ABSTRACT
Although epigenome-wide association studies (EWAS) have been successful in identifying DNA methylation (DNAm) patterns associated with disease states, any further characterization of etiologic mechanisms underlying disease remains elusive. This knowledge gap does not originate from a lack of DNAm–trait associations, but rather stems from study design issues that affect the interpretability of EWAS results. Despite known limitations in predicting the function of a particular CpG site, most EWAS maintain the broad assumption that altered DNAm results in a concomitant change of transcription at the most proximal gene. This study integrated DNAm and gene expression (GE) measurements in two cohorts, the Adolescent and Young Adult Twin Study (AYATS) and the Pregnancy, Race, Environment, Genes (PREG) study, to improve the understanding of epigenomic regulatory mechanisms. CpG sites associated with GE in cis were enriched in areas of transcription factor binding and areas of intermediate-to-low CpG density. CpG sites associated with trans GE were also enriched in areas of known regulatory significance, including enhancer regions. These results highlight issues with restricting DNAm-transcript annotations to small genomic intervals and question the validity of assuming a cis DNAm–GE pathway. Based on these findings, the interpretation of EWAS results is limited in studies without multi-omic support and further research should identify genomic regions in which GE-associated DNAm is overrepresented. An in-depth characterization of GE-associated CpG sites could improve predictions of the downstream functional impact of altered DNAm and inform best practices for interpreting DNAm–trait associations generated by EWAS.
KEYWORDS: Epigenetics, DNA methylation, gene expression, transcriptional regulation, multi-omics, data integration
Introduction
Epigenome-wide association studies (EWAS), aiming to test the theory that marks of DNA methylation (DNAm) are involved in the pathophysiology of disease, have successfully identified associations between complex traits and DNAm. Specific DNAm patterning has been associated with environmental exposures, as well as short- and long-term health outcomes [1–6]. Several attributes of DNAm potentially link this epigenetic mark to the development or progression of complex disease. Appropriate DNAm patterning is essential for normal development and ageing, and DNAm regulatory mechanisms are implicated in a multitude of molecular processes, such as cellular differentiation, X–inactivation, and genomic imprinting [7–10]. As an epigenetic mark, DNAm is both dynamic and persistent; modifiable by environmental exposures yet heritable during cell division, so that any alterations to DNAm patterns may be carried through future populations of cells [11–14]. Importantly, altered DNAm has been linked to downstream functional changes, particularly in the regulation of gene expression (GE) [15]. These properties suggest that DNAm may be contributing to mechanisms in which previous exposures and genetic predispositions can have lasting effects on disease risk. While EWAS methods are promising, their current utility beyond biomarker discovery is questionable due to study design limitations that impact the interpretability of results, particularly those stemming from the omission of GE measurements [14,16].
The canonical mechanism describes DNAm as a repressor of proximal transcription, in which DNAm within promoter regions is able to silence GE by either blocking the binding of essential transcriptional machinery or by recruiting chromatin modifying proteins that transition the local DNA conformation to a more heterochromatic state [9,17]. Despite accumulating evidence that suggests this model is overly simplistic when applied on a global scale, [17,18] many researchers rely on this paradigm to interpret an association between DNAm and a disease of interest [14,16,19]. In a typical EWAS, any significantly associated CpG sites (also known as differentially methylated positions or DMPs) are each mapped to their most proximal gene and the biological function of those genes is reported in the context of the tested phenotype. This method is problematic since the canonical DNAm mechanism applies specifically to CpGs within promoter regions whereas many DMPs are intergenic, within gene bodies, or downstream of the proposed gene. Given that this interpretation emphasizes the functional relevance of specific genes to disease biology, an argument can be made that current EWAS are primarily interested in examining a theory of DNAm-driven transcriptional regulation [14,16]. By inferring transcriptional activity from DNAm–trait associations, this approach relies on assumptions without directly testing for functional evidence. Given that accurately inferring the functional consequences of modified DNAm at any particular site is still very limited, this practice may lead to inaccurate conclusions about disease biology [18,20].
Accurately predicting the functional impacts of altered DNAm remains challenging, in part, due to the limited characterization of genome-wide DNAm–GE relationships [20]. DNAm often does not block transcription independently but rather works in concert with other regulatory elements to coordinate GE [21–23]. These regulatory mechanisms involve a complex crosstalk between DNAm, higher-order chromatin modifiers, and other epigenetic marks, further contributing to difficulties in determining the functional impact from DNAm measurements alone [21–23]. Moreover, linking genes to their putative regulatory regions is not always straightforward [24]. DMPs are often located outside of proximal regulatory elements, within intergenic or intronic regions with no known regulatory function. Since a frequently utilized approach for interpreting these results involves linking all DMPs to their nearest gene, any features of the genomic landscape beyond distance are disregarded. Even if CpG-GE pairs are identified, predicting the regulatory consequence of altered DNAm remains difficult as exceptions to the theory that describes DNAm as a proximal gene repressor have accumulated. For example, increased DNAm, particularly within the gene body, is frequently positively correlated with local transcription [25–30]. Although mechanisms linking hypermethylation to increased GE are still unclear, a recent study identified more transcription factors that preferred binding methylated sequences than those inhibited by DNAm [23]. These functional complexities suggest that assumptions regarding transcriptional activity should not be inferred by DNAm patterns alone. Instead, if the fundamental theory being explored is a mechanism of transcriptional regulation modulated by DNAm, measurements of GE should be included in the analysis [14,16].
Multi-omic studies integrating global DNAm and GE measurements can provide evidence for DNAm-driven transcriptional regulatory mechanisms. Measuring GE in parallel with DNAm could allow for not only a direct test of the association between DNAm and the outcome of interest, but also whether this relationship can be explained by a mediator, in this case, GE [31]. This approach tests the hypothesized mechanism while avoiding assumptions regarding the regulatory function of DNAm that typically cloud the interpretation of EWAS results. Although cross-sectional studies are still unable to eliminate the possibility of reverse causation (i.e., GE changes proceeding DNAm changes), they can provide a more comprehensive understanding of biological processes involved in disease. However, current studies often integrate DNAm and GE measures by discovering differentially methylated and differentially expressed genes separately, and regulatory mechanisms are inferred by the observation of overlaps between DNAm–trait and GE–trait associations at the gene level [32–39]. Since the relationship between DNAm and GE is not tested a priori, this method still relies on assumptions that DMPs strictly influence expression of the nearest transcript. A limitation impacting current studies is the lack of information on the extent that DNAm loci are associated with GE in cis or trans, which could vary by, for instance, cell type or developmental stage.
An extended EWAS approach integrating both DNAm and GE measurements holds promise in uncovering biological processes important to the development or progression of disease, however, a mechanistic interpretation requires prior knowledge regarding specific DNAm–GE relationships across the genome, which has yet to be resolved. Several studies have attempted to clarify this functional relationship by integrating DNAm and GE measurements [26,27,29,40,41]. Although a variety of complex relationships between DNAm and GE have been identified, including long-range associations, focus has been primarily placed on outlining proximal relationships and relatively few studies have examined distal associations on a genome-wide scale [26,41]. The objective of this study was to expand upon this work by cataloguing and characterizing the relationships between DNAm and both proximal and distal GE (i.e., cis and trans relationships, respectively) in peripheral blood, a tissue commonly assayed in EWAS. To identify attributes that replicate across disparate samples, analyses were conducted in two previously described cohorts, the Adolescent and Young Adult Twin Study (AYATS) [6,42], and the Pregnancy, Race, Environment, Genes Study (PREG) [43]. To our knowledge, this is the first study to test for genome-wide associations between DNAm and GE in two cohorts of the same tissue.
Methods
Study cohorts
Adolescent and Young Adult Twin Study (AYATS)
The AYATS study was designed to examine genetic and environmental contributions to internalizing pathways (e.g., depression and anxiety) during development. A sample of monozygotic twins were chosen for their adherence to the study’s inclusion criteria (e.g., 15–20 years of age, no current use of psychotropic medications) [6,42]. Peripheral blood collected from 141 participants at a single time point was assayed for both DNAm and GE. An overview of study characteristics and further demographic information can be accessed in the supplement (Supplementary Table S1).
Pregnancy, Race, Environment, Genes (PREG) study
The PREG Study is a prospective longitudinal study with the purpose of identifying how environmental determinants of health and DNAm remodelling relate to racial health disparities in perinatal health outcomes [43]. Of the 240 women who enrolled in the study, 177 met all birth and pregnancy inclusion criteria (e.g., mother and father self-identify as either both Caucasian or both African American) and no exclusion criteria (e.g., preeclampsia, fetal congenital anomaly, placental anomaly, fewer than 3 study time points completed). Peripheral blood samples were collected up to four times throughout pregnancy. Sample collection was scheduled during gestational weeks 0–15, 10–25, 20–40, and 37–42. DNAm was assessed at all time points, whereas GE was measured once at the final collection during weeks 37–42. Only those DNAm measurements from specimen simultaneously collected with GE were analysed in this study. A total of 151 women had concomitant DNAm and GE measured. An overview of study characteristics and further demographic information can be found in the supplement (Supplementary Table S2).
DNAm measurement and data processing
In both samples, DNAm and GE was measured from peripheral blood. The Infinium 450k HumanMethylation BeadChip assayed genome-wide DNAm and the Affymetrix HG-U133A 2.0 array measured GE. A description of platform characteristics as well as the methods used for measurement and preprocessing can be found in the supplement.
Association analysis
The relationship between all pairwise combinations of measured DNAm and GE (Table 1) was tested by linear regression in the R statistical environment (version 3.5) [44]. Log-transformed expression values (dependent variable) were regressed on DNAm M-values (independent variable), while covariates controlled for differences in cell type heterogeneity. Cell type proportions were derived from the Houseman algorithm, which estimates proportions for granulocytes, monocytes, CD8-positive T cells, CD4-positive T cells, B lymphocytes, and natural killer cells based on cell type-specific DNAm profiles [45]. Granulocytes were selected to account for overall differences in cell type proportions based on high correlations with other cell type estimates (absolute correlations ranged from 0.47 to 0.71), and included as a covariate in all models. Natural killer proportions were included in AYATS models exclusively to adjust for the atypical variation in this cellular fraction characteristic of depressed patients [6,46].
Table 1.
