Abstract
Sex ratio depends on sex determination mechanisms and is a key demographic parameter determining population viability and resilience to natural and anthropogenic stressors. There is increasing evidence that the environment can alter sex ratio even in genetically sex-determined species (GSD), as elevated temperature can cause female-to-male sex reversal (neomales). Alarmingly, neomales are being discovered in natural populations of several fish, amphibian and reptile species worldwide. Understanding the basis of neomale development is important for conservation biology. Among GSD species, it is unknown whether those with chromosomal sex determination (CSD), the most common system, will better resist the influence of high temperature than those with polygenic sex determination (PSD). Here, we compared the effects of elevated temperature in two wild zebrafish strains, Nadia (NA) and Ekkwill (EKW), which have CSD with a ZZ/ZW system, against the AB laboratory strain, which has PSD. First, we uncovered novel sex genotypes and the results showed that, at control temperature, the masculinization rate roughly doubled with the addition of each Z chromosome, while some ZW and WW fish of the wild strains became neomales. Surprisingly, we found that at elevated temperatures WW fish were just as likely as ZW fish to become neomales and that all strains were equally susceptible to masculinization. These results demonstrate that the Z chromosome is not essential for male development and that the dose of W buffers masculinization at the control temperature but not at elevated temperature. Furthermore, at the elevated temperature the testes of neomales, but not of normal males, contained more spermatozoa than at the control temperature. Our results show in an unprecedented way that, in a global warming scenario, CSD species may not necessarily be better protected against the masculinizing effect of elevated temperature than PSD species, and reveal genotype-by-temperature interactions in male sex determination and spermatogenesis.
Keywords: Global warming, Sex ratios, Sex-reversal, Neomales, Sex determination, Zebrafish
1. Introduction
Population sex ratio is a key ecological demographic parameter resulting from the action of sex determining mechanisms (Bull, 1985). These can be broadly classified into genetic (GSD) and environmental (ESD) sex determination, with the possibility of mixed systems (Nagahama et al., 2021). In ESD species, temperature is the main environmental factor influencing sex ratios (temperature-dependent sex determination; TSD). In vertebrates, TSD is present in reptiles (Valenzuela and Lance, 2004), fishes (Ospina-Alvarez and Piferrer, 2008) and amphibia (Nakamura, 2009). Sex ratios determine population viability and resilience to natural and anthropogenic stressors (Hays et al., 2017; Melbourne and Hastings, 2008). The response of TSD species to global warming is an issue of current intense debate (Consuegra and Rodríguez Lopez, 2016; Geffroy et al., 2021; Piferrer, 2016; Valenzuela et al., 2019), with no clear consensus as whether species will be able to adapt or not to fast climate change.
Most vertebrates have GSD. In theory, GSD species should be more resilient than ESD species to environmental influences. However, mounting evidence shows that the environment can influence sex determination also in GSD species. Thus, elevated temperature causes some genetic females to become phenotypic males, called neomales or sex reversals (Baroiller & D’Cotta, 2016; Nagahama, 2005; Pandian and Sheela, 1995). The presence of neomales alters sex ratios and can compromise the future of sensitive populations (Wedekind, 2019). In fish, neomales can be produced by thermal manipulation in GSD species where sex is determined by the action of a major sex determining gene located on sex chromosomes (chromosomal sex determination; CSD) (Capel, 2017), such as medaka (Oryzias latipes, XX/XY) (Matsuda et al., 1998) and Chinese tongue sole (Cynoglossus semilaevis, ZW/ZZ) (Shao et al., 2014), as well as in GSD species where sex is determined by the action of several loci of minor effect distributed across the genome (polygenic sex determination, PSD) (Nagahama et al., 2021) such as the European sea bass (Dicentrarchus labrax) (Geffroy et al., 2021; Saillant et al., 2002; Vandeputte et al., 2007). Neomales have also been produced in reptiles (Quinn et al., 2007), amphibians (Wallace et al., 1999) and in prawns (Levy et al., 2019).
Alarmingly, neomales are now being discovered also in natural populations of several fish species including Nile tilapia (Oreochromis niloticus) (Bezault et al., 2007) and medaka (Shinomiya et al., 2010), both with an XX/XY sex determination system, and reptiles such as the Australian bearded dragon (Pogona vitticeps, ZW/ZZ) (Castelli et al., 2020). In cobaltcap silverside (Hypoatherina tsurugae), a marine fish species with GSD and TSD sex determination, neomale proportions can change from 7% to 52% during a 3-year period (Miyoshi et al., 2020). This finding has been linked to exposure to abnormal conditions such as temperatures beyond the species’ usual thermal zone (Azaza et al., 2008; Sato et al., 2005).
The understanding of the genetic and environmental basis behind the presence of neomales in nature is then of great significance for conservation biology in a global warming scenario. However, not only the underlying mechanisms behind the appearance of neomales in a GSD species are far from clear, but also fundamental questions need to be answered. In particular, we need to know whether, among GSD species, those with CSD, i.e., with a sex-determining locus of strong effect, will better resist the influence of high temperature more than those with PSD. Alternatively, the sex-determining step that is sensitive to the environment might be downstream of the major sex determining gene and so temperature would be equally able to overcome either a CSD or a PSD system. To the best of our knowledge, such a comparative study, with the determination of proper reaction norms of sex ratio response to temperature, does not exist. Comparing species with different GSD systems, however, has the risk that possible observed differences can be due to the presence of unaccounted factors.
In this study we took advantage of the fact that different strains of zebrafish (Danio rerio) have different sex determining mechanisms to investigate the contributions of genetic vs. environmental factors to neomale production. Specifically, we compared two wild strains, Nadia (NA) and Ekkwill (EKW), which have CSD (Wilson et al., 2014), with the AB laboratory strain, which has PSD (Liew et al., 2012; Ribas et al., 2017). First, we developed new PCR primers for both NA and EKW that are closely linked to the sex determinant locus, sar4 (sex-associated region on chromosome-4; Wilson et al., 2014), and that allowed the identification of genotypic sex and novel sex genotypes not reported previously. Importantly, these primers not only distinguished neomales from genetic males, but also allowed a careful characterization of the reaction norms of the sex ratio response to temperature. Finally, we evaluated the effects of temperature on gonadal morphology to study differences at the cellular level between AB and NA strains comparing mature gonads of males, neomales and females. Results showed, surprisingly, that the two wild strains with CSD were as sensitive to elevated temperature-induced sex reversal as the lab strain with PSD.
Furthermore, neomales differed from regular males by possessing more sperm in their testes when exposed to elevated temperature. These results illuminate how environmental change may affect natural populations and suggest that fish species that have a GSD system with some environmental influence may not necessarily be protected from substantial perturbation of sex ratios as water temperatures rise due to global climate change.
2. Materials and methods
2.1. Fish and husbandry conditions
The zebrafish strains AB (ZDB-GENO-960809-7), Nadia (NA, ZDB-GENO-030115-2) and EkkWill (EKW, ZDB-GENO-990520-2) were used for temperature treatment experiments. Details regarding GSD in these strains, as well as breeding protocols and husbandry details, can be found in the Supplementary Information.
2.2. Temperature treatments and survival
Temperature treatments involved twelve different families grown until adulthood, three families each for EKW and AB and six families for NA. Offspring of each family were apportioned randomly into two groups with 20 larvae per tank in three technical replicates per group and cultured at 28 °C until 18 days post fertilization (dpf), when they were transferred into a programmable system at either 28 °C or a ramp up to 36 °C over a period of 16 days. Then they were held until 34 dpf to encompass the period of sex differentiation (Chen and Ge, 2013; Uchida et al., 2002; Wang et al., 2007), when they were returned to normal housing at 28 °C. The other three NA families experienced 34 °C for the 18–34 dpf period. Survival was recorded for each family at 18 dpf, 34 dpf, and 90 dpf. Temperature treatments and survival procedures are described in detail in the Supplementary Information.
2.3. Sampling
Adult fish were sampled at 90 dpf. Phenotypic sex was determined by gonad morphology after dissection under a dissecting microscope and sex ratio was calculated for each biological replicate. A fin clip provided material for genotypic sex determination. For histological analysis, five males and five females were randomly selected from each replicate tank and the portion of the body trunk containing gonads was fixed in Bouin’s solution for subsequent histological analysis. A total of 1218 fish were sampled. Sampling and histology details are described in the Supplementary Information.
