ABSTRACT
The rapid manufacturing of vaccines has increased hesitancy toward receiving the COVID-19 vaccines. Clarifying what to expect after vaccination and revealing the possible side effects will lower hesitancy toward receiving the COVID-19 vaccine and increase public awareness. This descriptive cross-sectional survey-based study was conducted in Jordan (August 2021) to collect data on the short-term side effects following the COVID-19 vaccines. An extensive literature review was conducted by the research team to assist in developing the first draft of the survey. The survey was tested for face and content validity and piloted test to improve readability and clarity. The survey was organized into two sections (demographics and perceived COVID-19 vaccines’ side effects). Data were analyzed using the Statistical Package for Social Science (SPSS). A total of 1,044 participants were enrolled in the study. The most received vaccine among the participants was Pfizer-BioNTech (51.1%). The most frequently reported side effects were sore arm at the injection site (84.65%), fatigue (84.48%), discomfort (65.43%), muscles/joint pain (61.38%), drowsiness (58.73%), and headache (58.38%). More side effects were significantly associated with being older (p = 0.046), having an allergy (p = 0.024) or rheumatoid arthritis (p = 0.023), and participants who take NSAIDs regularly (p = 0.029). Short-term side effects of COVID-19 vaccines seem to be mostly local or transient in nature. Older age and certain comorbidities may increase susceptibility to side effects.
KEYWORDS: COVID-19, pandemics, side effects, vaccine, immunization, Jordan
Introduction
The SARS-CoV-2 is a highly contagious virus that affects individuals in various ways. Transmission of SARS-CoV-2 occurs mainly by close contact with infected individuals via their respiratory droplets that can be expelled through coughing, sneezing, and breathing.1 The World Health Organization (WHO) authoritatively stated that the SARS-CoV-2 outbreak is a pandemic owing to its quick spread.2 Worldwide, governments pinned their hopes on the development of COVID-19 vaccines to prevent the spread of the virus. As a result, international alliances and governments urgently organized resources to develop multiple COVID-19 vaccines in the shortest possible time.3 By December 2020, four COVID-19 vaccines were approved by China, Russia, the United States, and the United Kingdom.
At the time of designing this study, there were four types of COVID-19 vaccines available in Jordan (Pfizer-BioNTech, Sinopharm, AstraZeneca, and Sputnik V). The Pfizer-BioNTech COVID-19 vaccine was authorized by the United States Food and Drug Administration in December 2020. It is a nucleic acid-based mRNA vaccine and has an efficacy of 95%, which can be reached after 1 week following the second dose.4,5 The Sinopharm vaccine is China’s first approved COVID-19 vaccine, and it has a 79% efficacy. It is an inactivated vaccine that introduces a dead copy of SARS-CoV-2 into the individual body.6,7 The AstraZeneca vaccine uses a viral vector that is a harmless and weakened virus that holds the genetic code for the SARS-CoV-2 spike protein.8 The Sputnik V vaccine is an adenoviral-based vaccine. It was approved by the Russian Ministry of Health in August 2020 as the world’s first registered combination vector COVID-19 vaccine.9
The COVID-19 vaccines have been successfully developed; however, the public trust in vaccines has arisen as a major challenge toward vaccination. Building public confidence in the vaccines is vital and highly dependent on the ability of governments to explain the benefits of vaccination, and to deliver the vaccines in a safe and effective manner.10 Jordan was among the first countries to adopt vaccination campaigns in January 2021, giving priority to health care providers and the elderly.11 The United Nations International Children’s Emergency Fund UNICEF has provided more than a million syringes in order to support Jordan’s national vaccination campaign. These donations have been handed over to the Ministry of Health (MoH) to be delivered to health centers across Jordan, where COVID-19 vaccines were provided for free and to all nationalities.12 The Jordanian government followed several measures in order to increase trust in COVID-19 vaccines, for example, the national vaccination campaign was carried out by the Jordanian MoH and the National Center for Security and Crises Management.13 Moreover, a youth volunteer work team was launched to combat the virus, that aimed to increase the vaccination rate, raise public awareness, and encourage citizens to register for the novel SARS-CoV-2 vaccine by providing effective and specific public education.14
Providing accurate data regarding COVID-19 vaccines is needed to increase public confidence in vaccines. Such data could be the transparent reporting of post-vaccination side effects. In Jordan, a cross-sectional study was conducted by Dar-Odeh et al. among 409 participants who were vaccinated with one of the following three vaccines: AstraZeneca Vaxzevria (AZ), Pfizer-BioNTeck (PB), and Sinopharm to explore side effects following the first or second dose of COVID-19 vaccine, no side effects were reported by 18% and 31% of the participants after the first and second doses, respectively; moreover, local side effects related to injection site were the most frequently reported side effect, while in terms of the systemic side effects, fatigue was the most frequently reported one.15 Another study was conducted using a validated questionnaire among vaccinated physicians and dentists in Jordan and Saudi Arabia to explore the long-term adverse effects associated with COVID-19 vaccines; among 498 participants, 80 participants reported long-term adverse effects that were mainly fatigue, menstrual disturbances, myalgia, arthralgia, dizziness, and headache. Pfizer-BioNTech, Sinopharm, and AstraZeneca vaccines were the most frequently administered vaccines. No correlation was found between the long-term side effects and gender, age, or medical history.16 In a retrospective cross-sectional study conducted by Almufty et al. among the Iraqi population to reveal side effects after receiving COVID-19 vaccines, the most reported side effects were fatigue, injection site reactions, fever, myalgia, headache, and chills. A statistically significant correlation was found between having side effects after vaccination and being a female, having a previous infection with COVID-19, having comorbidities, and being younger. In terms of severity, the study revealed that most symptoms were mild to moderate.17
Worldwide, governments encouraged populations to report any COVID-19 vaccine side effects. In the United States, vaccinated individuals were reporting the COVID-19 vaccine side effects to the Vaccine Adverse Event Reporting System (a government reporting system).18 In the United Kingdom, the side effects were reported to the Yellow card.19 In Jordan, the MoH advised individuals who received the COVID-19 vaccine to report any side effects through their platform by opening the link included in the SMS that was received upon registration.13 Nevertheless, the government’s main motive for reporting was to guarantee the safety of the vaccinated individual rather than delivering an early warning regarding the vaccine safety or providing any data on whether the vaccine requires an additional investigation.20 Thus, this cross-sectional study aims to collect data on the short-term side effects of the COVID-19 vaccines among the Jordanian population.