Study characteristics.
| AYATS | PREG | |
|---|---|---|
| N | 137 | 131 |
| Study phenotype | Internalizing disorders (e.g., early-onset major depression) |
Perinatal health outcomes (e.g., preterm birth) |
| Age | 16.96 (1.28) | 29.06 (4.99) |
| Sex (% female) | 97 (71%) | 131 (100%) |
| Ethnicity (% Caucasian) | 132 (97%) | 67 (51%) |
| Methylation probes tested | 445,120 | 421,729 |
| Expression probes tested | 10,913 | 12,249 |
Study characteristics were assessed after preprocessing and removal of poor quality samples.
Mean (standard deviation) or N (%).
Abbreviations. AYATS = Adolescent and Young Adult Twin Study; PREG = Pregnancy, Race, Environment, Genes cohort.
Additional covariates were selected to adjust for potential confounding influences specific to the characteristics of each cohort, while also maintaining a similar analytical approach across the two studies. Since PREG is a racially diverse sample (Table 1), ancestrally informative principal components were estimated from the DNAm data using the method described in Barfield et al. The third principal component was highly correlated with self-reported race and included as a covariate in the PREG cohort models [47]. A linear mixed-model framework was used to account for twin structure in the AYATS cohort [48]. The limma Bioconductor package was used to estimate within-family correlations from 1,000 randomly sampled CpGs in order to appropriately adjust model standard errors and account for the non-independence of twin pair DNAm observations [48].
Both DNAm and GE measurements were adjusted for technical artefacts prior to analysis (see supplement), so that variables related to slide or row effects were not included as covariates in subsequent analyses.
Although measurements were generated using the same technology in both cohorts, differing numbers of probes remained after quality control procedures (Table 1). A within-study Bonferroni correction was used to adjust for multiple testing at an alpha threshold of 0.05. While estimates of genomic inflation are typically used to identify spurious associations driven by artefacts in genome-wide association studies (GWAS), it has been recently suggested that inflated test statistics should be similarly reviewed in epigenetic studies [49]. To mitigate the presence of false positives, genomic inflation was assessed using the method described in Kennedy et al. [26]. Briefly, genomic inflation factors were calculated for each transcript, across all CpG associations, as the median (T-statistic)2/0.4549. Appropriate thresholds for test statistic inflation are not as well established in the epigenetics field. To facilitate cross-study comparisons, any transcript with an inflation factor > 2 was flagged for removal [26].
Every pairwise relationship between measured DNAm and GE was modelled and classified as either cis or trans, since molecular mechanisms linking proximal DNAm may differ from more long-range interactions. DNAm–GE pairs were in cis if the CpG site was located within a gene or 2,500 base pairs (bp) upstream. This extension is expected to capture important transcript-specific regulatory regions, given that many promoters are located up to 1 kilobase upstream of the transcriptional start site (TSS) [50]. CpG–transcript pairs located outside this range were categorized as trans relationships, with the rationale that more distal regulatory features (e.g., enhancers) may be responsible for the relationship between CpG methylation and transcript expression.
Characterization of results
CpG sites were mapped to biologically relevant annotations to test for feature enrichment among sites significantly associated with GE. Annotation selection was based on evidence that local CpG density, gene feature location, and proximity to regulatory elements are important characteristics that may impact the functional consequences of DNAm [17]. Transcription factor activity is regulated by DNAm, and a history of transcription factor binding also appears to influence the susceptibility of CpG methylation in specific locations [23,51–53]. Moreover, processes involving transcription factors were previously enriched among CpGs associated with GE [26,54]. Similarly, non-coding RNAs are regulated by methylation patterning, while also contributing to the regulatory activity of DNAm [55,56]. Chromatin states are accurately able to distinguish variable transcriptional activity by describing the specific patterns of histone modifications that impact the regulation of GE [57,58]. Histone modifications are intricately linked to both DNAm and GE, potentially serving to mediate the influence of methylation on transcription [17,57].
Selected features described local CpG densities (UCSC CpG island classifiers and HIL annotations) [59,60], genomic regions (UCSC knownGenes track, hg19 build) [61], chromatin states (ENCODE 15-state ChromHMM) [58], transcription factor binding (ENCODE TF ChIP-seq) [62], non-coding RNAs (GENCODE version 37) [63], and other annotations related to regulatory activity (i.e., FANTOM5-defined enhancer and ENCODE-defined insulator regions) [64]. All cell type-specific annotations (e.g., chromatin states, enhancer regions, etc.) were defined in the lymphoblastoid cell line GM12878. The specific gene feature annotations that were sourced using Bioconductor packages can be found on the Open Science Framework project page (https://osf.io/dk3cg/).
Enrichment analyses were performed separately for cis and trans groups. The proportion of significant findings annotated to each category was compared to the proportion of total number of tested CpG sites using Fisher’s exact test. Duplicate mappings were conserved for gene region annotations, so that individual CpG sites could be annotated to more than one gene region. Other annotations assigned each CpG to mutually exclusive categories (e.g., chromatin states and CpG classifiers). A Bonferroni correction for 20 enrichment tests was used to adjust for unique annotation categories examined (e.g., CpG density classifiers, chromatin states, transcription factor binding, etc.)
Results
Participant demographics and initial findings
After performing the preprocessing procedures described in the supplementary methods, all 137 of the remaining GE measurements also had corresponding DNAm of sufficient quality in AYATS. In PREG, 131 samples had both DNAm and GE that passed quality control. While the tissue and platforms were consistent across studies, these cohorts differed in other characteristics (Table 1). PREG was an older (aged 18–40 years) and more racially diverse sample, with 49% of participants identifying as African American (Supplementary Table S2). Notably, all participants in the PREG sample were pregnant women, while the AYATS sample consisted of both male (29%) and female (71%) adolescents (aged 15–20 years; Supplementary Table S1).
Genome-wide methylomic and transcriptomic data was generated using the Illumina HumanMethylation 450k BeadChip and Affymetrix HG-U133A 2.0 array, respectively. After performing quality control procedures separately in both cohorts, a differing number of probes were identified as poor quality. In AYATS, 40,392 DNAm probes and 10,809 GE probe sets were removed during preprocessing, while 63,783 DNAm and 9,473 GE measurements were removed in PREG (Table 1).
Associations between DNA methylation and gene expression
Overall findings
An overview of significant DNAm–GE relationships is presented in Table 2 (see the Open Science Framework project page at https://osf.io/dk3cg/ for summary statistics). Due to the differing number of measurements surviving quality control, associations with P-values < 9.68 ×10−12 (0.05 alpha corrected for 5,165,758,521 total tests) in PREG and P-values < 1.03 × 10−11 (0.05 alpha corrected for 4,857,594,560 tests) in AYATS were considered significant (Table 2). A total of 903 associations were identified in the AYATS cohort, 169 of which were in cis (4.72 × 10−61 < P < 1.01 × 10−11) and 734 in trans (2.64 × 10−61 < P < 1.03 × 10−11).
Table 2.
Overview of pairwise DNAm–GE Association results.
| Study | Significant associationsa |
Direction of Relationship |
Unique CpGs |
Unique transcriptsd | Mean adjustedR-squarede |
|---|---|---|---|---|---|
| AYATSb | |||||
| cis | 169 | 65% negative | 107 | 42 | 0.58 (0.11) |
|
trans PREGc |
734 | 78% negative | 266 | 96 | 0.48 (0.10) |
| cis | 121 | 79% negative | 87 | 31 | 0.46 (0.10) |
| trans | 258 | 49% negative | 156 | 43 | 0.40 (0.08) |
aAfter Bonferroni adjustment for total number of tests performed within cohort.
bP-value < 1.03× 10−11.
cP-value < 9.68× 10−12.
dDefined as unique by Entrez identifier.
eMean (standard deviation).
Abbreviations. AYATS = Adolescent and Young Adult Twin Study; PREG = Pregnancy, Race, Environment, Genes cohort.
Within the PREG sample, 379 DNAm–GE associations were statistically significant, of which 121 were cis (5.15 × 10−58 < P < 8.50 × 10−12) and 258 trans (2.86 × 10−53 < P < 9.51 × 10−12). Since GE probe sets measuring expression of the same gene were retained, some transcripts and CpG sites are represented more than once in the results. A total of 340 unique CpG sites and 105 unique genes comprised the 903 significant associations identified in AYATS, while 228 CpGs and 69 genes were unique in PREG across both cis and trans relationships (total n = 379). Across all categories (i.e., AYATS/PREG cis/trans), many significant relationships occurred between one transcript and one CpG site (Supplementary Figures S1 and S2), although instances in which a single CpG site was associated with multiple transcripts, and vice versa, were also common. Both positive and negative relationships were identified, although the majority of significant associations had negative coefficients (49% to 78% negative across tested categories; Table 2 and Figure 1). Effect sizes were relatively large throughout (adjusted R-squared range = 0.23–0.90), with cis DNAm explaining more GE variability on average (mean adjusted R-squared = 0.58 and 0.46 for AYATS and PREG, respectively) when compared to trans (mean adjusted R-squared = 0.48 and 0.40 for AYATS and PREG, respectively).
Figure 1.
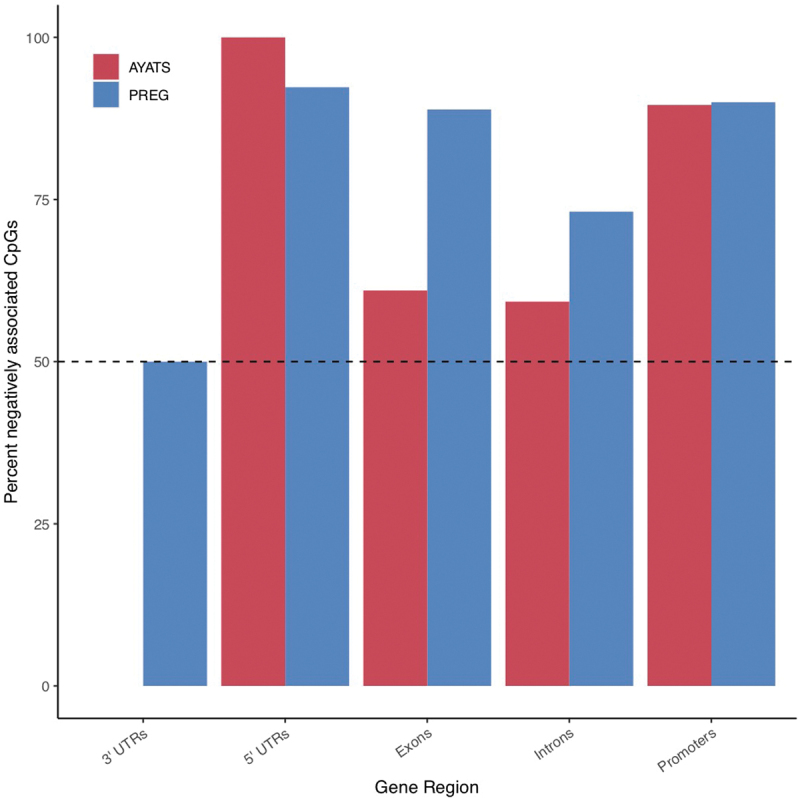
Percent negative CpG-GE associations by gene region.
