Abstract
An immunogenic aminopeptidase of Candida albicans was purified by high-performance liquid chromatography. It was then used for the development of an enzyme-linked immunosorbent assay to detect antibodies directed against this antigen in sera from patients with candidiasis. This enzyme specifically cleaves the l-Arg–7-amino-4-methyl-coumarin substrate at pH 7.4 and was detected in the crude extract of different C. albicans isolates. Sera used for this study were obtained from healthy blood donors or from patients with one of the following: systemic candidiasis, aspergillosis, cryptococcosis, toxoplasmosis, or malaria. The statistical analysis demonstrates significant differences between absorbency values obtained with sera from patients with candidiasis and with sera from the other groups (P = 0.000001). Diagnostic parameters show high diagnostic specificity of 97% and a sensitivity of 83% at a cutoff value of 0.425 and suggest the usefulness of this aminopeptidase for the diagnosis of systemic candidiasis.
The incidence of and mortality due to mycoses, particularly those caused by opportunistic fungi, have shown a marked increase in recent years, especially in patients whose immune defense mechanisms have been compromised by antibiotics, immunosuppressive therapy, or severe underlying diseases (cancers, AIDS, hematologic disorders) (5, 12, 14). Indeed, the high fatality rate due to these infections makes necessary prompt diagnosis and therapeutic measures. However, providing evidence of a deep-seated Candida infection is often a difficult challenge. Clinical signs are not pathognomonic (10). In most cases, serum antibody levels are not significant in seriously debilitated patients. Empiric antifungal therapy may also hamper fungus isolation. In addition, biopsy of deep tissues for histological confirmation is often impossible in the patients.
Many different procedures are used in the diagnosis of Candida infections. These include: (i) detection of circulating antigens; (ii) gas-liquid chromatography for the quantitation of arabinitol in serum; (iii) gas-liquid chromatographic quantitation of mannose in serum; and (iv) tests to detect antibodies against Candida species by using indirect immunofluorescence assays, electrosyneresis or Co-immunoelectrophoresis (15) and enzyme-linked immunosorbent assays (ELISA) (3, 4, 16, 17).
This latter method (ELISA) has already been developed. Several studies using specific antigens (9, 11, 13) have demonstrated that the presence of antibodies against purified antigens of Candida albicans correlated with invasive infections.
In this study, we developed a new ELISA to detect antibodies against C. albicans. The antigen we used consists of a purified C. albicans metallopeptidase that was expected to be specific to this yeast (6). It has been suggested that this antigen is cytoplasmic, but immunoelectron microscopical evidence indicates that it is located at the cell surface (7). In addition, sera from patients suffering from deep-seated C. albicans infections reacted positively to the metallopeptidase in Western blotting and immunoprecipitation analysis (unpublished data).
MATERIALS AND METHODS
Growth conditions and yeast extraction.
The C. albicans strain 2091 obtained from the Pasteur Institute (Paris, France) was grown for 48 h on Sabouraud dextrose agar slants at 37°C.
The cells were harvested in Tris-buffered saline (140 mM NaCl, 10 mM Tris-HCl [pH 7.2]), washed three times by centrifugation, and suspended to a final concentration of 109 cells/ml in the same buffer. The cells were disrupted for 10 min in an MSK cell homogenizer (B. Braun, Melsungen, Germany) with glass beads (0.45- to 0.55-mm diameter) with cooling under CO2.
Disrupted yeast cells were centrifuged at 100,000 × g for 30 min at 4°C, and the supernatant fluid was taken as the cytoplasmic extract and used for the assays. Protein concentration was determined as described by Bradford (2) with bovine serum albumin as a standard.
Enzyme purification.
A high-performance liquid chromatography system (Laboratoires Merck Clevenot, Nogent-sur-Marne, France) was used to purify the metallopeptidase in a two-step process involving ion exchange chromatography followed by gel filtration as described by El Moudni et al. (6). Ion exchange chromatography was carried out on a fractogel EMD-TMAE 650 column (150 by 10 mm) (Merck) equilibrated with 25 mM Tris-HCl (pH 7.4) by using a linear gradient from 0 to 0.5 M NaCl. The active fraction was concentrated on a Centricon 10 (Amicon) and injected into a TSK G3000 SWxL gel filtration column (300 by 7.8 mm) (Toyo Soda, Tokyo, Japan). This procedure yielded a purification of 36-fold and indicated a single protein of 52-kDa when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions (6).
Peptidase assays.