2.4. Sex genotyping NA and EKW fish
Primers for sex genotyping NA (Supplementary Information, Table S2 and Fig. S3) and EKW (Supplementary Information, Table S2, Fig. S4 and Fig. S5) were designed from sequence of sex-linked RAD-tags: accession SRP044635 (Wilson et al., 2014). Sex genotyping primers (NA-primers) for NA fish were available at the start of the experiment and thus they could be used not only to sex genotype the offspring but also the parents, which were genetic males (ZZ) crossed to genetic females (ZW) in all NA families; thus, no WW individuals appeared in the NA families. In EKW, sex genotyping primers (EKW-primers) were not available at the start of the experiments, so parents were selected according to phenotypic sex only, as with the AB strain. The version of sar4 in AB does not contain detectably different male vs. female alleles, so sex genotyping was not possible in AB fish. Raw data of phenotypic sex and genotypes were recorded, and then neomales were identified as a mismatch of genotypic and phenotypic sex (Data S1).
2.5. Histomorphometric analysis
To examine differences in the proportion of section surface area of spermatogenic cysts in the gonads, we selected AB and NA samples from fish exposed to either 28 °C or 36 °C from different families. In total, 53 fish were analyzed histologically. Testes from control and experimental groups were dissected out, and immediately fixed in Bouin’s fixative for 48 h. Subsequently, testes were dehydrated through a graded series of ethanol and embedded in paraffin, sectioned at 7 μm thickness per slide with a total of 7–8 slides per fish, and stained with hematoxylin & eosin (H&E). Cross section images (40× objective lens) were analyzed through IMAGEJ software (v. 1.8.0.112) (Rasband, 2011; Schneider et al., 2012) with grid plugging (46 × 33 = 1518 intersections or points on the histological field) to count the proportion of section surface area of different germ cell types. For each sample, four histological fields were counted, totaling 6072 intersections per animal. The final value (mean ± SEM) was obtained from the four histological fields of each sample. In testis, we calculated the proportion of section surface area of spermatogenic cysts containing different germ cell types: type A undifferentiated (Aund), type A differentiated spermatogonia (Adiff), type B spermatogonia (B), spermatocytes (Spc), spermatids (Spd), and spermatozoa (Spz). For the quantification of relative number of spermatozoa, histological images (40× objective lens) were aligned with the IMAGEJ optimized parameters (magnification, 8-bit image type). In this analysis, the background subtraction, threshold adjustment, and watershed separation of particles resulted in a black and white image of the highlighted spermatozoa that were used to count the relative number of spermatozoa in the control and treatment groups. The effects of elevated temperature on ovarian morphology in adult AB and NA zebrafish to 28 °C or 36 °C during sex differentiation were analyzed. The ovaries were dissected out and sampled following the methodology described for males. Using histological images (20× objective lens), we calculated the percent of different germ cell developmental stages in ovaries: primary growth (PG) oocytes, cortical alveolar (CA) oocytes, vitellogenic (Vtg) oocytes, postovulatory follicles (POFs), and atretic (A) oocytes. The proportion of each germ cell type was separately calculated for males and females. In males the relative number of spermatozoa was also estimated (Data S2). Further details of sampling protocols and histomorphmetric analysis are described in the Supplementary Information.
2.6. Statistical analyses
To calculate survival, the percent of fish alive at the end of each tested period were arcsine transformed and subjected to one-way ANOVA tests followed by a Tukey’s test to compare survival rates among three strains. The Student’s t-test was used to compare the effects of temperature in survival in each strain. Differences in sex ratio between temperature treatments, in genotypic frequencies and in neomale frequencies were assessed with the Fisher’s exact test with Yates’ correction when necessary. Because it was possible to obtain sex genotypes for NA and EKW strains but not for AB, we performed two separate Generalized Linear Mixed Model (GLMM-1 and GLMM-2) analyses with binomial distribution using the “lme4” package (v. 1.1–2.1) (Bates et al., 2015). In both analyses, temperature (continuous) and strain (categorical) were considered fixed effect factors; family and tank replicates were introduced as random effect factors, and the response variable was the proportion of males. GLMM-1 included the three strains (AB, NA and EKW) but no genotypic information, whereas GLMM-2 included only the NA and EKW strains but included sex genotype as a fixed categorical effect with five levels: W1W1, W1W2, ZW, ZW1 and ZW2. Individuals with the ZZ genotype were not included because they always develop as males regardless of temperature and W2W2 individuals were not found. In GLMM-1 and GLMM-2 analyses, fixed effects were introduced without an interaction term because the likelihood-ratio test showed no significant differences. To compare the proportion of germ cell types and the relative number of spermatozoa between treatments, the Student’s t-test was applied for each strain and group separately. Statistical analyses were carried out using R software (v. 3.0.2) (Team, 2013). Significant results were considered when P < 0.05. All graphs were created using the “ggplot2” package (v. 3.1.0) (Wickham et al., 2016). Further details on the statistical analyses performed are described in Supplementary Information.
3. Results
3.1. Survival before, during and after thermal treatment
To test whether any treatments increased lethality, we counted the number of animals alive at various stages of the experiment. From 4 to 18 dpf, all fish were reared at 28 °C and survival in the AB, NA and EKW strains was 69.7 ± 7.0%, 93.9 ± 3.2% and 88.6 ± 2.0% (mean ± SEM), respectively. Survival was significantly lower in AB than in NA and EKW (one-way ANOVA, F = 11.16, P < 0.001). Between 18 and 34 dpf, when half of the fish for each family were exposed to elevated temperature, survival at 28 °C and 36 °C in AB was 98.9 ± 1.6% and 93.0 ± 3.6%; in EKW, 98.5 ± 2.1% and 99.3 ± 0.9%, respectively; whereas in NA, 97.7 ± 0.7% at 28 °C and 97.6 ± 0.8% at 36 °C in one experiment, and 98.9 ± 0.8% at 28 °C and 100% at 34 °C in the other. Importantly, in all three strains, differences in survival between temperature treatments were not significant, indicating that elevated temperature did not cause mortality. Survival was >99% between 34 and 90 dpf for all strains (Table S1). Taken together, these results indicate that sex ratio results (see below) were not due to sex-specific or strain-specific differences in survival related to temperature.
3.2. Sex ratio response to temperature
For each of the three strains, different families had different sex ratios at 28 °C, suggesting both environmental and genetic components (Table S1). The environmental effect was unmistakable because the proportion of males increased with temperature in all but one of the twelve families.
For AB at 28 °C, the proportion of males varied over the three families, but at 36 °C the percent of males increased significantly (P < 0.001) in two of the three families (Fig. 1A). Summing the three AB families together gave an overall sex ratio increase from 49.5% males at 28 °C to 85% at 36 °C (χ2 = 35.4; P < 0.001) (Fig. S1A). Although the three EKW families showed different proportions of males at 28 °C, all families were significantly masculinized at 36 °C (Fig. 1B). Thus, the mean sex ratio was also significantly increased by temperature in EKW fish (χ2 = 35.4; P < 0.001) (Fig. S1B). In the NA strain, the sex ratio was male-skewed even at 28 °C, with less inter-family variation than AB and EKW strains (Fig. 1C). In contrast to EKW and AB, however, in the three NA families exposed to 36 °C, all individuals, surprisingly, developed as males (Fig. 1C). To attempt to find an intermediate rate of masculinization in NA, we reduced the temperature from 36 °C to 34 °C, but only family NA#5, which had the lowest proportion of males at 28 °C, had a significant (χ2 = 7.72; P < 0.01) increase in the proportion of males at 34 °C (Fig. 1C). Thus, masculinization was temperature-dependent for all strains, reaching 100% at 36 °C for NA (Fig. S2) and, unexpectedly, elevated temperature masculinized NA, to a greater extent than the AB strain.
Fig. 1. Sex ratio response to temperature in the AB, EKW and NA strains.

Percent male for the 28 vs. 36 °C comparison from three independent families each of A) AB strain (AB#1, solid square: n♂/♀ = 31/26 vs. 33/5; AB#2, solid circle: n♂/♀ = 9/23 vs. 31/10 and AB#3, solid triangle: n♂/♀ = 17/15 vs. 34/3 fish). B) EKW strain (EKW#1, solid square: n♂/♀ = 16/38 vs. 40/13; EKW#2, solid circle: n♂/♀ = 20/26 vs. 53/1 and EKW#3, solid triangle: n♂/♀ = 39/16 vs. 47/4 fish. C) Percent male from six independent NA families: three for the 28–34 °C comparison (NAD#4 open square: n♂/♀ = 45/13 vs. 46/14; NAD#5 solid triangle: n♂/♀ = 40/16 vs. 59/1 and NAD#6 open triangle: n♂/♀ = 51/6 vs. 54/4 fish) and three for the 28–36 °C comparison (NAD#1, open circle: n♂/♀ = 38/16 vs. 48/0; NAD#2, solid square: n♂/♀ = 48/9 vs. 57/0 and NAD#3, solid circle: n♂/♀ = 37/17 vs. 48/0 fish). A Fisher’s exact test was applied individually for each family comparing phenotypic proportions of males and females between treatments. In all strains, each family was split into three tanks per temperature (technical replicate). Solid and open points are used only for improved clarity due to overlapping symbols. Abbreviations: * = P < 0.05; ** = P < 0.01; *** = P < 0.001; N.S. = not significant.