With the exception of 3’ untranslated regions (UTRs), the majority of cis CpG-GE relationships were negative across gene regions in both the AYATS (red) and PREG (blue) cohorts. Promoters and 5’ UTRs had the highest fraction of negative associations, aligning with canonical descriptions of promoter DNAm as a repressor of local gene transcription.
AYATS Associations. The distribution of significant cis and trans connections is shown in Figure 2(a). On average, each significant cis CpG site was associated with 1.58 transcripts (median = 1, range = 1–4; Supplementary Figure S1). Trans CpGs were more likely to associate with multiple transcripts than cis (mean = 2.75, median = 1, range = 1–22). Effect sizes, defined by adjusted R-squared values, ranged from 0.23 to 0.90. Cis DNAm explained more variation in GE on average (Welch’s t-test P = 2.2 × 10−16). DNAm–GE relationships were predominantly negative (65% of cis and 78% of trans relationships; Table 2 and Figure 1).
Figure 2.
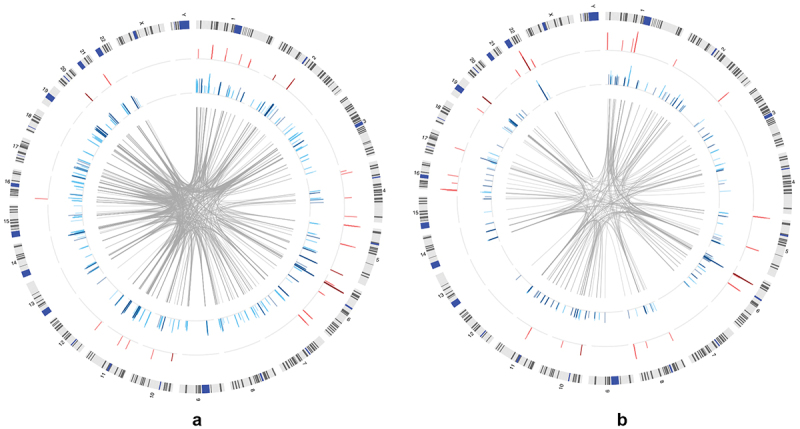
Distribution of significant connections between DNA methylation and transcript expression across the genome in the AYATS (2a) and PREG (2b) cohorts. The location of significant cis (red track) and trans relationships (blue track) across the genome (ideogram of human chromosomes, outer track) is shown. Bar graphs show the direction of the relationship (positive relationships are shown in the darker color) and the relative magnitude of the effect (height of bars; defined by adjusted R-squared values). Trans CpG-GE relationships often spanned chromosomes (location of associated CpG-GE pairs shown by center grey links).
PREG Associations. The distribution of significant cis and trans connections is shown in Figure 2b. On average, each significant cis CpG site is associated with 1.39 transcripts (median = 1, range = 1–4; Supplementary Figure S1). Significant trans CpGs were more likely to associate with multiple transcripts (mean = 1.65, median = 1, range = 1–11). Effect sizes ranged from 0.31 to 0.86, with cis DNAm explaining more variation in GE on average (Welch’s t-test P = 5.10 × 10−8). Cis DNAm–GE relationships were predominantly negative (79%) while trans relationships were split almost equally between positive and negative associations (Table 2).
Between study comparison
A total of 86 individual DNAm-transcript pairs replicated across cohorts (57 cis and 29 trans). In cis, 34% of significant CpG-GE pairs identified in AYATS were replicated, and 47% of those found in PREG overlapped with AYATS. In trans, only 4% of AYATS and 11% of PREG connections were replicated. Notably, all overlapping trans relationships consisted of same-chromosome CpG–transcript pairs. Replicated trans CpGs were located anywhere from 2,654 to 91,895 bp away from their associated gene (mean = 22,826 bp). On average, relationships that replicated across cohorts had stronger effects than those that were cohort-specific (Welch’s t-test P = 1.9 × 10−7).
Enrichment analyses characterizing those GE-associated CpG sites that replicated across cohorts identified similar attributes to the whole sets of significant CpGs. Replicated GE-associated CpG sites were significantly (P < 0.0025) enriched in South shores (P = 7.0 × 10−5), regions of transcription factor binding (P = 2.7 × 10−6), in chromatin states corresponding to areas flanking active transcription (chromatin state 3 P = 0.0008) and zinc-finger binding sites (chromatin state 4 P = 0.0024), and within introns (P = 3.5 × 10−7). These results were not significant when the replicated relationships were compared to all significant relationships rather than all tested relationships, indicating that similar proportions of replicated CpG sites and cohort-specific CpG sites were located within these regions.
Location relative to transcriptional start sites
Previous research has observed that methylation status at CpGs adjacent to gene TSS is more likely to correlate with proximal GE than that at CpG sites within other genomic regions [26,54,65]. To explore this topic further, CpG sites associated with GE in cis were mapped to their associated TSS, as defined by gene annotations from the UCSC hg19 build knownGene track [66]. This annotation defines the most 5’ TSS as the primary gene TSS. The location of cis CpG sites relative to the TSS of their associated gene is shown in Figure 3. Although many significant sites were located near the 5’ end of gene boundaries, these areas are also overrepresented in the 450k microarray. Overall, the relative proportion ([number GE-associated CpGs within 2500 bp/total number GE-associated CpGs]/[number microarray CpGs within 2500 bp/total number microarray CpGs]) of TSS-proximal CpGs was higher in the GE-associated CpG sites compared to the microarray background. Interestingly, this observation was driven by CpG sites located downstream of the TSS. The relative proportion of CpG sites 2500 bp upstream of the TSS was lower in GE-associated CpGs than was present on the microarray, while the opposite relationship was observed immediately downstream of the TSS (relative proportion upstream = 0.58 and 0.65; relative proportion downstream = 1.59 and 2.21 in AYATS and PREG, respectively).
Figure 3.
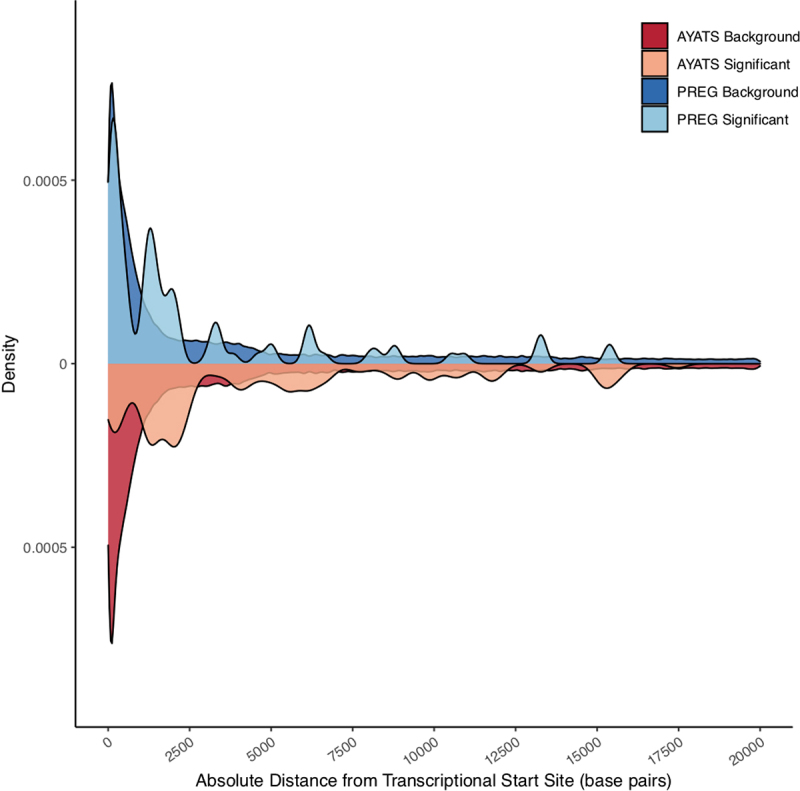
Absolute distance between CpG probes and transcriptional start sites (TSS) of proximal genes. A density plot depicting the absolute distance of DNA methylation microarray probes (darker colour) relative to the GE-associated CpG sites (lighter colour) from the transcriptional start sites of cis genes. Both the AYATS (red) and PREG (blue) cohorts showed enrichment in areas flanking active transcription. The proportion of GE-associated CpGs compared to the CpGs represented on the microarray was highest in areas directly downstream of the TSS.
Enrichment analyses
CpG sites were annotated by genomic regions, local CpG densities, chromatin states, bound transcription factors, and other related regulatory regions (e.g., insulator regions, regulatory RNAs). Enrichment tests were then performed within annotation type. An overview of results from enrichment analyses is outlined in Table 3 (AYATS) and Table 4 (PREG). Annotation categories with P-values <0.0025 exhibited significant depletion or enrichment, while P-values <0.05 were considered suggestive. A number of depleted and enriched categories overlapped between the two cohorts (underlined in Tables 3 and 4). Overall, regions of high CpG density were depleted across all groups (Supplementary Table S3 and Supplementary Figures S3 and S4) while annotations indicative of regulatory activity (e.g., transcription factor binding, enhancers) were enriched among GE-associated CpGs (Tables 3 and 4).
Table 3.
Results of AYATS Enrichment Analyses.a
| Annotation | cis | trans | ||
|---|---|---|---|---|
| Enriched | Depleted | Enriched | Depleted | |
| Chromatin Statesb | TxFlnk (3), ZNF/Rpts (8) |
Tx (4), TxWk (5), Quies (15) |
TssAFlnk(2), TxFlnk (3), TxWk (5), EnhG (6), Enh (7) |
TssA (1) Het (9), TssBiv (10), BivFlnk (11), ReprPC (13), ReprPCWk (14) |
| CpG Classifiers | South Shore | North shore, Island |
South shore, Open sea |
North shelf, North shore, Island |
| Gene Regions | 5’-UTRs, Promoters | Introns | 5’-UTRs, Promoters |
|
| Otherc | Enhancers, TF binding |
lncRNAs | Enhancers | lncRNAs, Insulators |
Abbreviations. UTR = untranslated region; TF = transcription factor; lncRNAs = long non-coding RNAs.