Methods
Study design
A descriptive cross-sectional study design (an online survey developed by the research team) was used to collect data on the side effects following receiving the COVID-19 vaccines available in Jordan. This study was web-based that was designed and filled using Google Forms. It was carried out over the period between August 8 and 18, 2021. The survey was written in the Arabic language, and the scientific terms for the symptoms were written and clarified in the public language.
Survey development
An extensive literature review was conducted to develop the first draft of the survey.5,21,22 To ensure face and content validity, the first draft of the survey was evaluated by three experts in the field. They evaluated questions comprehension, relevancy, and word clarity.
As a final point in the survey development, the survey was piloted with a group of 50 participants to improve readability and understandability, as well as to ensure its applicability to the Jordanian population. Internal consistency reliability was tested by cronbach’s alpha coefficient. The research team designed the survey to take less than 5 minutes to be completed.
The final version of the survey was organized into the following two main sections that addressed different topics of interest:
1. Demographic data (11 main quantitative items): gender, age, marital status, living place, nationality, educational level, profession, smoking, diseases/comorbidities, medications used, and previous infection with SARS-CoV-2 before getting vaccinated.
The participants were asked about 17 used medications/drug classes (common analgesics (for example, paracetamol), antihistamines, thyroid hormones, antibiotics, anti-hypertensives, nonsteroidal anti-inflammatory drugs (NSAIDs), anti-diabetics, cholesterol-lowering agents, anti-reflux medications, contraceptives, anti-depressants, anti-asthmatics, anti-coagulants, anti-epileptics, opioid analgesics, corticosteroids, and immunosuppressive agents), and 16 diseases/comorbidities (allergy, thyroid disease, chronic hypertension, bowel diseases, asthma, diabetes mellitus (type one), diabetes mellitus (type two), bone diseases, neurologic diseases, rheumatoid arthritis, cardiac diseases, blood diseases, renal diseases, cancer, hepatologic diseases, and chronic obstructive pulmonary disease).
Each of the previously mentioned medication/comorbidity was defined to the participants, for example, allergy was defined as the body’s reaction to a normally harmless substance such as pollen, and the NSAIDs were defined as regular use of NSAIDs (when these medications are taken more than three times a week for more than 3 months).
2. Side effects following COVID-19 vaccine (11 main quantitative items): the type of the received COVID-19 vaccine, the month in which the COVID-19 vaccine was received, whether side effects were experienced after the first dose, side effects after the first dose, and duration of side effects after the first dose. The last four questions were also asked regarding the second dose. Lastly, participants were requested to choose which dose was accompanied by more severe side effects (such as difficulty in breathing), and if they required hospitalization within 4 weeks after receiving the COVID-19 vaccine.
Study participants and survey implementation
To be eligible, participants were residents in Jordan and received at least the first dose of any COVID-19 vaccines that were available in Jordan.
The research team planned to recruit participants through social media platforms (mainly Facebook and WhatsApp). As a first step in participants’ recruitment, potential participants were asked by the research team (via WhatsApp) if they received a dose of the COVID-19 vaccine, if their answer was “Yes”, the objective of the study was briefly explained to the potential participants, and the link of the online survey was sent to them. Facebook was also used to recruit participants; researchers stated in the post that only vaccinated individuals are required to fill out the survey and a question on the first page about getting the COVID-19 vaccine along with the aim was needed to be answered “Yes” to move to the rest of the survey sections. All participants were able to open a link to view the ethical approval of the study and were informed that participation in this study is voluntary, and that their responses would be kept anonymous. Participants provided informed consent to participate in the research.