To parse out the effects of temperature and strain, we performed Generalized Linear Mixed Model (GLMM) analyses. GLMM-1 considered the three strains but no genotypic information and showed that temperature had a significant effect on the masculinization rate in all three strains. Furthermore, NA exhibited a higher rate of masculinization than either EKW or AB (Table 1).
Table 1.
Generalized linear mixed models (GLMM) with binomial errors with logit-link function to test effects of elevated temperature in masculinization in the AB, Nadia and EkkWill populations (GLMM-1) and on each genotype in Nadia and EkkWill strains (GLMM-2). Temperature, strain and genotype were set as fixed factors, whereas family and replicate as random factors.
| GLMM | Fixed effects | Coefficient | S. E.a | Z-valueb | Pr(>|Z|)c |
|---|---|---|---|---|---|
| GLMM-1 | Intercept | −7.26 | 0.73 | −10.00 | 2E−16 |
| Temperature | 0.25 | 0.02 | 11.63 | 2E−16 | |
| Strain: | |||||
| EkkWill | 0.11 | 0.20 | 0.52 | 0.6 | |
| Nadia | 1.39 | 0.22 | 6.44 | 1.23E−10 | |
| GLMM-2 | Intercept | −10.10 | 1.13 | −8.93 | <2E−16 |
| Temperature | 0.31 | 0.03 | 10.35 | <2E−16 | |
| Strain: | |||||
| Nadia | 1.38 | 0.53 | 2.35 | 0.02 | |
| Genotype: | |||||
| ZW1 | 1.44 | 0.6 | 2.39 | 0.02 | |
| ZW2 | 0.13 | 0.66 | 0.2 | 0.84 | |
| W1W2 | 0.46 | 0.66 | 0.69 | 0.49 |
GLMM-1. Temperature 28 °C and AB strain set as references (intercept). Number of observations: 1218; family: 12; and tank: 3. Intercepts of the random effects of the variance and standard deviation for family and tank are 0.11; 0.33 and 6.53 E-10; 2.569 E-5, respectively. GLMM-2. Temperature 28 °C, EkkWill strain and W1W1 genotype set as reference (intercept). Number of observations: 616; family: 6; and tank: 3. Intercepts of the random effects of the variance and standard deviation for family and tank are 0.26; 0.50 and 4.68 E-10; 2.15 E-5, respectively.
Notes:
= Standard Error of parameter estimate,
= Z-value estimate to standard error ratio,
= Pr (>|Z|) statistic for Z-value.
3.3. Validation of morphological and histological sex and identification of possible ovotestes
To validate the use of gonad morphology observed in a dissecting microscope to assign sex phenotype, we used histological sections to verify the gonadal sex of five males and five females for each of the three strains (30 fish). Morphological and histological gonadal sex was confirmed in all tested individuals for all three strains; thus, few errors were made in assigning phenotypic sex. To determine whether 90 dpf ZW or WW neomales formed testis like ZZ males or if, alternatively, neomale testes were in some way different, for example, retaining some oocytes, we genotyped individuals for chromosomal sex for NA and EKW fish. We examined 15–20 gonad-containing sections per fish from four fish ZW phenotypic females, four ZW neomales, four WW phenotypic females, and four WW neomales. Results revealed no ovotestes in any examined fish. We concluded that by 90 dpf phenotypic males containing at least one W chromosome exhibited complete sex reversal.
3.4. Genotypic frequencies and neomales in the NA strain
We wondered whether at least some of the male bias at the higher temperature could have occurred due to selective death of ZW females. Because only genotypic ZZ males and ZW females were used as parents, as verified by PCR genotyping (NA-primers), ZZ and ZW genotypes were expected at a 1:1 ratio; this result indeed was found at all three temperatures (Fig. S1C). Thus, observed phenotypic sex ratio distortions were not due to genotype-specific differential mortality due to temperature. Neomales increased significantly with temperature, ranging for NA from 28.6% in the 28 °C control to 42.7% at 34 °C and 53.6% at 36 °C (Fig. S1C).
3.5. Different EKW genotypes showed different rates of masculinization at elevated temperature
Analysis of temperature effects was more convoluted in the EKW strain because sex-genotyping EKW-primers became available only after EKW crosses were set up; thus, genotypes of EKW parents were initially unknown. Using primer pairs EKW-P1 and EKW-P2 together identified the EKW Z chromosome and distinguished two allelically different W chromosomes (i.e., W1 and W2). Further, based on the specific pattern of bands on the gel, they distinguished all possible genotypic combinations and their proportions in each cross. From genotypic frequencies found in the offspring of each family (Fig. S6), we could impute parental genotypes (Table S3). Unexpectedly, in all three EKW families, male parents were neomales. In family EKW#1, offspring were ZW2 and W1W2 in approximately a 1:1 ratio, both at 28 °C (31:23) and at 36 °C (27:26) (P > 0.05) as expected for parental genotypes of ZW1 female x W2W2 neomale or W2W2 female x ZW1 neomale (Table S3 and Fig. S6A). In family EKW#2, offspring frequencies were ZZ, ZW1 and W1W1 in a ~1:2:1 Mendelian ratio at 28 °C (8:26:12) and at 36 °C (14:30:10) (P > 0.05) as expected for parental genotypes of ZW1 male x ZW1 female (Table S3 and Fig. S6B). Finally, in family EKW#3, the genotypic frequencies of offspring were ZZ, ZW1, ZW2 and W1W2 in a ~1:1:1:1 Mendelian ratio, both at 28 °C (18:11:12:14) and at 36 °C (9:11:14:17) (P > 0.05), so the parental genotypes were ZW1 female x ZW2 neomale or ZW2 female x ZW1 neomale (Table S3 and Fig. S6C). None of the EKW families showed significant differences in genotypic frequencies between the 28 °C and 36 °C groups, again indicating no differential mortality of any genotype due to temperature.
To check for temperature-induced sex reversal in EKW, we quantified the number of neomales developing from the ZW1, ZW2, W1W1 and W1W2 genotypes for each family and temperature. ZZ individuals were males at all temperatures and were never sex-reversed to become phenotypic females. In family EKW#1, ZW2 and W1W2 genotypes had a significant increase of neomales (P < 0.001 for both) from 25.8% to 34.7% at 28 °C to 70.3% and 80.7% at 36 °C, respectively. In family EKW#2, ZW1 and W1W1 genotypes had a significant increase of neomales (P < 0.001 for both) from 43.3% to 8.3% at 28 °C to 96.6% and 100% at 36 °C, respectively. Finally, in family EKW#3, the ZW1, ZW2 and W1W2 genotypes had 81.8%, 41.6% and 50.0% neomales at 28 °C, and 100%, 92.8% and 82.4% at 36 °C, with a significant increase only in the ZW2 genotype (P < 0.05) (Fig. S7A). We compared all ZW to all WW fish at 28 °C vs. 36 °C ignoring both family origin and W allele. Results showed that the proportion of neomales was consistently and significantly higher at elevated temperature (P < 0.001) (Fig. S8). Thus, elevated temperature increased the number of neomales in all W-containing genotypes.
To evaluate the effect of temperature on sex determination, we calculated masculinization induced by elevated temperature above that occurring when fish were raised under control conditions. In each family and each genotype, we took neomale percent at 36 °C and subtracted it from that at 28 °C. Then, we averaged the increase of neomales due only to elevated temperature for each genotype among the different families. Results showed that for the ZW1 genotype, which was present in EKW#2 and EKW#3 families, the increase was 35.7 ± 17.7% (mean ± SD). For the ZW2 and W1W2 genotypes, which were present in both EKW#1 and EKW#3 families, the average increase was 47.9 ± 3.3% and 39.2 ± 6.8%, respectively. For the W1W1 genotype, which was present only in family EKW#2, the increase in neomales was 91.2% (Fig. S7B).
