ABSTRACT
Introduction
With 583 million inhabitants, the Eastern Mediterranean Region (EMR) is a worldwide hub for travel, migration, and food trade. However, there is a scarcity of data on the epidemiology of the hepatitis A virus (HAV).
Methods
The MEDLINE and grey literature were systematically searched for HAV epidemiological data relevant to the EMR region published between 1980 and 2020 in English, French, or Arabic.
Results
Overall, 123 publications were extracted. The proportion of HAV cases among acute viral hepatitis cases was high. HAV seroprevalence rate ranged from 5.7% to 100.0% and it was decreasing over time while the average age at infection increased.
Conclusion
In the EMR, HAV remains a significant cause of acute viral hepatitis. The observed endemicity shift will likely increase disease burden as the population ages. Vaccinating children and adopting sanitary measures are still essential to disease prevention; vaccinating at-risk groups might reduce disease burden even further.
KEYWORDS: Eastern Mediterranean Region, endemicity, hepatitis A, incidence, seroprevalence
Plain Language Summary
What is the context?
Hepatitis A is a viral liver disease caused by the hepatitis A virus.
It is generally transmitted by ingestion of contaminated food or water or through contact with an infected person.
Disease severity increases with age. Children under 6 years of age are usually asymptomatic, while adults are the most affected.
Limited information exists on the number of cases and transmission of hepatitis A in the Eastern Mediterranean region, which includes 21 countries and Palestine, as defined by the World Health Organization.
What is new?
We performed a literature review to summarize data on hepatitis A disease in the Eastern Mediterranean region over the last 40 years (1980-2020). As information for many countries is scarce or outdated, most of the data is from Egypt, Iran and Saudi Arabia.
- We found that:
- Hepatitis A virus is the most common cause of acute viral hepatitis.
- Hepatitis A exposure varied according to the country’s income level.
- Low- and middle-income countries showed a universal immunity to hepatitis A virus, although this is not the case anymore.
What is the impact?
Hepatitis A infections have decreased worldwide. Lower exposure to the virus has led to an increase in the susceptible population (including adolescent and adults).
Hepatitis A vaccination for children and high-risk groups such travelers should be considered in the Eastern Mediterranean region.
Introduction
Exposure to the hepatitis A virus (HAV) causes viral hepatitis which is characterized by inflammation of the liver. Globally, more than 100 million HAV infections and 30,000–35,000 deaths are reported annually.1 HAV is transmitted through the fecal-oral route, entering via the mouth and replicating in the liver.1 The ingestion of contaminated food or water, poor sanitation, and contact with an infected individual are the primary sources of infection.1,2 Clinically, HAV infection is similar to other types of acute hepatitis, with elevated levels of liver enzymes, dark-colored urine, and the onset of jaundice. It is accompanied by broad symptoms like fatigue, malaise, and abdominal pain.3 The severity and outcome of the disease is negatively correlated with the age at infection. Infected children under six years of age are usually asymptomatic (~70% cases), while older children and adults show symptoms of jaundice (~70% cases).3 The fatality rate increases with increasing age, from 0.1% (<15 years of age), to 0.3% (15–39 years of age) and 2.1% (≥40 years of age).4 Infection due to HAV can be diagnosed by serological testing in the presence of anti-HAV immunoglobulin M (IgM) and immunoglobulin G (IgG).5 The presence of IgM antibodies is indicative of a recent HAV infection, while the detection of IgG antibodies suggests previous exposure to HAV or vaccination, as IgG antibodies persist over time and confer lifelong immunity.3,5 The measurement of IgG antibodies is an indirect method of measuring seroprevalence, overall and by age, and can be used to assess the endemicity level (i.e., the circulation of the HAV) in a given population.2
Inactivated and live attenuated hepatitis A vaccines have proven to be immunogenic, well tolerated and safe in the target-vaccine population.6–8 The World Health Organization (WHO) recommends the inclusion of hepatitis A immunization into the national immunization schedule for children ≥1 year of age, taking into consideration the incidence of acute HAV cases, the endemicity level (high to moderate), and cost-effectiveness data.2 Notwithstanding this recommendation, the WHO states that vaccination should be part of a comprehensive plan for the prevention and control of viral hepatitis, including measures to improve hygiene, sanitation and outbreak control.2
Broader access to clean water and sanitation, and improved socio-economic conditions are changing the epidemiology of HAV infection.9,10 Due to globalization, rising income, and better infrastructure, low- to middle-income countries are undergoing a shift from high/intermediate to low HAV incidence rates, and high-income countries are now non-endemic to HAV infection.11 Importantly, countries reporting low or intermediate HAV endemicity, including those countries in transition from high to low HAV endemicity, are particularly susceptible to recurrent outbreaks of symptomatic disease.12
Given this context of evolving HAV epidemiology, the WHO Eastern Mediterranean Region (EMR) deserves attention. The EMR includes 21 member states and Palestine comprising nearly 600 million people.13 This region is comprised of middle-income (11) as well as high-income (6) and low-income (5) countries as classified by the World Bank (2017).14 In the last decade, EMR countries have documented a significant improvement in their socio-economic conditions. Advances in modern transportation and global accessibility, in particular, have boosted the travel and food industries. However, the EMR has also seen a rise in armed conflict, which has increased the rate of human migration and disease mobility. As a result, the EMR reports the highest global number of people displaced from their home countries.15 Refugees displaced from high endemicity countries represent a source of contagion for their new country, especially if their housing is crowded and with poor sanitation and hygiene conditions.
