovery of PP plastic to fuel-like liquid oil and solid char through thermal decomposition of pyrolysis process, helps in reducing the number of PP plastic wastes and produces good quality pyrolysis liquid oil and solid char to be used in fuel applications. This paper summarizes the pyrolysis process for massively produced PP plastic wastes, type of pyrolysis used and the main pyrolysis parameters affecting the product yields. Literature studies of pyrolysis of PP plastic and several key points to optimize solid char production for PP were thoroughly elaborated in this review paper.
Abbreviations: PP, polypropylene; PPE, personal protective equipment; COVID-19, coronavirus disease 2019; PE, polyethylene; PU, polyurethane; PVC, polyvinyl chloride; PS, polystyrene; PET, polyethylene terephthalate; WHO, World Health Organisation; MOH, Ministry of Health; HDPE, high density polyethylene; LDPE, low density polyethylene; min, minute; s, second; °C, degree Celsius; H2, hydrogen; CO, corban monoxide; CH4, methane; C5H12, pentane; PDO, plastic-derived oil; wt%, weight percentage; HHV, high heating value; LHV, low heating value; CO2, carbon dioxide; m, meter; g, gram
Keywords: Plastic waste treatment, Pyrolysis, Polypropylene, PPE, COVID-19, Soil and air pollution
Graphical abstract
1. Introduction
Global plastic production started in the early 1900s, where the first synthetic plastic had been invented in 1950. Annual global plastic demand has soared since the plastic industry started, from 1.5 million metric tons in 1950 to 3 billion metric tons in 2018 (Ritchie and Roser, 2018). Every year, the world generates 381 million tonnes of plastic waste, which is expected to double by 2034. Polypropylene (PP), polyethylene (PE), polyurethane (PU), polyvinyl chloride (PVC), polystyrene (PS), polyethylene terephthalate (PET), and phenolic resin are the most commonly used plastics, with PP and PE being widely used polymers in daily plastic goods, particularly disposable products such as plastic packaging, sterile medical uses, construction, and compostable plastic bottles (Giacovelli et al., 2018). This is due to the fact that polyolefins are highly resistant to microbial enzymes, light, and water due to their high molecular weight and hydrophobicity (Geyer et al., 2017). According to some researchers (Arkatkar et al., 2009; Chamas et al., 2020), the degradation rate of plastics is very low, it takes 60 to 1000 years to degrade under natural environment. Antioxidants and stabilizers, which are intended to extend the usable life of plastics, frequently inhibit the breakdown of plastic trash in the environment. In addition, colorants, plasticizers, and stabilizers are used as additives in these plastics, which comprised of cadmium and lead elements (Yaşar, 2004). Consequently, plastic wastes can account for up to 28% of total cadmium in urban solid waste and created an emerging threat to the environment.
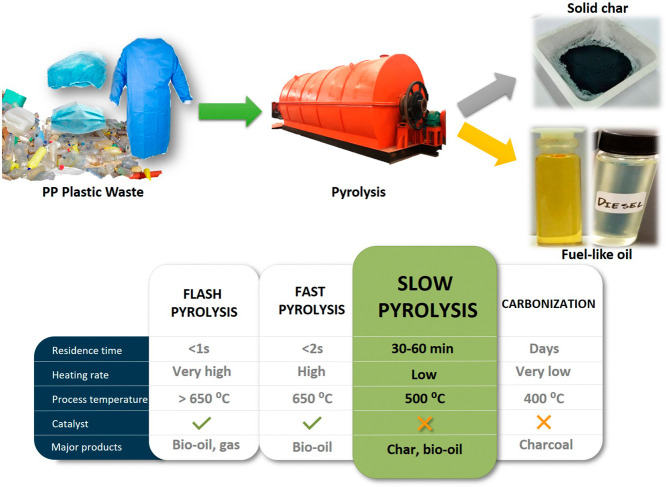
During the coronavirus disease 2019 (COVID-19) pandemic, over one million new cases of COVID-19 with total of 3.2 million deaths were reported by World Health Organisation (WHO) as of 6 May 2021 (WHO, 2021). With the aim of inhibiting the rapid COVID-19 spreading, hand washing should be done on a frequent basis, as should maintaining social distances and wearing a face mask in public areas. Personal protection equipment (PPE), such as ventilators, isolation gowns, hair and shoe nets, is used by frontline healthcare professionals (see Fig. 1 ), mainly made up of PP, about 72% (3M Personal Safety Division, 2018; MOH, 2020), as their main barrier from the viral and bacterial infections. As a result, PPE has led to an increase in plastic debris since the pandemic emerged. In response to high PPE demand among the public, health care workers, and service employees, single-use face mask manufacturing in China increased to 116 million per day in February 2020, over 12 times the usual amount (Bermingham and Tan, 2020). According to Ranney et al. (2020), an increase in new cases has resulted in a rapid accumulation of infectious used PPE in the municipal solid waste system. Furthermore, it has been reported that since the emergence of COVID-19, the manufacturing of PP-based PPEs and wastes has risen. Owing to the unprecedented output of debris from residences and health facilities, there appears mass waste production due to the hoarding of gloves, gowns, masks, and other protective equipment. Failure to adequately manage trash originated from healthcare institutions and residential areas will exacerbate the development of COVID-19 through secondary transmission. Plus, landfill dumping, open burning, and incineration might have an impact on air quality and health effects owing to toxic exposure (WHO, 2020a).
Fig. 1.
Frontline medical personnel mostly utilize PPE to treat COVID-19 patients.
Landfill strategy seemed unsuitable with the purpose to handle the lumped up of COVID-19 related medical waste which also were highly contagious. It was reported by Budiman and Ardiansyah (2020) in May 2020, the piling of COVID-19 medical waste collapsed the landfill's wall and tons of waste slipped into Cisadane river. Due to the improper waste management, the public water source had been contaminated as the medical waste fell into the main river (Budiman and Ardiansyah, 2020). Corburn et al. (2020) stated that improper landfilling and local burning of plastic and medical waste had increased Indian municipal waste issues the pandemic, and this situation aid the spread of the virus contagion. Therefore, there is a problem in handling unconventional waste in a sustainable manner with lowering carbon emissions, minimizing secondary virus transmission, and minimizing potential health risks. Furthermore, without conventional waste management strategy and waste emergency strategies to combat the epidemic, global might face catastrophic repercussions. Because of the negative environmental implications of plastic waste and its management practices, new ecologically friendly methods of plastic waste disposal should be developed.
Pyrolysis method had attracted the researchers' attention as it comprises of high waste decomposition, facile process and supports green waste management rather than current practices of incineration. Pyrolysis is a thermochemical process that converts plastic waste at high temperatures in a deoxygenated atmosphere into lower molecular weight compounds (Verma et al., 2019). While incineration of plastic waste generates mainly carbon dioxide, water, and unburned debris known as microplastics that occurs in the bottom ash (Yang et al., 2021). Pyrolysis emits very little pollutants. Pyrolysis of plastic produce primarily combustible low molecular weight compounds which basically comprised of gaseous substances; liquid and solid products including coke, char and carbon black (Harussani et al., 2020; Mohd Nurazzi et al., 2021). It would be a perfect application for converting plastic waste into usable materials (Worrel and Vesilind, 2012). There are several studies in the literature on the pyrolysis of plastic wastes and the use of their pyrolysis products (Harussani et al., 2021b, Harussani et al., 2020; Parku et al., 2020). The conversion of plastic wastes to hydrocarbon mixtures by pyrolysis has piqued the interest of many experts since it has the potential to reduce waste, extract chemicals, and substitute other fuels (Harussani et al., 2020; Sharuddin et al., 2016).
Conclusively, this review concentrated on the types and development of plastic pyrolysis technology, specifically in treating PP plastic, which have not been discussed by other review articles. Additionally, this review paper also provided a comprehensive overview of the optimization of product yields from PP pyrolysis, as well as the main influencing parameters in the PP plastic pyrolysis process that require attention to maximize the liquid oil and char production and improve the oil quality. Recent and future possible application of pyrolysis char product is expected to promote green product development and renewable energy generation for environment sustainability.
2. Polypropylene (PP)
PPEs are mainly made up of 72% of PP polymer (3M Personal Safety Division, 2018). PP is a thermoplastic material which produced by polymerizing of monomer propylene molecules. Fig. 1 shows the illustrations for the PPEs which mostly utilized by frontline healthcare personnel in isolation centres. Summary of the raw materials used in the manufacture of components of PPE can be outlined as Table 1 (WHO, 2020b). PP is a saturated polymer with a linear hydrocarbon chain that is chemically and thermally resistant, with melting point of 160 °C (Verbeek et al., 2020). Attributed with excellent and desirable physical, mechanical, and thermal properties in room-temperature really play a big role in widely applied of PP in plastic industry, furniture, storage boxes, car bumpers and stationery.
Table 1.
Raw materials of PPE components.
| PPE components | Raw materials |
|---|---|
| Facemasks | Polypropylene |
| Normal surgical masks | Polypropylene |
| Single use isolation gowns | Mainly polypropylene |
| Goggles | High quality polycarbonates |
| Coveralls | High density polyethylene |
| Hair nets | Polypropylene |
| Shoe covers | Polypropylene |
Adapted from WHO (2020b).
3. Pyrolysis approach
3.1. Pyrolysis of polypropylene
Pyrolysis is a method of thermochemical decomposition of substances such as plastic waste at high temperatures in an inert deoxygenated environment (Verma et al., 2019). In previously published study, as according to Xue et al. (2016), pyrolysis is a viable and effective waste management technique for the treatment of industrial and medical solid waste, and it is an alternative thermal disinfection approach that is available and practical for the treatment of COVID-19 associated waste. Due to the heat and/or pressure used, the waste pyrolysis plant's end products are mostly combustible lower molecular weight, short chain, and less complicated molecules (Kairytė et al., 2020). Generally, gaseous end-product will be yielded, the gases collected such as hydrogen, methane, and carbon monoxide. While, with high operating temperature, liquid outputs will be collected the most. Methanol, acetone, acetic acid, acetaldehyde as well as tar, solvent oil and other organic matter are various kind of liquid-like end products yielded (Verma et al., 2019). Other than that, solid end-products could be yielded mainly via low-temperature pyrolysis. Thus, coke, char and carbon black will be collected.
As the end-products of pyrolysis are collected and repurposed into usable outputs, it creates less pollution. This approach was mentioned as an excellent technology for the decomposition as well as transformation of plastic waste into usable by-products (Worrel and Vesilind, 2012). Vivero et al. (2005) emphasized that the conversion of plastic wastes to hydrocarbon blends by pyrolysis is the optimum method for decomposing and recycling PPE waste. It helps to reduce the amount of plastic trash generated, recover chemicals, and substitute fuels and other virgin materials. Table 2 summarizes the comparison of pyrolysis of various plastic waste, including PE, PVC, PS, PET and PP, regarding the solid yields, including char, coke or waxy residue.
Table 2.
Comparison of char yields (wt%) from the pyrolysis of plastic wastes (HDPE, LDPE, PS, PET, and PP) studied in literature works.
| Material | Processa | Char yields (wt%) | Ref. |
|---|---|---|---|
| HDPE | Batch reactor (T = 450 °C, rt = 60 min, catalyst HZSM-5) | 19.70 | (N Miskolczi et al., 2004) |
| HDPE | Semi-batch reactor (T = 300 °C, hr = 5 °C/min) | 2.34 | (Sogancioglu et al., 2017) |
| HDPE | Batch reactor (T = 440 °C, rt = 120 min) | 17.00 | (Sharma et al., 2014) |
| HDPE | Fixed bed reactor (T = 500 °C, hr = 5 °C/min, rt = 330 min) | 20.00 | (Al-Salem, 2019) |
| HDPE | Batch autoclave reactor (T = 490 °C, rt = 60 min) | 6.50 | (Palos et al., 2019) |
| LDPE | Semi-batch reactor (T = 300 °C, hr = 5 °C/min) | 10.12 | (Sogancioglu et al., 2017) |
| LDPE | Batch reactor (T = 300 °C, hr = 5 °C/min, rt = 360 min) | 10.80 | (Bow and Kurniawan, 2021) |
| PVC | Wire mesh reactor (T = 500 °C, hr = 1 °C/s) | 16.00 | (Zhou et al., 2016) |
| PS | Batch reactor (T = 425 °C, P = 1.6 MPa, hr = 10 °C/min) | 0.50 | (Onwudili et al., 2009) |
| PET | - (T = 500 °C, P = 1 atm, hr = 6 °C/min) | 9.00 | (FakhrHoseini and Dastanian, 2013) |
| PP | Batch reactor (T = 380 °C, P = 1 atm, hr = 3 °C/min) | 13.30 | (Sakata et al., 1999) |
| PP | Batch reactor (T = 740 °C, hr = 10 °C/min) | 1.60 | (Demirbas, 2004) |
| PP | Fluidized bed reactor (T = 703 °C, fr = 7 g/min, fast pyrolysis) | 6.90 | (Jung et al., 2010) |
| PP | Semi-batch reactor (T = 450 °C, P = 1 atm, hr = 25 °C/min, catalyst FCC) | 3.60 | (Abbas-Abadi et al., 2014) |
| PP | Horizontal steel reactor (T = 250 °C, hr = 20 °C/min, rt = 30 min) | 13.70 | (Ahmad et al., 2015) |
| PP | Fixed bed and batch reactor (T = 400 °C, hr = 5 °C/min, rt = 45 min) | 2.50 | (Sogancioglu et al., 2020) |
| PP | Fixed bed reactor (T = 900 °C, rt = 21 min) | 2.80 | (Liu et al., 2020) |
| PP | Microwave-assisted semi-batch reactor (T = 250 °C, HZSM-5 catalyst) | 1.54 | (Duan et al., 2017) |
| Real plastic waste | Batch reactor (T = 500 °C, hr = 20 °C/min, rt = 30 min) | 33.50 | (Adrados et al., 2012) |
T – temperature, P – pressure, fr – feed rate, hr – heating rate, rt – residence time.