The settings were adjusted so that each participant sent only one response. Still, sometimes some participants asked to be allowed to send more than one response, as they were also filling out a survey about their elderly relatives who could not handle the electronic surveys. Responses were carefully monitored by the research members and by a separate peer review team to ensure the quality of the data and the absence of controversy between the responses in different sections. In case a duplication of a survey was suspected by the team (same demographic information), the response to the second survey was removed. When necessary, communication between the researchers and the study participants was established by e-mails.
Sample Size
In order to calculate the sample size for this study, published tools were followed and a convenience sample design was implemented.23–25 Based on the Jordanian population in 2022 (10.1 million) and the number of vaccinated individuals, the sample size was calculated using the Epi Info software, with a 95% confidence level, 50% expected frequency, 5% acceptable margin of error, and design effect of 1.0. A minimum sample size of 384 was calculated.
The study data collection period was decided to be 10 days at the study design stage. This period allowed 1,044 subjects to complete the survey. Such sample size was allowed in the analysis as it increases the generalizability of the results.
Statistical analysis
Following reviewing the responses, data were coded and entered into a customized database using the Statistical Package for the Social Sciences (SPSS), Version 24.0 (IBM Corp., Armonk, New York, USA). Qualitative variables were presented as percentages.
Screening of factors (gender, age, previous infection with COVID-19, smoking, diseases/comorbidities, and using medications) affecting whether the participants experienced side effects after the first dose was carried out using simple logistic regression. Any variable that has a p-value <0.25 (in the simple logistic regression) was entered in the multiple logistic regression analysis to explore the variables that were significantly and independently associated with the side effects after the first dose of the COVID-19 vaccine. Variables were selected after ensuring their independence, where tolerance values >0.2 and Variance Inflation Factor (VIF) values <5 were checked to indicate the absence of multicollinearity between the independent variables in regression analysis. For multiple logistic regression, a variable that has a p-value less than 0.05 was considered statistically significant.
Results
A total of 1,044 individuals participated in this study. More than 80% of the participants were females (n = 847). Most of the participants were 18–39 years old (72.7%), married (61.2%), living in Amman (79.1%), have a Jordanian nationality (93.8%), have a bachelor’s degree (65.7%), employed (53.6%), and nonsmoker (66.7%). The detailed demographic characteristics of the study participants (n = 1,044) are shown in Table 1.
Table 1.
Demographic characteristics of the study sample at baseline (n = 1,044).
| Parameter | n (%) |
|---|---|
Gender, n (%)
|
197 (18.9%) 847 (81.1%) |
Age, n (%)
|
8 (0.8%) 367 (35.2%) 391 (37.5%) 206 (19.7%) 56 (5.4%) 16 (1.5%) |
Marital Status, n (%)
|
639 (61.2%) 359 (34.4%) 38 (3.6%) 8 (0.8%) |
Living place, n (%)
|
826 (79.1%) 218 (20.9%) |
Nationality, n (%)
|
979 (93.8%) 65 (6.2%) |
Educational level, n (%)
|
8 (0.8%) 57 (5.5%) 81 (7.8%) 686 (65.7%) 212 (20.3%) |
Profession, n (%)
|
560 (53.6%) 453 (43.4%) 31 (3.0%) |
Smoker, n (%)
|
320 (30.7%) 696 (66.7%) 28 (2.7%) |
A total of 384 (36.78%) participants reported having at least one disease or comorbidity. As shown in Table 2, allergy was the most reported comorbidity/disease among the participants (n = 193, 18.5%), followed by thyroid disease (n = 76, 7.3%), and chronic hypertension (n = 56, 5.4%). Furthermore, a total of 470 (45.02%) participants reported taking at least one medication at the time of filling in the survey. Analgesics (n = 276, 26.4%), followed by antihistamine (n = 91, 8.7%), were the most frequently reported taken drug classes.
Table 2.
Medications used and diseases/comorbidities among study participants (n = 1,044).
| Medication/Drug Class | n (%) | Disease/Comorbidity | n (%) |
|---|---|---|---|
| Common Analgesics (for example: Paracetamol) | 276 (26.4) | Allergy | 193 (18.5) |
| Antihistamines | 91 (8.7) | Thyroid Disease | 76 (7.3) |
| Thyroid Hormones | 69 (6.6) | Chronic hypertension | 56 (5.4) |
| Antibiotics | 66 (6.3) | Bowel Diseases | 54 (5.2) |
| Anti-hypertensives | 66 (6.3) | Asthma | 40 (3.8) |
| Nonsteroidal anti-inflammatory Drugs (NSAIDs) | 61 (5.8) | Diabetes mellitus—type two | 28 (2.7) |
| Anti-diabetics | 49 (4.7) | Bone Diseases | 28 (2.7) |
| Cholesterol-lowering agents | 45 (4.3) | Neurologic Diseases | 21 (2.0) |
| Anti-reflux medication | 41 (3.9) | Rheumatoid Arthritis | 19 (1.8) |
| Contraceptives | 36 (3.4) | Cardiac Diseases | 17 (1.6) |
| Anti-depressants | 34 (3.3) | Diabetes Mellitus—type one | 16 (1.5) |
| Anti-Asthmatics | 22 (2.1) | Blood Diseases | 14 (1.3) |
| Anti-coagulants | 21 (2.0) | Renal Diseases | 12 (1.1) |
| Anti-epileptics | 11 (1.1) | Cancer | 7 (0.7) |
| Opioid Analgesics | 11 (1.1) | Hepatologic Diseases | 7 (0.7) |
| Corticosteroids | 8 (0.8) | Chronic Obstructive Pulmonary Disease (COPD) | 3 (0.3) |
| Immunosuppressives | 8 (0.8) |
About one-third of the study participants (n = 350, 33.5%) reported that they were previously infected with the SARS-CoV-2 before getting vaccinated, while 597 participants (57.2%) did not get infected before vaccination. However, 9.3% of the participants (n = 97) were not sure.