There is limited information on the epidemiology of HAV disease in EMR countries, specifically in relation to shifts of HAV endemicity.16,17 This review aims to explore HAV epidemiology by collecting and summarizing the serological data from the EMR region. The review highlights the importance of the EMR as a globalized hub for travel, migration, and food trade to bring awareness toward the probability of future global outbreaks of HAV disease (Figure 1).
Figure 1.
Plain language summary.
Methods
A comprehensive review utilizing a systematic approach was performed to identify published literature on HAV incidence and seroprevalence in the WHO-EMR13 covering 22 countries according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.18 According to these guidelines, we defined search sources, search strategy, the inclusion, and exclusion criteria to identify and select relevant publications, and the scope of data extraction prior to the conduct of the review.
Search sources and strategy
The search was conducted in MEDLINE (via PubMed) and complemented with a search of gray literature sources such as Ministry of Health (MoH) websites and reports from universities. We developed a broad search strategy using free-text terms (”HAV”; “COUNTRY NAME”) and medical subject heading (MeSH) terms linked by Boolean operators.
Searches were limited to a period of 40 years, i.e., from 1980 to July 2020. The lower limit of the period was considered appropriate by the authors as it allows to observe shifts in the burden of disease, if any. The countries of interest, based on the geographic scope of this review, were limited to the WHO-EMR covering 22 countries. Searches were conducted in both English and the local language of each included country (Table 1).
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Hepatitis A disease (not limited to risk groups or specific ages) | Populations with chronic diseases or underlying comorbidities that are not representative of the general population |
| Intervention | Not restricted by intervention | N.A. |
| Comparator | Not restricted by comparator | N.A. |
| Outcome | Proportion of HAV among all acute viral hepatitis (HAV IgM) | N.A. |
| HAV seroprevalence (HAV IgG) | ||
| Study design | Primary peer-reviewed research observational studies | Non-primary research |
| Cohort studies | Systematic reviews | |
| Case-control studies | Meta-analyses | |
| Cross-sectional studies | Narrative reviews (without methods) | |
| Ecological studies | Predictions via modeling methods | |
| Outbreak investigations | Case reports | |
| Periodic surveys | Letter to editor | |
| Non-peer-reviewed research | Newspaper | |
| Reports from national and regional databases or websites | Editorial | |
| Comment | ||
| Opinions | ||
| Limits | ||
| Publication date | From 1980 onwards | All publications before 1980 |
| Geographic scope | Afghanistan, Bahrain, Djibouti, Egypt, Islamic Republic of Iran, Republic of Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syrian Arab Republic, Tunisia, United Arab Emirates, Yemen | All countries apart from those considered eligible |
| Language | English, French, Arabic | - |
Note: HAV, hepatitis A virus; IgG, immunoglobulin G; IgM, immunoglobulin M; N.A, not applicable.
Screening and selection
The identified publications were screened in two phases by two reviewers in an independent process using the inclusion and exclusion criteria listed in Table 1. The retrieved articles were initially screened by title and abstract for eligibility by one reviewer (AO, MK, YL, or OO) followed by a second step which included screening of the full text of articles using the eligibility criteria specified in Table 1. All discrepancies were discussed with an additional reviewer (SB).
Original research from non-interventional studies or from gray literature sources was included if it reported data on the occurrence of hepatitis A (defined as previous exposure to HAV confirmed by laboratory detection of HAV IgM) and seroprevalence of HAV (defined as previous exposure to HAV confirmed by laboratory detection of HAV IgG or total HAV immunoglobulin (Ig) in blood samples). Case reports and other publication formats such as commentaries, editorials, and letters were excluded from this review. Reviews and meta-analyses were consulted with the intention to screen their reference lists for eligible articles.
Data extraction and reporting
The information extracted from selected studies included study characteristics (year of publication, study design, main objective of the study and sample size), age group of the study population and case definition (e.g., laboratory confirmation methods). The occurrence of HAV (HAV cases expressed as a proportion of all acute viral hepatitis cases) and HAV seroprevalence (expressed as a percentage of patients with previous exposure to HAV measured according to the test kit specifications) were extracted and reported. When available, the same outcomes were reported and compared by age group, socioeconomic status, year, type of setting (rural versus urban), and acute viral hepatitis caused by other types (hepatitis B virus, hepatitis E virus, etc.).
Results
Included studies and their characteristics
Overall, the search yielded 315 publications (MEDLINE: n = 296; gray literature: n = 19). Of these, 157 were excluded at the title or abstract screening phase and 35 were further excluded after full-text review. Finally, a total of 123 publications for 22 countries in the EMR were included in the final review (Figure 2).
Figure 2.