Adapted and updated the information from Das and Tiwari (2018a).
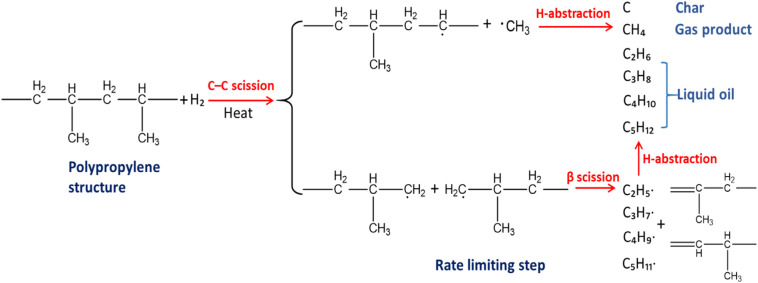
From published research works, it is reported that PP plastic wastes were slow pyrolysed with low heating rate of 5 °C/min. Sogancioglu et al. (2020) showed that in the pyrolysis experiment, more than 2.67% char product yields. Ahmad et al. (2015) studied the effect of pyrolysis temperature towards the production of fuel-like products from pyrolysis of PP and HDPE waste over range of low temperature, 250 °C to 400 °C. The highest total conversion of PP waste was achieved at 300 °C with 98.66. Whereas in terms of char yields, decreased yield of solid residue occurred along the increasing temperature, from 13.68% to 5.7%. The decreased output of char products at a higher temperature, according to Witkowski et al. (2016), is associated to the increased primary depolymerisation of plastics and secondary depolymerisation of yielded char as demonstrated in Fig. 2 . Note that the nitrogen gas used to avoid oxidisation happened towards the samples prior to the pyrolysis process.
Fig. 2.
Pyrolysis reaction for polypropylene (PP) polymer. Adapted from (Yan et al., 2015).
Similar trend of decreasing char yields along increasing pyrolysis temperature had been reported, including pyrolysis of PP waste, by Abbas-Abadi et al. (2014), Ahmad et al. (2015) and Harussani et al., 2021b, Harussani et al., 2021a, PE waste by Palos et al. (2019), and PVC plastic wastes by Miranda et al. (2012) and Adrados et al. (2012), refer Table 2. Brief literature review on pyrolysis of PP plastic waste has been discussed and compared according to the specified pyrolysis parameters and type of pyrolysis which the integrated strategy aids in achieving maximum economic and environmental advantages with minimum waste production.
3.2. Type of pyrolysis
Pyrolysis is classified into several categories based on the operating conditions, including the pyrolytic temperature, heating rate, and the time of residence of the volatile matter. Table 3 below summarizes that slow pyrolysis, fast pyrolysis, and flash pyrolysis are the three primary kinds of pyrolysis based on the heating rate. Pyrolysis, both fast and slow, is generally done in an inert environment (Zaman et al., 2017). Slow pyrolysis has a prolonged vapor residence period in the pyrolysis medium. This method is generally utilized to create the greatest quantity of char. Pyrolysis might well be classified into four types based on the operating environment: (1) hydro-pyrolysis, (2) oxidative pyrolysis, (3) catalytic pyrolysis and (4) vacuum-assisted pyrolysis. Hydro-pyrolysis is performed in the presence of hydrogen, whereas catalytic pyrolysis is the pyrolysis which assisted with catalysts to improve the process efficiency and by-products yields. While, some pyrolysis types depend on the heater system as the microwave or plasma pyrolysis (Martínez et al., 2013). In this section, the common type of pyrolysis practiced will be discussed regarding for plastic polymer polypropylene waste. Table 4 outlines the effect on desired product yields towards the different type of pyrolysis process on PP plastic waste.
Table 3.
Differentiation of pyrolysis processes based on main operating parameters.
| Pyrolysis methods | Residence time | Heating rate | Final temperature (°C) | Major products |
|---|---|---|---|---|
| Flash | <1 s | Very high (1000 °C/s) | 800–1000 | Oil |
| Fast | <5 s | High (10–200 °C/s) | 400–800 | Oil |
| Slow | 30–300 min | Low (<5 °C/s) | 300–600 | Char, oil, gas |
| Ultra-rapid | <0.5 s | Very high | >1000 | Gas |
| Hydro-pyrolysis | <10 s | High | <500 | Oil |
| Vacuum | 2–30 s | Medium | ~400 | Oil |
| Carbonization | Days | Very low | ~400 | Charcoal |
Adapted with permission from Zaman et al. (2017).
Table 4.
Pyrolysis of plastic PP using different type of pyrolysis.
| Plastic | Type of pyrolysis | Focused end products | Ref. |
|---|---|---|---|
| PP, PE | Slow | PDO for liquid fuel | (Das and Tiwari, 2018b) |
| PP | Slow | Char 10%, oil 76% and gas 14% | (Singh et al., 2019) |
| PP, PE waste | Slow | Liquid oil of C8-C28 aliphatic carbons | (Velghe et al., 2011) |
| PP waste | Slow (catalytic) | Solid oxide fuel cell, carbon nanotubes | (Cai et al., 2019) |
| PP | Slow | Carbon 25%, oil 33% and gas 38%, porous carbon sheets | (Ma et al., 2018) |
| PP, PET, PS waste | Slow (co-pyrolysis) | Pyrolysis oil | (Chhabra et al., 2020) |
| PP waste | Slow | Alkene/alkane rich gas products | (Ciliz et al., 2004) |
| PP waste | Slow | Liquid (oil/wax phase) yields 42% | (Maniscalco et al., 2021) |
| PP waste | Slow | 80% liquid fuel | (Santaweesuk and Janyalertadun, 2017) |
| PP waste | Slow | Char 5.7%, gases 5.6% and light and heavy oil fraction of 82.4% | (Parku et al., 2020) |
| PP | Slow | PDO and hydrocarbon gases | (Das and Tiwari, 2018a) |
| PP | Slow | Char 2.49%, oil 78.70%, gas 18.81% | (Sogancioglu et al., 2020) |
| PP | Fast | 50% of the feed mass, 40% oil and 10% char | (Kalargaris et al., 2018) |
| PP waste | Fast | Char 2.5%, gases 31.8% and light and heavy oil fraction of 60.5% | (Parku et al., 2020) |
| PP | Fast | Char 2%, oil 7% and gas 91% | (Singh et al., 2019) |
| PP | Fast | High quality biofuel | (Ojha and Vinu, 2015) |
| PP | Fast | 51.9% organic carbon basis, 0.2% char residue | (Erdoğan et al., 2014) |
| PP, lignin | Fast (non-catalytic co-pyrolysis) | High hydrocarbons originating from PP pyrolyzates | (Ryu et al., 2020) |
| PP from facemask | Catalytic | Formations of syngas, C1-2 hydrocarbons | (Jung et al., 2021) |
| PP, lignin | Catalytic (fast co-pyrolysis) | Larger amounts of BTEXs, OMAHs, small molecular hydrocarbons (C3 ~ C7) | (Ryu et al., 2020) |
| PP, seaweed | Catalytic | Large pore size, strong Brönsted acid sites bio-oil | (Kim et al., 2017) |
| PP | Plasma | Low emission rate, waste residue is inert and sterile | (Datta et al., 2018) |
| PP waste | Plasma | Synthesis gas and fuels | (Munir et al., 2019) |
3.2.1. Slow pyrolysis
Slow pyrolysis refers to pyrolytic breakdown that occurs at a lower operating temperature, with a low heating rate and extended solid and vapor residence times. Longer stays lead to secondary conversion of primary products, which is beneficial for the production of coke, tar, and char (Martínez et al., 2013). Carbonization is another name for slow pyrolysis. According Singh et al. (2019), the slow pyrolysis heating rate with lengthy time of feedstock residence optimize char production, whereas high heating rate of fast pyrolysis produces very little char. A significantly lower non-isothermal heating rate promoted the synthesis of H2, CO, CH4, and C5H12, whereas rapid pyrolysis produces predominantly gases (Bennett, 2007). It is recorded that slow pyrolysis of PP products with 10 °C/min heating rate yielded 10% solid char, higher than that of fast pyrolysis, with 2% char. Another claim is slow pyrolysis also yielded higher amount of oil product rather than fast pyrolysis, shown by previous work (Das and Tiwari, 2018a; Parku et al., 2020; Singh et al., 2019).
Das and Tiwari (2018b) had showed in his work that thermally decomposed PP and PE wastes with low temperature (~400 °C) gave output of light hydrocarbon liquid fractions (C6–C20). Subsequently, we discovered that utilizing slow pyrolysis, there are less works on char production from PP waste than from plastic-derived oil. Slow pyrolysis, however, actually results in higher char yields (Ahmad et al., 2015). Sogancioglu et al. (2020) studied the effect of pyrolysis temperature on the solid pyrolysis product of pyrolysis of PP plastic wastes. The yielded char was used as filler material in epoxy composite for structural applications. PP identified to have the highest carbon content, verifying its carbonaceous nature.
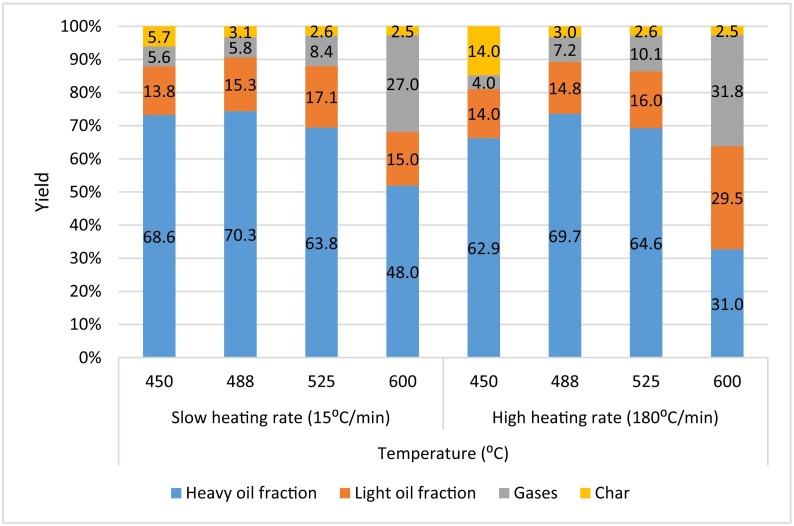
Harussani et al. (2021a) slow pyrolysed disposed single use PP isolation gowns under different low pyrolysis temperatures, increasing char yields had been recorded along decreasing process temperature. Two degradation mechanisms occurred in the process: (1) end chain scission, results in monomer units, and (2) random scission, results in the bulk of hydrocarbon products. Parku et al. (2020) studied the effect of slow and fast pyrolysis of PP waste with heating rate of 15 °C/min and 180 °C/min, respectively, under atmospheric pressure in a bench-scale reactor. From Fig. 3 , char yields are highest (14 wt%) with lower gas yields, via fast pyrolysis with lower temperature. Contradict to previous claim, Parku et al. (2020) had observed that fast pyrolysis approach successfully yielded higher char amounts, increasing with 145%. However, thermal decomposition with low temperature under atmospheric condition was proven to enhance the char and liquid oil yields, while, higher temperature lead to improve gases yields. All condensable materials have higher heating values (HHV), which are obtained under milder conditions. The lower HHVs obtained at higher temperatures were clarified by increased aromatic activity.
Fig. 3.
Effects of temperature and heating rate on liquid, gases and char yields for atmospheric conditions. Reproduced with copyright permission from (Parku et al., 2020), Elsevier.
3.2.2. Fast pyrolysis
In contrary, this type of pyrolysis considers a rapid thermal decomposition with higher heating rates and/or high process temperature for rapid products quenching, admired for liquid product formation. Liquid fuel with higher calorific value was yielded, based on Singh et al. (2019). They had studied the effect of residence time towards PP waste using fast pyrolysis technique. Jung et al. (2020) had thermally decomposed PP and PE waste under high process temperature around 668–746 °C. Approximately, 2 wt% of char residue had been yielded compared to about 32 wt% and 66 wt% of oil and gas yields, respectively. This is due to the higher heating rate, 20 °C/min that causes the char residue to interact with the volatile matters to generate secondary compounds. Thus, it promotes the carbon to be converted into pyrolysis oil and gas products, about 91% of gas yielded.
However, Jung et al. (2010) discovered that increase in process temperature in isothermal process, producing char with higher surface area, 192 m2/g. According to Ojha and Vinu (2015), treated microcrystalline cellulose and PP were mixed and decomposed via fast pyrolysis with high heating rate. It was recently showed that maximum char formation for feed composition of cellulose and PP is 50:50, 14.32% and 40.21% of char and hydrocarbons yields, respectively. The hydrocarbon vapors from pure PP are thought to react with the char formed by the burnt cellulose under isothermal conditions. Thus, condensed ring aromatic compounds were formed, which persisted in the solid residue after the pyrolysis process was completed. With increasing pyrolysis temperature, the quantity of char reduced while the production of aromatic hydrocarbons increased.
3.2.3. Catalytic pyrolysis
Recently, the formation of aromatic hydrocarbons via catalytic pyrolysis of waste plastic, including PP (Abbas-Abadi et al., 2014; Jung et al., 2021; Kim et al., 2017; Ryu et al., 2020), PE (Lee et al., 2002; N Miskolczi et al., 2004), or PS (Shah and Jan, 2014; Williams and Bagri, 2004) was reported by many researchers. Commonly, PP waste was thermally decomposed with aid from catalysts in to enhance the yield of the by-products. Plus, presence of catalyst does speed up chemical reaction via lowering the activation energy of the process, leads to energy saving and widely applied in industries and researches for the aforementioned particular reasons.
