Abstract
Purpose
Educational interventions have already been shown to positively affect awareness of clinical trials (CTs) among medical students. We aimed to explore basic knowledge and attitudes about CTs among medical students in terms of educational interventions that should be reflected in their further involvement in performing CTs and their role in raising awareness about CTs.
Methods
This cross-sectional, self-report anonymous online survey involved undergraduate medical students of the Medical Faculty University of Sarajevo enrolled in classes held within the Department of Pharmacology and Toxicology in the academic year 2015–2016. To include all accessible subjects for better representation of the whole population, consecutive sampling was applied.
Results
Among 142 students who completed questionnaire, 50% of them expressed partial or full agreement with the questionnaire statement that they were satisfied with the available information on CTs. Only 38% said they would participate in a CT, 21% would not, while 41% were not sure. Positive correlations were detected for composite subscale scores of agreement with questionnaire statements conveying the student’s knowledge about ethical and legal aspects of CTs and their perception about reliability/integrity and impact of CTs on medical practice.
Conclusion
Students have knowledge of the basic design and ethical aspects of CTs. Positive attitudes toward the impact of CTs on medical practice were shown in students of higher years of study, where educational intervention of additional knowledge of CTs was inserted and those students expressed better knowledge of CTs. However, no significant impact was detected between knowledge and willingness to participate in CTs, irrespective of years of study, reflecting the third of students that would participate in CTs. Changes in medical curricula led to the change in students’ knowledge and attitudes regarding CTs as well as their involvement in CTs.
Keywords: students, knowledge, education, questionnaire, intervention
Introduction
Clinical trials (CTs) are prospective biomedical research studies used as a gold standard for discovery of new drugs. They are recognized as highly important for improvement of the quality in general health care. Continuous and comprehensive efforts are applied to enhance CT integrity.1 Therefore, the guidelines for Good Clinical Practice (GCP), an international ethical and scientific quality standard accepted by a majority of regulatory authorities in the world, are used to tightly regulate the conduct of CTs.
Therapeutic guidelines, including but not limited to the guides for treatment of malignant diseases, are frequently based on results of randomized CTs conducted in high-income countries, but the globalization trend of CTs that is perceived to raise health-care standards, support capacity of development, and investment in low- and middle-income countries.2–4 However, while rising the number of externally financed CTs to developing countries, there is a need to address several concerns: protection of the patients participating in CTs from exploitation; balancing of implementation of locally driven trials to adapt therapeutic approaches to the country’s income settings; the lack of trained investigators, as CTs are continuously growing more complex and it is essential to enable further increase in the number of CTs performed locally; the lack of skilled health-care personnel and need for adequate training to enable them to respond to the challenges of both an ethical and scientific nature; and the lack of motivation to participate in CTs are outlined as some of the barriers for conducting of CT in developing countries.2–8
In addressing some of the concerns, as medical curricula are usually not directed towards wider education on this topic and only the basic information is shared within several subjects,9 education of medical students and medical professionals about CTs has been proposed as an educational intervention (EI).8,9 EI as a specific type of knowledge insertion has already been shown to positively affect awareness of CTs among medical students.10 In a study of Abushouk et al, a positive attitude towards CTs prevailed among medical students, but the lack of time, lack of adequate mentoring, and lack of knowledge were recognized as main barriers to their engagement in CTs.7
Consistently with internationalization and migration of clinical research from high- to low- and middle-income countries (mainly Asian), Bosnia and Herzegovina (B&H) has been gradually opening to sponsored and international CTs in the last two decades.11,12 According to the site of the B&H Agency for Medicinal Products and Medical Devices (www.almbih.gov.ba), the number of approved CTs per year varied from nine to 25 during 2012–2018 (mainly phase III and IV studies). Conduct of international CTs in B&H was enabled due to available medical infrastructure and a considerable pool of treatment-naive patients that in consequence usually produce high enrollment rates in our investigational sites. However, as the current medical curricula in B&H, only the basic information on CTs is shared, we aimed to explore the basic knowledge about and attitudes toward CTs among medical students in terms of education interventions, which should be reflected in both their further involvement in conducting CTs and their role in raising awareness about CTs.