3.6. Reaction norms
Reaction norms for masculinization of different sexual phenotypes as a function of environmental temperature are shown in Fig. 2. For ZZ fish of both NA and EKW strains, temperature had no effect on sex phenotype – they were always males (Fig. 2A and B). For ZW and WW fish of both strains, the rate of masculinization was proportional to temperature (Fig. 2A and B). EKW had two genotypically distinct W chromosomes. At both the control and elevated temperatures, ZW1 fish were more masculinized than ZW2 fish, suggesting that W2 might have a stronger feminizing effect than W1. The W1W2 genotype was also better at resisting the effects of high temperature than was the W1W1 genotype, suggesting that the two EKW-W chromosomes are not only genotypically distinguishable, but also functionally distinct. Note, however, that at the control temperature, W1W1 fish were more resistant to masculinization than were W1W2 fish, as if the heterozygous genotypes experienced more sex-reversing stress than the homozygous W1W1 genotype. These observations were confirmed by a second GLMM analysis (GLMM-2), which included only the NA and EKW strains and sex genotype as a fixed categorical effect with five levels: W1W1, W1W2, ZW, ZW1 and ZW2 (Table 1). We then considered results without distinguishing the two EKW W chromosomes from each other. At the control temperature, from a baseline of 25% masculinization, the substitution of one Z for one W (e. g., ZZ vs. ZW) doubled the masculinization rate to about 50% and replacing the remaining W with a Z (ZW vs. WW) doubled the masculinization rate again to 100% (Fig. 2C). This finding suggests antagonisms between the Z and W or that the W is only partially ‘dominant’ over the Z. Notice that in EKW strain we did not test the 34 °C temperature (34 °C temperature was designed for NA to get at least some females at elevated temperatures). For EKW, however, from the reaction norms plotted, it is not clear whether these differences would be significant. We speculated that at 34 °C, both genotypes would have a similar masculinization rate, as is shown at 36 °C. At the elevated temperature, however, the ZW and WW genotypes had the same high rate of masculinization (Fig. 2C), suggesting that high temperature cancels out the feminizing effect of the extra W or that the masculinizing effect of the Z is less effective at high temperature.
Fig. 2. Reaction norms of the masculinization rate as a function of genotype and environmental temperature.

A) ZZ and ZW genotypes of NA. B) ZZ, ZW1, ZW2, W1W2, and W1W1 genotypes of EKW strain. C) EKW without distinguishing the two W chromosomes. Data shown as mean ± SD of all families tested within each strain.
3.7. Histomorphometric changes in gonads due to elevated temperature
To determine if elevated temperature not only caused sex reversal but also led to abnormal gonad development, we examined histological sections of the gonads of 90 dpf fish.
Considering males, histomorphometric analysis showed that the testis of AB and NA males adults had similar proportions of germ cells at different stages of development regardless of temperature (Fig. 3A and Fig. 4), showing that the two strains appear to have similar spermatogenic dynamics. In testes of NA neomales, however, the proportion of the gonad containing type B spermatogonia (B), spermatocytes (Spc), and spermatids (Spd) was significantly lower in 36 °C fish compared to 28 °C fish, whereas the proportion of the gonad containing spermatozoa (Spz) was significantly higher in heated fish (Figs. 3A and 4). The Spz number per field in NA neomales was significantly lower (P < 0.05) than that in NA males at 28 °C but was significantly higher (P < 0.001) in NA neomales at 36 °C when compared to NA neomales at 28 °C (Figs. 3B and 4). Thus, NA neomales had fewer Spz than regular NA males at the control temperature but at the high temperature had more Spz than NA neomales at 28 °C.
Fig. 3. Effects of elevated temperature on testis and ovary morphology in adult AB and NA zebrafish exposed to 28°C and 36°C during sex differentiation.

A) Percent of different germ cell developmental stages in testes of AB males (n28° C = 3, n36° C = 5), NAZZ males (n28° C = 7, n36° C = 7) and NAZW neomales (n28° C = 7, n36° C = 8). AStudent’s t-test was used to compare the number of each germ cell type between temperature treatments. Abbreviations: type A undifferentiated spermatogonia (Aund), type A differentiated spermatogonia (Adiff), type B spermatogonia (B), spermatocytes (Spc), spermatids (Spd), and spermatozoa (Spz). B) Spermatozoa number per field for AB (n28°C = 3, n36° C = 5), NAZZ males (n28° C = 7, n36° C = 7) and NAZW neomales (n28° C = 7, n36° C = 8). A Student’s t-test was used to compare the number of spermatozoa between different groups. Boxes include values between the lower and upper quartiles, the upper whisker = min (max(x), Q3 + 1.5 *IQR), the lower whisker = max (min(x), Q1 – 1.5 * IQR), where IQR = third quartile (Q3) – first quartile (Q1). The line inside the boxplots indicates the median. C) Percent of different germ cell developmental stages in ovaries of AB (n28° C = 7, n36° C = 4), and NAZW females (n28° C = 6). Note: NAZW females at 36 °C were not available due to 100% masculinization in all families. A Student’s t-test was used to compare the number of each germ cell type between the two temperatures. Abbreviations: primary growth (PG) oocytes, cortical alveolar (CA) oocytes, vitellogenic (Vtg) oocytes, postovulatory follicles (POFs), and atretic (A) oocytes. Data shown as mean ± SEM. * = P < 0.05; ** = P < 0.01; *** = P < 0.001; N.S. = not significant. For sample size in each group see Table S4. For stage descriptions see Supplementary Information and Fig. 4. For detailed information of zebrafish germ cells (Leal et al., 2009).
Fig. 4.
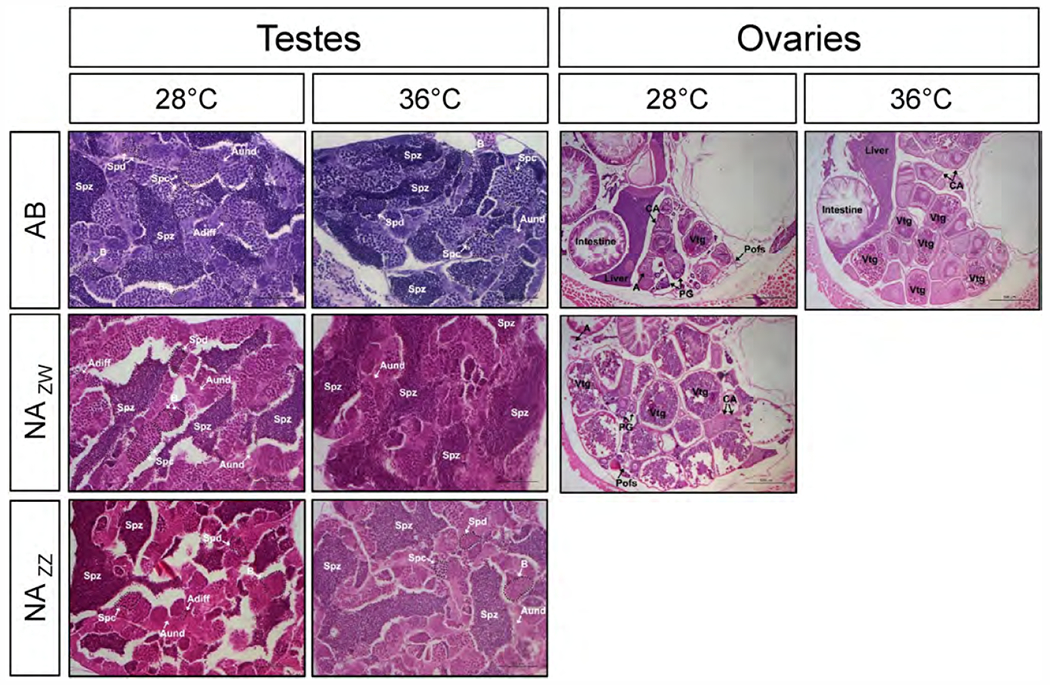
Histological sections of adult zebrafish gonads in AB and Nadia strains. Testes of AB males, NAZW neomales and NAZZ males and ovaries of AB and NAZW females exposed to 28 °C (control) and 36 °C (high temperature) during sex differentiation. Abbreviations for males: type A undifferentiated spermatogonia (Aund), differentiated spermatogonia (Adiff), type B spermatogonia (B), spermatocytes (Spc), spermatids (Spd) and spermatozoa (Spz); for females: primary growth oocytes (PG), cortical alveolar oocytes (CA), vitellogenic oocytes (Vtg), postovulatory follicles (Pofs) and atretic oocytes (A). Staining: Hematoxylin and Eosin. Scale bar = 500 μm. Note: high temperature resulted in 100% males in NA, so no NAZW females were available for analysis.
Concerning ovaries, in AB females, histomorphometric analysis showed that the proportion of post-ovulatory follicles was significantly lower at 36 °C than at 28 °C (Figs. 3C and 4). In contrast, differences in primary growth (PG), cortical alveolar (CA) oocytes, vitellogenic (Vtg) and atretic (A) oocytes were not statistically significant for AB females cultured at different temperatures (Figs. 3C and 4). Because none of the ZW fish of the NA strain became females at the elevated temperature, no ovaries were available from this genotype for histomorphometric analyses.
4. Discussion
4.1. Sex ratio response to temperature
To test the hypothesis that zebrafish strains with a major sex-determining locus would resist environmental influences on sex development, we exposed juveniles of two strains with a ZZ/WZ sex chromosome system (NA, EKW) and one strain with a PSD system (AB) to elevated temperature during the thermolabile period. Results showed, surprisingly, that all three strains exhibited significant masculinization, ruling out the hypothesis. Furthermore, both the NA and EKW strains displayed family-dependent sex ratios at the control temperature and a family-dependent sex ratio response to elevated temperatures as previously described in domesticated zebrafish i.e., AB and DDR strains (Hosseini et al., 2019; Ribas et al., 2017). This family-dependent response would be expected from intra-strain variation in genetic modifiers of sex ratios.