The most received COVID-19 vaccine among Jordanians was Pfizer-BioNTech (51.1%), followed by Sinopharm (35.2%), AstraZeneca (12.8%), and Sputnik V (1.0%).
Regarding the time of receiving the first dose, the highest percentage (n = 238, 22.8%) was recorded in May 2021, while the lowest percentage was reported in February 2021 (n = 20, 1.9%). Table 3 shows the percentages of vaccinated participants in each month (from January to August 2021) among the study participants.
Table 3.
The percentage of vaccines (first dose) received each month (from January and August 2021) among the study participants (n = 1,044).
| The month | n (%) |
|---|---|
| January 2021 | 25 (2.4) |
| February 2021 | 20 (1.9) |
| March 2021 | 151 (14.5) |
| April 2021 | 203 (19.4) |
| May 2021 | 238 (22.8) |
| June 2021 | 230 (22) |
| July 2021 | 122 (11.7) |
| August 2021 | 55 (5.3) |
More than half of the participants (n = 567, 54.3%) documented that they experienced side effects of the COVID-19 vaccines after the first dose (Figure 1). Table 4 shows the side effects among those participants (n = 567). Participants were asked about 23 side effects, the most frequently reported side effects after receiving the first dose were, sore arm at the injection site (84.65%), fatigue and tiredness (84.48%), discomfort (65.43%), muscles/joint pain (61.38%), drowsiness (58.73%), and headache (58.38%). Regarding the duration of the side effects after the first dose of the COVID-19 vaccine, more than 92% (n = 520) reported that the duration of their side effects was 1 week or less.
Figure 1.
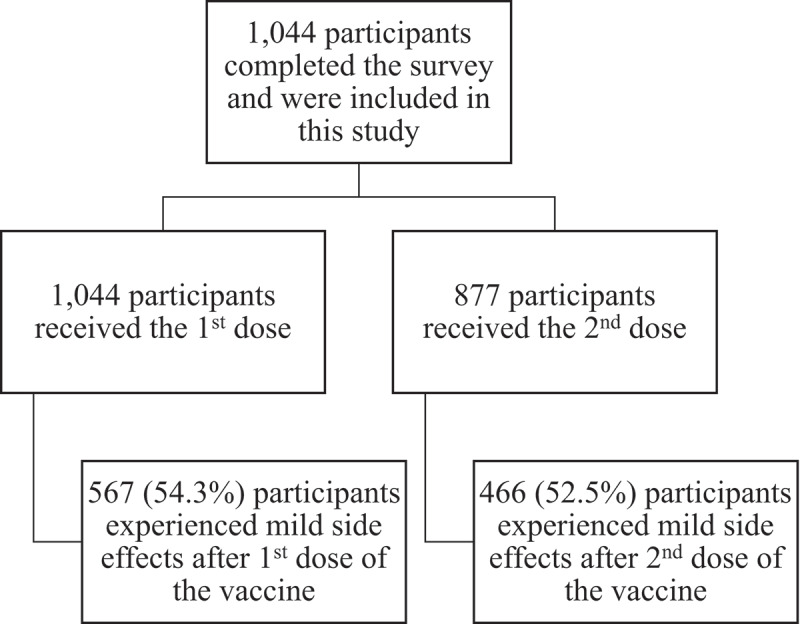
Flow chart of study participants (n = 1,044).
Table 4.
The side effects among the participants after the first (n = 567) and the second dose (n = 466) of COVID-19 vaccine.