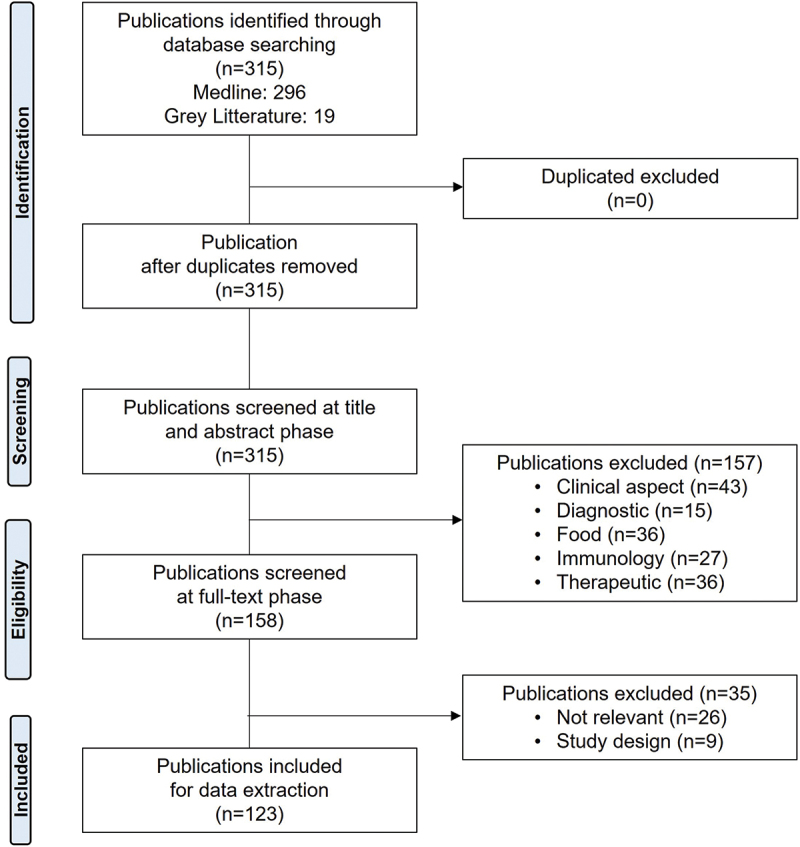
PRISMA flow diagram showing the study research and selection process.
Among the 123 publications which provided data on hepatitis A disease for the 21 countries in the EMR and Palestine (Table 2), the distribution of publications by country was: Saudi Arabia (n = 30),19–46 followed by Iran (n = 28),47–74 Egypt (n = 19),75–93 Pakistan (n = 8),94–101 Lebanon (n = 6),102–107 Tunisia (n = 6),108–113 Iraq (n = 4),114–117 Kuwait (n = 3),118–120 Somalia (n = 3),121–123 Djibouti (n = 2),124,125 Jordan (n = 2),126,127 Syria (n = 2),128,129 UAE (n = 2),130,131 Yemen (n = 2),132,133 Afghanistan (n = 1),134 Libya (n = 1),135 Morocco (n = 1),136 Palestine (n = 1),137 Qatar (n = 1)138 and Sudan (n = 1) (Figure 3).139
Table 2.
Demographic characteristics and HAV vaccination status of the 22 EMR countries.
| COUNTRY | Geographic region | World bank classification (2017)14 | GAVI eligibility (2017)140 | HAV vaccination in NIP | Year of vaccination implementation | Availability of vaccine (private/public) | Recommendation status | Reimbursement |
|---|---|---|---|---|---|---|---|---|
| Afghanistan | Asia | LIC | Yes | No | N.A. | Private | - | No |
| Bahrain | Middle East | HIC | No | Yes | 2012 | Public | 15 and 24 months High risk groups and travellers141 |
Reimbursed |
| Djibouti | Middle East | LMIC | NIP through GAVI support | No | - | - | No | - |
| Egypt | Africa | LMIC | No | No | - | Private | - | No |
| Iran, Islamic Republic Of | Asia | UMIC | No | No | - | Private | - | No |
| Iraq | Middle East | UMIC | No | High risk group | - | Private | - | No |
| Jordan | Middle East | UMIC | No | No | - | Private | - | No |
| Kuwait | Middle East | HIC | No | No | - | Private | Citizens born prior to 1990 and healthcare personnel141 | No |
| Lebanon | Middle East | UMIC | No | No | - | Private | - | No |
| Libya | Africa | UMIC | No | No | - | - | No | - |
| Morocco | Africa | LMIC | No | No | - | Private | 18 and 24 months | In private market: covered by insurance |
| Oman | Middle East | HIC | No | Yes | 2020 | Both | 13 and 24 months | Reimbursed |
| Pakistan | Asia | LMIC | Yes | No | N.A. | Private | * | No |
| Palestine | Middle East | LIC | No | No | - | Private | 18 and 24 months | No |
| Qatar | Middle East | HIC | No | Yes | 2012 | Both | 12 and 18 months | Reimbursed |
| Saudi Arabia | Middle East | HIC | No | Yes | 2008 | Both | 18 and 24 months | In private market: covered by insurance, Public: FoC for Saudi, non-Saudi and illegal immigrants |
| Somalia | Asia | LIC | Yes | No | - | - | - | - |
| Sudan, Republic of | Africa | LIC | Yes | No | - | - | - | No |
| Syrian Arab Republic | Middle East | LMIC | Yes | No | - | Private | - | No |
| Tunisia | Africa | LMIC | No | Yes | 2019 | Both | 12 months and 6 years | In private market: covered by insurance, Public: FoC |
| United Arab Emirates | Middle East | HIC | No | No | - | Private | High risk groups and travelers141 | No |
| Yemen | Middle East | LIC | Yes | No | - | - | - | - |
*No local recommendations. Recommended by international bodies for HAV in case people are traveling to Pakistan.