Bolded items. P-value <0.0025 (Bonferroni corrected for 20 tests)
Underlined items. Concordance across both the AYATS and PREG study.
aP-value < 0.05.
bENCODE ChromHMM 15-state model; 1 = Active transcriptional start site (TSS), 2 = Flanking active TSS, 3 = Flanking strong transcription, 4 = Strong transcription, 5 = Weak transcription, 6 = Genic enhancer, 7 = Active enhancer, 8 = Zinc-finger genes and repeats, 9 = Heterochromatin, 10 = Bivalent/poised TSS, 11 = Flanking bivalent TSS, 12 = Bivalent Enhancers, 13 = Polycomb-repressed, 14 = Weak Repressed Polycomb, 15 = Quiescent.
cFANTOM5-defined enhancers, transcription factor binding sites derived from ENCODE TF ChIP-seq, GENCODE long non-coding RNAs.
Table 4.
Results of PREG Enrichment Analyses.a
| Annotation | cis | trans | ||
|---|---|---|---|---|
| Enriched | Depleted | Enriched | Depleted | |
| Chromatin Statesb | TssA (1), ZNF/Rpts (8) |
TssAFlnk (2), Tx (4) | TssAFlnk(2), TxFlnk (3), Enh (7), EnhBiv (12) |
TxWk (5), Het (9), ReprPCWk (14),Quies (15) |
| CpG Classifiers | South Shore | North shore, South shore | Island | |
| Gene Regions | Exons | Promoters, 3’-UTRs | ||
| Otherc | TF binding | Enhancers, TF binding |
Abbreviations. UTR = untranslated region; TF = transcription factor; lncRNAs = long non- coding RNAs.
Bolded items. P-value <0.0025 (Bonferroni corrected for 20 tests)
Underlined items. Concordance across both the AYATS and PREG study.
aP-value < 0.05.
bENCODE ChromHMM 15-state model; 1 = Active transcriptional start site (TSS), 2 = Flank- ing active TSS, 3 = Flanking strong transcription, 4 = Strong transcription, 5 = Weak tran- scription, 6 = Genic enhancer, 7 = Active enhancer, 8 = Zinc-finger genes & repeats, 9 = Heterochromatin, 10 = Bivalent/poised TSS, 11 = Flanking bivalent TSS, 12 = Bivalent En- hancers, 13 = Polycomb-repressed, 14 = Weak Repressed Polycomb, 15 = Quiescent.
cFANTOM5-defined enhancers, transcription factor binding sites derived from ENCODE TF ChIP-seq, GENCODE long non-coding RNAs.
Characterization of cis connections
South shore regions (i.e., shore regions located downstream from a CpG island) were either significantly (P < 0.0025) or suggestively (P < 0.05) enriched across all groups (AYATS/PREG and cis/trans; Figure 4). In addition to CpG classifiers, HIL annotations were used to describe local CpG density [60]. Regions of low CpG density were enriched in the AYATS cohort, while high-density regions were consistently depleted across AYATS and PREG (Table S3 and Figure S3). CpG islands are often associated with promoters, and both of these annotations were depleted in AYATS but were neither significantly enriched or depleted in PREG (Figures 4 and 5). Transcription factor binding sites, defined by significant peaks identified in ChIP-seq analyses of 134 transcription factors in lymphoblastoid cells [62], were enriched in both cohorts (Figure 6). Chromatin state characteristics, which assign a function to genomic regions based on the presence of specific histone methylation marks, also showed some concordance between the two studies (Figure 7). Specifically, zinc-finger genes and repeats were consistently found to be enriched, whereas areas of strong transcription were consistently depleted. Regions flanking active transcription were more variably assigned, with one category found to be enriched in AYATS (areas flanking strong transcription) and another depleted in PREG (areas flanking active TSS).
Figure 4.
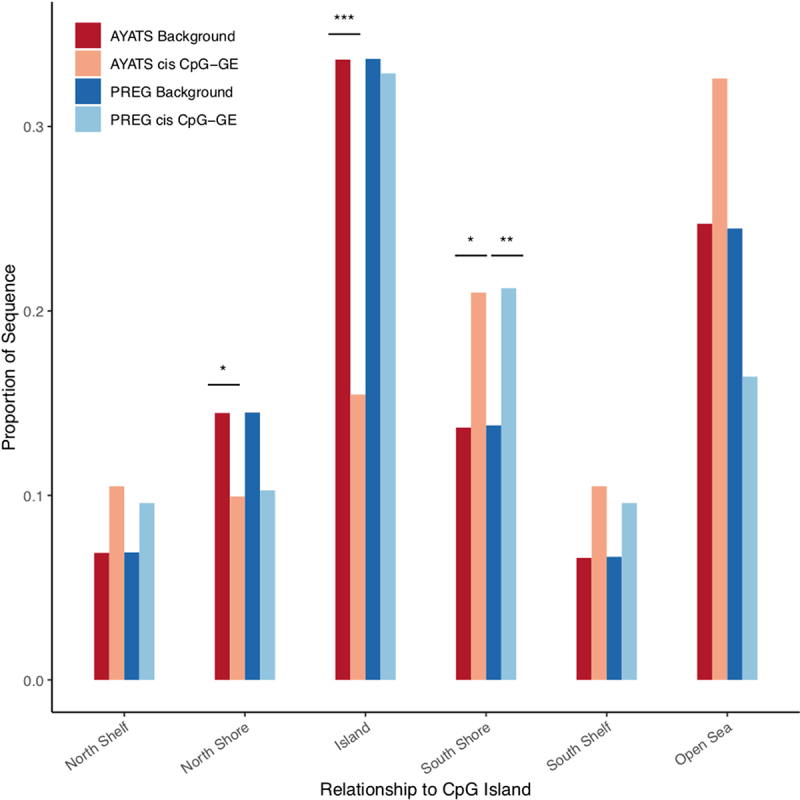
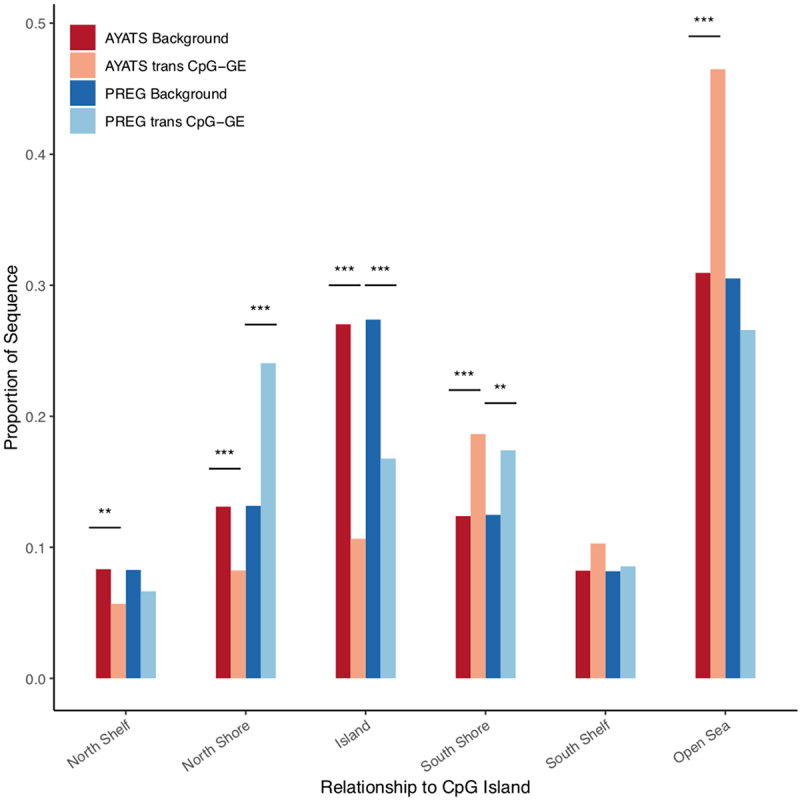
Enrichment for CpG classifiers in cis CpG–transcript relationships. CpG classifiers based on the distribution around CpG island regions were defined by the UCSC hg19 knownGene track. Islands and regions directly upstream from islands were depleted in AYATS. However, downstream regions bordering islands (South shores), were significantly enriched in both cohorts (***P < 0.0005; ** P < 0.005; * P < 0.05).
Figure 5.
Enrichment for gene regions in cis CpG–transcript relationships. Gene regions were annotated based on the UCSC hg19 knownGene track. GE-associated CpG sites were depleted in 5’ untranslated regions (UTRs) and in promoters in the AYATS cohort only. (*** P < 0.0005; **P < 0.005; * P < 0.05).
Figure 6.
Enrichment for additional regulatory annotations in cis CpG-transcript relationships. Sites of transcription factor binding, as defined by ENCODE TF ChIP-seq annotations, were significantly enriched across cohorts. FANTOM5 enhancers were enriched in AYATS (*** P < 0.0005; ** P < 0.005; * P < 0.05).
Figure 7.
Enrichment for ENCODE chromatin states in cis CpG–transcript relationships. The 15-state ChromHMM model was used to determine regional chromatin states. Overall, GE-associated CpGs were depleted in transcriptionally active regions but enriched at zinc-finger binding sites (*** P < 0.0005; ** P < 0.005; * P < 0.05). Abbreviations: 1 = Active transcriptional start site (TSS), 2 = Flanking active TSS, 3 = Flanking strong transcription, 4 = Strong transcription, 5 = Weak transcription, 6 = Genic enhancer, 7 = Active enhancer, 8 = Zinc-finger genes & repeats, 9 = Heterochromatin, 10 = Bivalent/poised TSS, 11 = Flanking bivalent TSS, 12 = Bivalent Enhancers, 13 = Polycomb-repressed, 14 = Weak Repressed Polycomb, 15 = Quiescent.