Methods
Study Design and Sample
This study was designed as a cross-sectional, self-report online survey among undergraduate medical students of the Medical Faculty University of Sarajevo enrolled in classes held within the Department of Pharmacology and Toxicology in the academic year 2015–2016, ie, in their first, third, fourth and fifth year. Ethics approval was not required, as an anonymized approach was applied. According to the department’s informations, numbers of all students enrolled per study year were: 177 in year 1, 95 in year 3, 102 in year 4, and 88 in year 5. The questionnaire was distributed to these students. To include all accessible subjects for better representation of the whole population, the consecutive sampling technique was applied.
Data Collection
Students were asked to complete the anonymous electronic questionnaire. The questionnaire consisted of five subscales: 1, Aspects of design of CTs; 2, Ethical and legal aspects of CTs; 3, Attitudes about integrity of clinical research; 4, Attitudes about impact of CTs on medical practice; and 5, Availability of information on CTs. Questions were developed by experts with experience of monitoring in CTs. Some of the questions were created based on reviews of studies exploring knowledge pf and attitudes of medical students towards CTs.10,13 Validation of the questionnaire construct was done through a review of experts engaged in the CTs. Also, the questionnaire was piloted among students prior to implementation (n=10). Validation and standardization of the questionnaire was performed through the pilot study.
The questionnaire items used a five-point Likert scale: 1, strongly disagree; 2, partially disagree; 3; neither agree nor disagree; 4, partially agree; and 5, strongly agree. Answers to assess:
Knowledge about the aspects of design of CTs (domains 1 and 2) and ethical and legal aspects of CTs.
Attitudes regarding aspects of design of CTs (domains 3–5), integrity of clinical research, and impact of CTs on medical practice.
Availability of information regarding CTs (domain 5).
Composite scores were evaluated for each section of the questionnaire, along with a composite score for questions assessing knowledge. The composite score for items related to knowledge about CTs was constructed from items 1, 2, and 6–11. Apart from questions related to demographic characteristics of respondents, one question evaluated if students or their closer relatives had been enrolled in any CTs. Finally, students were requested to assess their satisfaction with information available to them about CTs and express their willingness to participate in CTs. Survey completion was defined as reaching the last question and clicking Submit, with nonresponse permitted. The reliability of the scale was satisfying, since Cronbach’s α=0.817 (number of items 22) for the whole scale.
Statistical Analysis
Statistical analysis was done using SPSS version 23, with p<0.05 considered statistically significant. Reliability of the questionnaire was calculated as a measure of internal consistency (Cronbach’s α coefficient). Statistical significance was assessed with Spearman’s  assessment for ordinal data.
assessment for ordinal data.
Results
The sample consisted of 142 respondents (48 male) with a mean age of 22.9±2.59 years. The overall response rate was 30.08%, and for year 3 students, the response rate was slightly higher than 56%. There were no missing answers to the questionnaire statements. Demographic characteristics of our sample are presented in Table 1. As a measure of reliability of the scale, Cronbach’s α coefficient was calculated as a measure of internal consistency of our data. Before this calculation, two inverse items were recoded (“A patient participating in a CT is always receiving an investigational drug” and “A patient may be enrolled in a CT without being informed about his/her participation”).
Table 1.
Demographic characteristics (n=142)
| n (%) | |
|---|---|
| Sex | |
| Male | 48 (33.8%) |
| Female | 94 (66.2%) |
| Year of study | |
| First | 47 (33.1%) |
| Third | 54 (38.0%) |
| Fourth | 24 (16.9%) |
| Fifth | 17 (12.0%) |
| Age, years | |
| 18–21 | 70 (49.3%) |
| 22–25 | 63 (44.4%) |
| 26–29 | 6 (4.2%) |
| 30–33 | 3 (2.1%) |
Percentages of agreement with questionnaire statements are shown in Table 2. To the question of whether they or their close relatives had been enrolled in any CTs, a majority of respondents answered no (n=123, 86.6%), while 15 respondents answered positively (10.6%) and four were unsure (2.8%). Half the respondents (50%) expressed partial or full agreement with the statement that they were satisfied with the available information about CTs. However, only 38% of students would participate in a CT, 21% would not, while 41% were not sure.