The enzyme activity was monitored as previously described (6) by using a 20 mM concentration of the fluorogenic substrate l-Arg–7-amino-4-methyl-coumarin (l-Arg-AMC). Briefly, 10 μl of freshly prepared yeast extract or purified enzyme was incubated with 190 μl of the substrate in 50 mM Tris-buffered saline (pH 7.2). After incubation for 15 min at 37°C, the reaction was stopped by adding 1 ml of ethanol. The fluorescence at 440 nm was measured, with excitation at 380 nm for AMC.
ELISA.
Four positive sera and four negative sera were initially tested to determine the optimal dilution (1:100) without background reactivities and potential nonspecificities.
Antigen (0.25 μg) was applied in 100 μl of coating buffer (carbonate-bicarbonate at 0.15 M [pH 9.6]) to each well of a polystyrene microtiter plate (Nunc, Roskilde, Denmark).
The plate was incubated at 37°C for 3 h, saturated with bovine serum albumin (3%) in phosphate-buffered saline (PBS) and then washed three times (5 min each) with PBS containing 0.05% (wt/vol) Tween 20 (PBS-T). All serum samples were diluted to 1:100 in PBS-T and incubated for 2 h at 37°C. After incubation, the plate was washed three times with PBS-T and treated with the peroxidase-labelled anti-human immunoglobulins (immunoglobulins G, A, and M) diluted in PBS-T for 2 h at 37°C. The reaction was developed by using o-phenylenediamine as chromogen (Sigma), and the absorbency at 492 nm was read.
Serum samples.
The patient sera included in the present study were divided into four groups on the basis of clinical findings and standard serological tests (immunofluorescence assays and Co-immunoelectrophoresis) and were confirmed by positive C. albicans blood cultures. Group I consisted of 10 healthy blood donors, group II consisted of 10 patients with toxoplasmosis or plasmodium infections, group III consisted of 16 patients with aspergillosis or cryptococcosis, and group IV consisted of 40 patients with confirmed invasive candidiasis.
Statistical analysis.
Absorbency values observed in different populations were compared by the nonparametric analysis of variance method of Kruskal-Wallis. In this study, the P value was considered not significant at P ≥ 0.01.
The relative operating characteristic (ROC) curve is constructed with the sensibility and the false positive value (8). The ROC curve was used to determine directly the diagnostic parameters, such as sensitivity, specificity, predictive positive value, and predictive negative value at different cutoff values.
RESULTS
SDS-PAGE (Fig. 1) shows the electrophoretic patterns of C. albicans cytosolic extract (Fig. 1, lane A) performed in parallel with the purified metallopeptidase (Fig. 1, lane B). The enzyme activities in the cytosolic extract or the purified C. albicans metallopeptidase were determined by using the l-Arg-AMC substrate.
FIG. 1.
SDS-PAGE analysis of the C. albicans cytosolic extract stained with Coomassie brilliant blue R250 (lane A) and the purified metallopeptidase stained by the silver procedure (lane B). The electrophoresis was carried out under reducing conditions. Numbers on the left are calibration standards as follows: α2-macroglobulin (180 kDa); β-galactosidase (120 kDa); fructose-6-phosphate kinase (88 kDa); pyruvate kinase (55 kDa); and fumarase (44 kDa).
In this ELISA, we examined 76 sera from patients with candidiasis (n = 40), with aspergillosis or cryptococcosis (n = 16), and with toxoplasmosis or plasmodium infections (n = 10) and sera from healthy blood donors (n = 10).
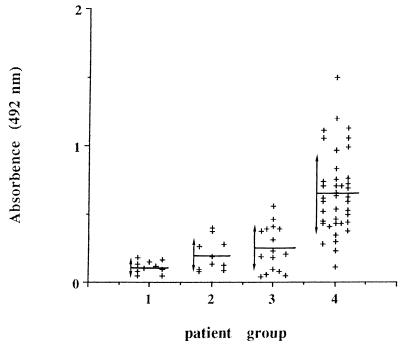
The results (means and standard deviations) obtained with different sera are shown in Fig. 2. The mean absorbency value for the patients with invasive candidiasis is 0.650; however, the mean absorbency values for the other populations are between 0.112 and 0.250.
FIG. 2.
Absorbency values determined by an ELISA performed with different sera. Means (horizontal line) and standard deviations (vertical line with arrows) for different populations are shown. Groups: 1, healthy blood donors; 2, patients with toxoplasmosis or malaria; 3, patients with aspergillosis or cryptococcosis; 4, patients with candidosis.
Statistical analysis using the analysis of variance showed significant differences between absorbency values obtained with sera of the group with invasive candidiasis and those obtained with sera of the three other groups (P = 0.000001). In addition, there was no significant difference between absorbency values obtained with sera of the three other populations (P > 0.02).