| Side Effect | n (%) After the first dose |
n (%) After the second dose |
|---|---|---|
| Sore arm at the injection site | 480 (84.65) | 376 (80.69) |
| Fatigue and tiredness | 479 (84.48) | 374 (80.25) |
| Discomfort | 388 (65.43) | 281 (60.30) |
| Muscles/joint pain | 348 (61.38) | 265 (56.86) |
| Drowsiness | 333 (58.73) | 243 (52.15) |
| Headache | 331 (58.38) | 264 (56.65) |
| High temperature | 261 (46.03) | 175 (37.55) |
| Chills | 252 (44.44) | 178 (38.20) |
| Flu-like symptoms | 200 (35.27) | 145 (31.33) |
| Injection site swelling | 182 (32.10) | 137 (29.40) |
| Redness at the injection site | 142 (25.04) | 110 (23.61) |
| Hair loss | 127 (22.40) | 99 (21.24) |
| Gastrointestinal symptoms (such as nausea, vomiting, and diarrhea) | 115 (20.28) | 86 (18.45) |
| Tachycardia or heart palpitations | 106 (18.70) | 75 (16.09) |
| Chest pressure/pain | 105 (18.52) | 78 (16.73) |
| Shortness of breath | 100 (17.63) | 71 (15.23) |
| Anorexia | 89 (15.70) | 58 (12.45) |
| Runny nose | 80 (14.11) | 64 (13.73) |
| Sore throat | 71 (12.52) | 49 (10.52) |
| Cough | 54 (9.52) | 36 (7.73) |
| Eye pain | 53 (9.35) | 42 (9.01) |
| Loss or change in the sense of taste or smell | 39 (6.88) | 21 (4.51) |
| Lymphadenopathy | 38 (6.70) | 33 (7.01) |
| Number of side effects per participant | 4.50 ± 2.596 | 3.43 ± 4.858 |
Of the study participants (n = 1,044), 877 (85%) participants received the second dose. Regarding the side effects after the second dose; 466 participants out of the 877 (52.5%) documented that they experienced side effects (Figure 1). As shown in Table 4, the most frequently reported side effects after the second dose were, sore arm at the injection site (80.69%), fatigue and tiredness (80.25%), discomfort (60.30%), muscles/joint pain (56.86%), headache (56.65%), and drowsiness (52.15%). More than 85% (n = 397) reported that the duration of their side effects after the second dose was 1 week or less.
In general, the mean number of side effects per participant after the second dose was lower (3.43 ± 4.858) than the first dose (4.50 ± 2.596); and a slightly lower proportion of participants (52.5%) reported COVID-19 vaccine side effects after the second dose compared to the first dose (54.3%).
More than half (51.5%) of the participants (who received both doses) documented that the side effects of the second dose were more severe than the first one, while 17.5% documented that there was no difference between the two doses.
Regarding the severe side effects (difficulty in breathing, swelling of the face and neck, tachycardia, and rash) that required hospitalization within 4 weeks of the vaccination, only 2.5% of the participants documented that they experienced these side effects.
Multiple logistic regression of factors affecting whether the participants experienced side effects after the first dose highlighted that being older (p-value = 0.046), having an allergy (p-value = 0.024), rheumatoid arthritis (p-value = 0.023), and taking NSAIDs regularly (p-value = 0.029) significantly affected the appearance of the side effects after the first dose (Table 5).
Table 5.
Assessment of factors affecting whether the participants (n = 567) experienced side effects after the first dose.
| Parameter | Appearance of side effects after the first dose |
|||
|---|---|---|---|---|
| OR | P-value# | OR | P-value$ | |
Gender
|
Reference 1.318 |
0.081* |
1.307 |
0.103 |
Age
|
Reference 0.579 |
0.027* |
0.571 |
0.046** |
| Got infected before vaccinated | 1.343 | 0.026* | 1.289 | 0.063 |
| Smoker | 0.996 | 0.957 | —– | —— |
| Allergy | 1.748 | 0.001* | 1.512 | 0.024** |
| Asthma | 1.273 | 0.462 | —– | —– |
| Blood Diseases | 3.126 | 0.082* | 1.645 | 0.480 |
| Bone Diseases | 1.803 | 0.150* | 0.976 | 0.959 |
| Bowel Diseases | 2.510 | 0.004* | 1.856 | 0.071 |
| Cancer | 1.123 | 0.880 | —– | —– |
| Cardiac Diseases | 1.205 | 0.707 | —– | —– |
| Chronic Hypertension | 0.664 | 0.138* | 0.626 | 0.324 |
| Neurologic Diseases | 0.924 | 0.858 | —– | —– |
| Diabetes Mellitus—type one | 1.868 | 0.260 | —– | —– |
| Diabetes mellitus—type two | 0.970 | 0.937 | —– | —– |
| Renal Diseases | 1.180 | 0.779 | —– | —– |
| Hepatologic Diseases | 0.963 | 0.924 | —– | —– |
| Rheumatoid Arthritis | 7.341 | 0.008* | 6.241 | 0.023** |
| Thyroid Disease | 0.984 | 0.947 | —– | —– |
| Chronic Obstructive Pulmonary Disease (COPD) | 0.963 | 0.924 | —– | —– |
| Common Analgesics (for example: paracetamol) | 1.248 | 0.118* | 0.920 | 0.607 |
| Antihistamines | 1.615 | 0.036* | 1.013 | 0.963 |
| Thyroid Hormones | 0.912 | 0.712 | —– | —– |
| Antibiotics | 1.152 | 0.582 | —– | —– |
| Anti-hypertensives | 0.684 | 0.138* | 0.983 | 0.969 |
| Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | 2.719 | 0.001* | 2.091 | 0.029** |
| Anti-diabetics | 1.475 | 0.200* | 1.519 | 0.197 |
| Cholesterol-lowering agents | 0.797 | 0.456 | —– | —– |
| Anti-reflux medications | 2.701 | 0.007* | 1.935 | 0.101 |
| Contraceptives | 0.938 | 0.851 | —– | —– |
| Anti-depressants | 2.064 | 0.058* | 1.435 | 0.379 |
| Anti-asthmatics | 1.825 | 0.193* | 1.181 | 0.736 |
| Anti-coagulants | 1.376 | 0.482 | —– | —– |
| Anti-epileptics | 1.010 | 0.987 | —– | —– |
| Opioid Analgesics | 2.261 | 0.230* | 1.197 | 0.814 |
| Corticosteroid | 2.540 | 0.255 | —– | —– |
| Immunosuppressives | 0.905 | 0.818 | —– | —– |
#Using simple logistic regression, $Using multiple logistic regression, *A variable that entered the multiple logistic regression analysis after it was significant at 0.25 significant level at the simple logistic regression, **Significant at 0.05 significance level.