Table 2.
Percentages of agreement with questionnaire statements
| Statementsa | 1 | 2 | 3 | 4 | 5 | M | v |
|---|---|---|---|---|---|---|---|
| |||||||
| a) A patient participating in a CT is always receiving an investigational drug. | 52.8 | 13.4 | 14.8 | 12.0 | 7.0 | 1 | 1.80 |
| b) The use of a placebo allows a fair comparison of observed effects in control and experimental groups. | 3.5 | 2.8 | 6.3 | 19.0 | 68.3 | 5 | 0.97 |
| c) When a patient enrolled in a CT, is given placebo his/her standard therapy can be excluded. Due to this, a placebo is used only if the safety of the patient is not compromised. I think that is acceptable. | 3.5 | 7.7 | 9.9 | 26.1 | 52.8 | 5 | 1.23 |
| d) CTs are often double-blinded. I think that is acceptable. | 9.9 | 5.6 | 11.3 | 21.8 | 51.4 | 5 | 1.75 |
| e) The randomization of patients on an investigational drug or placebo is acceptable. | 5.6 | 4.9 | 10.6 | 21.8 | 57.0 | 5 | 1.35 |
| |||||||
| a) The safety of experimental drug can be explored in CTs. | 11.3 | 7.0 | 13.4 | 35.2 | 33.1 | 4 | 1.69 |
| b) Strict compliance with legal regulations and GCP is obligatory. | 1.4 | 1.4 | 0.7 | 7.0 | 89.4 | 5 | 0.42 |
| c) Government authorities monitor the safety during the conduct of CTs in their territory. | 2.1 | 9.2 | 35.9 | 26.1 | 26.8 | 4 | 1.08 |
| d) Potential risks and planned study procedures are listed on the informed consent form that the patient signs before enrollment in the CT. | 2.1 | 0 | 5.6 | 4.9 | 87.3 | 5 | 0.56 |
| e) Available standard and alternative treatments are explained in the informed consent form that the patient signs before enrollment in the CT. | 0 | 2.1 | 4.9 | 18.3 | 74.6 | 5 | 0.46 |
| f) A patient may be enrolled in a CT without being informed about his/her participation. | 71.8 | 14.1 | 4.2 | 7.7 | 2.1 | 1 | 1.06 |
| g) Emphasis is placed on the protection of the patient’s privacy. | 0.7 | 2.1 | 6.3 | 13.4 | 77.5 | 5 | 0.60 |
| |||||||
| a) The conduct of large multicenter CTs is justified because their results are a reliable source of evidence in medicine. | 1.4 | 2.8 | 20.4 | 40.1 | 35.2 | 4 | 0.80 |
| b) A conflict of interest is present to a great extent in international multicenter CTs sponsored by pharmaceutical companies. | 1.4 | 2.8 | 12.0 | 43.0 | 40.8 | 4 | 0.74 |
| c) The results of CTs should be published even if they indicate that the investigational treatment or drug is not successful. | 2.1 | 4.2 | 7.7 | 22.5 | 63.4 | 5 | 0.91 |
| |||||||
| a) The most up-to-date medical treatments are based on evidence from CTs | 0.7 | 2.8 | 9.9 | 43.0 | 43.7 | 4 | 0.65 |
| b) Conduct of international multicenter CTs within health-care institutions improves the standards of health care delivered to the patient. | 2.1 | 2.1 | 16.2 | 47.9 | 31.7 | 4 | 0.76 |
| c) Conduct of international multicenter CTs within health-care institutions positively affects health-care professionals’ awareness of up-to-date therapeutic guidelines | 1.4 | 1.4 | 12.0 | 38.0 | 47.2 | 4 | 0.70 |
| |||||||
| a) Information about multicenter sponsored CTs can be obtained at www.clinicaltrials.org free of charge. | 1.4 | 1.4 | 69.0 | 9.2 | 19.0 | 3 | 0.74 |
| b) International multicenter sponsored CTs are being conducted in Bosnia and Herzegovina. | 7.7 | 5.6 | 71.8 | 5.6 | 9.2 | 3 | 0.79 |
| c) Clinical studies conducted in B&H are published on the website of the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina | 1.4 | 2.8 | 80.3 | 7.0 | 8.5 | 3 | 0.46 |
| I am satisfied with the current information I have about CTs | 11.3 | 19.7 | 19.0 | 38.7 | 11.3 | 3 | 1.46 |
Notes: aQuestions were on a five-point Likert scale: 1, strongly disagree; 2, partially disagree; 3 neither agree nor disagree; 4, partially agree; 5, strongly agree.