FoC, free of charge; GAVI, the vaccine alliance; HIC, high-income countries; LIC, low-income countries; LMIC, low- middle- income countries; N.A., Not applicable; NIP, national immunization programs; UMIC, Upper-middle income countries; WHO-EMR, World Health Organization—Eastern Mediterranean Region.
Figure 3.
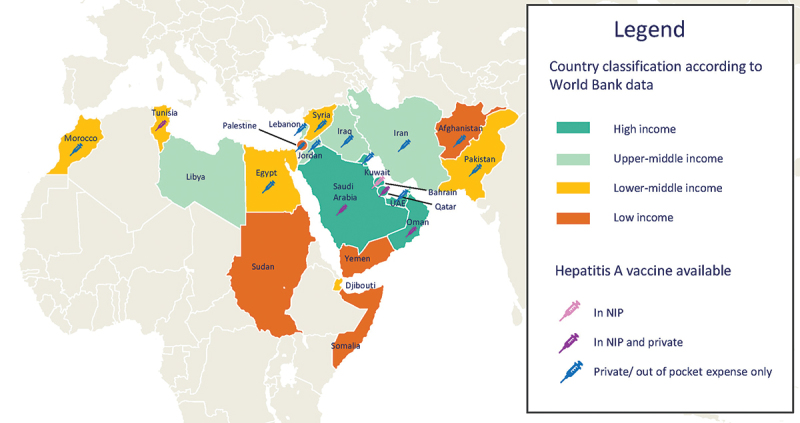
Classification of included countries by income level and hepatitis a vaccination status.
Notes: NIP, national immunization program; UAE: United Arab Emirates.
No study dealing with HAV could be identified for Bahrain and Oman. Among the countries included in this review, childhood hepatitis A vaccination has been implemented in the national immunization programs (NIP) of Bahrain, Oman, Qatar, Saudi Arabia, and Tunisia and only for high-risk groups in Iraq (Table 2). In most countries, however, hepatitis A vaccination is available in the private market (Table 2 and Figure 3).
Main findings from the review
Occurrence of HAV among acute viral hepatitis cases
A total of 41 studies provided data on HAV occurrence among all acute viral hepatitis cases. Overall, the proportion of HAV cases among acute viral hepatitis cases was large and ranged from 1.5% to 97.0% (Table 3). One study reported an increase in the proportion of HAV from 2001–2004 (40.2%) to 2014–2017 (89.7%); and reported a reduction in the proportion of patients infected with HAV before five years of age and an increase in the proportion of patients infected in an older age group.89 In patients with acute viral hepatitis, coinfection with hepatitis B, C, and E was documented in nine studies83,86,87,91,92,98,116,120,133 (Table 3).
Table 3.
Occurrence of HAV among acute viral hepatitis cases (41 studies).
| Studies by country | Data period, year(s) | Study population (number, age restrictions) | HAV, % (n) |
|---|---|---|---|
| Djibouti | |||
| Coursaget et al., 1998125 | 1992–1993 | 111 pts, 2–65 y | 33% (37) |
| Egypt | |||
| Fouad et al., 201885 | 2015–2017 | 268 pts, 1–18 y | 97% (260) |
| Talaat et al., 201989 | 2014–2017 | 9,321 pts, all ages | 93.4% (7,806) |
| Hasan et al., 201686 | 2007–2008 | 123 pts, 2–18 y | 13.8% (17) |
| Eldin et al., 201084 | 2007–2008 | 235 pts, 1–65 y | 8.1% (19) |
| Meky et al., 200688 | 2002–2005 | 47 community residents, 2–77 y | 8.5% (4) |
| Talaat et al., 201090 | 2001–2004 | 5,909 pts, all ages | 28.5% (1,684) |
| Zakaria et al., 200792 | 2001–2002 1983 |
200 pts, all ages 235 pts, all ages |
34% (68) 2.1% (5) |
| Hyams et al., 199287 | 1987–1988 | 73 outpatients, ≤13 y | 41% (30) |
| Divizia et al., 199983 | 1993 | 202 hospitalized pts, 1–73 y | 10.4% (21) |
| Youssef et al., 201391 | n.r. | 33 hospitalized children | 33% (11) |
| Zaki Mel et al., 200893 | n.r. | 162 children | 34.1% (n.r) |
| Darwish et al., 199282 | n.r. | 200 adult pts, 20–40 y | 4.5% (9) |
| Iran | |||
| Karimi et al., 201557 | 2010 | 70 pts | 68.6% (48) |
| Iraq | |||
| Al-Naaimi et al., 2012114 | 2010–2011 | 2,629 pts, all ages | 44.8% (1,206) |
| Turky et al., 2011117 | 2005–2006 | 2,975 pts, all ages | 41% (1,219) |
| Marcus et al., 1993115 | n.r. | 107 pts, 1.5–65 y | 40.2% (43) |
| Rassam et al., 1989116 | n.r. | 253 hospitalized pts, 3–65 y | 15% (39) |
| Kuwait | |||
| Al-Kandari et al., 1986120 | 1983–1984 | 1,788 pts, all ages | 1.5% (26) |
| Al-Kandari et al., 1987119 | 1980–1984 | 52 pregnant pts, 15–44 y | 11.5% (6) |
| Lebanon | |||
| Shamma’a et al., 1984106 | 1980–1981 | 93 pts, >12 y | 35.5% (33) |
| Pakistan | |||
| Khan et al., 201199 | 2007–2008 | 89 pts, all ages | 6.1% (4) |
| Ahmed et al., 201097 | 1987–2007 | 346 outpatients, all ages | 3.5% (12) |