Abbas-Abadi et al. (2014) works on PP with fluid catalytic cracking (FCC) catalyst in a stirred reactor. It is observed that the increasing amount of FCC catalyst applied leads to the lower yields of condensed product but higher non-condensable product. While, the coke yield is increasing, 14% coke collected under 0.6 FCC/PP ratio. Kim et al. (2017) investigated the catalytic co-pyrolysis of PP and biomass waste in a fixed-bed reactor using a variety of catalysts such as HZSMS-5, Pt/mesoporous MFI, and mesoporous Al-SBA-16. The quantities of oxygenates, acids, and wax species were considerably reduced by catalytic upgrading, while the amount of aromatics and light hydrocarbons in the gasoline and diesel ranges were considerably increased, suitable for bio-oil applications. Thus, it indicates that the catalysts used improved the performance of the thermal cracking process plus the number of by-products yielded.
3.2.4. Plasma pyrolysis
Extreme heat provided by the plasma allows it to safely and reliably dispose of all forms of waste, which were pyrolysed into CO, H2, and hydrocarbons (Nema and Ganeshprasad, 2002). Thus, it suits to be used in managing infectious medical waste as thermally stable bacteria are killed by the plasma environment. Gases produced by the pyrolysis leads to a high temperature, around 1200 °C. During the plasma pyrolysis process, the hot gases are quenched from 500 to 700 °C to avoid recombination reactions of gaseous molecules, which hinder the generation of dioxins and furans. The results of the gas analysis demonstrate that the toxic gases released during combustion are far within the emission limitations set by the Central Pollution Control Board (CPCB).
Datta et al. (2018) stated that this approach generates plasma energy by using plasma torches. In the plasma state, the ionized gas can conduct electric current, then, the electric energy is transformed to heat energy due to its high resistance. Residues are generated at the end of process, include carbon black, glass aggregates, and metallic residues (Bano et al., 2017). Tang et al. (2003) stated that the percentage of hydrogen and carbon monoxide in the gaseous liquid could well be produced up to 40% at the optimal experimental power input of 35.2 kVA and feed rate of 60 g/min. Thus, utilization of plasma pyrolysis for fast decomposition of medical waste is suggested rather than gaseous combustion (Wang et al., 2020). This approach has produced low emission rates, inert residuals, volume reductions of about 95%, and mass reductions of 90%. However, high energy consumed and costly hindered the plasma pyrolysis application.
4. Pyrolytic parameters
Summary on the effect of various process parameters towards the yields of final products had been denoted in Table 5 . Depending on the material composition, pyrolysis temperature, residence duration, catalysts employed, and other operational parameters, a variety of products will be produced (Hidalgo et al., 2016). The importance of reaction parameters in the thermal degradation process cannot be understated. Pyrolysis operation parameters have an impact on the generation of oil, char, and gaseous products. Optimal process parameters will result in the highest yields of pyrolysis by-products. Thus, by modifying and regulating a set of process parameters, the intended end-products may be effectively produced. In this section, the discussions of the operating conditions are revised as follows.
Table 5.
Pyrolysis of PP with specific process parameters.
| Sample | Process parameters |
Final products (wt%) |
Ref.a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of reactors | Temperature (°C) | Residence time (min) | Heating rate (°C/min) | Particle size (mm) | Catalyst | Solid | Liquid | Gas | ||
| PP | Auger and fluidized bed reactor | 718.2 | 10 | 5 | 0.4–1.5 | – | 1.67 | 33.83 | 63.4 | 1 |
| PP waste | Lab scale borosilicate glass reactor | 450 | 90 | – | – | Zeolite | Wax | 80.82 | – | 2 |
| 65 | – | – | 86.40 | – | ||||||
| PP and biomass waste | Co-pyrolysis | 450 | 50 | 5 | 5 | – | 23.20 | 57.41 | 19.4 | 3 |
| PP | Tubular electrical furnace semi batch pyrolysis | 325 | 10 | 5 | 5 | – | Wax | 54.02 | 29.8 | 4 |
| PP waste | Batch autoclave reactor | 500 | 60 | 5 | 2–5 | – | – | 95 | 5.0 | 5 |
| PP | Conical spouted bed reactor | 450 | 18 | – | 0.6–1 | – | Wax | 92 | 8.0 | 6 |
| PP, PE, PS, PET waste | Batch reactor | 500 | 30 | 20 | – | – | 33.5 | 40.9 | 25.6 | 7 |
| PP | Batch reactor | 740 | 10 | – | – | – | 1.6 | 48.8 | 49.6 | 8 |
| PP | Batch reactor pyrolysis | 350 | 180 | – | – | – | – | 54.5 | – | 9 |
| PP, PS | Fixed bed pyrolysis reactor | 700 | 60 | 25 | – | 0.4 | 83.6 | 16.0 | 10 | |
| PP, APP | Fixed bed pyrolysis reactor | 425 | 150 | 5 | 0.2 | – | – | 93 | 7.0 | 11 |
| PP waste | Batch reactor pyrolysis | 400 | 60 | – | – | – | 1.84 | 88.86 | 9.3 | 12 |
| PP waste | Fixed-bed reactor co-pyrolysis | 500 | 30 | 5 | 30 | – | 15.6 | 6.3 | 40.0 | 13 |
| PP waste | Fixed-bed reactor | 250 | 60 | – | – | – | – | 8 | – | 14 |
| PP | Fluidised bed pyrolysis | 520 | 300 | 3 | 0.18 | HUSY | 3.44 | 3.75 | 89.5 | 15 |
| PP | Steel micro reactor | 300 | – | – | – | – | 1.34 | 69.8 | 28.8 | 16 |
| PP | Batch reactor pyrolysis | 380 | 30 | 3 | 1 | – | 13.3 | 80.1 | 6.6 | 17 |
| PP, PE, PA, PS waste | Horizontal tube reactor | 500 | 72 | – | 4–5 | – | 85.3 | 10.9 | 3.4 | 18 |
| PP | Pyrolysis reactor | 500 | – | 6 | – | – | 0.12 | 82.12 | 18.0 | 19 |
Ref. – 1 - (Park et al., 2019); 2 - (Sonawane et al., 2015); 3 - (Sajdak and Muzyka, 2014); 4 - (Das and Tiwari, 2018a); 5 - (Williams and Slaney, 2007); 6 - (Aguado et al., 2002); 7 - (Adrados et al., 2012); 8 - (Demirbas, 2004); 9 - (Wong and Broadbelt, 2001); 10 - (Williams and Williams, 1999); 11 - (Ballice and Reimert, 2002); 12 - (Martynis et al., 2019); 13 - (Kusrini et al., 2019); 14 - (Bow and Pujiastuti, 2019); 15 - (Lin and Yen, 2005); 16 - (Ahmad et al., 2015); 17 - (Sakata et al., 1999); 18 - (Norbert Miskolczi et al., 2004); 19 - (FakhrHoseini and Dastanian, 2013).
4.1. Temperature of process
Temperature is the main parameter affecting the pyrolysis. Optimum temperature controls the breaking of the polymer chain of the plastic. During the increasing temperature, the molecules of the materials start to vibrate and tend to evaporate from the surface of the materials. This tends to occur when the van der Waals force-induced energy across the polymer chains rises exceeding the C—C bond enthalpy, thus, carbon chain breaks (Das and Tiwari, 2018b; Sobko, 2008). In addition, higher operating temperatures of pyrolysis produces better non-condensable gases (syngas, synthetic gas) yields, while lower temperatures are recommended for high yields of solid product, such as charcoal, bio-coal, and char.
Harussani et al. (2021a) has studied the effect of temperature towards PP degradation phase. According to the thermal analysis, PP deterioration temperature started at 400 °C, lower than the HDPE as reported by Marcilla et al. (2005). Theoretically, PP degraded quicker than HDPE because half of the carbon in the PP chain is tertiary carbon, which promotes the production of tertiary carbocation during deterioration (Jung et al., 2010). Panda et al. (2010) explained that the event happened due to recombination of the by-products via retrogressive reunion leading to char formation. Thus, the coke and carbonaceous formation yields more than the liquid products at this phase, 5.7% at 400 °C. However, maximum char yield occurred at low temperature of 250 °C, with 13.68% solid residue collected. Ahmad et al. (2015) studied the effect of temperature on the thermal cracking of polyolefinic polymers PP and HDPE. As the temperature increasing, the total yield was increased from 86% at 250 °C to 99% at 300 °C. This indicates that PP easily degrades due to its branching structure plus it has higher proportion of tertiary carbons in its polymer chains, leads to thermal cleavage of C—C bonds (Aguado et al., 2000). However, the overall conversion start decreasing as the process temperature increased, 300–350 °C.
4.2. Residence time
Residence time referred to the time length for the sample will be in the pyrolysis chamber, affecting the yields of end-products. Prolonged time of residence increases the degradation of the primary product contributing to the formation of more thermal stable products like the light molecular weight hydrocarbons and non-condensable gas (Sharuddin et al., 2016), as it affects the degree of thermal conversion of the solid and gas products. Miskolczi et al., 2004, Miskolczi et al., 2004 developed a research on thermal cracking of PE and PP for fuel-like hydrocarbons yield using horizontal tubular reactor. The degradation of PP had been studied at different residence times, 36 min, 54 min, and 72 min. The higher the residence time affecting the yields of liquid and solid residue products, the residue yields decreased. As stated by Martínez et al. (2013), the residence time effect also linked with process temperature and the heating rate of the pyrolysis. The longer pyrolysis time commonly used for thermally cracking larger size of particles, as increasing temperature or heating rate contributes to a shorter reaction time.
4.3. Heating rate
Heating rate influences the reaction rate and defines the temperature profile of the particles (Martínez et al., 2013). Increasing the heating rate leads to higher temperatures for weight loss in thermal analysis. This is due to the rate of degradation increases as the heat transfer and the kinetic of the decomposition changes, thus, the decomposition of particles delayed a bit. Based on Singh et al. (2019), fast pyrolysis must be equipped with higher heating rate in order to increase the volatiles yield and also to allow the secondary reactions for higher gas fraction yields. Besides, low heating rates was discovered to yield oil with solid product formation. Thus, slow pyrolysis optimises the carbonaceous char yields. However, in term of surface area, fast pyrolysis produced char with higher surface area, approximately 200 m2/g. Jung et al. (2010) mentioned that slower heating rate causing a slower volatiles formation and allowing the gases to penetrate in a channelized manner creating less reaction surface.
4.4. Presence of catalysts
Chemical catalysts act to accelerate the chemical reactions of the pyrolysis system. Catalysts are extensively utilized in manufacturing and are being explored to enhance the product distribution and selectivity. Several lists of catalysts were commonly used in fast pyrolysis of PP plastic, including kaolin, silica, alumina, zeolites (beta, USY, ZSM, HZSM, etc.), and other non-zeolite catalysts (FCC, MCM, HMOR). As a result, catalytic degradation is used to create output with high economic value, such as automobile fuel, diesel, and gasoline, as well as C2–C4 olefins (Elordi et al., 2009). The activation energy of the mechanism is lowered as well as the optimised process temperature as the catalyst applied in the pyrolysis process, thereby accelerating the rate of reaction. Other than that, the presence of catalyst during pyrolysis reaction help in yielding high quality products via low energy consumed, which low-cost process is important for industrial applications (Sharuddin et al., 2016).
Jung et al. (2020) investigated CO2-assisted catalytic pyrolysis towards PP-based disposable facemask. Ni/SiO2 catalyst had been applied in this process. The control the ratio of H2/CO helps in improve the selectivity of by-products such as chemicals, saturated hydrocarbons, olefins, and alcohols. While, the catalytic pyrolysis stage contributed to substantial conversion of longer chain (≥C2) hydrocarbons into H2 and CH4. Luo et al. (2000) stated that F9 and Silica/Alumina (SA) catalysts had been used to degrade the HDPE and PP samples inside fluidised bed reactor under nitrogen atmosphere. SA produced better amount of liquid yields however lower yields of gases and solid product compared to F9.
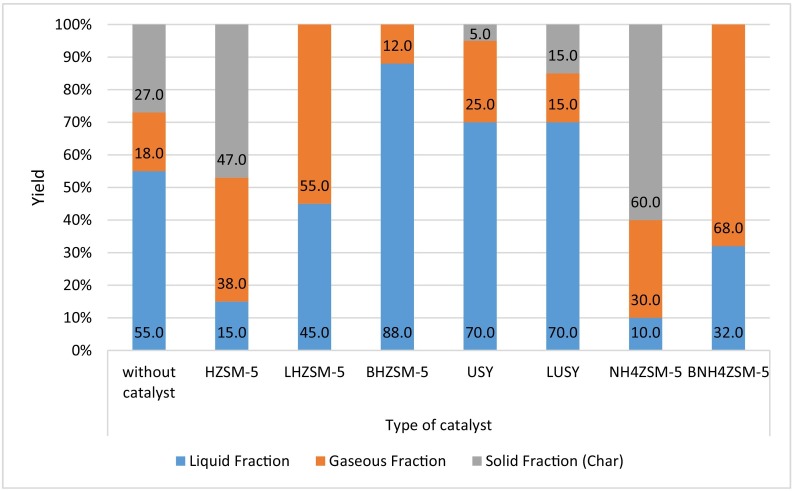
Based on Santos et al. (2018), thermal cracking of plastic waste consisted of PE and PP assisted with catalyst BHZSM-5, USY, BUSY and NH4ZSM-5 had resulted in higher yields of liquid fraction. The pyrolysis was done under nitrogen flux at temperature of 450 °C for 30 min inside a tubular reactor. Fig. 4 above displays the best catalyst for liquid yields is BHZSM-5 zeolite with almost 90% of liquid fraction, BNH4ZSM-5 zeolite showed the best catalytic performance for gaseous fraction production, ~70% of gases yields and LHZSM-5 catalyst with over 50% of solid residue yields, favourite for char yields. It is concluded, zeolite with higher mesoporous volume was preferred for higher liquid product yields, while zeolite with lower volume of mesoporous admired for gaseous fraction production. As a conclusion, the catalyst was an essential aspect that required attention since it impacted the end product distribution in plastic pyrolysis, specifically PP.