Abbreviations: M, Median; v, variance.
From Table 2, we can see that respondents had been introduced to basic design and ethical aspects of CTs. For a majority of respondents, the answers to the questionnaire statements exploring design and ethical aspects of CTs were distributed within predicted fashion. Participants were aware of experimental design and use of placebo in CTs (items 1 and 2), obligation to follow GCP/local regulations and evaluation of safety of investigational drugs (items 6–8), and the informed consent process (items 9–11).
A majority of participants’ answers regarding “availability of information regarding CTs” were distributed around the strongly disagree to neither agree not disagree range for all three of the domain (items 19–21). Half the respondents expressed satisfaction with the current information that they possessed about CTs. A positive correlation was detected for the composite domain scores of agreements to questionnaire statements conveying the student’s knowledge about ethical and legal aspects of CTs and their perceptions of reliability/integrity and impact of CTs on medical practice (Table 3).
Table 3.
Spearman’s correlation coefficient 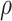 (n=142) for composite scores of questionnaire sections
(n=142) for composite scores of questionnaire sections
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|
| 1. Ethical and legal aspects of CTs (domain score) | – | 0.112 | 0.276** | 0.100 | 0.105 | 0.116 | 0.214* |
| 2. Integrity of CTs (domain score) | – | 0.582** | 0.153 | 0.196* | 0.198* | 0.163 | |
| 3. Impact on medical practice (domain score) | – | 0.295** | 0.205* | 0.221** | −0.055 | ||
| 4. Availability of information regarding CTs (domain score) | – | 0.257** | 0.104 | −0.080 | |||
| 5. Satisfaction with availability of information about CTs | – | 0.133 | −0.130 | ||||
| 6. Year of study | 0.152 | ||||||
| 7. Willingness to participate in CTs | – |
Notes: *P≤0.05 (two-tailed); **P≤0.01 (2-tailed).
Statistical significance of correlations for composite scores of domains is presented in Table 3. A statistically significant correlation was noted for the domain “Ethical and legal aspects of CT” with willingness to participate in CTs (r=0.214, p=0.01). Composite scores of domains assessing attitudes about integrity of CT and impact of CTs on medical practice strongly positively correlated with students’ satisfaction with availability of information on CTs (r=0.196, p=0.019; r=0.205, p=0.014 respectively), and year of study (r=0.198, p=0.018; r=0.221, p=0.008, respectively).
Positive correlation was not detected for the composite score of knowledge and willingness to participate in CTs (Table 4). Significant positive correlations were detected between items questioning composite score of knowledge with year of study (r=0.310, p=0.0001) and satisfaction with availability of information about CTs (r=0.188, p=0.025). Students or relatives’ prior enrollment in CTs positively correlated with willingness to participate in CTs (r=0.211, p=0.012).
Table 4.