Characterization of trans connections
Like cis CpG-GE pairings, CpGs associated with GE in trans were overall depleted in areas of high CpG density (Table S3 and Figure S4) and within the promoter regions of genes, while South shore regions and regions of intermediate CpG density were enriched (Tables 3–4 and Figures 8–9). Again, GE-associated CpG sites were overrepresented in areas of known regulatory importance, such as sites of transcription factor binding and enhancer regions (Figure 10). With the exception of lncRNAs, which were depleted in AYATS, noncoding RNAs were neither over- or underrepresented. The chromatin state analysis highlighted distinct differences between cis and trans results. Chromatin states reflecting enhancer regions were enriched in both AYATS and PREG, as were areas flanking sites of active transcription. Repressed states, including heterochromatic regions and polycomb-repressed regions, were consistently depleted (Figure 11).
Figure 8.
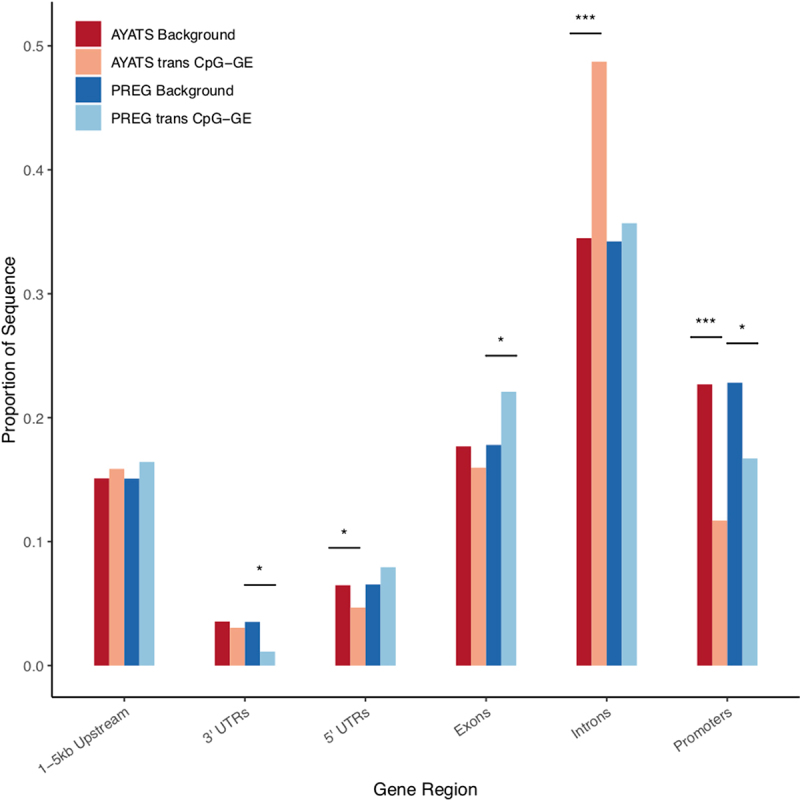
Enrichment for CpG classifiers in trans CpG–transcript relationships. CpG classifiers based on the distribution around CpG island regions were defined by the UCSC hg19 knownGene track. Islands were depleted while downstream regions bordering islands were significantly enriched in both cohorts. The North shore region directly upstream of CpG islands was more variable, with significant CpGs showing depletion in AYATS and enrichment in PREG (*** P < 0.0005; **P < 0.005; * P < 0.05).
Figure 9.
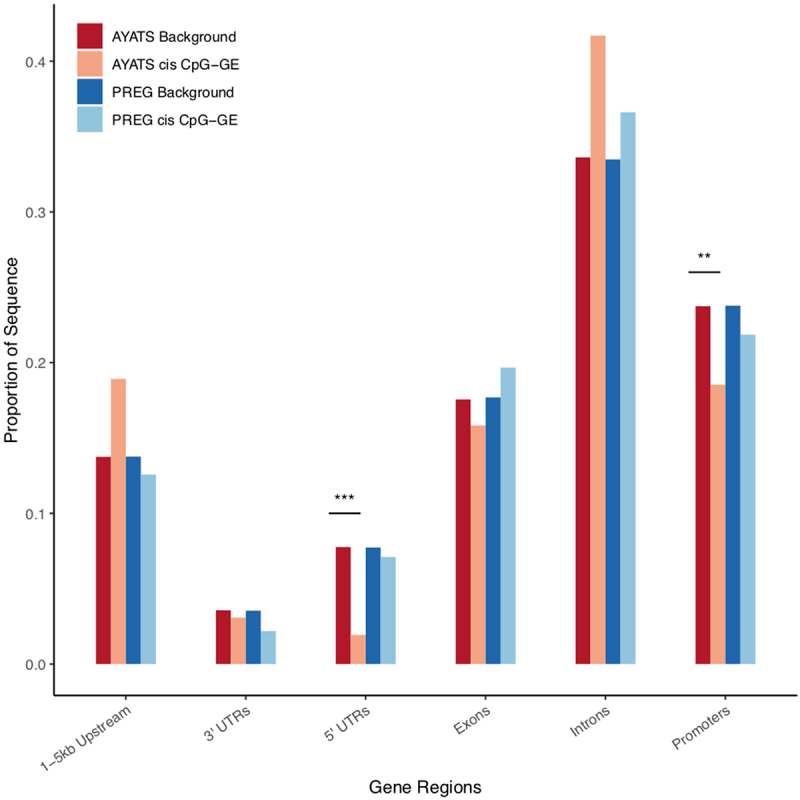
Enrichment for gene regions in trans CpG–transcript relationships. Gene regions were annotated based on the UCSC hg19 knownGene track. GE-associated CpG sites were depleted in 3’ untranslated regions (PREG), 5’ untranslated regions (AYATS), and in promoters (AYATS and PREG). Exons and introns were enriched in PREG and AYATS, respectively (***P < 0.0005; **P < 0.005; *P < 0.05).
Figure 10.
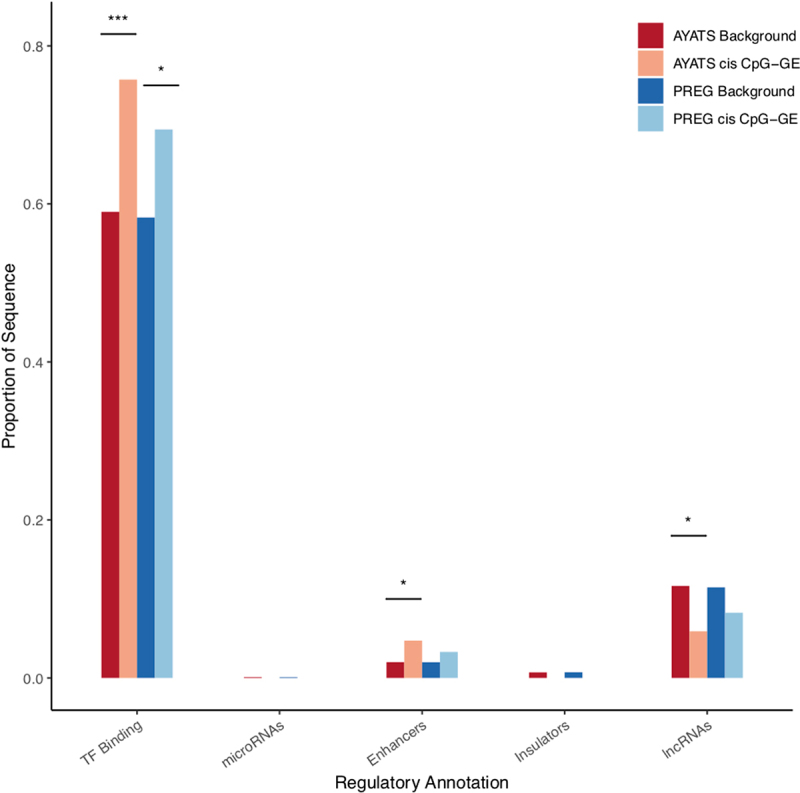
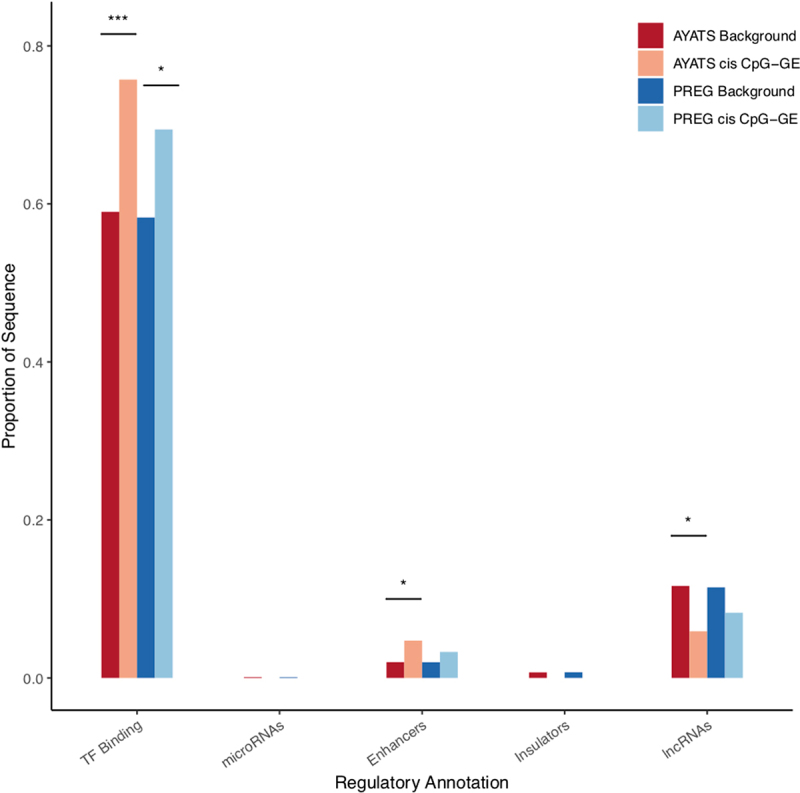
Enrichment for additional regulatory annotations in trans CpG–transcript relationships. Sites of transcription factor binding, as determined by ENCODE TF ChIP-seq, were significantly enriched in the PREG cohort. Enhancers were enriched across cohorts, and insulator regions were depleted in AYATS (*** P < 0.0005; **P < 0.005; *P < 0.05).
Figure 11.