| Waheed-uz-Zaman et al., 2006101 | 2003–2004 | 626 pts, all ages | 40.6% (252) |
| Syed et al., 2003100 | 1994–1999 | 658 pts, 11 y and over | 64.4% (424) |
| Haider et al., 199498 | 1991 | 93 hospitalized pts, all ages | 5.4% (5) |
| Qatar | |||
| Glynn et al., 1985138 | 1981 | 126 hospitalized pts, 13–52 y | 5.5% (7) |
| Saudi Arabia | |||
| Al-Tawfiq et al., 200832 | 2000–2005 | 1,214 pts, 1–94 y | 10% (120) |
| Memish et al., 200343 | 1999–2001 • 1999: • 2000: • 2001: |
3,490 pts, all ages • 1,194 pts • 1,039 pts • 1,257 pts |
8.2% (286) • 6.7% (80) • 6.9% (72) • 10.7% (134) |
| Fathalla et al., 200040 | 1987–1999 | 683 pediatric pts | 65% (641) |
| Ayoola et al., 200137 | 1997–1998 | 246 pts, all ages | 37% (91) |
| Arif et al., 199534 | 1993–1994 | 133 pts, all ages | 38.3% (51) |
| Yohannan et al., 199046 | 1987 | 47 pts, <12 y | 72% (34) |
| Al-Majed et al., 199028 | n.r. | 23 pts, all ages | 82.6% (19) |
| Al-Knawy et al., 199727 | n.r. | 132 hospitalized pts, >3 y | 81.8% (108) |
| Sudan | |||
| Hyams et al., 1991139 | 1987–1988 | 80 outpatients, <14 y | 33.8% (27) |
| Syria | |||
| Al-Azmeh et al., 1999129 | 1995–1998 | 193 pts, >12 y | 53.9% (104) |
| Tunisia | |||
| Neffatti et al., 2017110 | 2014–2015 | 92 pts, 1-62 y | 21.7% (20) |
| Gharbi-Khelifi et al., 2012112 | 2006–2008 | 400 pts, 1-60 y | 19.8% (79) |
| Hellera et al., 2014113 | 2004–2005 | 105 pts, 15–65 y | 34.3% (36) |
| Yemen | |||
| Gunaid et al., 1997133 | n.r. | 78 pts, ≥13 y | 5.1% (4) |
HAV, hepatitis A virus; n, number of study participants who were anti-HAV positive; n.r., not reported; pts, patients; y, years.
HAV seroprevalence
A total of 77 studies provided data on HAV seroprevalence. HAV seroprevalence ranged from 3% to 100%, depending on the age of the study population (Table 4). Overall, the EMR region has an intermediate level of HAV seroprevalence, and the data show a remarkable consistency. While seroprevalence studies from before the year 2000 showed nearly universal immunity among the general population in many countries of the EMR, after the year 2000, seroprevalence rates reveal that more adolescents and adults remain susceptible to HAV, although with significant variation within the region.
Table 4.
Seroprevalence of HAV (77 studies).
| Studies by country | Data period, year(s) | Study population (number, age restrictions) | HAV seroprevalence (IgG), % (n*) | ||
|---|---|---|---|---|---|
| Afghanistan | |||||
| Carmoi et al., 2009134 | 2008 | 102 anicteric pts, 5–65 y | 99% (101) • <15 y: 91.7% • ≥15 y: 100% |
||
| Djibouti | |||||
| Fox et al., 1988124 | 1987 | 400 healthy adults | 98.5% (394) | ||
| Egypt | |||||
| El-Karaksy et al., 200877 and El-Karaksy et al., 200678 | 2004 | 101 children with chronic liver disease (CLD), <18 y | 85.1% (86) • <5 y: 62.1% • ≥5 y: 94.4% |
||
| Al-Aziz et al., 200875 | 2002–2003 | 296 children with minor illnesses, 2.5–18 y | 61.4% (181) • 2.5–6 y: 53.1% • 6–9 y: 56.4% • 9–18 y: 73.8% |
||
| Salama et al., 200781 | 2003–2004 | 426 children with minor medical problems,3–18 y | 86.2% (367)
|
||
| Darwish et al., 199676 | 1994 | 155 healthy community residents, 1–67 y | 100% (155) • 1–3 y: 100% • ≤67 y: 100% |
||
| Kamel et al., 199579 | 1992 | 1,259 healthy community residents, all ages | 97.2% (1224) • 0–4 y: 92.7% • 5–9 y: 97.8% • 10–14 y: 97.9% • 15–19 y: 97.5% • 20–24 y: 96.6% • 25–29 y: 97.9% • 30–34 y: 95.5% • 35–39 y: 100% • 40–44 y: 93.3% • 45–49 y: 100% • 50–54 y: 97.6% • 55–59 y: 93.3% • 60–64 y:100% • 65–69 y: 100% • >70 y: 100% |
||
| Omar et al., 200080 | n.r. | 228 community residents, preschool children | 26.3% (60) | ||
| Iran | |||||
| Mirzaei et al., 201660 | 2014–2015 | 108 hemophilic pts, 4–85 y | 77.8% (84) | ||