Spearman correlation coefficient  (n=142) for composite score of knowledge
(n=142) for composite score of knowledge
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Year of study | – | 0.310** | 0.152 | 0.058 | 0.133 |
| 2. Composite score for knowledge | – | 0.144 | 0.100 | 0.188* | |
| 3. Students’ or their close relatives’ prior enrollment in CTs | – | 0.211* | −0.130 | ||
| 4. Willingness to participate in CTs | – | −0.039 | |||
| 5. Satisfaction with availability of information about CTs | – |
Notes: *P≤0.05 (two-tailed); **P≤0.01 (two-tailed).
Discussion
The results of this study suggest that our medical students have knowledge of the basic design and ethical aspects of CTs, but are not aware of CTs being conducted locally (85.1% disagree/neither agree nor disagree) and where to find that information (84.5% disagree/neither agree nor disagree). Furthermore, 50% of the respondents expressed partial or full agreement with the questionnaire statement: “I am satisfied with the current information I have about CTs.” This may imply that our students do not find information about locally conducted CTs as relevant as those about study design and ethical aspects of CTs. Probably, our students are unaware of the fact that multicenter CTs are conducted in the region, which strongly suggests a need to include this information in the current curricula. As expected, students with better scores in the domain “Availability of information regarding CT” were more satisfied with the information they possessed about CTs (r=0.257, p=0.002).
Engagement of institutions and individuals in CTs is associated with improvement in health-care performance.14 The distribution of answers to our questionnaire suggests confidence in the benefit of multicenter CTs being conducted in local health-care institutions in terms of impact on the standards of health care (79.6% partially/strongly agree) and awareness of health professionals of up-to-date therapeutic guidelines and approaches (85.2% partially/strongly agree). Our results suggest that respondents who expressed more positive attitudes towards impact of CTs on medical practice also expressed more agreement with the statements assessing both the attitude about integrity of CTs (r=0.582, p=0.0001) and statements assessing awareness of availability of information about CTs conducted locally and internationally (r=0.295, p=0.0001). Furthermore, positive attitudes toward impact of CTs on medical practice increased in both students who were in higher years of study (r=0.221, p=0.008) and those that expressed better knowledge of ethical and legal aspects of CT (r=0.276, p=0.001). Could these correlations imply that increased availability of information about and visibility of CT conduct affect the perception of its influence on the health-care system?
The significant positive correlation of composite score of knowledge with year of study (r=0.310, p=0.0001) and answers to questions assessing satisfaction with availability of information about CTs (r=0.188, p=0.025) may imply that students in higher years of study possess more knowledge about CTs and are more confident in their knowledge. Surprisingly, no significant correlation was detected between knowledge and willingness to participate in CT. Less than half (38%) the students expressed willingness to participate in a CT. Next to patients’ knowledge/provided information, safety and costs were factors identified as the main barriers to participation in CTs, the decisions to take part in this type of research may be strongly influenced by an emotional component from both the potential subjects’ and researchers’ points of view.15–17 The “guinea pig” perception of potential subjects in CTs and the role of the pharmaceutical industry in initiating and sponsoring CTs have both been shown to contribute to negative public attitudes towards CTs.18–21 This could be indicative of misconceptions and lack of adequate information about CTs in the general population. Poor recruitment to CTs has been recognized as an issue that could influence CT implementation.16,17,22 Poor recruitment has recently partially shifted the focus towards investigating patients’ attitudes to CTs. On the other hand, a study that used convenience sampling showed that 92% of patients with cancer made decisions to participate in high-risk CTs prior to informed consent conferences taking place.23
Nevertheless, negative attitudes towards CTs are not frequent within the population of health-care professionals engaged in CTs as investigators. Qualitative research that used a sample of 39 study nurses indicated their overall positive attitude towards CTs, despite ethical challenges they sometimes faced, especially with regard to the enrollment of terminally ill patients.24 In this study, two statistically significant positive correlations in terms of willingness to participate in CTs were detected: prior enrollment in CTs of a student or clos relative (r=0.211, p=0.012), and the domain score of knowledge about ethical and legal aspects of CT (r=0.214, p=0.01).