Fig. 4.
By-products distribution yields from PE:PP pyrolysis at 450 °C for 30 min aided with various kind of zeolite catalysts. Reproduced with copyright permission from (Santos et al., 2018), Elsevier.
4.5. Type of reactors
Numerous types of pyrolysis reactors have been designed to convert a diverse variety of biomass into three product categories: liquid, solid and gas. The yields of specific products rely on the reactor design and operating parameters. Various methods have been proposed for the classification of the process and equipment designs. In this subsection, the common type of reactors applied in thermal cracking of plastic polymer PP, specifically, will be reviewed. Table 6 displays the key points of several types of reactors.
Table 6.
Summary of advantages and disadvantages of different type of reactors.
| Type of reactor | Key points |
Ref. | |
|---|---|---|---|
| Advantages | Disadvantages | ||
| Batch reactor | High conversion of the reactants | Variability of product from batch to batch, high labour costs and the difficulty of large scale production | (Kassargy et al., 2017) |
| Semi batch reactor | Flexibility of adding reactants during reaction | High labour cost and suitable for small scale production | (Das and Tiwari, 2018a) |
| Fluidized bed reactor | Provide better access to the catalyst, good heat transfer, low operating cost | Complex design | (Park et al., 2019) |
| Fixed bed reactor | Facile process | Irregular particle size will lead problem during feeding process | (Chattopadhyay et al., 2016) |
| Rotary kiln reactor | Continuous process, easily handle large amount of feedstock, low cost | Complex design | (Efika et al., 2012) |
| Conical spouted bed reactor | Enable to mix thoroughly a wide range of particle sizes, bigger particles and densities. | Complex design, high operating cost | (Arabiourrutia et al., 2017) |
4.5.1. Batch reactor
Pyrolysis batch reactor is basically a closed system, without reactants introduction allowed (Sharuddin et al., 2016) (Fig. 5 ). High conversion in a batch reactor can indeed be accomplished by leaving the reactants for a prolonged period of time, which is one of its benefits (Kim and Kim, 2004; Panda et al., 2010) However, the hindrances of this type of reactor are the variability of product from batch to batch, high labour costs for each batch processing and the unsuitability for massive industrial scale of production (Fogler, 2010). In addition, pyrolysis that was done using batch or semi-batch reactor usually performed at 300–800 °C of temperature range either for thermal or catalytic pyrolysis (Williams and Slaney, 2007).
Fig. 5.
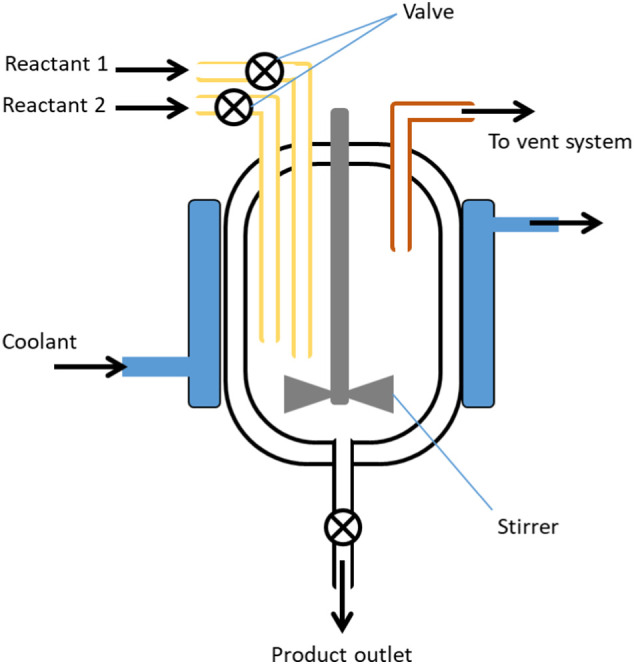
Batch reactor with stirrer.
Anene et al. (2018) had carried out thermal pyrolysis of LDPE and PP plastic waste using laboratory scale batch reactor in a N2 atmosphere at temperature of 460 °C. Overall conversion of PP and LDPE into liquid products was approximately 86 wt% and 94 wt%, respectively which the liquid oil yielded hydrocarbon distribution were dispersed within C7-C40 carbon. Sogancioglu et al. (2020) used batch reactor to study the thermal pyrolysis of PP heating rate used is 5 °C/min at different final temperatures. The authors found that the PP pyrolysis at 700 °C produced the highest pyrolysis conversion ratio with 97.83%. The PP char yielded discovered to have higher aromatic structure plus the highest surface area, 22 m2/g better than the other char samples.
4.5.2. Semi-batch reactor
Some researchers, Singh et al. (2019), Das and Tiwari (2018a), Adrados et al. (2012) as well as Yan et al. (2015), preferred to apply semi-batch reactors in plastic pyrolysis as it allows addition of reactant as well as removal of by-products during the process. As stated by Singh et al. (2019), semi-batch reactor that equipped with different heating rates of 10 and 20 °C/min at 500 °C during slow pyrolysis of mixed plastic waste, such as HDPE, PP, PET and PS. As the heating rate increased the oil yields increased too, while, the gas and char yields decreased, from 10% to 8.5% for char residue and for gas from 14.2% to 10.5%. Yan et al. (2015) investigated the thermal cracking of PP and LDPE plastic wastes using a semi-batch reactor, under atmospheric pressure at operating temperature of 460 °C. The thermal pyrolysis of both samples yielded higher liquid fraction for about 84%. The oil yields produced from the pyrolysis of PP contained 58 wt% gasoline range hydrocarbons, while for LDPE, contained 21 wt% of gasoline range hydrocarbons.
Accordingly, it was concluded from the literature study abovementioned that the batch or semi-batch reactors are the finest reactors that can be used in thermal pyrolysis to achieve highest liquid yields, since the parameters can indeed be easily managed. Moreover, batch operation was not ideal for large-scale production as it requires large operational costs for the recharging of feedstock and therefore was more suitable for laboratory studies.
4.5.3. Fixed-bed reactor
Despite of many drawbacks of fixed-bed reactor, such as the problem during feeding process as the particle size and shape of feedstock plastics are irregular plus the available surface area of the catalyst for the pyrolysis is also limited. Tekin et al. (2012) stated that the catalyst is palletized and packed in a static bed. As a result, the accessible surface area of the catalyst for the reaction to reach is small. Several studies, however, elected to employ a fixed-bed reactor for the PP plastic pyrolysis (Bow and Pujiastuti, 2019; Kusrini et al., 2019; Tekin et al., 2012). There are also a few researchers utilized fixed-bed reactor due to its simpler design for the plastic pyrolysis (Achilias et al., 2007; Chattopadhyay et al., 2016; Gutierrez and Palza, 2015; Zanella et al., 2013). Zanella et al. (2013) chose to use fixed-bed reactor to study co-pyrolysis of coffee and PP wastes into fuels. With residence time of 3 h and fixed heating rate 5 °C/min, the thermal cracking of the substances relations with the operating temperature, 360–420 °C, had been investigated. It is concluded that as the operating temperature increased, the thermal degradation of the waste improved, particularly from 360 to 380 °C, where the percentage of degraded material doubles, becoming >90 wt% at 420 °C.
Chattopadhyay et al. (2016) had studied the catalytic co-pyrolysis of plastic HDPE, PP and PET along with biomass using fixed-bed reactor. The catalysts used were cobalt based alumina, ceria and ceria-alumina. From the pyrolysis, the plastic is rapidly degraded from 320 to 380 °C. Gaseous products yielded the highest during co-pyrolysis of 5:1 ratio of biomass/plastic assisted by catalyst of 40% Co/30% CeO2/30% Al2O3 thus it shows the catalysts aided the thermal cracking process. Besides, HDPE producing higher yield of char residue rather than PP waste, with 20.0 wt% of PP char, supported by previous works (Achilias et al., 2007; Elordi et al., 2009) (Fig. 6).
Fig. 6.
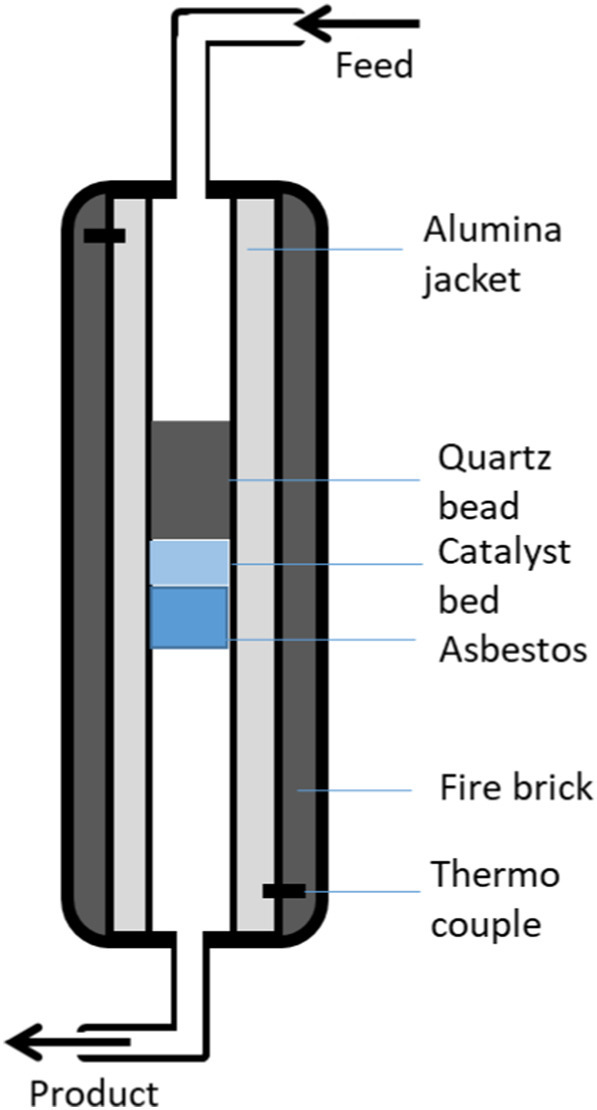
Fixed-bed reactor.
4.5.4. Fluidized bed reactor
Numerous studies recommended fluidized bed reactors over fixed bed reactors in catalytic cracking of polyolefins (Jung et al., 2010; Lin and Yen, 2005; Luo et al., 2000; Park et al., 2019) (Fig. 7). Jung et al. (2010) had been practiced the thermal degradation of PP and PE input via fluidized bed reactor as it ensures a fixed temperature with greater mass and heat transfer, thus, leads to less time of residence. The PP and PE were thermally degraded with temperature range of 650 °C until 750 °C. Thus, 43 wt% of fuel-like liquid products had been yielded from the PP pyrolysis, while, more than 60 wt% of oil yields from the PE fraction. Moreover, the BTX aromatics in PP fraction is much higher than in PE fraction, with 53 wt% compared to 32 wt% of oil yields, respectively. According to Luo et al. (2000), the research on the correlation between HDPE and PP samples in catalytic decomposition in a fluidized bed reactor had been studied, with the assist from silica–alumina (SA) catalyst. According to Luo et al., PP generated 87 wt% of liquid oil yields, whereas HDPE produced lower oil yields of 85 wt% at constant operating temperature of 500 °C. This result was expected since HDPE had higher strength properties than PP.
Fig. 7.
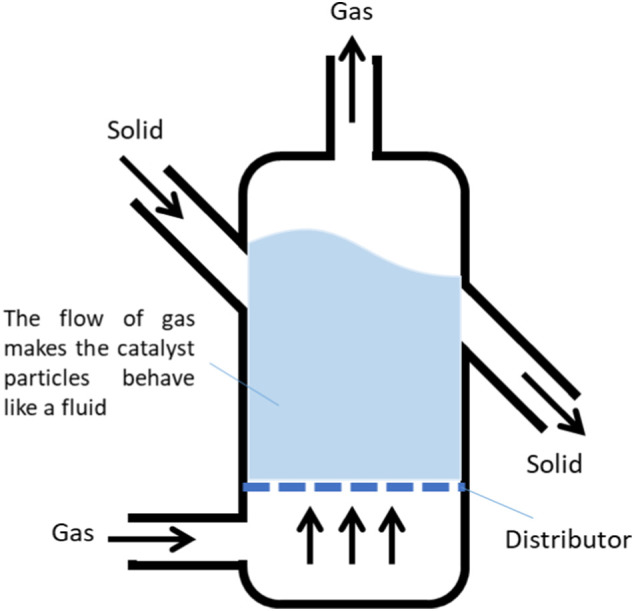
The schematic diagram of thermal and catalytic pyrolysis equipped with fluidized bed reactor. Reproduced with copyright permission from (Luo et al., 2000), Elsevier.
As a result, the fluidized bed reactor is the ideal reactor to conduct catalytic plastic pyrolysis, since the catalyst can be reused several times without the need for discharging, knowing that the catalyst is a very costly material in the industry. In addition, it is more versatile than the batch reactor, as for continuous operation, repeated loading of feedstock could be evaded. Indeed, this type of reactor is the best reactor in treating large-scale operation due to its large operating capacity for a single run.