4.2. Spontaneous production of neomales
In fish, neomales can occur spontaneously (Nanda et al., 2003; Sissao et al., 2019; Wilson et al., 2014) or can be environmentally induced by a variety of stressors, (Abozaid et al., 2011; Lawrence et al., 2008; Liew et al., 2012; Piferrer and Anastasiadi, 2021; Santos et al., 2017; Shang et al., 2006; Walker-Durchanek, 1980). Neomales can also be induced by treatment with androgens or aromatase inhibitors (Lee et al., 2017; Rahaman et al., 2020; Takatsu et al., 2013), with morpholinos targeting the dead end protein-1 (dnd) gene (Siegfried and Nüsslein-Volhard, 2008), or by knock-out mutations in genes involved in gonadal development (see review Kossack and Draper, 2019). When using AB zebrafish or other species with polygenic sex determination, the frequency of neomales is usually calculated from the increase in the frequency of males at high temperature minus the frequency of males at low temperature because no markers have been available to assign genetic sex (Anastasiadi et al., 2018; Palaiokostas et al., 2015; Ribas et al., 2017). In NA and EKW strain zebrafish, in contrast, sex chromosome-specific primers can identify genotypic sex; hence, we could distinguish between genotypic males and neomales in the phenotypic male population in these strains.
In our experiments, we took care to avoid the masculinizing effects of high density (Ribas et al., 2017a; 2017b). However, multiple environmental factors present in the “standard” laboratory conditions can potentially affect sex ratios. In the NA strain, the offspring of ZZ males mated to ZW females provided Mendelian ratios of ZZ and ZW genotypes. Even at 28 °C, however, the number of ZZ males (mean = 48.1 ± 0.5% of the total population) plus the number of ZW neomales (mean = 28.5 ± 3.5%) explained the highly skewed phenotypic male sex ratios observed in all NA families (mean = 77.0 ± 2.5%). This result indicates that nearly half of the individuals with the ZW genotype developed into males even at 28 °C, considered the optimal temperature for laboratory-adapted strains (Kimmel et al., 1995). Although NA has chromosomal sex determination, there is variation in sex ratio at the control temperature and also a GxE interaction, as reported from different families (Hosseini et al., 2019; Ribas et al., 2017). From our results, the lack of response to elevated temperature in some families (i. e., NA#4 and NA#6) is not due to bad timing since all fish were treated during the thermosensitive period, but rather to differences in genetic background apart from the major chromosomal sex determining locus.
In EKW, sex genotyping primers were not available at the start of the experiments, so crosses were carried out according to phenotypic sex only, as with AB. Thus, the genotypic sex of the EKW parents was initially unknown. After we developed primers to genotype sex in EKW fish, we discovered that EKW families also produced neomales at 28 °C. Genotyping showed, importantly, that the EKW population has two distinct W chromosomes distinguishable by PCR, which provided EKW neomales with several different genotypes (i.e., ZW1, ZW2, W1W1, and W1W2; the W2W2 genotype was not produced in these crosses). Differences in sex ratios among families can then be attributed to the parental genotypes of each individual cross that gave different genotypic frequencies in the offspring. This explanation clarifies differences in the percent of genetic males (ZZ) and neomales (ZW and WW) found among the three EKW families.
The presence of EKW WW neomales provides a model for the idea that domestication of the AB strain resulted in the loss of the Z chromosome, leaving AB with only WW genotypes, some of which become fertile neomales and others of which become females. We therefore cannot discard the possible influences on sex determination from as yet undiscovered or already known loci, such as factors on chromosomes 3, 5, and 16 (Anderson et al., 2012; Sharma et al., 1998). The presence of secondary loci modifying sex ratios in stocks genetically manipulated for use in mutagenesis experiments (AB by gynogenesis and TU by inbreeding), however, does not necessarily exclude the possibility that strains in wild populations possess a ZW/ZZ system. Our results are consistent with the idea that genetic elements in sar4 may be epistatic over other loci involved in sex determination in strains derived from wild populations because in all NA and EKW crosses, 100% of ZZ individuals developed as males. Additional loci with or without environmental interactions might affect the likelihood of sex reversal in fish carrying the W chromosome, and those loci might provide an alternative polygenic mechanism in highly domesticated strains.
Given that stress masculinizes many fish species (Fernandino et al., 2013; Geffroy and Wedekind, 2020; Goikoetxea et al., 2017; Hattori et al., 2020), uncovering the actual underlying mechanism that generates neomales should provide useful insights on sexual development in zebrafish in particular and in poikilothermic vertebrates in general where instances of sex reversal are frequent (Takatsu et al., 2013). In fact, environmentally-induced sex reversals are associated with dosage (i.e., below or above a certain threshold during gonadal development) of sex genes which lead to differentiation toward one sex or the other (Quinn et al., 2007). The regualtion of gene expression of sex genes are associated by epigenetic changes, integrating environmental information into chromatin changes (Piferrer, 2013; Turner, 2009). In fish, environmental cues, as thermal exposure, can determine sex in GSD and ESD species through differential DNA methylation of key sex genes (Navarro-Martín et al., 2011; Piferrer et al., 2019; Shao et al., 2014; Xiong et al., 2020).
In zebrafish, inbreeding can result in masculinization in fully domesticated (i.e., AB, TU, TL) (Lawrence et al., 2008; Monson and Sadler, 2010; Nasiadka and Clark, 2012) and wild zebrafish (Brown et al., 2015). In our study, the NA and EKW broodstock fish had been in captivity in the Institute of Neuroscience from small initial founding populations, thus likely resulting in some inbreeding despite efforts to avoid it (Wilson et al., 2014). Phylogenetic analysis of different zebrafish strains shows that NA and EKW cluster together, perhaps indicating origin from nearly locations in West Bengal (Suurvali et al., 2020). Domesticated strains are also inbred, but they have had decades of selection by fish workers that could have selected for allele frequencies at loci other than the major sar4 sex determining sequence that tend to yield populations containing both sexes.
4.3. Response of different sex chromosome genotypes to thermal stress
The fraction of neomales increased significantly in both NA and EKW populations exposed to elevated temperature. High temperature also induces neomales in medaka (XX/XY) (Sato et al., 2005) and half-smooth tongue sole (ZW/ZZ) (Shao et al., 2014), indicating that even in species with a strong GSD mechanism phenotypic sex can be affected by environmental conditions. In medaka and half-smooth tongue sole, only one female genotype (XX and ZW, respectively) was shown to be masculinized by elevated temperature. In the EKW strain, we identified different SNPs in different W chromosomes. Our study is thus the first to show that four different female genotypes can be masculinized: ZW1, ZW2, W1W1 and W1W2. Furthermore, in NA families, we observed temperature-dependent masculinization, and surprisingly, all ZW fish developed as males at 36 °C but not at 34 °C.
One important finding of this study is that the sex ratio response to elevated temperature in NA and EKW strains resembled the response of the domesticated AB strain, as evidenced by similar sex ratio reaction norms. Aside from the ZZ genotype, which always produced males, the ZW genotype of the NA strain produced both males and females in the offspring, due to the W allele of sar4 without excluding the possibility of additional loci contributing to sex determination (Bradley et al., 2011; Nagabhushana and Mishra, 2016). In contrast, in the EKW strain, we identified genotypes that varied in the dosage of the Z and W chromosome (ZW vs. WW) and in SNP content in the W chromosome (W1 vs. W2). In the EKW strain, a small fraction of fish with ZW and WW genotypes represented heat-resistant chromosomally female individuals that developed as females. The question arises whether some of the allelic combinations of these genotypes have an intrinsic resistance to masculinization by temperature or if the response was by chance. For instance, the W1W1 genotype, which occurred only in the offspring of EKW#2, showed a lower rate of neomales (8.3%) at control conditions when compared to the ZW1 genotype (43.3%), suggesting that a Z allows more heat-induced masculinization than does a W1 when the second homolog is a W1, but only at the normal rearing temperature. This apparent contradiction might be related to homozygosity vs. heterozygosity. At the control temperature, the heterozygotes (W1W2 and ZW1) have some type of incompatibility that is not acting in the homozygous (W1W1), and that incompatibility leads to stress and the high rate of masculinization for the heterozygous genotypes. But at the high temperature, the homozygous genotype (W1W1) for some reason could be more stressful, leading to the high masculinization rate. We did not tested these genotypes at 34 °C. However, based on the reactions norms both genotypes we expected similar masculinization rate as is shown at high temperature. A similar situation was described in the half-smooth tongue sole, with a SNP (A vs. T) located on the Z chromosome (Jiang and Li, 2017). Thus, in sole, while the genotype ZTW was strongly associated with neomales, the genotype ZAW always developed as females (Jiang and Li, 2017).