Raising the visibility of CTs should be emphasized when developing education interventions. Researchers have already stressed the need to enhance training on research and CTs in medical curricula.6–8,10,13 The lack of adequately trained health-care professionals has been recognized in parts of the world with high growth in CT implementation.6 Specific areas of deficient knowledge are recognized, and these results could be useful as general guidance for medical curriculum updates. Moreover, improving students’ knowledge could positively affect wider-community attitudes towards CT.
Conclusion
Our medical students have knowledge of the basic design and ethical aspects of CTs, but they are not aware of or sufficiently informed about CTs being conducted locally or where to find that information. Positive attitudes towards the impact of CTs on medical practice were evident. Positive attitudes toward impact of CTs on medical practice was shown in students in higher years of study, where education intervention about additional knowledge on CTs is included, and those students expressed better knowledge of CTs. However, no significant impact was detected between knowledge and willingness to participate in CTs, regardless of years of study (with or without basic knowledge), reflecting the one third of students that would participate in CTs.
The increase in visibility of CTs and the availability of relevant information regarding ethical and legal enforcement standards can be of crucial importance for the development of this type of research, including the recruitment of future clinicians as investigators. This process should start with implementing changes in medical curricula, while follow-up of changes in students’ knowledge and attitudes regarding CTs and involvement in CTs and education of the wider community should be evaluated. It is certain that beyond teaching of the benefits that CTs bring to science, community, and health care, medical curricula need to be expanded to cover the relevant sources of information about the scope of implementation of CTs locally and internationally.
Limitations and Strengths
This study was designed as a self-reported survey and the response rate was low (30%), so generalizability cannot be determined. In addition, each domain of the questionnaire should be further correlated with participation in CTs in detail, but the results could be a primary step in more comprehensive studies on the subject.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or all this areas, took part in drafting, revising or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for aspects of the work.
Disclosure
The authors report no conflicts of interest in this work. The abstract of this paper was presented at the EACPT Focus Meeting 2016 as a conference talk with interim findings. The abstract was published in “Presented Abstracts” in Clinical Therapeutics, volume 38, number 10S from October 2016: https://www.clinicaltherapeutics.com/article/S0149-2918(16)30468-4/fulltext.
References
- 1.Gupta A. Fraud and misconduct in clinical research: a concern. Perspect Clin Res. 2013;4(2):144–147. doi: 10.4103/2229-3485.111800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang T, Siribaddana S. Clinical trials have gone global: is this a good thing? PLoS Med. 2012;9(6):e1001228. doi: 10.1371/journal.pmed.1001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph PD, Caldwell PH, Tong A, Hanson CS, Craig JC. Stakeholder views of clinical trials in low- and middle-income countries: a systematic review. Pediatrics. 2016;137(2):e20152800. doi: 10.1542/peds.2015-2800 [DOI] [PubMed] [Google Scholar]
- 4.Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health. 2018;17(1):37. doi: 10.1186/s12939-018-0748-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dal-Ré R, Jha V, Lv J, Chaudhury RR, Wang Y, Perkovic V. International trials in middle-income countries: different local scenarios require different ethical approaches. J R Soc Med. 2016;109(2):47–51. doi: 10.1177/0141076815608854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhodi DK, Thakkar KB, Billa G, et al. Knowledge, attitude and practices of medical students and teachers towards clinical research in a tertiary care hospital in Mumbai – cross sectional survey. J Contemp Med Edu. 2013;1(4):238–244. doi: 10.5455/jcme.20130608045519 [DOI] [Google Scholar]