4.5.5. Rotary kiln reactor
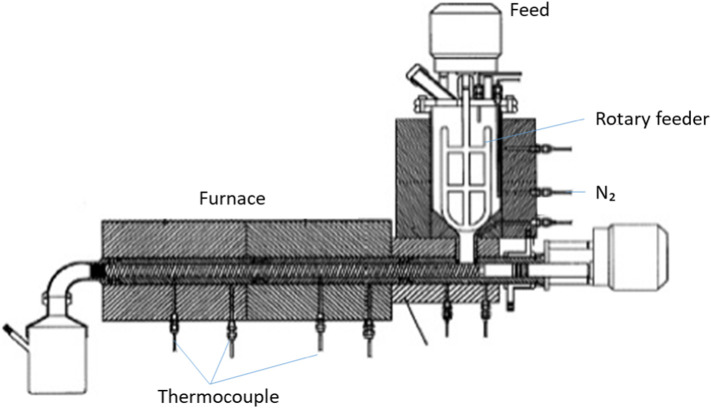
Rotary kiln reactor basically was designed with 2% of slant in the direction of the secondary combustion chamber, the place where plastic waste is incinerated (Bujak, 2015), as shown in Table 6. Lower calorific value (LCV) and the moisture content of feedstock determined the length of the incineration (Behzadi and Farid, 2006). Non-condensable gases were combusted when pyrolysis oils were condensed in a shell-and-tube condenser. At the screw kiln reactor port (Fig. 8), the stable char was restored. In the study of Zhang et al. (2020), rotary kiln reactor had been utilized. The inner diameter and length of the 316 stainless steel rotary kiln pyrolyzer are 300 mm and 65 mm, respectively. The rotation speed of the reactor, controlled by a transducer, may be varied between 0 and 20 rpm. The gas-liquid separation apparatus consisted of four glass condensers, used to collect the liquid oil, was chilled to 15 °C by coolant pump. In this study, pyrolysis of PP waste at 500 °C was carried out and produced high liquid oil yields, 75 wt% with 5 wt% char yields, higher than chars yielded from pyrolysed mixture of plastic wastes and PS.
Fig. 8.
Rotary kiln reactor. Reproduced with copyright permission from (Serrano et al., 2001), Elsevier.
4.5.6. Conical spouted bed reactor
Conical spouted bed reactors offer effective mixing and can accommodate a wide range of particle sizes, bigger particulates, and particle densities (Fogler, 2010) (Fig. 9 ). For their catalytic cracking of plastic PP, several researchers employed a conical spouted bed reactor (Aguado et al., 2002; Elordi et al., 2011) which mainly focused on the production of oils having high calorific values. Elordi et al. (2011) reported that a conical spouted bed reactor was suitable for the olefin production due to the short residence time of volatiles in the reactor. HZSM-5 zeolite (SiO2/Al2O3 = 30) was agglomerated with bentonite and alumina to create the catalyst for the process. The experiment was carried out in a continuous mode at temperatures ranging from 450 to 570 °C. Pyrolysis of waste plastic yields mainly liquid and gas products that have high heat density and also contain value-added chemicals.
Fig. 9.
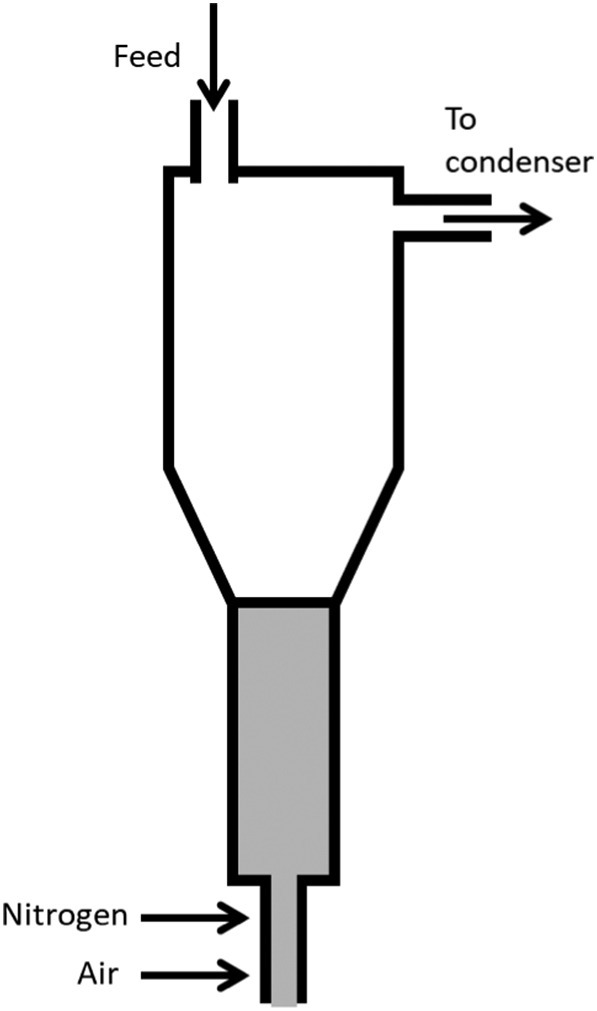
Conical spouted bed reactor.
Arabiourrutia et al. (2012) studied the significant effect of the operating conditions on the characteristics of waxes yield. Due to the obvious low residence times and high heating speeds, this technology has a high selectivity for waxes, which reduces secondary reactions and increases the yield of primary pyrolysis materials (waxes). As the temperature rises, more waxes are fractured into a liquid or gaseous form, resulting in a reduction in the amount of wax produced. Unfortunately, a variety of practical difficulties such as catalyst feeding, catalyst entrapment, and end-product yield, have been noted during the operation of this reactor, making it less suitable (Fogler, 2010). Furthermore, its complex design, which necessitates the use of many pumps in the operation, renders it unfavourable due to the high operational costs.
5. Recent application of pyrolysis by-products
5.1. Scenario on pyrolysis approach of PPE waste
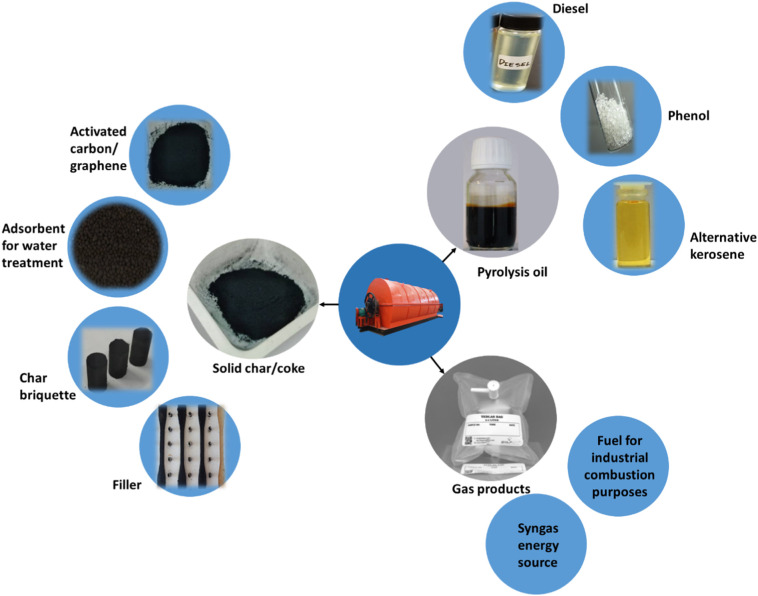
In pyrolysis, there are several difficulties and areas for development that must be addressed and improved in order to get optimum advantages. Although pyrolysis oil contains more energy than coal and some other fuels, pyrolysis is an energy-intensive process in and of itself, and the oil product requires more energy to refine (Inman, 2012), implying that pyrolysis oil may not be significantly better than conventional diesel in terms of greenhouse gas emissions. Much detailed research studies on mass and energy balance across the whole process's boundaries are needed to confirm this. To overcome these process energy requirements, more advanced technologies can be developed using the integration of renewable energies such as solar or hydro with pyrolysis-based polymer products, utilization of catalysts during the combustion process and also rebuild facile reactor design, to achieve maximum economic and environmental benefits (Torres and De-la-Torre, 2021). However, main by-products yielded including hydrocarbon gases, refinery oil and char residue were collected and reused into new products which were further commercialised. Fig. 9 summarizes the various applications of solid, liquid and gaseous products produced via pyrolysis of plastic wastes influenced by different process conditions. Although the research of plastic waste conversion into sustainable liquid and gaseous fuels has been around for over a decade, those focusing on medical PPE under the COVID-19 context remain preliminary (Fig. 10).
Fig. 10.
Pyrolysis oil and char products yielded were utilized into several applications. Reproduced with copyright permission from (Harussani et al., 2021a, Harussani et al., 2021b; Venderbosch and Heeres, 2011).
5.2. Recent application of the plastic pyrolysis by-products
5.2.1. Gaseous products
Recent studies have evaluated pyrolysis approach for the conversion of PP plastic into gaseous products as a possible replacement of natural gas. Pyrolysis gas from plastics, unlike natural gas, includes a combination of alkanes, alkenes, dienes, and alkynes. The removal of unsaturated hydrocarbons is required for the operation of gas engines, gas turbines, and fuel cells. Unsaturated hydrocarbons can produce soot, rubber-like compounds, and gums during burning due to their high reactivity (Veksha et al., 2018). Thus, the gaseous hydrocarbons suitable to be utilized in several applications including as sustainable gaseous fuels (Jung et al., 2021; Park et al., 2019), and precursors for chemical syntheses for hydrogen and carbon nanotubes (CNT) production (Liu et al., 2011; Veksha et al., 2018).
Jung et al. (2021) studied the pyrolysis of PP-based surgical face masks in a tubular reactor to obtain gaseous fuels. Catalytic pyrolysis substantially increased the concentration of gas fuels, especially for H2, which increased up to 55.1 mol%. Veksha et al. (2018) run a study on the pyrolysis of mixed plastics with nickel loaded calcium carbonate catalysts (Ni—Ca). Depending on the temperature, Ni—Ca dosage, and HCl concentration in the pyrolysis gas, about 99% of unsaturated hydrocarbons were degraded into hydrogen and multi-walled CNTs during the process, whereas alkane conversion was kept below 10%, indicating the excellent selectivity of the method. Whereas, Borsodi et al. (2016) had used Fe and Co catalysts to enhance the conversion of pyrolysis gases into CNTs via chemical vapor deposition which were then incorporated into LDPE matrix as reinforcement. However, gases produced from pyrolysis of some plastic waste such as PVC are toxic, and therefore pyrolysis emission treatment technology has to be further refined such as via dechlorination (Jung et al., 2021) to achieve maximum environmental benefits.
5.2.2. Liquid oil
Liquid oil produced from pyrolysis of plastic waste is as an important renewable material for the generation of chemicals and fuel. From previous works, catalytic pyrolysis process was shown as the best pyrolysis approach to convert plastic into liquid hydrocarbons (Cai et al., 2019; Jung et al., 2021; Kim et al., 2017; Ryu et al., 2020). The aromatic, olefin, and naphthalene chemicals present in petroleum products are abundant in liquid oil generated by catalytic pyrolysis of several types of plastic feedstock (Miandad et al., 2019). Moreover, the HHV of the produced liquid oil has been found for about 44 MJ/kg which is very close to the energy value of conventional diesel yielded from pyrolysis of PS, PP and PE plastic using the natural zeolite (AA-NZ) catalyst. Thus, the pyrolysis liquid oil produced from various plastic wastes has the potential to be used as an alternative source of energy.
Lee et al. (2015) and Rehan et al. (2016) had discovered the potential of pyrolysis liquid oil as fuels in a diesel engine, used in the production of electrical energy. In kerosene application, Saptoadi and Pratama (2015) effectively employed pyrolytic liquid oil as potential substitute precursor in a kerosene stove. Furthermore, the aromatic compounds generated can be utilized as a polymerization raw material in a variety of chemical industries (Sarker and Rashid, 2013; Shah and Jan, 2015). According to Das and Tiwari (2018b), the liquid yield through slow pyrolysis of polyolefin PP composed with short chain hydrocarbons with high octane number and the research octane number (RON) value, ~92. Thus, the plastic-derived oil suitable to be reused as high-quality gasoline blend components and the high values of H/C ratio of the oils will ensure clean burning which good for application of liquid fuel. Panda et al. (2010) also studied the conversion of PP waste into useful gasoline, diesel or kerosene range chemicals using thermal catalytic pyrolysis. Moreover, various researchers (Lee et al., 2015; Nileshkumar et al., 2015) had utilized the yielded liquid oil as transportation fuel after mixing with diesel fuels at various ratios. The research focused at the prospects of produced liquid oil in terms of engine performance and vehicle exhaust emissions, and concluded that a 20:80% blend ratio of pyrolytic liquid oil and conventional diesel, equivalent engine performance outcomes as conventional diesel.
The pyrolysis oil's high aromatic concentration is beneficial, as some aromatic chemicals like benzene, toluene, and styrene may be processed and marketed in an established market. Some aromatic hydrocarbons, on the other hand, are recognized carcinogens that can harm human health and the environment. To guarantee minimum environmental effect, pyrolysis oil produced from diverse plastic kinds must be thoroughly cleaned before use in any application. As a result, careful consideration is required in this area (Miandad et al., 2019).
5.2.3. Char
There are several lists of char potential applications is extended also to less traditional materials (Martín-Lara et al., 2021), including char-based fuel briquettes (Harussani et al., 2021b), raw materials for fabrication of graphene and its derivatives (Miandad et al., 2019), nano-catalysts, adsorbent materials, nano-fillers for composite application (Sogancioglu et al., 2020), sensors (Chaudhary et al., 2021; Spanu et al., 2020) and supercapacitors (Pandey et al., 2021; Vivekanandhan, 2018).
5.2.3.1. Graphene and carbon nanotubes production
Pandey et al. (2021) documented the upcycling of waste plastics into high-value graphene nanosheets (GN) and their potential use in dye-sensitive solar cells (DSSCs) and supercapacitors. To obtain GNs, waste plastics are degraded using two-step pyrolysis. The photo-anode with polymeric electrolyte DSSC manufacturing had a high fill factor of 87% and a high open-circuit voltage of 0.77 V. The application of GNs as an active layer material of supercapacitor electrodes resulted in a substantial amount of specific capacitance, energy density and power density, for about 398 F/g, 40 Wh/kg and 1000 W/kg, respectively. In another study, multi-walled carbon nanotubes (MWCNTs) were successfully produced through the combustion of PP using catalyst of nickel compounds and organic-modified montmorillonite (OMMT) nanoclay. Yao et al. (2018) discovered that Ni—Fe catalyst and optimised operational parameters including catalyst temperatures and steam to plastic ratios, affect the yield of MWCNTs. It should be emphasized that the Ni(0), which was produced as a result of the reduction of nickel compounds with the help of hydrocarbon gases coming from PP degradation as well as hydrogen, is the real active site of MWCNT generation (Jiang et al., 2007). However, Mishra et al. (2012) had used PP plastic waste as precursor for synthesizing MWCNTs via chemical vapor deposition approach using nickel catalyst, suggesting that MWCNTs can be used for further optoelectronic applications.