None of the three EKW families had a genetic male father (ZZ); all, by chance, were ZW neomales. The fact that none of the three males taken at random from our stock of EKW were ZZ males suggests that during its laboratory culture, the EKW strain may be gradually losing its Z chromosome and becoming an all-WW stock, which our results show produces both males and females and which is likely the situation in the AB strain (Postlethwait and Braasch, 2020). Under the hypothesis that the W chromosome has a dominant incompletely penetrant female determining gene or that the Z has a largely recessive anti-female effect, we expected that individuals possessing two W chromosomes (WW) would more strongly resist the masculinizing effects of elevated temperature compared to fish with only one W chromosome (ZW). Our GLMM-2 results clearly showed, however, that EKW fish with the W1W1 or W1W2 genotypes had similar susceptibility to temperature as fish with the ZW genotype. Surprisingly, the ZW1 genotype of EKW (like the ZW genotype of NA) had a tendency to develop as males. Taken together, our results with the EKW strain suggest that for the rate of sex reversal, fish showed genotype-dependent differences at the control temperature but genotype-independent responses to elevated temperature, suggesting that the threshold of the temperature-sensitive labile trait is crossed between 28 °C and 36 °C.
4.4. Histomorphometric analyses reveal temperature-related germ cell proportions
Exposure to elevated temperature during sex differentiation also significantly altered the proportion of different germ cell types of adult NA neomales but not of adult NA males or adult males of the AB strain compared to each strain at the control temperature. High temperature reduced the percentage of type B spermatogonia, spermatocytes and spermatids in the mature testis of both NA males and neomales compared to their respective controls reared at 28 °C, but effects were significant in only neomales. Likewise, in high temperature-exposed NA fish, the proportion of the testes containing spermatozoa was higher in neomales than in males.
In fish and mammals, temperature is considered to be an important modulator of reproduction, including aspects related to testicular function and spermatogenesis (Borg, 1982; Quintana, 2004). The reduction of testicular germ cells by apoptosis after exposure to elevated temperature was observed in Nile tilapia (Alvarenga and França, 2009) and also in mammals including humans (Shaha et al., 2010). The increase in the proportion of testis cross section occupied by sperm in NA neomales exposed to high temperature might be explained as an adaptation or compensatory mechanism that copes with a stressful situation where temperatures might be higher once the fish reached maturity. This phenomenon was described in Gambusia holbrooki males exposed to high temperature, which produced about three times more sperm than those kept in much colder water (Adriaenssens et al., 2012). Nevertheless, a higher proportion of spermatozoa in neomales does not necessarily mean higher reproductive success because in Atlantic salmon (Salmo salar), sperm of XX neomales was not as effective at fertilizing eggs as was sperm of XY males (de Castro Assis et al., 2018). In contrast, in Nile tilapia, temperature did not alter the frequency of spermatocytes and spermatozoa between males and temperature-induced neomales (Sun et al., 2016). One possibility is that mating frequency in neomales is lower when compared to that of normal males resulting in sperm accumulation in the testes and differences in other stages due to feedback mechanisms. Alternatively, the higher proportion of sperm in the testis of NA neomales might help explain the fixation of W chromosomes in laboratory populations if ZW males had greater fitness than ZZ males at elevated temperature, causing W chromosomes to increase in frequency and be more likely to become fixed in small populations.
The ovaries of AB strain zebrafish exposed to elevated temperature contained a non-significant decrease in the percent of oocytes at the cortical alveolar stage, and a significant reduction of postovulatory follicles. Similar results were found also in adult females of Japanese flounder (Paralichthys olivaceus) exposed to cold temperature. Effects on pre-vitellogenic and vitellogenic stages in high temperature-treated Nile tilapia females has also been observed (Sun et al., 2016). In the present study, the effects of temperature on oogenesis were visible (less frequent post-ovulatory follicles with elevated temperature), although macroscopically, the gonads had the same appearance as at the control temperature.
5. Conclusions
We hypothesized that the ZW/ZZ genetic sex determination mechanism in the NA and EKW zebrafish strains should buffer sex-ratio effects of high temperature displayed by highly manipulated strains like AB. In contrast to this prediction, NA and EKW zebrafish, which have a W chromosome that is necessary but not sufficient for making females, had a similar or perhaps even greater susceptibility to the masculinizing effects of elevated temperature during gonadal development than AB, which relies on weak polygenes or the environment for sex determination. NA and EKW also showed genotype-by-environment interactions in sex ratio response, suggesting that genetic factors in addition to sar4 may contribute to phenotypic sex, so the system in these strains currently appears to involve a major sex determination locus with polygenic and environmental modifiers. We hypothesize that: 1) the sex determination system in wild zebrafish in nature involves only (or primarily) the Z and W alleles at the sar4 locus; 2) the W chromosome increases in frequency in laboratory culture due to stress-induced production of neomales; 3) the Z chromosome is easily lost in culture due to reproductively successful ZW males and genetic drift; and 4) that alleles, likely pre-existing, that increase the likelihood of male development may then increase in frequency due to positive selection in the stress of laboratory culture. This scenario might gradually reduce the importance of the major female-determining mechanism on the W, which furthermore would lead to a reduction in the frequency of the Z chromosome. Further research is needed to determine whether ZW or WW individuals that become neomales have genetic modifiers different from those ZW or WW fish that become females. Finally, we recognize as a limitation the fact that our study involves a single species, albeit with different strains with different sex determining mechanisms and thus ideal for answering the question that prompted this study. Nevertheless, in a broader sense, our results show that: 1) strains with a major sex-determining locus may not necessarily be well protected from the effects of elevated temperature, 2) that genotype-by-temperature interactions act on male sex determination, and 3) that elevated temperature can affect gametogenesis with possible functional fitness consequences. Further study can provide access to the molecular genetic basis for neomale production. It can also help better anticipate consequences of global warming on natural fish populations by showing to which extent the sex ratio in a system with strong genetic factors would evolve in response to environmental change.
Supplementary Material
Acknowledgements
We thank Tim Mason, Trevor Enright, John Dowd, and the University of Oregon fish facility staff for their valuable help in setting up these experiments with special care.
Funding
This study was supported by the Spanish Ministry of Science and Innovation grant AGL2016–787107-R “Epimark” to FP and NIH grants R01 GM085318 and 5R35 GM139635 to JHP. With funding from the Spanish government through the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S). AV was supported by Spanish government scholarships (BES-2014-069051) with two short-term stay grants (EEBB-I-18-12947 and EEBB-I-17-12342) performed in the JHP lab. LR was supported by RYC2018-024017-I. RHN and MSR were supported by São Paulo Research Foundation (FAPESP 14/07620–7 and 17/15793–7).
Footnotes
Declaration of competing interest The authors declare no competing interests.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.113549.