| Hesamizadeh et al., 201653 | 2014 | 559 volunteer blood donors, >18 y | 70.7% (395) • 18–27 y: 26.7% • 28–37 y: 59.8% • 38–47 y: 91.2% • >47 y: 94.8% |
||
| Hosseini Shokouh et al., 201574 | 2012–2014 | 270, healthy medical students, 18–30 y | 34.8% (94) | ||
| Vasmehjani et al., 201573 | 2012–2013 | 159, CLD pts, 21–68 y | 79.2% (126) • 21–30 y: 28.6% • 31–40 y: 91.4% • 41–50 y: 93.9% • <50 y: 95%) |
||
| Izadi et al., 201655 | 2011–2013 | 1,554, healthy soldiers, 18–60 y | 80.3% (1,248) • <20 y: 72.2% • 20–30 y: 79.1% • >30 y: 92.4% |
||
| Farajzadegan et al., 201452 | 2003–2013 | 11,857 cumulative population of 16 studies (systematic review), all ages | 51%–66% | ||
| Jahanbakhsh et al., 201856 | 2012 | 569 homeless adults, 18–60 y | 94.3% (nr) • <42 y: 90.3% • ≥42 y: 98.1% |
||
| Asaei et al., 201548 | 2011–2012 | 1,030, healthy individuals, 0.5–95 y | 66.2% (682) • 6–15 y: 18.3% • 16–29 y 79.4% • 30–55 y: 94.3% • ≥56 y: 98.2% |
||
| Bayani et al., 201350 | 2011–2012 | 466 healthy healthcare workers | 71% (330) • 20–29 y: 57.8% • 30–39 y: 77.1% • >40 y: 86.3% |
||
| Rabiee et al., 201363 | 2011 | 1,813, healthy university students | 39.8% (722) | ||
| Shoaei et al., 201269 | 2010–2011 | 117, chronic hepatitis C pts | 94.9% (111) • ≤30 y: 93.1% • 31–40 y: 93.3% • 41–50 y: 100% • 51–60 y: 100% • ≥61 y: 100% |
||
| Vakili et al., 201472 | 2010 | 1,028, healthy 1st year medical students,17-27 y | 68.5% (704) | ||
| Saffar et al., 201267 | 2010 | 984, community residents, 1–30 y | 19.2% (189) • 1–2.9 y: 5.7% • 3–6.9 y: 9.1% • 7–10.9 y: 20.4% • 11–17.9 y: 34.8% • 18–30 y: 68.4% |
||
| Mostafavi et al., 201662 and Hoseini et al., 201654 | 2009–2010 | 2,494, national health survey participants,10–18 y | 50.4%–78.8% across provinces 64% (1,597) |
||
| Sofian et al., 201070 | 2009 | 1,065, pediatric hospital pts, 0.5–20 y | 61.6% • 0.5–1.9 y: 61.5% • 2–5.9 y: 51.7% • 6–10.9 y: 52.9% • 11–15.9 y: 65.2% • 16–20 y: 85.0% |
||
| Taghavi et al., 201171 | 2008–2009 | 1,050, pre-marriage lab analysis, 15–63 y | 88.2% (927) • <20 y: 79.3% • 20–30 y: 91.3% • >30 y: 99% |
||
| Ramezani et al., 201164 | 2008 | 351, blood donors, 17–60 y | 94.9% (333) | ||
| Saneian et al., 201468 | 2007 | 361, healthy medical students | 75.3% (272) | ||
| Alian et al., 201147 | 2007 | 1,034, community residents, 1–25 y | 38.9% (402) • 1–5 y: 8.9% • 5–15 y: 15.8% • 15–25 y: 64.3% |
||
| Mohebbi et al., 201261 | 2006–2007 | 551, community residents, 1–83 y | 90.0% (496) • <30 y: 85.7% • 30–60 y: 90.7% • >60 y: 93.9% |
||
| Merat et al., 201059 | 2006 | 1,869, community residents, 18–65 y | 86% | ||
| Davoudi et al., 201051 | 2005–2006 | 247 HIV+, 5–74 y | 96.3% (238) | ||
| Ataei et al., 200849 | 2006 | 816, community residents, >6 y | 8.3% | ||
| Roushan et al., 200765 | 2004–2005 | 392, HBsAg+ pts, 10–70 y | 82.1% (332) • 10–19 y: 59.4% • 20–29 y: 89.8% • >29 y: 97.5% |
||
| Mehr et al., 200458 | 2002 | 1,018, children in pediatric hospital, 0.5–15 y | 22.3% (227) | ||
| Saberifiroozi et al., 200566 | n.r. | 204, pts in liver clinic, adults | 98% (200) | ||
| Jordan | |||||
| Hayajneh et al., 2015127 | 2008 | 3,066, community residents, 0–85 y | 51% • ≤1 y: 24% • 1–2 y: 26% • 2–4 y: 32% • 5–9 y: 44% • 10–14 y: 63% • 15–19 y: 78% • >20 y: 94% |
||
| Kuwait | |||||
| Alkhalidi et al., 2009118 | 2003–2004 | 2,851, healthy adults | 28.6% (816) • 18–27 y: 24.2% • 28–40 y: 51% • 41–60 y: 56.5% |
||
| Lebanon | |||||
| Melhem et al., 2015104 | 2012–2013 | 283, blood donors | 72% • 19–29 y: 60% • 30–39 y: 77% • 40–49 y: 94% • 50–59 y: 91% |
||
| Bizri et al., 2006102 | 1999–2000 | 902, school children, 14–18 y |
|