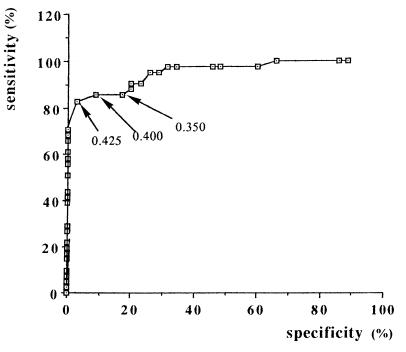
Applying the ROC procedure (Fig. 3), we evaluated the specificity and sensitivity of this ELISA at different cutoff values (0.350, 0.4, and 0.425) (Table 1). The higher specificity and sensitivity (83 and 97.15%, respectively) were obtained at an absorbence of 0.425. The predictive positive value and predictive negative values were 97 and 82%, respectively.
FIG. 3.
Determination of cutoff absorbency value by ROC curves.
TABLE 1.
Comparison of diagnostic values of the Candida metallopeptidase antibody ELISAs at different cutoff values
| Cutoff value (absorbance at 492 nm) | Sensitivity (%) | Specificity (%) | False positives (%) | False negatives (%) | Predictive value (%)
|
|
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 0.350 | 87.80 | 80 | 20 | 12 | 83 | 84 |
| 0.400 | 85.36 | 91.43 | 8.57 | 15 | 92 | 84 |
| 0.425 | 83 | 97.15 | 2.85 | 17 | 97 | 82 |
DISCUSSION
The diagnosis of invasive candidiasis remains difficult. The common problem is properly identifying the immunocompromised patient who needs an antifungal treatment while avoiding the unnecessary exposure of uninfected patients to this therapy.
Immunodiagnosis of candidiasis infections is without doubt an important diagnostic tool in the early detection of the infections. However, previous reports of antibody detection assays with crude antigens for the detection of deep-seated candidiasis always showed that the test suffered from suboptimal sensitivity and/or specificity (11). The lack of specificity is due to the fact that most healthy patients already have antibodies directed against cell wall components of C. albicans. Therefore, it is conceivable that the use of a purified and well-defined antigen rather than a crude extract provides a more specific test for systemic candidiasis. Several studies have reported a high sensitivity and specificity obtained by using an antibody detection assay with purified antigens (1, 8, 9, 18).
In this study, we purified an antigen of C. albicans and coated it onto well plates in an attempt to detect specific antibodies directed against this antigen. This antigen has recently been isolated and characterized as an intracellular C. albicans metallopeptidase. This enzyme is specific for the fluorogenic substrate l-arginine-7-amino-4-methylcoumarin at pH 7.2. This antigen was purified by high-performance liquid chromatography and gave a single protein band at 52 kDa by SDS-PAGE. Similar activity was also demonstrated in other Candida spp. However, the rabbit polyclonal antibody developed against this antigen recognizes and immunoprecipitates only the C. albicans metallopeptidase. This enzyme is recognized by sera from patients suffering from candidiasis and is released in vitro in the culture supernatant (unpublished data).
Statistical analysis showed significant differences between absorbency values obtained with sera of patients with candidiasis and those obtained with sera provided by patients with aspergillosis, cryptococcosis, toxoplasmosis, or malaria or by healthy patients (P = 0.000001). The Candida metallopeptidase antibody ELISA had diagnostic values at a cutoff point of 0.425 of 97.15% (specificity) and 83% (sensitivity). The positive predictive value and the negative predictive value were 97 and 82%, respectively.
This metallopeptidase ELISA gave satisfactory results. The results we obtained were better than those obtained with the other antigens (Table 2). Regarding this result, further studies should be undertaken using sera from patients with superficial candidiasis, to discriminate the disseminated candidiasis from the superficial ones, and sera from patients suffering from deep-seated candidiasis caused by other Candida species. This test, using a specific antigen of C. albicans, could therefore be useful in this case.
TABLE 2.
Comparison of diagnostic values of different Candida antibody ELISAs
| Authors (reference) | Antigen(s) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Greenfield et al. (9) | 54-kDa cytoplasmic antigen | 25 | 97.8 |
| Zöller et al. (19) | 29- to 47-kDa cytoplasmic antigens | 81.5 | 96.4 |
| Van Deventer et al. (18) | 47-kDa enolase antigen | 53a | 78a |
| 50b | 86b | ||
| Berdin et al. (1) | 48-kDa enolase antigen | 73 | 95.5 |
| Ag 3D9 mannoprotein | 78 | 82 | |
| This study | 52-kDa metallopeptidase | 83 | 97.15 |
Value for immunocompromised patients.
Value for immunocompetent patients.
ACKNOWLEDGMENTS
We thank R. Robert and A. Marot-Leblond (University of Angers) for fruitful discussions. We also thank M. L. Dardé (University of Limoges) and D. Chabasse (University of Angers) for the generous gift of the human sera from candidosis patients. The help of E. Delpech for the technical assistance was particularly appreciated.