References
- Abozaid H, Wessels S, Horstgen-Schwark G, 2011. Effect of rearing temperatures during embryonic development on the phenotypic sex in zebrafish (Danio rerio). Sex. Dev 5 (5), 259–265. 10.1159/000330120. [DOI] [PubMed] [Google Scholar]
- Adriaenssens B, van Damme R, Seebacher F, Wilson RS, 2012. Sex cells in changing environments: can organisms adjust the physiological function of gametes to different temperatures? Global Change Biol. 18 (6), 1797–1803. 10.1111/j.1365-2486.2012.02672.x. [DOI] [Google Scholar]
- Alvarenga E. R. de, França L. R. de, 2009. Effects of different temperatures on testis structure and function, with emphasis on somatic cells, in sexually mature Nile Tilapias (Oreochromis niloticus). Biol. Reprod 80 (3), 537–544. 10.1095/biolreprod.108.072827. [DOI] [PubMed] [Google Scholar]
- Anastasiadi D, Vandeputte M, Sanchez-Baizan N, Allal F, Piferrer F, 2018. Dynamic epimarks in sex-related genes predict gonad phenotype in the European sea bass, a fish with mixed genetic and environmental sex determination. Epigenetics 13 (9), 988–1011. 10.1080/15592294.2018.1529504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Rodríguez-Marí A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH, 2012. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One 7 (7), e40701. 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaza MS, Dhraïef MN, Kraïem MM, 2008. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Therm. Biol 33 (2), 98–105. 10.1016/j.jtherbio.2007.05.007. [DOI] [Google Scholar]
- Baroiller J-F, D’Cotta H, 2016. The reversible sex of gonochoristic fish: insights and consequences. Sex. Dev 10 (5–6), 242–266. 10.1159/000452362. [DOI] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J. Stat. Software 67 (1), 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bezault E, Clota F, Derivaz M, Chevassus B, Baroiller J-F, 2007. Sex determination and temperature-induced sex differentiation in three natural populations of Nile tilapia (Oreochromis niloticus) adapted to extreme temperature conditions. Aquaculture 272, S3–S16. 10.1016/j.aquaculture.2007.07.227. [DOI] [Google Scholar]
- Borg B, 1982. Seasonal effects of photoperiod and temperature on spermatogenesis and male secondary sexual characters in the three-spined stickleback, Gasterosteus aculeatus L. Can. J. Zool 60 (12), 3377–3386. 10.1139/z82-427. [DOI] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JR, 2011. An SNP-based linkage map for zebrafish reveals sex determination loci. Genet. 1 (1), 3–9. 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AR, Owen SF, Peters J, Zhang Y, Soffker M, Paull GC, Hosken DJ, Wahab MA, Tyler CR, 2015. Climate change and pollution speed declines in 10.1073/pnas.1416269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ, 1985. Sex determining mechanisms: an evolutionary perspective. Experientia 41 (10), 1285–1296. 10.1007/BF01952071. [DOI] [PubMed] [Google Scholar]
- Capel B, 2017. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev. Genet 18 (11), 675–689. 10.1038/nrg.2017.60. [DOI] [PubMed] [Google Scholar]
- Castelli MA, Whiteley SL, Georges A, Holleley CE, 2020. Cellular calcium and redox regulation: the mediator of vertebrate environmental sex determination? Biol. Rev 95 (3), 680–695. 10.1111/brv.12582. [DOI] [PubMed] [Google Scholar]
- Chen W, Ge W, 2013. Gonad differentiation and puberty onset in the zebrafish: evidence for the dependence of puberty onset on body growth but not age in females. Mol. Reprod. Dev 80 (5), 384–392. 10.1002/mrd.22172. [DOI] [PubMed] [Google Scholar]
- Consuegra S, Rodríguez Lopez CM, 2016. Epigenetic-induced alterations in sex-ratios in response to climate change: an epigenetic trap? Bioessays 38 (10), 950–958. 10.1002/bies.201600058. [DOI] [PubMed] [Google Scholar]
- de Castro Assis LH, de Nobrega RH, Gomez-Gonzalez NE, Bogerd J, Schulz RW, 2018. Estrogen-induced inhibition of spermatogenesis in zebrafish is largely reversed by androgen. J. Mol. Endocrinol 60 (4), 273–284. 10.1530/JME-17-0177. [DOI] [PubMed] [Google Scholar]
- Fernandino JI, Hattori RS, Moreno Acosta OD, Strüssmann CA, Somoza GM, 2013. Environmental stress-induced testis differentiation: androgen as a by-product of cortisol inactivation. Gen. Comp. Endocrinol 192, 36–44. 10.1016/j.ygcen.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Geffroy B, Besson M, Sanchez-Baizan N, Clota F, Goikoetxea A, Sadoul B, Ruelle F, Blanc M-O, Parrinello H, Hermet S, Blondeau-Bidet E, Pratlong M, Piferrer F, Vandeputte M, Allal F, 2021. Unraveling the genotype by environment interaction in a thermosensitive fish with a polygenic sex determination system. Proc. Natl. Acad. Sci. Unit. States Am 118 (50), e2112660118 10.1073/pnas.2112660118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffroy B, Wedekind C, 2020. Effects of global warming on sex ratios in fishes. J. Fish. Biol 97 (3), 596–606. 10.1111/jfb.14429. [DOI] [PubMed] [Google Scholar]
- Goikoetxea A, Todd EV, Gemmell NJ, 2017. Stress and sex: does cortisol mediate sex change in fish? Reproduction 154 (6), R149–R160. 10.1530/REP-17-0408. [DOI] [PubMed] [Google Scholar]
- Hattori RS, Castañeda-Cortes DC, Arias Padilla LF, Strobl-Mazzulla PH, Fernandino JI, 2020. Activation of stress response axis as a key process in environment-induced sex plasticity in fish. Cell. Mol. Life Sci 77, 4223–4236. 10.1007/s00018-020-03532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays GC, Mazaris AD, Schofield G, Laloe J-O, 2017. Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc. Biol. Sci 284 (1848), 20162576. 10.1098/rspb.2016.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S, Brenig B, Tetens J, Sharifi AR, 2019. Phenotypic plasticity induced using high ambient temperature during embryogenesis in domesticated zebrafish, Danio rerio. Reprod. Domest. Anim 54 (3), 435–444. 10.1111/rda.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Li H, 2017. Single locus maintains large variation of sex reversal in half-smooth tongue sole (Cynoglossus semilaevis). Genet. 7 (2), 583–589. 10.1534/g3.116.036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Dev. Dynam 203 (3), 253–310. 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kossack ME, Draper BW, 2019. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top. Dev. Biol 134, 119–149. 10.1016/bs.ctdb.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsevier. Lawrence C, Ebersole JP, Kesseli RV, 2008. Rapid growth and out-crossing promote female development in zebrafish (Danio rerio). Environ. Biol. Fish 81 (2), 239–246. 10.1007/s10641-007-9195-8. [DOI] [Google Scholar]
- Leal MC, Cardoso ER, Nobrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW, 2009. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod 81 (1), 177–187. 10.1095/biolreprod.109.076299. [DOI] [PubMed] [Google Scholar]
- Lee SLJ, Horsfield JA, Black MA, Rutherford K, Fisher A, Gemmell NJ, 2017. Histological and transcriptomic effects of 17α-methyltestosterone on zebrafish gonad development. BMC Genom. 18 (1), 557. 10.1186/s12864-017-3915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy T, Rosen O, Manor R, Dotan S, Azulay D, Abramov A, Sklarz MY, Chalifa-Caspi V, Baruch K, Shechter A, Sagi A, 2019. Production of WW males lacking the masculine Z chromosome and mining the Macrobrachium rosenbergii genome for sex-chromosomes. Sci. Rep 9 (1), 12408. 10.1038/s41598-019-47509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L, 2012. Polygenic sex determination system in zebrafish. PLoS One 7 (4). 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Matsuda C, Hamaguchi S, Sakaizumi M, 1998. Identification of the sex chromosomes of the medaka, Oryzias latipes, by fluorescence in situ hybridization. Cytogenet. Genome Res 82 (3–4), 257–262. 10.1159/000015113. [DOI] [PubMed] [Google Scholar]
- Melbourne BA, Hastings A, 2008. Extinction risk depends strongly on factors contributing to stochasticity. Nature 454 (7200), 100–103. 10.1038/nature06922. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Hattori RS, Strüssmann CA, Yokota M, Yamamoto Y, 2020. Phenotypic/genotypic sex mismatches and temperature-dependent sex determination in a wild population of an Old World atherinid, the cobaltcap silverside Hypoatherina tsurugae. Mol. Ecol 29 (13), 2349–2358. 10.1111/mec.15490. [DOI] [PubMed] [Google Scholar]
- Monson CA, Sadler KC, 2010. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish 7 (2), 189–197. 10.1089/zeb.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushana A, Mishra RK, 2016. Finding clues to the riddle of sex determination in zebrafish. J. Biosci 41 (1), 145–155. 10.1007/s12038-016-9593-1. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, 2005. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol. Biochem 31 (2–3), 105–109. 10.1007/s10695-006-7590-2. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Chakraborty T, Paul-Prasanth B, Ohta K, Nakamura M, 2021. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev 101 (3), 1237–1308. 10.1152/physrev.00044.2019. [DOI] [PubMed] [Google Scholar]
- Nakamura M, 2009. Sex determination in amphibians. Semin. Cell Dev. Biol 20 (3), 271–282. 10.1016/j.semcdb.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Nanda I, Hornung U, Kondo M, Schmid M, Schartl M, 2003. Common spontaneous sex-reversed XX males of the medaka Oryzias latipes. Genetics 163 (1), 245–251. 10.1093/genetics/163.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiadka A, Clark MD, 2012. Zebrafish breeding in the laboratory environment. ILAR J. 53 (2), 161–168. 10.1093/ilar.53.2.161. [DOI] [PubMed] [Google Scholar]