||
| Kalaajieh et al., 2000103 | 1996–1998 | 740, pediatric clinic pts, 0.5–15 y | 29.3% (217) • 0.5–6 y: 14.7–21% • 7–15 y: 37.6–40.1% |
||
| Sacy et al., 2005105 | 1999–2000 | 606, healthy volunteers visiting or working in four hospitals, 1–30 y | 43.2% (262) • 1–5 y: 10.5% • 6–10 y: 27.7% • 11–15 y: 57.4% • 16–20 y: 70.1% • 21–30 y: 78.1% |
||
| Shamma’a et al., 1982107 | n.r. | 772, mixed sample of pts | • Lebanese adults: 97.7% (474/485) • Pediatric group: 79.5% (136/171) • Foreign adults: 38.8% (45/116) |
||
| Libya | |||||
| Gebreel et al., 1983135 | 1979–1981 | 400, school children, 3–18 y | 60%–100% | ||
| Morocco | |||||
| Bouskraoui et al., 2009136 | 2005–2006 | 150, children, 0.5-14 y | 51% • ≤6 y: 45.2% • >6–14 y: 70.3% |
||
| Palestine | |||||
| Yassin et al., 2001137 | n.r. | 396, school children, 6–14 y | 93.7% • 6 y: 87.8% • 14 y: 97.5% |
||
| Pakistan | |||||
| Aziz et al., 200795 | 2002–2004 | 380, children from squatter settlements,<18 y | ≥14 y: 100% | ||
| Agboatwalla et al., 199494 | 1990–1991 | 258, healthy children (239) and adults (19) | 55.8% (144) • <5 y: 41% (98/239) • 30–50 y: 100% (19/19) |
||
| Hamid et al., 200296 | n.r. | 233, adult outpatients with CLD | • 97.8% (228) | ||
| Saudi Arabia | |||||
| Alshabanat et al., 201331 | 2006–2010 | 44,679, viral hepatitis pts, all ages | 17% (7,566) | ||
| Al-Faleh et al., 200821 | 2007–2008 | 1,357, school children, 16–18 y | 18.6% (253) | ||
| El-Gilany et al., 201039 | 2006–2007 | 950, children attending regular vaccination schedule, 1–6 y | 33.8% (321) | ||
| Almuneff, et al., 200629 | 2001–2005 | 4,006, healthcare workers | 67% | ||
| Almuneef et al., 200630 | 2005 | 2,399, all ages | 28.9% (694) • <8 y: 7.1% • 8–11 y: 14.5% • 12–15 y: 30.6% • ≥16 y 52% |
||
| Jaber, 200641 | 2004 | 527, aged 4–14 y | •28.7% | ||
| Al-Ghamdi et al., 200426 | 2003 | 650, children − 1st year primary school | 8.2% (53) | ||
| Fathalla et al., 200040 | 1987–1999 | 11,674, healthy children and adults (18–50 y) | 86% (10,029) • children: 65% • adults: 78.8% Detailed in children: • <6 y: 3% • 6–<8 y: 62% • 8–<10 y: 71% • 10–<12 y: 83% • 12–<18 y: 93% |
||
| Al-Faleh et al., 199924 | 1997 | 5,355, community residents, children 1–12 y | 25% (1,331) • 1–2 y: 16% • 3–4 y: 22% • 5–6 y: 25% • 7–8 y: 29% • 9–10 y: 34% • 11–12 y: 34% |
||
| Khalil et al., 199842 | 1995–1996 | 592, children in regular appointments or inpatient care, <16 y | 30.2% (179) • 0.5–2 y: 12.5% • 3–4 y: 14.7% • 5–6 y: 20.3% • 7–8 y: 40.4% • 9–10 y: 32% • 11–12 y: 44.3% • 13–15 y: 48.6% |
||
| Al Rashed, 199723 | 1989 | 4,375, community residents, children, 1–10 y | 52.4% | ||
| Ashraf et al., 198635 | 1985 | 55, hemodialysis pts, all ages | 100% | ||
| Ashraf et al., 198636 | 1984–1985 | 395, healthy blood donors or minor illness pts, all ages | 89% (353) • <0.5 y 65.5% • 0.5–2 y: 60% • 3–5 y: 83.3% • 6–12 y: 97.8% • >13 y: 100% |
||
| Babaeer et al., 201138 | n.r. | 1,050, pts, >2 y | 33.1% (348) • 2–5 y: 17% • 6–9 y: 21.1% • 10–14 y: 28.8% • 15–19 y: 27.2% • 20–24 y: 34.3% • 25–29 y: 38.2% • 30–34 y: 47.7% • >35 y: 49.2% |
||
| Al-Faleh et al., 201022 | n.r. | 1,157, school children, 16–18 y |
|
||
| Arif, 199633 | n.r. | 1,418, community residents, all ages | 68.0% (964)
|
||
| Ramia, 198645 | n.r. | 1,015, Riyadh residents, all ages | 82.5% (837) • <1 y: 67.9% • 1–4 y: 38.6% • 5–9 y: 61.3% • 10–15 y: 81.5% • 16–19 y: 83.5% • 20–29 y: 91% • 30–39 y: 93.5% • ≥40 y: 95% |
||
| Somalia | |||||
| Hassan-Kadle et al., 2018121 | [4 studies published from 1984 to1994] | Participants in the 4 studies, all ages | 90.2% • <1 y: 61.5% • 1–10 y: 91.9% • 11–19 y: 96.3% • 20–39 y: 91.3% • ≥40 y: 87% |