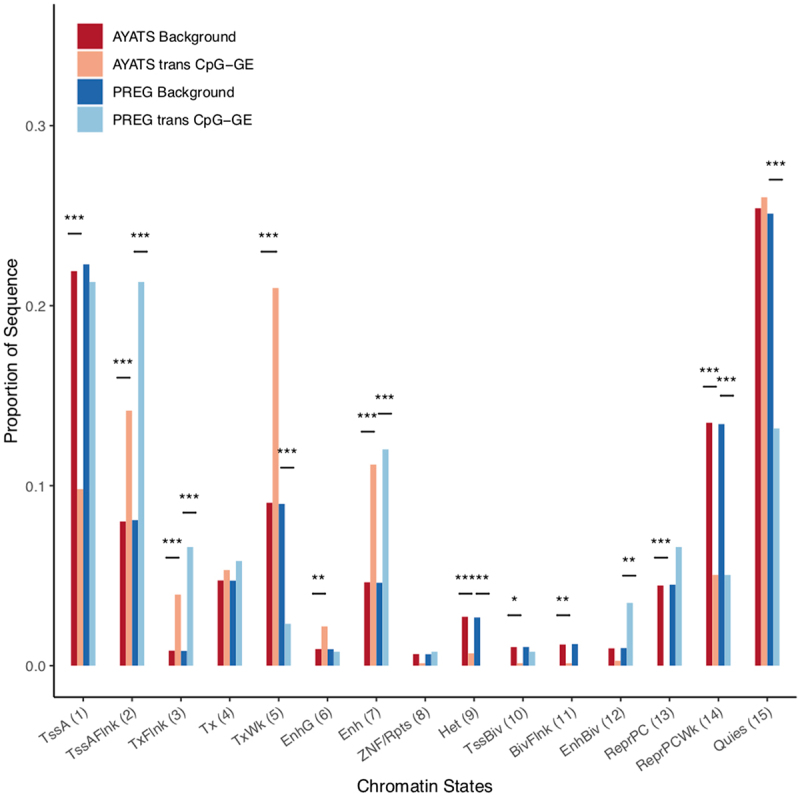
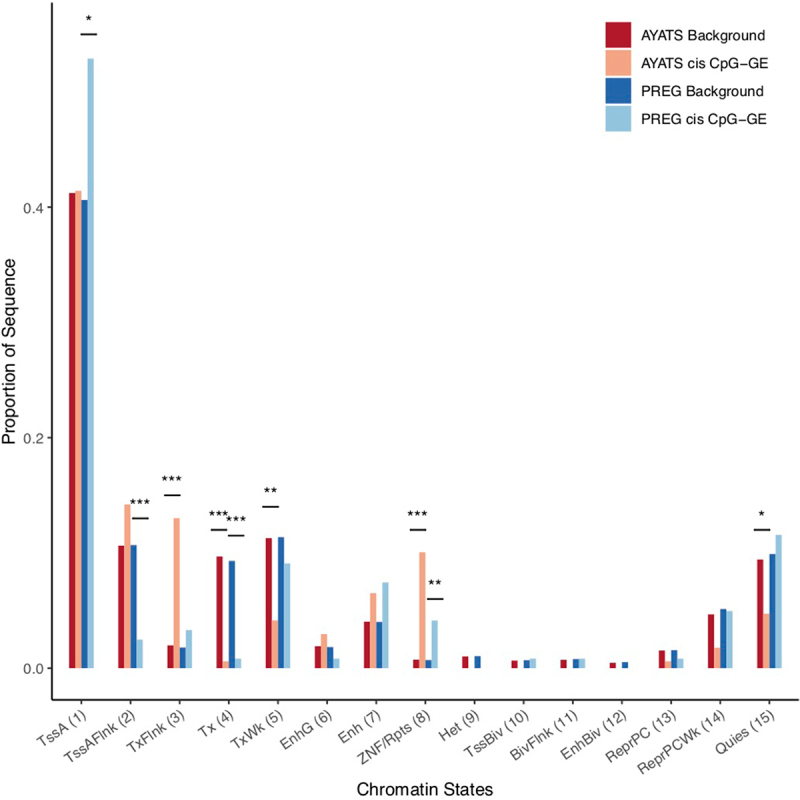
Enrichment for ENCODE chromatin states in trans CpG–transcript relationships. The 15-state ChromHMM model was used to determine regional chromatin states. Overall, GE-associated CpGs were depleted in repressive states but enriched at enhancers and areas flanking actively transcribed genes (***P < 0.0005; **P < 0.005; *P < 0.05). Abbreviations: 1 = Active transcriptional start site (TSS), 2 = Flanking active TSS, 3 = Flanking strong transcription, 4 = Strong transcription, 5 = Weak transcription, 6 = Genic enhancer, 7 = Active enhancer, 8 = Zinc-finger genes & repeats, 9 = Heterochromatin, 10 = Bivalent/poised TSS, 11 = Flanking bivalent TSS, 12 = Bivalent Enhancers, 13 = Polycomb-repressed, 14 = Weak Repressed Polycomb, 15 = Quiescent.
Functional enrichment analysis
The Gene Ontology (GO) Consortium and the Kyoto Encyclopaedia of Genes and Genomes (KEGG) were used to assess overrepresented gene functions and pathways within significant cis results (see supplement for more information). Terms with a false discovery rate (FDR) <0.05 were deemed significant [67]. Common themes were uncovered in both cohorts (Supplementary Tables S4–S11), and include functions related to the activation and regulation of immune response and cellular detoxification. A total of 33 significantly enriched GO terms overlapped between the two cohorts, and all significant KEGG pathways identified in AYATS (n = 31) were also found in PREG (n = 39). However, these consistencies were supported by relatively few genes.
Discussion
Although genome-wide epigenetic studies aim to uncover the role of DNAm in disease development and progression, they often do not utilize an experimental framework that provides evidence for a mechanistic relationship. Most EWAS operate under the assumption that DNAm influences proximal gene transcription. However, the absence of measured GE makes relying on this interpretation difficult, especially as mounting evidence suggests that DNAm does not always follow a canonical cis relationship [17]. Given the complicated network of interactions between DNAm, GE, higher-order chromatin modifiers, and other regulatory elements, it is challenging to draw accurate conclusions about the downstream functional effects of altered DNAm without, at minimum, integrating concomitant measurements of GE [14,17,22,62]. To investigate the relationship between DNAm and GE further, this study tested genome-wide associations between DNAm and GE in peripheral blood collected from two cohorts. Both proximal and distal relationships were identified, highlighting potential inaccuracies in the current functional interpretation of trait-associated CpG sites within the frequently adopted EWAS framework.
Across cohorts, DNAm was significantly associated with both proximal (cis) and distal (trans) GE. The primary findings of this study align with other reports of long-range DNAm–GE relationships, adding to the growing body of literature questioning the accuracy of current EWAS interpretations [18,26,68]. The effect sizes detected among DNAm–GE pairs were relatively large, with DNAm predicting 42–50% percent of GE variability on average. Although this result is likely influenced by a lack of statistical power to detect more attenuated relationships, it reiterates that while DNAm may not be an appropriate proxy for changes in GE, strong links between the two measurements exist. Approximately 23% of connections identified in PREG were also significant in the AYATS cohort, suggesting a consistent programme of gene regulation even among the disparate cohorts tested. While this proportion is similar to DNAm–GE connections identified in peripheral blood and isolated monocytes [26], discrepancies between the two cohorts could be related to differences in statistical power or differences in demographic and clinical features (i.e., genetic ancestry, developmental stage, etc.). On average, cis connections were more likely to replicate between studies and account for larger proportion of GE variability when compared to within-cohort trans associations. Larger samples are likely necessary to detect more subtle cis and trans CpG-GE pairings and provide a balanced assessment of the expected replication across samples.
Interpreting DNAm–disease relationships is hindered not only by limitations in identifying DNAm–GE pairs but also by challenges in predicting the precise functional impact of altered DNAm on an associated gene’s expression. Both negative and positive relationships between cis and trans DNAm–GE pairs were identified (Figure 1). Although DNAm is usually considered a repressive mark, inhibiting GE by either blocking transcription factor binding or by promoting a more condensed DNA conformation [17], positive DNAm–GE relationships could be explained by several mechanisms. Within genomic regulatory elements, transcription factors with repressive, rather than activating properties, may bind unmethylated sequences [25]. Furthermore, many transcription factors actually exhibit an increased affinity for heavily methylated sites [23]. Besides influencing the binding affinity of regulatory proteins, DNAm patterns may also reflect a history of transcription factor binding, a phenomenon that cannot be separately identified by a classic EWAS design [69]. In recent years, speculation has emerged regarding potential alternative roles of DNAm in the cell, including theories that DNAm may serve to direct splicing regulation or in maintaining genomic stability within specific regions [4,70–73]. Although this study found that the associations were predominantly negative across the majority of gene regions (Figure 1), these findings agree with other reports that strong positive DNAm–GE relationships exist [26,27,54]
Given the large effect size distribution of detected associations and the modest number of participants in each cohort, it was expected that only a small proportion of DNAm–GE connections would reach statistical significance. Instead of only focusing on individual connections, this study sought to outline genome-wide trends by identifying attributes of GE-associated CpG sites. Despite the modest number of DNAm–GE pairs overlapping across cohorts, GE-associated CpG sites displayed similar annotation characteristics (Tables 3 and 4). Annotations uniquely characterized attributes of cis and trans GE-associated CpGs, indicating that separate paradigms may exist for proximal and distal connections. In general, DNAm within intermediate CpG density regions were more likely to be associated with GE. Regions of intermediate CG density are more variable compared with low- or high-density regions, and appear more dynamic across tissues and developmental stages [74,75]. Conversely, CpG sites in high-density regions, which are most often associated with CpG islands and promoter regions, were consistently depleted. Interestingly, both transcription factor binding and chromatin states in regions near active TSS were enriched, with those regions directly downstream of the TSS particularly characterized by a high proportion of GE-associated CpGs (Figure 3). Other studies have noted a similar relationship with DNAm located in the first intron, while also observing high transcription factor activity typical of intronic enhancers within these areas [65,76,77]. Although mechanisms of DNAm transcriptional inactivation usually focus on the hypermethylation of CpG sites within promoter and island regions, these results agree with other studies showing enrichment for “off-island” DNAm among GE-associated CpG sites [26,74]
The DNAm regulatory paradigm is predicated on the importance of DNAm in promoter regions, yet in this study CpG associations in promoters were depleted. Results from this study instead reiterated the significance of DNAm within enhancer regions and suggest that additional chromatin areas should be considered in addition to promoter-associated DNAm [26,29]. Multiple enhancer definitions (i.e., enhancer-like chromatin states and enhancer annotations generated from cap analysis of gene expression [CAGE]) were enriched within trans results. Enhancers have an established role in long-range gene regulation [8,78], often looping over more proximal genes to interact with those farther away [79]. Enhancer regions are often characterized by intermediate DNAm and chromatin accessibility, demonstrate greater DNAm variability than promoters [57,74], and exhibit ongoing de novo methylation and demethylation activity [53]. The high rate of DNAm remodelling within enhancers, coupled with the strong DNAm–GE relationships found within these regions, align with hypotheses that suggest environmental exposures can influence complex disease risk through epigenetic mechanisms of transcriptional dysregulation. While mapping enhancers to their putative genes is a fundamental aim in identifying transcriptional regulatory networks, current methods are still under development [79], adding to the uncertainty in predicting the downstream functional effects of DNAm within these distal regulatory regions. Further challenges arise from evidence that many genes actually interact with multiple enhancers, and that these compounded interactions can result in additive effects on target GE [79].