5.2.3.2. Adsorbent material
Pyrolysis char can be exploited for a variety of environmental applications. Considering the features and qualities of the char are dependent on the composition of the materials to be pyrolysed, the application of the solid product generated also depended on it (Martín-Lara et al., 2021). Due to its high characteristics including surface area and porosity, this product is most commonly used as an adsorbent material.
Several researchers activated the char via steam and thermal activation to enhance the Brunauer–Emmett–Teller (BET) surface area and lower the pore size of the char (Heras et al., 2014). Char can be utilized as a raw material to create activated carbon and acts as adsorbent materials. Martín-Lara et al. (2021) had been studying the slow co-pyrolysis of PP, PS and PE waste to produce char products. The pyrolysis solid product is described and tested for its potential as a lead adsorbent in aqueous media with 26 mg/g adsorption capacity. Bernardo (2011) upgraded the plastic char with biomaterial and carried out the adsorption (3.6–22.2 mg/g) of methylene blue dye from wastewater. Miandad et al. (2018) used the char obtained from pyrolysis of PS plastic waste to synthesize a novel carbon-metal double-layered oxides (C/MnCuAl-LDOs) nano-adsorbent for the adsorption of Congo red (CR) in wastewater. In some cases, this char is treated to improve these surface properties and increase its adsorbent capacity.
5.2.3.3. Fillers in engineering applications
Another application of interest is the one proposed by Sogancioglu et al. (2020), which used solid pyrolysis product, char as reinforcement materials in epoxy composite. From the pyrolysis of PP waste, char residue with higher aromatic structure had been yielded which efficient in improve the hardness of epoxy composites. The mechanical improvement of epoxy composites associating the functionalization of char also reported by other researchers (Ahmetli et al., 2013; Yousef et al., 2021). Thus, the composite materials could be applied in the aircraft, automobile and also microelectronics as it enhanced the mechanical strength and electrical conductivities of the composites.
In civil engineering applications, the chars have the potential to be used as carbon-reducing additives in concrete. Within cementitious materials, pulverized chars operate as a micro-filler, which filled the pore gaps and increased the material strength and stiffness (Wijesekara et al., 2021). Based on recent scenarios, there are limited works of plastic-derived char being used in civil engineering applications, whereas biochars were widely used due to their effective water absorption characteristics (Cuthbertson et al., 2019; Gupta and Kua, 2018), which aid in hydration processes and strength development. However, some studies had discovered that plastic derived chars have similar BET surface areas as the biochars (Wijesekara et al., 2021). Thus, there will be a surge of demand in using plastic derived chars within that concrete and cement industries for future development.
5.2.3.4. Fuel briquettes
Similar scenarios as in civil engineering applications, biochars were also more dominant in fuel briquette applications than plastic derived char. However, several researchers, in this recent years, had interests in utilizing the char for fuel applications (Harussani et al., 2021b; Jamradloedluk and Lertsatitthanakorn, 2014; Saptoadi et al., 2016). Amidst of COVID-19 pandemic, PP plastic waste which vigorously generated from used PPE were treated wisely via thermally pyrolysis in order to be utilized into proper outputs of solid char which could be exploited into fuel as reported by Harussani et al. (2021b). The findings indicate that char reinforced with natural sugar palm starch briquettes presented excellent combustion characteristics, about 2 MJ/g, with satisfactory mechanical strength which made the briquette composites are suitable for domestic and commercial uses (Harussani et al., 2021b). Another work, Jamradloedluk et al. (2014) had adopted fast pyrolysis method to convert HDPE plastic waste into briquettes. The utilization of these briquettes is classified by its fuel characteristics. Thus, various applications of chars derived from plastic waste were a paramount alternative to reuse and reduce the plastic waste existed in global, thus, successfully promotes green energy recovery technology for the environment sustainability.
6. Future prospects
Utilization of single-use plastic products including packaging, service ware and medical equipments had been recommended by the governments as an efficient way to hinder the virus transmission amidst COVID-19 pandemic. Thus, most of the governments enforced the use of PPE also for domestic activities which leads to high production of plastic waste and exacerbate plastic pollution (Akhbarizadeh et al., 2021). Common approaches of PPE waste, as aforementioned in previous section, are open landfilling (Budiman and Ardiansyah, 2020; Nzediegwu and Chang, 2020) and incineration method (Hicks, 2020; Singh et al., 2020; Tan, 2019; Yang et al., 2021). Jain et al. (2020) proposed that PPE kits associated with the COVID-19 pandemic could follow a repurposing strategy based on pyrolysis and the production of biofuels (Torres and De-la-Torre, 2021). Pyrolysis is an interesting disposal alternative for plastic wastes, including those from medical use (Qin et al., 2018), without the need for material-based segregation (Demirbas, 2004; Moreira et al., 2017). According to the researchers, the high PP content in medical PPE, which including face masks and isolation gowns, could result in substantial yields of liquid and gas fuels, as demonstrated in previous studies with PP wastes (Ahmad et al., 2015; Martynis et al., 2019).
Furthermore, recent study on pyrolysis shows the application of plasma technology in pyrolysis system. Cold plasma pyrolysis offers effective approach in dealing with infectious medical plastic waste with the aid from high pyrolytic temperature, and complex cooling system. This technique had utilized the excited hot electrons, generated from two electrodes to efficiently break down the chemical bonds of plastics in a controlled condition and much lesser time than the conventional pyrolysis (Diaz-Silvarrey et al., 2018; Yao et al., 2021). However, this approach is an energy-consuming strategy due to its advanced cooling system as higher process temperature was employed to thermally crack the plastic waste into lighter hydrocarbons.
Low cost, facile process and energy saving technology in treating massive plastic waste production is paramount to be practiced globally. In the recent years, researchers had discovered new technique of hydrothermal processing in decomposing plastic waste using hot compressed aqueous medium at elevated pressures to produce monomer and chemicals, including hydrochar and crude oil, utilized in plastic production and other applications (Hongthong et al., 2020). In addition, hydrothermal processing helps in yield of lower oxygen content and improved energy liquid crude oil with enhanced heating value, low processing costs and its storage. Thus, this alternative will help in reducing plastic pollution and other environmental disturbance.
7. Conclusions
The present review has provided a detail summary of pyrolysis process of polypropylene (PP)-based plastic waste as alternative step to decompose it, key parameters in generation of desired by-products, and numerous lists of their possible applications, especially on their char by-products. From the literature review, recent study demonstrated promising results on the increased yield amount of the pyrolysis by-products; liquid oil, gaseous and solid char products. As of conventional personal protective equipments (PPE) was commonly made up of PP plastic, there are high demand towards the plastic production. Thus, this scenario leads to the increasing of plastic waste and contributed to environmental pollution.
Pyrolysis is one of the best alternatives as the PP waste was converted into high-quality char which suitable for various applications. Hence, many researchers have recognized that fast and catalytic pyrolysis technique can significantly optimize the quantity and quality of pyrolysis oil which suits to be utilized in fuel applications. From an economic point of view, mainly slow pyrolysis approach is useful to maximize the yield of solid products of carbonaceous char and coke. This is due to the lower process temperature (300–600 °C) and heating rate (below 5 °C/min) with longer residence time (1–5 h), applied to completely decompose the waste prior to end product extraction. Since, PP plastic waste, are easy to find and available in abundant amounts during this COVID-19 pandemic globally, pyrolysis approach has huge potential for development of proper infectious plastic waste management. In addition, comprehensive overview regarding to the various possible applications of char by-products, including adsorbents in water treatment, nano-catalysts, nano-fillers and raw materials for graphene and activated carbon fabrication, were discussed in this article. The important key point is that this pyrolysis approach helps in controlling the virus spreading via waste disposal, reduces environmental pollution as well as solves the rise in energy demand.
CRediT authorship contribution statement
M.M. Harussani: Data Curation, Investigation, Writing - original draft. S.M. Sapuan: Conceptualization, Resources, Supervision. U. Rashid: Writing - Review & Editing, Validation A. Khalina: Visualization, Data curation. R.A. Ilyas: Conceptualization, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors have warmly recognized that the Ministry of Higher Education (MOHE) which financed this project through Post COVID-19 Special Research Grant 2020 (Vote No. 5540346) as well as Universiti Putra Malaysia by providing the research facilities.
Funding
This work was supported by the Ministry of Higher Education (MoHE) for funding this project via Post COVID-19 Special Research Grant 2020 [Vote No. 5540346] and also Universiti Putra Malaysia.
Editor: Damia Barcelo
References
- 3M Personal Safety Division . 2018. Technical Specification Sheet: 3MTM Particulate Respirator 8210, N95. [Google Scholar]
- Abbas-Abadi M.S., Haghighi M.N., Yeganeh H., McDonald A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis. 2014;109:272–277. [Google Scholar]
- Achilias D.S., Roupakias C., Megalokonomos P., Lappas A.A., Antonakou ?.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP) J. Hazard. Mater. 2007;149:536–542. doi: 10.1016/j.jhazmat.2007.06.076. [DOI] [PubMed] [Google Scholar]
- Adrados A., De Marco I., Caballero B.M., López A., Laresgoiti M.F., Torres A. Pyrolysis of plastic packaging waste: a comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012;32:826–832. doi: 10.1016/j.wasman.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Aguado J., Serrano D.P., Escola J.M., Garagorri E., Fernandez J.A. Catalytic conversion of polyolefins into fuels over zeolite beta. Polym. Degrad. Stab. 2000;69:11–16. [Google Scholar]
- Aguado R., Olazar M., San José M.J., Gaisán B., Bilbao J. Wax formation in the pyrolysis of polyolefins in a conical spouted bed reactor. Energy Fuel. 2002;16:1429–1437. [Google Scholar]
- Ahmad I., Khan M.I., Khan H., Ishaq M., Tariq R., Gul K., Ahmad W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy. 2015;12:663–671. [Google Scholar]
- Ahmetli G., Kocaman S., Ozaytekin I., Bozkurt P. Epoxy composites based on inexpensive char filler obtained from plastic waste and natural resources. Polym. Compos. 2013;34:500–509. [Google Scholar]
- Akhbarizadeh R., Dobaradaran S., Nabipour I., Tangestani M., Abedi D., Javanfekr F., Jeddi F., Zendehboodi A. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian Gulf: an emerging source of secondary microplastics in coastlines. Mar. Pollut. Bull. 2021;168 doi: 10.1016/j.marpolbul.2021.112386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salem S.M. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC) Process Saf. Environ. Prot. 2019;127:171–179. [Google Scholar]
- Anene A.F., Fredriksen S.B., Sætre K.A., Tokheim L.-A. Experimental study of thermal and catalytic pyrolysis of plastic waste components. Sustainability. 2018;10:3979. [Google Scholar]
- Arabiourrutia M., Elordi G., Lopez G., Borsella E., Bilbao J., Olazar M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis. 2012;94:230–237. [Google Scholar]
- Arabiourrutia M., Elordi G., Olazar M., Bilbao J. Pyrolysis. InTech; Rijeka: 2017. Pyrolysis of polyolefins in a conical spouted bed reactor: a way to obtain valuable products; pp. 285–304. [Google Scholar]
- Arkatkar A., Arutchelvi J., Bhaduri S., Uppara P.V., Doble M. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegradation. 2009;63:106–111. [Google Scholar]
- Ballice L., Reimert R. Classification of volatile products from the temperature-programmed pyrolysis of polypropylene (PP), atactic-polypropylene (APP) and thermogravimetrically derived kinetics of pyrolysis. Chem. Eng. Process. Process Intensif. 2002;41:289–296. [Google Scholar]
- Bano K., Kuddus M., Zaheer M.R., Zia Q., Khan M.F., Gupta A., Aliev G. Microbial enzymatic degradation of biodegradable plastics. Curr. Pharm. Biotechnol. 2017;18:429–440. doi: 10.2174/1389201018666170523165742. [DOI] [PubMed] [Google Scholar]