- Navarro-Martín L, Viñas J, Ribas L, Díaz N, Gutierrez A, Di Croce L, Piferrer F, 2011. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 7 (12) 10.1371/journal.pgen.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina-Alvarez N, Piferrer F, 2008. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One 3 (7). 10.1371/journal.pone.0002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaiokostas C, Bekaert M, Taggart JB, Gharbi K, McAndrew BJ, Chatain B, Penman DJ, Vandeputte M, 2015. A new SNP-based vision of the genetics of sex determination in European sea bass (Dicentrarchus labrax). Genet. Sel. Evol 47 (1), 68. 10.1186/s12711-015-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandian TJ, Sheela SG, 1995. Hormonal induction of sex reversal in fish. Aquaculture 138 (1–4), 1–22. 10.1016/0044-8486(95)01075-0. [DOI] [Google Scholar]
- Piferrer F, 2013. Epigenetics of sex determination and gonadogenesis. Dev. Dynam 242 (4), 360–370. 10.1002/dvdy.23924. [DOI] [PubMed] [Google Scholar]
- Piferrer F, 2016. Altered sex ratios in response to climate change-Who will fall into the (epigenetic) trap? (Comment on DOI 10.1002/bies.201600058). Bioessays 38 (10), 939. , 939. [DOI] [PubMed] [Google Scholar]
- Piferrer F, Anastasiadi D, 2021. Do the offspring of sex reversals have higher sensitivity to environmental perturbations? Sex. Dev. 1–14. 10.1159/000515192. [DOI] [PubMed] [Google Scholar]
- Piferrer F, Anastasiadi D, Valdivieso A, Sanchez-Baizan N, Moraleda-Prados J, Ribas L, 2019. The model of the conserved epigenetic regulation of sex. Front. Genet 10 10.3389/fgene.2019.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait JH, Braasch I, 2020. Zebrafish genetics in the Zebrafish in Biomedical Research. Elsevier, pp. 25–39. 10.1016/B978-0-12-812431-4.00003-8. [DOI] [Google Scholar]
- Quinn AE, Georges A, Sarre SD, Guarino F, Ezaz T, Graves JAM, 2007. Temperature sex reversal implies sex gene dosage in a reptile. Science 316 (5823), 411. 10.1126/science.1135925, 411. [DOI] [PubMed] [Google Scholar]
- Quintana L, 2004. Temperature induces gonadal maturation and affects electrophysiological sexual maturity indicators in Brachyhypopomus pinnicaudatus from a temperate climate. J. Exp. Biol 207 (11), 1843–1853. 10.1242/jeb.00954. [DOI] [PubMed] [Google Scholar]
- Rahaman Md M., Kumagai R, Tokumoto T, 2020. Rapid induction of female-to-male sex change in adult zebrafish by injection of an aromatase inhibitor. Zebrafish 2020, 1864. 10.1089/zeb.2020.1864. [DOI] [PubMed] [Google Scholar]
- Rasband WS, 2011. Imagej US National Institutes of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij/. [Google Scholar]
- Ribas L, Liew WC, Díaz N, Sreenivasan R, Orban L, Piferrer F, 2017. Heat-induced masculinization in domesticated zebrafish is family-specific and yields a set of different gonadal transcriptomes. Proc. Natl. Acad. Sci. U.S.A 114 (6), E941–E950. 10.1073/pnas.1609411114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas L, Valdivieso A, Díaz N, Piferrer F, 2017a. Appropriate rearing density in domesticated zebrafish to avoid masculinization: links with the stress response. J. Exp. Biol 220 (6), 1056–1064. 10.1242/jeb.144980. [DOI] [PubMed] [Google Scholar]
- Ribas L, Valdivieso A, Díaz N, Piferrer F, 2017b. Response to “The importance of controlling genetic variation – remarks on ‘Appropriate rearing density in domesticated zebrafish to avoid masculinization: links with the stress response”. J. Exp. Biol 220 (21), 4079–4080. 10.1242/jeb.167437. [DOI] [PubMed] [Google Scholar]
- Saillant E, Fostier A, Haffray P, Menu B, Thimonier J, Chatain B, 2002. Temperature effects and genotype-temperature interactions on sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool 292 (5), 494–505. 10.1002/jez.10071. [DOI] [PubMed] [Google Scholar]
- Santos D, Luzio A, Coimbra AM, 2017. Zebrafish sex differentiation and gonad development: a review on the impact of environmental factors. Aquat. Toxicol 191, 141–163. 10.1016/j.aquatox.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Sato T, Endo T, Yamahira K, Hamaguchi S, Sakaizumi M, 2005. Induction of female-to-male sex reversal by high temperature treatment in medaka, Oryzias latipes. Zool. Sci 22 (9), 985–988. 10.2108/zsj.22.985. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9 (7), 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaha C, Tripathi R, Mishra DP, 2010. Male germ cell apoptosis: regulation and biology. Phil. Trans. Biol. Sci 365 (1546), 1501–1515. 10.1098/rstb.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang EH, Yu RM, Wu RS, 2006. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio). Environ. Sci. Technol 40 (9), 3118–3122. 10.1021/es052257. [DOI] [PubMed] [Google Scholar]
- Shao C, Li Q, Chen S, Zhang P, Lian J, Hu Q, Sun B, Jin L, Liu S, Wang Z, 2014. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 24 (4), 604–615. 10.1101/gr.162172.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Sharrna O, Tripathi N, 1998. Female heterogamety in Danio rerio (cypriniformes: cyprinidae). Proc. Natl. Acad. Sci. India Sect. B (Biol. Sci.) 68, 123–126. https://eurekamag.com/research/003/140/003140361.php. [Google Scholar]
- Shinomiya A, Otake H, Hamaguchi S, Sakaizumi M, 2010. Inherited XX sex reversal originating from wild medaka populations. Heredity 105 (5), 443–448. 10.1038/hdy.2010.51. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Nüsslein-Volhard C, 2008. Germ line control of female sex determination in zebrafish. Dev. Biol 324 (2), 277–287. 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Sissao R, D’Cotta H, Baroiller J-F, Toguyeni A, 2019. Mismatches between the genetic and phenotypic sex in the wild Kou population of Nile tilapia Oreochromis niloticus. PeerJ 7, e7709. 10.7717/peerj.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L-X, Wang Y-Y, Zhao Y, Wang H, Li N, Ji XS, 2016. Global DNA methylation changes in Nile tilapia gonads during high temperature-induced masculinization. PLoS One 11 (8), e0158483. 10.1371/journal.pone.0158483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurvali J, Whiteley AR, Zheng Y, Gharbi K, Leptin M, Wiehe T, 2020. The laboratory domestication of zebrafish: from diverse populations to inbred substrains. Mol. Biol. Evol 37 (4), 1056–1069. 10.1093/molbev/msz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K, Miyaoku K, Roy SR, Murono Y, Sago T, Itagaki H, Nakamura M, Tokumoto T, 2013. Induction of female-to-male sex change in adult zebrafish by aromatase inhibitor treatment. Sci. Rep 3 (1), 3400. 10.1038/srep03400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC, 2013. R: A Language and Environment for Statistical Computing.https://www.R-project.org/.
- Turner BM, 2009. Epigenetic responses to environmental change and their evolutionary implications. Phil. Trans. Biol. Sci 364 (1534), 3403–3418. 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T, 2002. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol 205 (6), 711–718. 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- Valenzuela N, & Lance V (Eds.). (2004). Temperature-dependent Sex Determination in Vertebrates . Smithsonian Books. [Google Scholar]
- Valenzuela N, Literman R, Neuwald JL, Mizoguchi B, Iverson JB, Riley JL, Litzgus JD, 2019. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep 9 (1), 4254. 10.1038/s41598-019-40597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte M, Dupont-Nivet M, Chavanne H, Chatain B, 2007. A polygenic hypothesis for sex determination in the European sea bass Dicentrarchus labrax. Genetics 176 (2), 1049–1057. 10.1534/genetics.107.072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Durchanek R, 1980. Induction of Germ Line Mutations by Gamma-Irradiation of Zebrafish Embryos. Master’s thesis. Department of Biology, University of Oregon, Eugene, OR. [Google Scholar]
- Wallace M, Badawy GMI, Wallace BMN, 1999. Amphibian sex determination and sex reversal. Cell. Mol. Life Sci 55 (7), 901. 10.1007/s000180050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L, 2007. The timing and extent of «juvenile ovary» phase are highly variable during zebrafish testis differentiation. J. Fish. Biol 70 (sa), 33–44. 10.1111/j.1095-8649.2007.01363.x. [DOI] [Google Scholar]
- Wedekind C (2019). Population consequences of releasing sex-reversed fish: Applications and concerns. En H.-P Wang, F. Piferrer, S.-L. Chen & Z.-G. Shen (Eds.), Sex Control in Aquaculture (pp. 179–188). John Wiley & Sons, Ltd. 10.1002/9781119127291.ch8. [DOI] [Google Scholar]
- Wickham H, Chang W, Wickham MH, 2016. Package ‘ggplot2. Create elegant data visualisations using the grammar of graphics. Version 2 (1), 1–189. [Google Scholar]
- Wilson CA, High SK, McCluskey BM, Amores A, Yan Y, Titus TA, Anderson JL, Batzel P, Carvan MJ, Schartl M, Postlethwait JH, 2014. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics 198 (3), 1291–1308. 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Wang S, Gui J-F, Mei J, 2020. Artificially induced sex-reversal leads to transition from genetic to temperature-dependent sex determination in fish species. Sci. China Life Sci 63 (1), 157–159. 10.1007/s11427-019-1568-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