Discussion
This study aims to investigate and provide data regarding COVID-19 vaccines’ side effects among the Jordanian population.
Separating myth from reality, worldwide, there is no existing vaccine that is considered 100% free from side effects.26 After receiving the vaccine, the process of building immunity in the individual’s body can cause side effects, this may indicate that the body is developing the desired immunity;27 thus, the Jordanian MoH asked individuals who received the COVID-19 vaccine to report any side effects through their platform by opening the link included in the SMS that was received upon registration.13
In an effort to encourage the public in receiving the COVID-19 vaccine, Jordan’s King Abdullah II and the Crown Prince Hussein received the COVID-19 vaccine in front of cameras because the success of the vaccination campaign depends on the population’s conception and awareness of the benefits and risks associated with the vaccine.5 The king reported in an interview that he got tired and had trouble sleeping for a couple of days after receiving the vaccine. “But that is a small price to pay compared with actually catching the virus”, he said.28
The COVID-19 vaccine hesitancy is complicated, including a plethora of underlying concerns, for example, concerns about the speed of vaccine development have been documented,29 in addition to concerns regarding the safety, effectiveness, and side effects of the COVID-19 vaccine.30 Results of the present study showed that the reported side effects are consistent with those classified as common side effects of the COVID-19 vaccines according to the United States Centers for Disease Control and Prevention.31 A cross-sectional sectional study (preprint) reported that fewer side effects associated with the COVID-19 vaccine are preferable.32 Studies have revealed that there is huge variability in public awareness and perception of COVID-19, as well as a variety in the acceptance rate of receiving the COVID-19 vaccine.33–35 Thus, increasing public awareness (such as documentation of the COVID-19 vaccines’ side effects) could affect COVID-19 vaccine uptake and decrease hesitancy toward receiving the COVID-19 vaccines, which in turn would pave the way toward controlling the pandemic.
More than half of the recruited participants in this study received the Pfizer-BioNTech vaccine (51.1%). This was similar to another cross-sectional study conducted in Jordan between March 13 and April 23, 2021, by Omiesh et al. where the majority of the participants (40.6%) received the Pfizer vaccine.36 On the other hand, a study conducted in Jordan from 9 to April 15, 2021, documented that the most frequently received vaccine among 2,213 Jordanian participants was the Sinopharm (38.2%).37 This can be explained by the fact that in Jordan people did not get to choose what vaccine they will receive. Furthermore, the type of vaccine received each time was dependent on its availability because Jordan received variously funded and donated shipments from the European Union and China as a part of the World Health Organization’s COVAX program.38–40
In the current study, the participants mainly reported mild side effects after the COVID-19 vaccines. Sore arm at the injection site, fatigue and tiredness, discomfort, muscles/joint pain, drowsiness, and headache were the most reported side effects, and mainly lasted for a week or less after receiving the COVID-19 vaccine. Similar side effects were reported in other studies, such as the one conducted by Omeish et al. in Jordan where the most reported side effects were injection site pain, followed by fatigue, and myalgia,36 Klugar et al. in Germany where the injection site pain was the most frequently documented side effect associated with the mRNA-based vaccine, while headache and fatigue were associated with the viral vector-based vaccine,41 and Saeed et al. in the United Arab Emirates where injection site pain and fatigue were the most frequently reported side effects after the Sinopharm COVID-19 vaccine.7
In the current study, the side effects were significantly higher among older participants; this is similar to the findings of Omeish et al. where higher participants’ age was associated with an increased probability of having side effects after the COVID-19 vaccine,36 but contrary to the findings of Saeed et al. where side effects after receiving the Sinopharm vaccine were more prevalent among younger participants (>49-year-olds compared to ≤49).7 In the study conducted by Klugar et al.,41 age played a role in the prevalence and severity of side effects; however, this was dependent on the type of vaccine received, and it was found that younger people were more affected by side effects after receiving mRNA-based vaccines, but less affected after receiving vector-based vaccine when compared with older people.
Commonly, the body’s immune system distinguishes the spike protein of the virus after the first dose and initiates a greater response after the second dose.42 In the current study, side effects after the first and the second dose were reported. The reported number of side effects per participant after the first dose (4.50 ± 2.596) was higher than the second dose (3.43 ± 4.858); however, no statistically significant was found.