- Behzadi S., Farid M. Liquid fuel from plastic wastes using extrusion-rotary kiln reactors. Feed. Recycl. Pyrolysis Waste Plast. Convert. Waste Plast. Into Diesel Other Fuels. 2006:531–548. [Google Scholar]
- Bennett G.F. In: Feedstock Recycling and Pyrolysis of Waste Plastics. Scheirs J., Kaminsky W., editors. Vol. 2006. John Wiley & Sons Ltd; Chichester, West Sussex, England: 2007. 815 pp., Price: US $360.00, ISBN: 0-470-02152-7. [Google Scholar]
- Bermingham F., Tan S.L. South China Morning Post. Vol. 12. 2020. Coronavirus: China’s mask-making juggernaut cranks into gear, sparking fears of over-reliance on world’s workshop. [Google Scholar]
- Bernardo M.M.S. 2011. Physico-chemical Characterization of Chars Produced in the Co-pyrolysis of Wastes and Possible Routes of Valorisation. [Google Scholar]
- Borsodi N., Szentes A., Miskolczi N., Wu C., Liu X. Carbon nanotubes synthetized from gaseous products of waste polymer pyrolysis and their application. J. Anal. Appl. Pyrolysis. 2016;120:304–313. [Google Scholar]
- Bow Y., Kurniawan S. 4th Forum in Research, Science, and Technology (FIRST-T1-T2-2020) Atlantis Press; 2021. Study of temperature and use of catalysts in the pyrolysis of LDPE plastic waste on the quantity of oil fuel products produced; pp. 24–28. [Google Scholar]
- Bow Y., Pujiastuti L.S. IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2019. Pyrolysis of polypropylene plastic waste into liquid fuel; p. 12128. [Google Scholar]
- Budiman Y.C., Ardiansyah T. TheJakartaPost; 2020. In Indonesia, Coronavirus Floods Cisadane River with Extra Hazard: Medical Waste.https://www.thejakartapost.com/news/2020/09/01/in-indonesia-coronavirus-floods-cisadane-river-with-extra-hazard-medical-waste.html [Google Scholar]
- Bujak J.W. Thermal utilization (treatment) of plastic waste. Energy. 2015;90:1468–1477. [Google Scholar]
- Cai W., Liu P., Chen B., Xu H., Liu Z., Zhou Q., Yu F., Liu M., Chen M., Liu J. Plastic waste fuelled solid oxide fuel cell system for power and carbon nanotube cogeneration. Int. J. Hydrog. Energy. 2019;44:1867–1876. [Google Scholar]
- Chamas A., Moon H., Zheng J., Qiu Y., Tabassum T., Jang J.H., Abu-Omar M., Scott S.L., Suh S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020;8:3494–3511. [Google Scholar]
- Chattopadhyay J., Pathak T.S., Srivastava R., Singh A.C. Catalytic co-pyrolysis of paper biomass and plastic mixtures (HDPE (high density polyethylene), PP (polypropylene) and PET (polyethylene terephthalate)) and product analysis. Energy. 2016;103:513–521. [Google Scholar]
- Chaudhary S., Kumari M., Chauhan P., Chaudhary G.R. Upcycling of plastic waste into fluorescent carbon dots: an environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021;120:675–686. doi: 10.1016/j.wasman.2020.10.038. [DOI] [PubMed] [Google Scholar]
- Chhabra V., Bambery K., Bhattacharya S., Shastri Y. Thermal and in situ infrared analysis to characterise the slow pyrolysis of mixed municipal solid waste (MSW) and its components. Renew. Energy. 2020;148:388–401. [Google Scholar]
- Ciliz N.K., Ekinci E., Snape C.E. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004;24:173–181. doi: 10.1016/j.wasman.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Corburn J., Vlahov D., Mberu B., Riley L., Caiaffa W.T., Rashid S.F., Ko A., Patel S., Jukur S., Martínez-Herrera E. Slum health: arresting COVID-19 and improving well-being in urban informal settlements. J. Urban Heal. 2020:1–10. doi: 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D., Berardi U., Briens C., Berruti F. Biochar from residual biomass as a concrete filler for improved thermal and acoustic properties. Biomass Bioenergy. 2019;120:77–83. [Google Scholar]
- Das P., Tiwari P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018;128:69–77. [Google Scholar]
- Das P., Tiwari P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel. Waste Manag. 2018;79:615–624. doi: 10.1016/j.wasman.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Datta P., Mohi G.K., Chander J. Biomedical waste management in India: critical appraisal. J. Lab. Phys. 2018;10:6. doi: 10.4103/JLP.JLP_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirbas A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis. 2004;72:97–102. [Google Scholar]
- Diaz-Silvarrey L.S., Zhang K., Phan A.N. Monomer recovery through advanced pyrolysis of waste high density polyethylene (HDPE) Green Chem. 2018;20:1813–1823. [Google Scholar]
- Duan D., Wang Y., Dai L., Ruan R., Zhao Y., Fan L., Tayier M., Liu Y. Ex-situ catalytic co-pyrolysis of lignin and polypropylene to upgrade bio-oil quality by microwave heating. Bioresour. Technol. 2017;241:207–213. doi: 10.1016/j.biortech.2017.04.104. [DOI] [PubMed] [Google Scholar]
- Efika C.E., Wu C., Williams P.T. Syngas production from pyrolysis–catalytic steam reforming of waste biomass in a continuous screw kiln reactor. J. Anal. Appl. Pyrolysis. 2012;95:87–94. [Google Scholar]
- Elordi G., Olazar M., Lopez G., Amutio M., Artetxe M., Aguado R., Bilbao J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis. 2009;85:345–351. [Google Scholar]
- Elordi G., Olazar M., Lopez G., Artetxe M., Bilbao J. Continuous polyolefin cracking on an HZSM-5 zeolite catalyst in a conical spouted bed reactor. Ind. Eng. Chem. Res. 2011;50:6061–6070. [Google Scholar]
- Erdoğan C., Ballice L., Saglam M., Yüksel M. Fast pyrolysis and co-pyrolysis of Göynük oil shale (Turkey) and polypropylene in free falling reactor (FFR) Oil Shale. 2014:31. [Google Scholar]
- FakhrHoseini S.M., Dastanian M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013;2013 doi: 10.1155/2013/487676. (2013) [DOI] [Google Scholar]
- Fogler H.S. Pearson Education; 2010. Essentials of Chemical Reaction Engineering: Essenti Chemica Reactio Engi. [Google Scholar]
- Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacovelli C., Zamparo A., Wehrli A., Alverson K. United Nations Environ. Program. Nairobi. 2018. Single-use plastics: a roadmap for sustainability; p. 90. [Google Scholar]
- Gupta S., Kua H.W. Effect of water entrainment by pre-soaked biochar particles on strength and permeability of cement mortar. Constr. Build. Mater. 2018;159:107–125. [Google Scholar]
- Gutierrez O., Palza H. Effect of carbon nanotubes on thermal pyrolysis of high density polyethylene and polypropylene. Polym. Degrad. Stab. 2015;120:122–134. [Google Scholar]
- Harussani M.M., Sapuan S.M., Khalina A., Ilyas R.A., Hazrol M.D. Proceedings of the 7th Postgraduate Seminar on Natural Fibre Reinforced Polymer Composites 2020. Institute of Tropical Forest and Forest Products (INTROP) Universiti Putra Malaysia; Serdang, Selangor: 2020. Review on green technology pyrolysis for plastic wastes; pp. 50–53. [Google Scholar]
- Harussani M.M., Sapuan S.M., Khalina A., Rashid U., Tarique J. International Symposium on Applied Sciences and Engineering ISASE2021. Office of International Affairs, Atatürk University; Erzurum, TURKEY, Erzurum, Turkey: 2021. Slow pyrolysis of disinfected COVID-19 non-woven polypropylene (PP) waste; pp. 310–312. [Google Scholar]
- Harussani M.M., Sapuan S.M., Rashid U., Khalina A. Development and characterization of polypropylene waste from personal protective equipment (PPE)-derived char-filled sugar palm starch biocomposite briquettes. Polymers (Basel) 2021;13:1707. doi: 10.3390/polym13111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras F., Jimenez-Cordero D., Gilarranz M.A., Alonso-Morales N., Rodriguez J.J. Activation of waste tire char by cyclic liquid-phase oxidation. Fuel Process. Technol. 2014;127:157–162. [Google Scholar]
- Hicks R. eco-business; 2020. Singapore’s Zero-waste Plan Aims to Raise Domestic Recycling Rate From 22 to 30 per cent by 2030 [WWW Document] [Google Scholar]
- Hidalgo J.P., Gerasimov N., Hadden R.M., Torero J.L., Welch S. Methodology for estimating pyrolysis rates of charring insulation materials using experimental temperature measurements. J. Build. Eng. 2016;8:249–259. [Google Scholar]
- Hongthong S., Leese H.S., Chuck C.J. Valorizing plastic-contaminated waste streams through the catalytic hydrothermal processing of polypropylene with lignocellulose. ACS Omega. 2020;5:20586–20598. doi: 10.1021/acsomega.0c02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman M. Cooking up fuel. Nat. Clim. Chang. 2012;2:218–220. [Google Scholar]
- Jain S., Yadav Lamba B., Kumar S., Singh D. Strategy for repurposing of disposed PPE kits by production of biofuel: pressing priority amidst COVID-19 pandemic. Biofuels. 2020:1–5. [Google Scholar]
- Jamradloedluk J., Lertsatitthanakorn C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014;69:1437–1442. [Google Scholar]
- Jiang Z., Song R., Bi W., Lu J., Tang T. Polypropylene as a carbon source for the synthesis of multi-walled carbon nanotubes via catalytic combustion. Carbon N. Y. 2007;45:449–458. [Google Scholar]
- Jung S.-H., Cho M.-H., Kang B.-S., Kim J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010;91:277–284. [Google Scholar]
- Jung S., Lee S., Dou X., Kwon E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021;405 doi: 10.1016/j.cej.2020.126658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Lee S., Dou X., Kwon E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2020;405 doi: 10.1016/j.cej.2020.126658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairytė A., Kremensas A., Vaitkus S., Czlonka S., Strakowska A. Fire suppression and thermal behavior of biobased rigid polyurethane foam filled with biomass incineration waste ash. Polymers (Basel) 2020;12:683. doi: 10.3390/polym12030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalargaris I., Tian G., Gu S. Experimental characterisation of a diesel engine running on polypropylene oils produced at different pyrolysis temperatures. Fuel. 2018;211:797–803. [Google Scholar]
- Kassargy C., Awad S., Burnens G., Kahine K., Tazerout M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis. 2017;127:31–37. [Google Scholar]
- Kim S.-S., Kim S. Pyrolysis characteristics of polystyrene and polypropylene in a stirred batch reactor. Chem. Eng. J. 2004;98:53–60. [Google Scholar]
- Kim Y.-M., Lee H.W., Choi S.J., Jeon J.-K., Park S.H., Jung S.-C., Kim S.C., Park Y.-K. Catalytic co-pyrolysis of polypropylene and Laminaria japonica over zeolitic materials. Int. J. Hydrog. Energy. 2017;42:18434–18441. [Google Scholar]
- Kusrini E., Supramono D., Muhammad I.A., Pranata S., Wilson D.L., Usman A. Effect of polypropylene plastic waste as co-feeding for production of pyrolysis oil from palm empty fruit bunches. Evergreen. 2019;6:92–97. [Google Scholar]
- Lee K.-H., Noh N.-S., Shin D.-H., Seo Y. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polym. Degrad. Stab. 2002;78:539–544. [Google Scholar]
- Lee S., Yoshida K., Yoshikawa K. Application of waste plastic pyrolysis oil in a direct injection diesel engine: for a small scale non-grid electrification. Energy Environ. Res. 2015;5:18. [Google Scholar]
- Lin Y.-H., Yen H.-Y. Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym. Degrad. Stab. 2005;89:101–108. [Google Scholar]
- Liu J., Jiang Z., Yu H., Tang T. Catalytic pyrolysis of polypropylene to synthesize carbon nanotubes and hydrogen through a two-stage process. Polym. Degrad. Stab. 2011;96:1711–1719. [Google Scholar]
- Liu X., Burra K.G., Wang Z., Li J., Che D., Gupta A.K. On deconvolution for understanding synergistic effects in co-pyrolysis of pinewood and polypropylene. Appl. Energy. 2020;279 [Google Scholar]
- Luo G., Suto T., Yasu S., Kato K. Catalytic degradation of high density polyethylene and polypropylene into liquid fuel in a powder-particle fluidized bed. Polym. Degrad. Stab. 2000;70:97–102. [Google Scholar]
- Ma J., Liu J., Song J., Tang T. Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide. RSC Adv. 2018;8:2469–2476. doi: 10.1039/c7ra12733b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco M., La Paglia F., Iannotta P., Caputo G., Scargiali F., Grisafi F., Brucato A. Slow pyrolysis of an LDPE/PP mixture: kinetics and process performance. J. Energy Inst. 2021;96:234–241. [Google Scholar]
- Marcilla A., Garcia-Quesada J.C., Sanchez S., Ruiz R. Study of the catalytic pyrolysis behaviour of polyethylene–polypropylene mixtures. J. Anal. Appl. Pyrolysis. 2005;74:387–392. [Google Scholar]
- Martín-Lara M.A., Piñar A., Ligero A., Blázquez G., Calero M. Characterization and use of char produced from pyrolysis of post-consumer mixed plastic waste. Water. 2021;13:1188. [Google Scholar]
- Martínez J.D., Puy N., Murillo R., García T., Navarro M.V., Mastral A.M. Waste tyre pyrolysis–a review. Renew. Sust. Energ. Rev. 2013;23:179–213. [Google Scholar]
- Martynis M., Winanda E., Harahap A.N. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2019. Thermal pyrolysis of polypropylene plastic waste into liquid fuel: reactor performance evaluation; p. 12047. [Google Scholar]
- Miandad R., Kumar R., Barakat M.A., Basheer C., Aburiazaiza A.S., Nizami A.S., Rehan M. Untapped conversion of plastic waste char into carbon-metal LDOs for the adsorption of Congo red. J. Colloid Interface Sci. 2018;511:402–410. doi: 10.1016/j.jcis.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Miandad R., Rehan M., Barakat M.A., Aburiazaiza A.S., Khan H., Ismail I.M.I., Dhavamani J., Gardy J., Hassanpour A., Nizami A.-S. Catalytic pyrolysis of plastic waste: moving toward pyrolysis based biorefineries. Front. Energy Res. 2019;7:27. [Google Scholar]
- Miranda R., Sosa C., Bustos D., Carrillo E., Rodríguez-Cantú M. Characterization of pyrolysis products obtained during the preparation of bio-oil and activated carbon. IntechOpen. 2012:77–92. doi: 10.5772/3346. [DOI] [Google Scholar]