- 7.Abushouk AI, Hatata AN, Omran IM, et al. Attitudes and perceived barriers among medical students towards clinical research: a cross-sectional study in an Egyptian medical school. Hindawi publishing corporation. J Biomed Educ. 2016:7. doi: 10.1155/2016/5490575 [DOI] [Google Scholar]
- 8.Eley DS, Jensen C, Thomas R, Benham H. What will it take? Pathways, time and funding: Australian medical students’ perspective on clinician-scientist training. BMC Med Educ. 2017;17(1):242. doi: 10.1186/s12909-017-1081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanarek NF, Kanarek MS, Olatoye D, Carducci MA. Removing barriers to participation in clinical trials, a conceptual framework and retrospective chart review study. Trials. 2012;13:237. doi: 10.1186/1745-6215-13-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priyadarshini DM, Bagewadi HG, Patil BV, Dass AP. Knowledge, attitude and perceptions of 3rd term medical students towards clinical trials in a medical college in southern India. Indian J Pharm Pharmacol. 2017;4(3):125–129. doi: 10.18231/2393-9087.2017.0028 [DOI] [Google Scholar]
- 11.Minisman G, Bhanushali M, Conwit R, et al. Implementing clinical trials on an international platform: challenges and perspectives. J Neurol Sci. 2012;313(1–2):1–6. doi: 10.1016/j.jns.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drain PK, Robine M, Holmes KK, Bassett IV. Trial watch: global migration of clinical trials. Nat Rev Drug Discov. 2014;13(3):166–167. doi: 10.1038/nrd4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhury S, Pradhan R, Dubey L, et al. Knowledge and perception regarding clinical trials among doctors of government medical colleges: a questionnaire-based study. Perspect Clin Res. 2016;7(2):94–99. doi: 10.4103/2229-3485.179433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annette B, Hanney S, Jones T, Soper B. Does the engagement of clinicians and organizations in research improve healthcare performance: a three-stage review. BMJ Open. 2015;5:e009415. doi: 10.1136/bmjopen-2015-009415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn R. Surveys identify barriers to participation in clinical trials. JNCI J Natl Cancer Inst. 2000;92(19):1556–1558. doi: 10.1093/jnci/92.19.1556 [DOI] [PubMed] [Google Scholar]
- 16.Sood A, Prasad K, Chhatwani L, et al. Patients’ attitudes and preferences about participation and recruitment strategies in clinical trials. Mayo Clin Proc. 2009;84(3):243–247. doi: 10.1016/S0025-6196(11)61141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron P, Pond GR, Xu RY, Ellis PM, Goffin JR. A comparison of patient knowledge of clinical trials and trialist priorities. Curr Oncol. 2013;20(3):e193–e205. doi: 10.3747/co.20.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sameer S, Chopra AM. Industry funding of clinical trials: benefit or bias? JAMA. 2003;290(1):113–114. doi: 10.1001/jama.290.1.113 [DOI] [PubMed] [Google Scholar]
- 19.Lexchin J, Bero LA, Djulbegovic B, Otavio C. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167. doi: 10.1136/bmj.326.7400.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yavchitz A, Boutron I, Bafeta A, et al. Misrepresentation of randomized controlled trials in press releases and news coverage: a cohort study. PLoS Med. 2012;9(9):e1001308. doi: 10.1371/journal.pmed.1001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grover S, Xu M, Jhingran A, et al. Clinical trials in low and middle-income countries -Successes and challenges. Gynecol Oncol Rep. 2017;19:5–9. doi: 10.1016/j.gore.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramers-Verhoeven WC, Perrone F, Oliver K. Exploratory research into cancer patients’ attitudes towards clinical trials. Ecancer. 2014;8:432. doi: 10.3332/ecancer.2014.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon-Dorcy K, Drevdahl DJ. “I had already made up my mind”: patients and caregivers’ perspectives on making the decision to participate in research at a US cancer referral center. Cancer Nurs. 2011;34(6):428–433. doi: 10.1097/NCC.0b013e318207cb03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godskesen TE, Petri S, Eriksson S, et al. When nursing care and clinical trials coincide: a qualitative study of the views of Nordic oncology and hematology nurses on ethical work challenges. J Empir Res Hum Res Ethics. 2018;13(5):475–485. doi: 10.1177/1556264618783555 [DOI] [PubMed] [Google Scholar]