In this study, it was found that individuals suffering from allergies, rheumatoid arthritis, and those who consume NSAIDs as pain relievers on a regular basis were more affected by the COVID-19 vaccines’ side effects. There was no significant difference in side effects between male and female participants, which indicates that gender did not play a role in the prevalence or severity of COVID-19 vaccines’ side effects; this data was consistent with other studies.7,36,41 On the contrary, some studies documented opposite results that stated that the reported side effects were more frequent in females than in males.7,36,41,43,44 However, in the study conducted in Germany by Klugar et al., side effects were almost equally distributed between males and females, but females suffered more from the viral vector-based vaccine.41 This could explain why in this study no statistical significance was found for gender, as most of the participants received mRNA vaccines.
The differences between studies conducted regarding the effect of age, health status, medication intake, and being affected by side effects can be due to the differences in the types of COVID-19 vaccines received by individuals and could also be due to the difference in the race of participants in the different studies.
This study comes with limitations, such as recall bias, the side effects were self-reported with no validation, and the recruitment method was via social media that may limit population representation. The self-perceived nature of data collected was also another limitation; however, the researchers overcome this limitation as much as possible by making the survey anonymous, being careful when framing the survey, using neutrally worded questions, avoiding hypothetical questions, as well as making sure that the answer options are not leading, and using simple precise language. Moreover, participants were not asked about the onset of symptoms that could have added value to the outcomes of the study, allowing assessment of the risk windows; future studies would benefit from collecting such data in order to assess temporal associations and account for recall bias.
Another limitation of the study is that the research team did not add a measure to the survey that would prevent the same participant from answering the survey more than once; yet as clarified in the methods section, responses were carefully monitored by the research members and related actions were taken when necessary. In the current study, no details regarding the different COVID-19 vaccines were provided; identifying side effects regarding each type of the COVID-19 vaccine is the future study directions.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical Approval
Ethical approval for this study was obtained from the Faculty of Pharmacy, Applied Science Private University (Approval Number: 2021-PHA-31).
References
- 1.The World Health Organization . Coronavirus disease (COVID-19) Q&As. [Google Scholar]
- 2.The World Health Organization . Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 3.Jordan : This is how we prepare for the third wave of coronavirus. https://www.skynewsarabia.com/middle-east/1433707-الأردن-نستعد-لموجة-كورونا-الثالثة.
- 4.U.S. Food and Drug Administration . FDA briefing document. Pfizer-BioNtech COVID-19 vaccine. https://www.fda.gov/media/144245/download.
- 5.El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, Alghamdi BS, Alahmadi AA, Hassan NA, Sindi N, et al. Minor to moderate side effects of Pfizer-Biontech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. int J Gen Med. 2021;14:1–9. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuters . Sinopharm’s COVID-19 vaccine 79% effective, seeks approval in China. 2020. https://www.reuters.com/article/us-health-coronavirus-china-vaccine/sinopharms-covid-19-vaccine-79-effective-seeks-approval-in-china-idUSKBN2940C8.
- 7.Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO.. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Australian Government . AstraZeneca vaccine. https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/astrazeneca.
- 9.Cazzola M, Rogliani P, Mazzeo F, Matera MG. Controversy surrounding the Sputnik V vaccine. Respir Med. 2021;187(August):106569. doi: 10.1016/j.rmed.2021.106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Organization for Economic Co-operation and Development . Enhancing public trust in COVID-19 vaccination: the role of governments. 2021. doi: 10.1787/eae0ec5a-en. [DOI] [Google Scholar]
- 11. Anadolu Agency. Jordan begins COVID-19 vaccine rollout. 2021. https://www.aa.com.tr/en/latest-on-coronavirus-outbreak/jordan-begins-covid-19-vaccine-rollout/2108497.
- 12.UNICEF . UNICEF donates 1.3 million syringes to support national COVID-19 vaccination campaign. 2021. https://www.unicef.org/jordan/press-releases/unicef-donates-13-million-syringes-support-national-covid-19-vaccination-campaign.
- 13.UNHCR . COVID-19 vaccine. https://help.unhcr.org/jordan/en/frequently-asked-questions-unhcr/covid-19-vaccine/.
- 14.Ministry of Interior . Launching the youth volunteer work team to combat the novel coronavirus. 2022. https://moi.gov.jo/Ar/NewsDetails/إطلاق_فريق_العمل_التطوعي_الشبابي_لمكافحة_فيروس_كورونا_المستجد.
- 15.Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, Nourwali I, Qasem F, Dar-Odeh N. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;9(6):577. doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dar-Odeh N, Abu-Hammad O, Qasem F, Jambi S, Alhodhodi A, Othman A, et al. Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum Vaccin Immunother doi: 10.1080/21645515.2022.2039017.2022. [DOI] [PMC free article] [PubMed]
- 17.Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr Clin Res Rev. 2020;15(5):102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccine adverse event reporting system (VAERS). https://vaers.hhs.gov/.
- 19.Yellow card. https://yellowcard.mhra.gov.uk/.