- Mishra N., Das G., Ansaldo A., Genovese A., Malerba M., Povia M., Ricci D., Di Fabrizio E., Di Zitti E., Sharon M. Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J. Anal. Appl. Pyrolysis. 2012;94:91–98. [Google Scholar]
- Miskolczi Norbert, Bartha L., Deak G., Jover B. Thermal degradation of municipal plastic waste for production of fuel-like hydrocarbons. Polym. Degrad. Stab. 2004;86:357–366. [Google Scholar]
- Miskolczi N., Bartha L., Deák G., Jover B., Kallo D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis. 2004;72:235–242. [Google Scholar]
- MOH . Minist. Heal. Malaysia; 2020. The Infection Prevention and Control (IPC) Measures in Managing Patient Under Investigation (PUI) or Confirmed Corona Virus Disease (COVID-19) [Google Scholar]
- Mohd Nurazzi N., Muhammad Asyraf M.R., Khalina A., Abdullah N., Sabaruddin F.A., Kamarudin S.H., Ahmad S., Mahat A.M., Lee C.L., Aisyah H.A., Norrrahim M.N.F., Ilyas R.A., Harussani M.M., Ishak M.R., Sapuan S.M. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: an overview. Polymers (Basel) 2021;13:1047. doi: 10.3390/polym13071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira R., dos Reis Orsini R., Vaz J.M., Penteado J.C., Spinacé E.V. Production of biochar, bio-oil and synthesis gas from cashew nut shell by slow pyrolysis. Waste Biomass Valoriz. 2017;8:217–224. [Google Scholar]
- Munir M.T., Mardon I., Al-Zuhair S., Shawabkeh A., Saqib N.U. Plasma gasification of municipal solid waste for waste-to-value processing. Renew. Sust. Energ. Rev. 2019;116 [Google Scholar]
- Nema S.K., Ganeshprasad K.S. Plasma pyrolysis of medical waste. Curr. Sci. 2002:271–278. [Google Scholar]
- Nileshkumar K.D., Jani R.J., Patel T.M., Rathod G.P. Effect of blend ratio of plastic pyrolysis oil and diesel fuel on the performance of single cylinder CI engine. Int. J. Sci. Technol. Eng. 2015;1:195–203. [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161 doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha D.K., Vinu R. Fast co-pyrolysis of cellulose and polypropylene using py-GC/MS and py-FT-IR. RSC Adv. 2015;5:66861–66870. [Google Scholar]
- Onwudili J.A., Insura N., Williams P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: effects of temperature and residence time. J. Anal. Appl. Pyrolysis. 2009;86:293–303. [Google Scholar]
- Palos R., Gutiérrez A., Vela F.J., Maña J.A., Hita I., Asueta A., Arnaiz S., Arandes J.M., Bilbao J. Assessing the potential of the recycled plastic slow pyrolysis for the production of streams attractive for refineries. J. Anal. Appl. Pyrolysis. 2019;142 [Google Scholar]
- Panda A.K., Singh R.K., Mishra D.K. Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew. Sust. Energ. Rev. 2010;14:233–248. [Google Scholar]
- Pandey S., Karakoti M., Surana K., Dhapola P.S., SanthiBhushan B., Ganguly S., Singh P.K., Abbas A., Srivastava A., Sahoo N.G. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 2021;11:1–17. doi: 10.1038/s41598-021-83483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.-B., Jeong Y.-S., Kim J.-S. Activator-assisted pyrolysis of polypropylene. Appl. Energy. 2019;253 [Google Scholar]
- Parku G.K., Collard F.-X., Görgens J.F. Pyrolysis of waste polypropylene plastics for energy recovery: influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process. Technol. 2020;209 [Google Scholar]
- Qin L., Han J., Zhao B., Wang Y., Chen W., Xing F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrolysis. 2018;136:132–145. [Google Scholar]
- Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- Rehan M., Nizami A.S., Shahzad K., Ouda O.K.M., Ismail I.M.I., Almeelbi T., Iqbal T., Demirbas A. Pyrolytic liquid fuel: a source of renewable electricity generation in Makkah. Energy Sources, Part A. 2016;38:2598–2603. [Google Scholar]
- Ritchie H., Roser M. Our World Data; 2018. Plastic Pollution. [Google Scholar]
- Ryu S., Lee H.W., Kim Y.-M., Jae J., Jung S.-C., Ha J.-M., Park Y.-K. Catalytic fast co-pyrolysis of organosolv lignin and polypropylene over in-situ red mud and ex-situ HZSM-5 in two-step catalytic micro reactor. Appl. Surf. Sci. 2020;511 [Google Scholar]
- Sajdak M., Muzyka R. Use of plastic waste as a fuel in the co-pyrolysis of biomass. Part I: the effect of the addition of plastic waste on the process and products. J. Anal. Appl. Pyrolysis. 2014;107:267–275. [Google Scholar]
- Sakata Y., Uddin M.A., Muto A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis. 1999;51:135–155. [Google Scholar]
- Santaweesuk C., Janyalertadun A. The production of fuel oil by conventional slow pyrolysis using plastic waste from a municipal landfill. Int. J. Environ. Sci. Dev. 2017;8:168. [Google Scholar]
- Santos B.P.S., Almeida D., Maria de Fatima V.M., Henriques C.A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel. 2018;215:515–521. [Google Scholar]
- Saptoadi H., Pratama N.N. Utilization of plastics waste oil as partial substitute for kerosene in pressurized cookstoves. Int. J. Environ. Sci. Dev. 2015;6:363. [Google Scholar]
- Saptoadi H., Rohmat T.A., Sutoyo . AIP Conference Proceedings. AIP Publishing LLC; 2016. Combustion of char from plastic wastes pyrolysis; p. 30006. [Google Scholar]
- Sarker M., Rashid M.M. Waste plastics mixture of polystyrene and polypropylene into light grade fuel using Fe2O3 catalyst. Int. J. Renew. Energy Technol. Res. 2013;2:17–28. [Google Scholar]
- Serrano D.P., Aguado J., Escola J.M., Garagorri E. Conversion of low density polyethylene into petrochemical feedstocks using a continuous screw kiln reactor. J. Anal. Appl. Pyrolysis. 2001;58:789–801. [Google Scholar]
- Shah J., Jan M.R. Effect of polyethylene terephthalate on the catalytic pyrolysis of polystyrene: investigation of the liquid products. J. Taiwan Inst. Chem. Eng. 2015;51:96–102. [Google Scholar]
- Shah J., Jan M.R. Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J. Taiwan Inst. Chem. Eng. 2014;45:2494–2500. [Google Scholar]
- Sharma B.K., Moser B.R., Vermillion K.E., Doll K.M., Rajagopalan N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014;122:79–90. [Google Scholar]
- Sharuddin S.D.A., Abnisa F., Daud W.M.A.W., Aroua M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016;115:308–326. [Google Scholar]
- Singh N., Tang Y., Zhang Z., Zheng C. COVID-19 waste management: effective and successful measures in Wuhan, China. Resour. Conserv. Recycl. 2020;163 doi: 10.1016/j.resconrec.2020.105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Ruj B., Sadhukhan A.K., Gupta P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: product yield analysis and their characterization. J. Energy Inst. 2019;92:1647–1657. [Google Scholar]
- Sobko A.A. Doklady Physics. Springer; 2008. Generalized van der Waals-Berthelot equation of state; pp. 416–419. [Google Scholar]
- Sogancioglu M., Yel E., Ahmetli G. Behaviour of waste polypropylene pyrolysis char-based epoxy composite materials. Environ. Sci. Pollut. Res. 2020;27:3871–3884. doi: 10.1007/s11356-019-07028-3. [DOI] [PubMed] [Google Scholar]
- Sogancioglu M., Yel E., Ahmetli G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017;165:369–381. [Google Scholar]
- Sonawane Y.B., Shindikar M.R., Khaladkar M.Y. Use of catalyst in pyrolysis of polypropylene waste into liquid fuel. Int. Res. J. Environ. Sci. 2015;4:24–28. [Google Scholar]
- Spanu D., Binda G., Dossi C., Monticelli D. Biochar as an alternative sustainable platform for sensing applications: a review. Microchem. J. 2020;105506 [Google Scholar]
- Tan A. The Straitstimes. 2019. Singapore aims to send one-third less waste to Semakau Landfill by 2030: Amy Khor [WWW Document] [Google Scholar]
- Tang L., Huang H., Zhao Z., Wu C.Z., Chen Y. Pyrolysis of polypropylene in a nitrogen plasma reactor. Ind. Eng. Chem. Res. 2003;42:1145–1150. [Google Scholar]
- Tekin K., Akalin M.K., Kadi Ç., Karagöz S. Catalytic degradation of waste polypropylene by pyrolysis. J. Energy Inst. 2012;85:150–155. [Google Scholar]
- Torres F.G., De-la-Torre G.E. Face mask waste generation and management during the COVID-19 pandemic: an overview and the Peruvian case. Sci. Total Environ. 2021;786:1–11. doi: 10.1016/j.scitotenv.2021.147628. 147628, [DOI] [Google Scholar]
- Veksha A., Giannis A., Oh W.-D., Chang V.W.-C., Lisak G. Upgrading of non-condensable pyrolysis gas from mixed plastics through catalytic decomposition and dechlorination. Fuel Process. Technol. 2018;170:13–20. [Google Scholar]
- Velghe I., Carleer R., Yperman J., Schreurs S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrolysis. 2011;92:366–375. [Google Scholar]
- Venderbosch R.H., Heeres H.J. Pyrolysis oil stabilisation by catalytic hydrotreatment. Biofuel’s Eng. Process Technol. 2011:387–411. [Google Scholar]
- Verbeek J.H., Rajamaki B., Ijaz S., Sauni R., Toomey E., Blackwood B., Tikka C., Ruotsalainen J.H., Balci F.S.K. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020;(4):1–147. doi: 10.1002/14651858.CD011621.pub4. CD011621, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Budiyal L., Sanjay M.R., Siengchin S. Processing and characterization analysis of pyrolyzed oil rubber (from waste tires)-epoxy polymer blend composite for lightweight structures and coatings applications. Polym. Eng. Sci. 2019;59:2041–2051. [Google Scholar]
- Vivekanandhan S. Biochar supercapacitors: recent developments in the materials and methods. Green Sustain. Adv. Mater. Appl. 2018:223. [Google Scholar]
- Vivero L., Barriocanal C., Alvarez R., Diez M.A. Effects of plastic wastes on coal pyrolysis behaviour and the structure of semicokes. J. Anal. Appl. Pyrolysis. 2005;74:327–336. [Google Scholar]
- Wang Jiao, Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang Junqi, Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262:1–10. doi: 10.1016/j.envpol.2020.114665. 114665, [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Heal. Organ; 2021. WHO Coronavirus (COVID-19) Dashboard [WWW Document]https://covid19.who.int/ URL. (accessed 6.22.21) [Google Scholar]
- WHO . World Health Organization; 2020. Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, The Virus That Causes COVID-19: Interim Guidance. [Google Scholar]
- WHO . World Heal. Organ; 2020. Rational use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations During Severe Shortages [WWW Document]https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages URL. (accessed 5.19.21) [Google Scholar]
- Wijesekara D.A., Sargent P., Ennis C.J., Hughes D. Prospects of using chars derived from mixed post waste plastic pyrolysis in civil engineering applications. J. Clean. Prod. 2021;128212 [Google Scholar]
- Williams P.T., Bagri R. Hydrocarbon gases and oils from the recycling of polystyrene waste by catalytic pyrolysis. Int. J. Energy Res. 2004;28:31–44. [Google Scholar]
- Williams P.T., Slaney E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007;51:754–769. [Google Scholar]
- Williams P.T., Williams E.A. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuel. 1999;13:188–196. [Google Scholar]
- Witkowski A., Stec A.A., Hull T.R. SFPE Handbook of Fire Protection Engineering. Springer; 2016. Thermal decomposition of polymeric materials; pp. 167–254. [Google Scholar]
- Wong H.-W., Broadbelt L.J. Tertiary resource recovery from waste polymers via pyrolysis: neat and binary mixture reactions of polypropylene and polystyrene. Ind. Eng. Chem. Res. 2001;40:4716–4723. [Google Scholar]
- Worrel W.A., Vesilind P.A. CENGAGE Learn; Stamford, USA: 2012. Solid Waste Engineering. [Google Scholar]
- Xue Y., Kelkar A., Bai X. Catalytic co-pyrolysis of biomass and polyethylene in a tandem micropyrolyzer. Fuel. 2016;166:227–236. [Google Scholar]
- Yan G., Jing X., Wen H., Xiang S. Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuel. 2015;29:2289–2298. [Google Scholar]
- Yang Z., Lü F., Zhang H., Wang W., Shao L., Ye J., He P. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123429. [DOI] [PubMed] [Google Scholar]
- Yao D., Zhang Y., Williams P.T., Yang H., Chen H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018;221:584–597. [Google Scholar]
- Yao L., King J., Wu D., Chuang S.S.C., Peng Z. Non-thermal plasma-assisted hydrogenolysis of polyethylene to light hydrocarbons. Catal. Commun. 2021;150 [Google Scholar]
- Yaşar H. World of Plastics. Vol. 1. Ankara, TMMOB Mech. Eng. Publ; 2004. pp. 59–63. [Google Scholar]
- Yousef S., Eimontas J., Subadra S.P., Striugas N. Functionalization of char derived from pyrolysis of metallised food packaging plastics waste and its application as a filler in fiberglass/epoxy composites. Process Saf. Environ. Prot. 2021;147:723–733. [Google Scholar]
- Zaman C.Z., Pal K., Yehye W.A., Sagadevan S., Shah S.T., Adebisi G.A., Marliana E., Rafique R.F., Johan R.Bin. Pyrolysis. BoD–Books on Demand. 2017. Pyrolysis: a sustainable way to generate energy from waste; p. 1. [Google Scholar]
- Zanella E., Della Zassa M., Navarini L., Canu P. Low-temperature co-pyrolysis of polypropylene and coffee wastes to fuels. Energy Fuel. 2013;27:1357–1364. [Google Scholar]
- Zhang Y., Ji G., Chen C., Wang Y., Wang W., Li A. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln. Fuel Process. Technol. 2020;206 [Google Scholar]
- Zhou J., Gui B., Qiao Y., Zhang J., Wang W., Yao H., Yu Y., Xu M. Understanding the pyrolysis mechanism of polyvinylchloride (PVC) by characterizing the chars produced in a wire-mesh reactor. Fuel. 2016;166:526–532. [Google Scholar]