This study serves to improve understanding of the relationships between DNAm and GE across the genome and cautions that, in the absence of concomitant GE measurements, EWAS should interpret DNAm–trait associations with care. Overall, these results identified that strong distal relationships between DNAm and GE are prevalent across the genome, highlighting issues with restricting DNAm-transcript annotations to small genomic intervals only [26,29]. The results from this study underscore concerns in predicting the biological mechanisms underlying disease from DNAm measurements alone and question the validity of assuming a cis DNAm–GE pathway without considering relevant features of the surrounding genomic landscape. EWAS relying on a DNAm-mediated transcriptional regulatory mechanism to interpret DNAm–trait associations may reach inaccurate conclusions about disease pathoetiology, as this approach fails to consider that the downstream effect of DNAm is likely context-dependent and may be impacted by the attributes of specific CpG sites. While modified EWAS that incorporate GE information by performing a differential expression analysis (i.e., testing GE-disease associations) alongside testing for DNAm–disease associations avoid relying on assumptions of altered GE, biologically relevant information may be lost with this study design since a priori assumptions link CpG sites to putative genes and distal DNAm–GE relationships remain uninvestigated.
Based on these results, epigenetic research should continue moving towards multi-omic approaches that integrate DNAm with other levels of data (e.g., GE, genotypes, transcription factor binding) to study complex traits. Although DNAm-GE relationships are highly complex, the integration of DNAm with data outlining regional chromatin architecture and transcription factor activity may assist in predicting the functional impact of altered DNAm [26,80]. However, as an emerging and heterogeneous field, several obstacles can interfere with the implementation and interpretation of multi-omic studies [81–84]. Standardized analytical pipelines have yet to be developed, leading to difficulties in cross-study comparisons and in assessing rigour, and it may be some time before multi-omic studies generate replicable findings [83]. Even as some of these issues are mitigated, determining how to meaningfully but accurately decipher results from DNAm-only studies remains paramount. EWAS have generated a wealth of information outlining relationships between DNAm, diseases, and environmental exposures [19]. Researchers should reconsider how best to interpret these findings moving forward, given the emerging understanding of the complexity of epigenetic regulatory mechanisms. Currently, only a handful of studies have tested genome-wide associations between GE and DNAm [26,29,41,68,74,85], but variability in the methodologies used has led to difficulties in determining the replicability and generalizability of identified relationships. Although cross-study comparisons are challenging, several consistent themes have emerged from this modest body of literature. This study replicates the overrepresentation of GE-associated CpGs within enhancers and at transcription factor binding sites, as well as the depletion within islands and promoter regions[26]. By further cataloguing specific genome-wide DNAm–GE associations and identifying attributes of GE-associated CpG sites, results from this study can be used to inform EWAS design and the interpretation of DNAm–trait associations. Moving forward, continued examination of DNAm–GE relationships in large, diverse cohorts should be prioritized to advance our understanding of the role of DNAm within the cell and disease biology.
Strengths and Limitations
To our knowledge, this was the first study to assess the global relationship between peripheral blood DNAm and GE in both a primary and replication sample. However, results of this study should be considered in the context of the following limitations. First, both DNAm and GE were measured by microarray technologies that provided coverage within well-characterized locations, but were unable to assay the full extent of RNA and CpG sites in the genome [86]. GE was measured on a gene-level, rather than transcript-level, array platform, so that specific transcript variants were not analysed individually. Future analyses with more comprehensive measurements, particularly those that utilize technology with transcript-level resolution (e.g., RNA-seq), are necessary for confirming genome-wide trends. Second, only relationships of relatively large effect size were detected in this study (adjusted R-squared range = 0.23–0.90). Especially given that a conservative multiple testing correction was applied, it is assumed that many more DNAm–GE connections exist but were undetected in this study, which could influence the results of the feature enrichment tests. Third, both cohorts were analysed cross-sectionally, a study design that is unable to provide evidence for causation or directionality [14,16]. Mechanisms of reverse causation, in which changes to DNAm occur in response to modified GE, have been observed [87]. Therefore, it is unknown whether changes to DNAm are actually proceeding changes in GE as described in the canonical mechanism. Fourth, some annotations were derived from experiments conducted on a well-described lymphoblastoid cell line (GM12787), which was selected based on data that supports the genetic and functional similarity to mature blood cells (i.e., T cells and B cells) [88]. One benefit of using this approach is that annotations were kept consistent across the different functional enrichment categories (e.g., chromatin landscapes, enhancer definitions, etc.). It remains important to consider that this study focused on the association between DNAm at individual CpG sites and GE. In actuality, regional changes in DNAm may be co-regulating GE in some instances, and future studies can examine how regions of CpGs work in concert to regulate GE [68,89]. Finally, this study only investigated DNAm and GE in the peripheral blood and may not generalize to other tissues [65]. Future analyses with more comprehensive measurements in alternative tissues will be crucial for characterizing genome-wide trends across cell types.
Supplementary Material
Funding Statement
This work was supported by the National Center for Advancing Translational Sciences [UL1TR000058]; National Institute of Mental Health [MH106924]; National Institute of Mental Health [MH101518]; National Institute on Minority Health and Health Disparities [P60MD002256]; Brain and Behavior Research Foundation [21976].
Abbreviations
| AYATS | Adolescent and Young Adult Twin Study |
| DMPs | Differentially methylated positions |
| DNAm | DNA methylation |
| EWAS | Epigenome-wide association study |
| FDR | False discovery rate |
| GE | Gene expression |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopaedia of Genes and Genomes |
| PREG | Pregnancy, Race, Environment, Genes study |
| TSS | Transcriptional start site |
| UTRs | Untranslated regions |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2022.2079293
Author contributions
All authors assisted with the design of the study. EL cleaned the data, performed the analyses, and wrote the initial draft of the manuscript. RRN, VV, BR, JL and TY provided substantive feedback and revisions, and TY and RRN planned and secured funding for the PREG and AYATS studies.
Data availabilty
Sharing PREG and AYATS study data is limited by Institutional Review Board agreements and participant consent forms, which restrict openly sharing individual-level measures. Anyone interested in data access or collaboration is encouraged to contact Dr. Timothy P. York (timothy.york@vcuhealth.org) or Dr. Roxann Roberson-Nay (roxann.robersonnay@vcuhealth.org) for more information.
Informed consent and ethical approvals
Both the AYATS and PREG study received Virginia Commonwealth University Institutional Review Board approval and obtained written participant consent for each participant.
References
- [1].Kaur G, Begum R, Thota S, et al. A systematic review of smoking-related epigenetic alterations. Arch Toxicol. 2019. Oct;93(10):2715–2740. [DOI] [PubMed] [Google Scholar]
- [2].Anderson OS, Sant KE, Dolinoy DC.. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012. Aug;23(23):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vinkers CH, Kalafateli AL, Rutten BPF, et al. Traumatic stress and human DNA methylation: a critical review. Epigenomics. 2015;7(4):593–608. [DOI] [PubMed] [Google Scholar]
- [4].Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019. Oct;20(10):590–607. [DOI] [PubMed] [Google Scholar]
- [5].York TP, Eaves LJ, Neale MC, et al. The contribution of genetic and environmental factors to the duration of pregnancy. 3rd. Am J Obstet Gynecol. 2014. May;210(5):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Roberson-Nay R, Lapato DM, Wolen AR, et al. An epigenome-wide association study of early-onset major depression in monozygotic twins. Transl Psychiatry. 2020. Aug;10(10):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Bustos C, Ramos E, Young JM, et al. Tissue-specific variation in DNA methylation levels along human chromosome 1. Epigenetics Chromatin. 2009. June;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. [DOI] [PubMed] [Google Scholar]
- [9].van der Maarel SM. Epigenetic mechanisms in health and disease. Ann Rheum Dis. 2008. Dec;67(3):iii97–100. [DOI] [PubMed] [Google Scholar]
- [10].Zhang X, Gierman HJ, Levy D, et al. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC Genomics. 2014. June;15(15):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hannon E, Knox O, Sugden K, et al. Characterizing genetic and environmental influences on variable DNA methylation using monozygotic and dizygotic twins. PLoS Genet. 2018. Aug;14(8):e1007544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teh AL, Pan H, Chen L, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014. July;24(7):1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Dongen J, Ehli EA, Slieker RC, et al. Epigenetic variation in monozygotic twins: a genome-wide analysis of DNA methylation in buccal cells. Genes (Basel). 2014. May;5(2):347–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lappalainen T, Greally JM. Associating cellular epigenetic models with human phenotypes. Nat Rev Genet. 2017. July;18(7):441–451. [DOI] [PubMed] [Google Scholar]
- [15].Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb). 2010. July;105(1):4–13. [DOI] [PubMed] [Google Scholar]
- [16].Birney E, Smith GD, Greally JM. Epigenome-wide association studies and the interpretation of disease -omics. PLoS Genet. 2016. June;12(6):e1006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schübeler D. Function and information content of DNA methylation. Nature. 2015. Jan;517(7534):321–326. [DOI] [PubMed] [Google Scholar]
- [18].Teschendorff AE, Relton CL. Statistical and integrative system-level analysis of DNA methylation data. Nat Rev Genet. 2018. Mar;19(3):129–147. [DOI] [PubMed] [Google Scholar]
- [19].Michels KB, Binder AM, Dedeurwaerder S, et al. Recommendations for the design and analysis of epigenome-wide association studies. Nat Methods. 2013. Oct;10(10):949–955. [DOI] [PubMed] [Google Scholar]
- [20].Zhong H, Kim S, Zhi D, et al. Predicting gene expression using DNA methylation in three human populations. PeerJ. 2019. May;7:e6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Du J, Johnson LM, Jacobsen SE, et al. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015. September;16:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu H, Chen Y, Lv J, et al. Quantitative epigenetic co-variation in CpG islands and co-regulation of developmental genes. Sci Rep. 2013;3(1):2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yin Y, Morgunova E, Jolma A, et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science. 2017;356(6337). 10.1126/science.aaj2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Doane AS, Elemento O. Regulatory elements in molecular networks. Wiley Interdiscip Rev Syst Biol Med. 2017. May;9(3). 10.1002/wsbm.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith J, Sen S, Weeks RJ, et al. Promoter DNA hypermethylation and paradoxical gene activation. Trends Cancer Res. 2020. May;6(5): 392–406. [DOI] [PubMed] [Google Scholar]