- 20. UNHCR. COVID-19 vaccine. https://help.unhcr.org/jordan/en/frequently-asked-questions-unhcr/covid-19-vaccine/ The National Vaccination Campaign to have to pay any fees.
- 21.The World Health Organisation . Vaccines and immunization: What is vaccination? https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination?adgroupsurvey=%7Badgroupsurvey%7D&gclid=CjwKCAjwoP6LBhBlEiwAvCcthB3a_79sbLP10DG-wVCJ3uLAY-d6mwfYY5irn1mr6bREZqT6Ga4srBoCXGQQAvD_BwE
- 22.Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10:1428 doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227(2):309–313. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
- 24.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–126. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taherdoost H. Determining sample size; how to calculate survey sample size. J Econ Manag Syst. 2017. [Google Scholar]
- 26.Kimmel SR. Vaccine adverse events: separating myth from reality. Am Fam Physician. 2002;66:2113–2120. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention . 2020. What to expect at your appointment to get vaccinated for COVID-19. https://stacks.cdc.gov/view/cdc/99715.
- 28.News TN . Coronavirus: Jordan’s King Abdullah recounts his vaccine experience. https://www.thenationalnews.com/mena/jordan/coronavirus-jordan-s-king-abdullah-recounts-his-vaccine-experience-1.1157080.
- 29.Jennings W, Stoker G, Bunting H, Valgarðsson VO, Gaskell J, Devine D, McKay L, Mills MC. Lack of trust, conspiracy beliefs, and social media use predict COVID-19 vaccine hesitancy. Vaccines. 2021;9(6):593. doi: 10.3390/vaccines9060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batteux E, Mills F, Jones LF, Symons C, Weston D. The effectiveness of interventions for increasing COVID-19 vaccine uptake: a systematic review. Vaccines. 2022;10(3):386. doi: 10.3390/vaccines10030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention . Possible side effects. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html.
- 32.Yuen SWH, Yue RPH, Lau BHB, Chan CLW, S-M N. When to be vaccinated? What to consider? Modelling decision-making and time preference for COVID-19 vaccine through a conjoint experiment approach. medRxiv doi: 10.1101/2021.06.05.21258416. 2021. [DOI] [PubMed]
- 33.Adedeji-Adenola H, Olugbake OA, Adeosun SA, Yunusa I. Factors influencing COVID-19 vaccine uptake among adults in Nigeria. PLoS One. 2022;17(2):e0264371. doi: 10.1371/journal.pone.0264371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enitan SS, Oyekale AO, Akele RY, Olawuyi KA, Olabisi EO, Nwankiti AJ, et al. Assessment of knowledge, perception and readiness of Nigerians to participate in the COVID-19 vaccine trial. Int J Vaccines Immun doi: 10.16966/2470-9948.123. 2020. [DOI] [Google Scholar]
- 35.Batarseh YS, ElHajji FWD, Shammas S, Darwish RM, Fakhoury R, Al Haj Ahmad M, et al. Perception and attitude of the public on vaccine practices and pharmacists as immunizers in Jordan. J Pharm Heal Serv Res. 2021;12:114–121. doi: 10.1093/jphsr/rmaa009. [DOI] [Google Scholar]
- 36.Omeish H, Najadat A, Al-Azzam S, Tarabin N, Abu Hameed A, Al-Gallab N, et al. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum Vaccines Immunother. 2021;1–8. doi: 10.1080/21645515.2021.1981086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, Mohamud R. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):1–23. doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arab News. Jordan secures 3 million doses of COVID-19 vaccine. 2021. https://www.arabnews.com/node/1786701/middle-easthttps://www.unicef.org/jordan/press-releases/first-shipment-european-union-funded-covid-19-vaccines-covax-facility-arrived-jordan.
- 39.Times TJ China donates 500,000 doses of sinopharm vaccines to Jordan. 2022. https://jordantimes.com/news/local/china-donates-500000-doses-sinopharm-vaccines-jordan.
- 40.South EN Jordan receives a new shipment of COVID19 vaccines from COVAX facility. 2021. https://www.euneighbours.eu/en/south/stay-informed/news/jordan-receives-new-shipment-covid19-vaccines-covax-facility.
- 41.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt H, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. 2021;1–21 doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elterman K, Aungst C. Why are pfizer and moderna vaccines’ side effects worse after the second shot? 2021. https://www.goodrx.com/conditions/covid-19/why-side-effects-worse-after-second-covid-19-shot.
- 43.Di Resta C, Ferrari D, Viganò M, Moro M, Sabetta E, Minerva M, et al. The gender impact assessment among healthcare workers in the SARS-CoV-2 vaccination—An analysis of serological response and side effects. Vaccines. 2021;9(5):1–13 doi: 10.3390/vaccines9050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elnaem MH, Taufek NHM, Ab Rahman NS, Mohd Nazar NI, Zin CS, Nuffer W, Turner CJ. COVID-19 vaccination attitudes, perceptions, and side effect experiences in Malaysia: do age, gender, and vaccine type matter? Vaccines. 2021;9(10):1–15. doi: 10.3390/vaccines9101156. [DOI] [PMC free article] [PubMed] [Google Scholar]