This work was partly supported by grants from Pfizer Laboratories.
REFERENCES
- 1.Berdin B, Boux de Casson-Raimbeau F, Marot-Leblond A, Robert R, Senet J M. Etude préliminaire évaluant l’intérêt de l’utilisation d’antigène purifié (Ag3D9, Ag48) pour le sérodiagnostic des candidoses profondes par méthode immuno-enzymatique ELISA (Enzyme Linked Immunosorbent Assay) J Mycol Med. 1995;5:140–144. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.De Repentigny L, Marr L D, Keller J W, Carter A W, Kuykendall R J, Kaufman L, Reiss E. Comparison of enzyme immunoassay and gas-liquid chromatography for the rapid diagnosis of invasive candidiasis in cancer patients. J Clin Microbiol. 1985;21:972–979. doi: 10.1128/jcm.21.6.972-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Repentigny L. Serological techniques for diagnosis of fungal infection. Eur J Clin Microbiol Infect Dis. 1989;8:362–375. doi: 10.1007/BF01963470. [DOI] [PubMed] [Google Scholar]
- 5.Drouhet E, Dupont B. Mycoses in AIDS patients: an overview. In: Vanden Bossche H, Mackenzie D W R, Cauwenbergh G, Van Cutsem J, Drouhet E, Dupont B, editors. Mycoses in AIDS patients. New York, N.Y: Plenum Press; 1990. pp. 27–53. [Google Scholar]
- 6.El Moudni B, Rodier M H, Barrault C, Ghazali M, Jacquemin J L. Purification and characterisation of a metallopeptidase of Candida albicans. J Med Microbiol. 1995;43:282–288. doi: 10.1099/00222615-43-4-282. [DOI] [PubMed] [Google Scholar]
- 7.El Moudni B, Rodier M H, Babin P, Jacquemin J L. Metallopeptidase in Candida albicans: immunolocalization in yeast. J Mycol Med. 1997;7:5–9. [Google Scholar]
- 8.Erdreich L, Lee E T. Use of relative operating characteristic analysis in epidemiology. Am J Epidemiol. 1981;144:649–662. doi: 10.1093/oxfordjournals.aje.a113236. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield R A, Bussey M J, Stephens J L, Jones J M. Serial enzyme-linked immunosorbent assays for antibody to Candida antigens during induction chemotherapy for acute leukemia. J Infect Dis. 1983;148:275–283. doi: 10.1093/infdis/148.2.275. [DOI] [PubMed] [Google Scholar]
- 10.Grillot R, Lebeau B, Pinel C, Fricker H, Ambroise-Thomas P. Diagnostic biologique des mycoses systémiques en oncohématologie. J Mycol Med. 1991;1:4–10. [Google Scholar]
- 11.Jones J M. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3:32–45. doi: 10.1128/cmr.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maksymiuk A W, Thongprasert S, Hopfer R. Systemic candidiasis in cancer patients. Am J Med. 1984;77:20–27. [PubMed] [Google Scholar]
- 13.Mitsutake K, Khono S, Miyazaki H, Maesaki S, Koga H. Detection of Candida enolase antibody in patients with candidiasis. J Clin Lab Anal. 1994;4:207–210. doi: 10.1002/jcla.1860080405. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller M A. Epidemiology and control of fungal infections. Clin Infect Dis. 1994;19:S8–S13. doi: 10.1093/clinids/19.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 15.Poulain D, Pinon J M. Diagnosis of systemic candidiasis: development of Co-counterimmunoelectrophoresis. Eur J Clin Microbiol. 1986;5:420–426. doi: 10.1007/BF02075698. [DOI] [PubMed] [Google Scholar]
- 16.Reiss E, Morrison C J. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993;6:311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugar A M. Problems in the diagnosis of invasive candidiasis in the immunocompromised patient. In: Richardson R G, editor. Opportunistic fungal infections: focus on fluconazole. Royal Society of Medicine Services International Congress and Symposium Series. London, England: Royal Society of Medicine Services Limited; 1989. pp. 17–24. [Google Scholar]
- 18.Van Deventer A J M, Van Vliet H J A, Hop W C J, Goessens W H F. Diagnostic value of anti-Candida enolase antibodies. J Clin Microbiol. 1994;32:17–23. doi: 10.1128/jcm.32.1.17-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zöller L, Krämer I, Kappe R, Sonntag H-G. Enzyme immunoassay for invasive Candida infections: reactivity of somatic antigens of Candida albicans. J Clin Microbiol. 1991;29:1860–1867. doi: 10.1128/jcm.29.9.1860-1867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]