- [26].Kennedy EM, Goehring GN, Nichols MH, et al. An integrated -omics analysis of the epigenetic landscape of gene expression in human blood cells. BMC Genomics. 2018. June;19(19):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wagner JR, Busche S, Ge B, et al. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014. Feb;15(2):R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009. Nov;462(7271):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aran D, Hellman A. DNA methylation of transcriptional enhancers and cancer predisposition. Cell. 2013. July;154(1):11–13. [DOI] [PubMed] [Google Scholar]
- [30].Varley KE, Gertz J, Bowling KM, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013. Mar;23(3):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang Y-T. Integrative modeling of multi-platform genomic data under the framework of mediation analysis. Stat Med. 2015. Jan;34(1):162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mamrut S, Avidan N, Truffault F, et al. Methylome and transcriptome profiling in myasthenia gravis monozygotic twins. J Autoimmun. 2017. Aug;82:62–73. [DOI] [PubMed] [Google Scholar]
- [33].Gillberg L, Perfilyev A, Brøns C, et al. Adipose tissue transcriptomics and epigenomics in low birthweight men and controls: role of high-fat overfeeding. Diabetologia. 2016. Apr;59(4):799–812. [DOI] [PubMed] [Google Scholar]
- [34].Tian W, Li Y, Zhang J, et al. Combined analysis of DNA methylation and gene expression profiles of osteosarcoma identified several prognosis signatures. Gene. 2018. Apr;650:7–14. [DOI] [PubMed] [Google Scholar]
- [35].Song D, Qi W, Lv M, et al. Combined bioinformatics analysis reveals gene expression and DNA methylation patterns in osteoarthritis. Mol Med Rep. 2018. June;17(6):8069–8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miao L, Yin R-X, Zhang Q-H, et al. Integrated DNA methylation and gene expression analysis in the pathogenesis of coronary artery disease. Aging (Albany NY). 2019. Mar;11(5):1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Z, Wu X, Wang Y. A framework for analyzing DNA methylation data from Illumina Infinium HumanMethylation450 BeadChip. BMC Bioinformatics. 2018. Apr;19(S5):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang Y, Fang L, Zang Y, et al. Identification of core genes and key pathways via integrated analysis of gene expression and DNA methylation profiles in bladder cancer. Med Sci Monit. 2018. May;24:3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li H, Wang F-L, Shan L-P, et al. Comprehensive analysis of gene expression and DNA methylation for human nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2019. Sept;276(9): 2565–2576. [DOI] [PubMed] [Google Scholar]
- [40].Bell JT, Pai AA, Pickrell JK, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 2011. Jan;12(1):R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van Eijk KR, de Jong S, Boks MPM, et al. Genetic analysis of DNA methylation and gene expression levels in whole blood of healthy human subjects. BMC Genomics. 2012. Nov;13(13):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cecilione JL, Rappaport LM, Hahn SE, et al. Genetic and environmental contributions of negative valence systems to internalizing pathways. Twin Res Hum Genet. 2018. Feb;21(21):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lapato DM, Moyer S, Olivares E, et al. Prospective longitudinal study of the pregnancy DNA methylome: the US Pregnancy, Race, Environment, Genes (PREG) study. BMJ Open. 2018. May;8(5):e019721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].R Core Team . “R: a language and environment for statistical computing. Vienna Austria: ”: R Foundation for Statistical Computing; 2016. [Google Scholar]
- [45].Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012. May;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maes M, Meltzer HY, Stevens W, et al. Natural killer cell activity in major depression: relation to circulating natural killer cells, cellular indices of the immune response, and depressive phenomenology. Prog Neuropsychopharmacol Biol Psychiatry. 1994. July;18(18):717–730. [DOI] [PubMed] [Google Scholar]
- [47].Barfield RT, Almli LM, Kilaru V, et al. Accounting for population stratification in DNA methylation studies. Genet Epidemiol. 2014. Apr;38(3): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Guintivano J, Shabalin AA, Chan RF, et al. Test-statistic inflation in methylome-wide association studies. Epigenetics. 2020. May;15(11):1163–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goñi JR, Pérez A, Torrents D, et al. Determining promoter location based on DNA structure first-principles calculations. Genome Biol. 2007;8(12):R263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brandeis M, Frank D, Keshet I, et al. Sp1 elements protect a CpG island from de novo methylation. Nature. 1994. Sept;371(6496):435–438. [DOI] [PubMed] [Google Scholar]
- [52].Mummaneni P, Yates P, Simpson J, et al. The primary function of a redundant sp1 binding site in the mouse aprt gene promoter is to block epigenetic gene inactivation. Nucleic Acids Res. 1998. Nov;26(22):5163–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Feldmann A, Ivanek R, Murr R, et al. Transcription factor occupancy can mediate active turnover of DNA methylation at regulatory regions. PLoS Genet. 2013. Dec;9(12):e1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xie -F-F, Deng F-Y, Wu L-F, et al. Multiple correlation analyses revealed complex relationship between DNA methylation and mRNA expression in human peripheral blood mononuclear cells. Funct Integr Genomics. 2018. Jan;18(18):1–10. [DOI] [PubMed] [Google Scholar]
- [55].Zhao Y, Sun H, Wang H. Long noncoding RNAs in DNA methylation: new players stepping into the old game. Cell Biosci. 2016. July;6(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yu L, Xia K, Cen X, et al. DNA methylation of noncoding RNAs: new insights into osteogenesis and common bone diseases. Stem Cell Res Ther. 2020. Mar;11(11):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roadmap Epigenomics Consortium AK, Meuleman W, Ernst J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015. Feb;(518):317–330. 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012. Feb;9(9):215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987. July;196(2):261–282. [DOI] [PubMed] [Google Scholar]
- [60].Price ME, Cotton AM, Lam LL, et al. Additional annotation enhances potential for biologically-relevant analysis of the Illumina Infinium HumanMethy- lation450 BeadChip array. Epigenetics Chromatin. 2013. Mar;6(6):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cavalcante RG, Sartor MA. annotatr: genomic regions in context. Bioinformatics. 2017. Aug;33(15):2381–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Project Consortium ENCODE. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012. Sept;489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res. 2012. Sept;22:1760–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Andersson R, Gebhard C, Miguel-Escalada I, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014. Mar;507(7493):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Anastasiadi D, Esteve-Codina A, Piferrer F. Consistent inverse correlation between DNA methylation of the first intron and gene expression across tissues and species. Epigenetics Chromatin. 2018. June;11(11):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Carlson M, Maintainer BP. TxDb.Hsapiens.UCSC.hg19.knownGene: annotation package for TxDb object(s), 2015. R package version 3.2.2.
- [67].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- [68].Cheng J, Wei D, Ji Y, et al. Integrative analysis of DNA methylation and gene expression reveals hepatocellular carcinoma-specific diagnostic biomarkers. Genome Med. 2018. May;10(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhu H, Wang G, Qian J. Transcription factors as readers and effectors of DNA methylation. Nat Rev Genet. 2016. Aug;17(9):551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010. July;466(7303):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lev Maor G, Yearim A, Ast G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015. May;31(5):274–280. [DOI] [PubMed] [Google Scholar]
- [72].Irizarry RA, Ladd-Acosta C, Wen B, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009. Feb;41(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007. Apr;8(4):272–285. [DOI] [PubMed] [Google Scholar]
- [74].Liu Y, Ding J, Reynolds LM, et al. Methylomics of gene expression in human monocytes. Hum Mol Genet. 2013. Dec;22(24):5065–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Doi A, Park I-H, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009. Dec;41(12):1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vanderkraats ND, Hiken JF, Decker KF, et al. Discovering high-resolution patterns of differential DNA methylation that correlate with gene expression changes. Nucleic Acids Res. 2013. Aug;41(14):6816–6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schlosberg CE, VanderKraats ND, Edwards JR. Modeling complex patterns of differential DNA methylation that associate with gene expression changes. Nucleic Acids Res. 2017. May;45(9):5100–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011. Feb;144(3):327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schoenfelder S, Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat Rev Genet. 2019. Aug;20(8):437–455. [DOI] [PubMed] [Google Scholar]
- [80].Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016. Sept;8(9):1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pinu FR, Beale DJ, Paten AM, et al. Systems biology and multi-omics integration: viewpoints from the metabolomics research community. Metabolites. 2019. Apr;9(4):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Haas R, Zelezniak A, Iacovacci J, et al. Designing and interpreting ‘multi- omic’ experiments that may change our understanding of biology. Curr Opin Syst Biol. 2017. Dec;6:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Subramanian I, Verma S, Kumar S, et al. Multi-omics data integration, interpretation, and its application. Bioinform Biol Insights. 2020. Jan;14(14):1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bersanelli M, Mosca E, Remondini D, et al. Methods for the integration of multi-omics data: mathematical aspects. BMC Bioinformatics. 2016. Jan;17(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Taylor DL, Jackson AU, Narisu N, et al. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc Natl Acad Sci U S A. 2019. May;116(22):10883–10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bibikova M, Barnes B, Tsan C, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011. Oct;98(4):288–295. [DOI] [PubMed] [Google Scholar]
- [87].Pacis A, Mailhot-Léonard F, Tailleux L, et al. Gene activation precedes DNA demethylation in response to infection in human dendritic cells. Proc Natl Acad Sci U S A. 2019. Apr;116(14):6938–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sie L, Loong S, Tan EK. Utility of lymphoblastoid cell lines. J Neurosci Res. 2009. July;87(9):1953–1959. [DOI] [PubMed] [Google Scholar]
- [89].Yong W-S, Hsu F-M, Chen P-Y. Profiling genome-wide DNA methylation. Epigenetics Chromatin. 2016. June;9(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