||
| Bile et al., 1992122 | n.r. | 672, children in 2 residential institutions, <18 y | By institution: • 96% (Shebeli) • 59% (Societe Organization Sociale) |
||
| Mohamud et al., 1992123 | n.r. | 593, 0-83 y | •90% | ||
| Syria | |||||
| Antaki et al., 2000128 | n.r. | 849, all ages | 89% (754) • 1–5 y: 50% • 6–10 y: 81% • 11–15 y: 95% • 16–20 y: 94% • 21–30 y: 97% • 31–40 y: 98% • 41–50 y: 100% |
||
| Tunisia | |||||
| Neffatti et al., 2017110 | 2014–2015 | 216 pregnant women, 19-46 y | 98.6% (212) | ||
| Louati et al., 2009109 | 2000 & 2007 | 376 blood donors, 18–30 y | 2000 | 2007 | |
| 18–20 y | 91.9% | 80.6% | |||
| 21–25 y | 93.7% | 84.9% | |||
| >26 y | 99.2% | 92.1% | |||
| Total | 94.9% | 85.9% | |||
| Rezig et al., 2008111 | n.r. | 2,482, community residents, children and young adults | 87.9% (2,180) • 5- <10 y: 83.9% • 10–15: 90.5% • 16–25 y: 91.9% |
||
| Letaief et al., 2005108 | 2002 | 2,400, school children, 5–20 y | 60% • 5–10 y: 44% • 10–15 y: 58% • 15–20 y: 83% |
||
| United Arab Emirates | |||||
| Sheek-Hussein et al., 2012130 | 2011–2012 | 261, healthy medical students | 21% | ||
| Sharar et al., 2008131 | 2004–2005 | 367 children attending hospital, 1–12 y | 20.1% (74) • 1–6 y: 10.2% • 6–12 y: 31.5% |
||
| Yemen | |||||
| Bawazir et al., 2010132 | 2005 | 538, pts attending hospitals, all ages | 86.6% (466) • 0–1 y: 53% • <18 y: 80.8% • ≥18 y: 98.8% |
||
CLD, chronic liver disease; HAV, hepatitis A virus; HBsAg, surface antigen of the hepatitis B virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; n, the number of study participants who were anti-HAV positive (* if available); n.r. not reported; pts, patients; y, year(s).
Main observations from the different countries are summarized in Table 4. In Afghanistan, a high seroprevalence (99%) was documented; HAV seroprevalence was higher among individuals >15 years of age compared to those <15 years of age (100% versus 91.7%).134 A study from 1987, in Djibouti, reported a prevalence of 98.5%.124 Seroprevalence surveys conducted in Egypt in the 1990s76,79 generally depicted a high immunity rate among children ≤5 years of age with 97.2–100% anti-HAV antibody prevalence. Studies from Egypt in the 2000s showed that 61.4%75 to 86.2%77,81 of children ≤6 years of age had immunity, and that 85.1% of patients with chronic liver disease had immunity.77,78 Studies from Iran indicate that most children and teenagers are susceptible to hepatitis A infection47,48,65,67,70 (Table 4). One study from Jordan provides strong evidence for continuous transition of HAV epidemiology toward intermediate endemicity, with increasing proportions of susceptible adolescents and adults.126,127 A study conducted in Lebanon in the early 1980s highlighted that 79.5% of children had anti-HAV antibodies.107 Studies conducted in 1999 and 2000 showed that more than half of teenagers had immunity, and about 20% of young adults remained susceptible to infection.102–105 Studies in Pakistan in the 1980s, 1990s, and 2000s indicate that more than half of children acquire immunity by their preschool years and nearly all adolescents and adults are immune.94–96 Earlier seroprevalence surveys conducted in Saudi Arabia generally reported high proportions of children and teenagers with acquired immunity,23,36,40,45 but noted lower seroprevalence in urban areas.33,42 In the same population, studies after the 2000s generally report lower immunity levels21,30,41 (Table 4). Studies from Kuwait,118 Tunisia,108 and the United Arab Emirates131 conducted in the 2000s show 10.2 to 31.5%131 HAV seroprevalence in children, and immunity in only 21% of young adults.130 In Morocco, the high overall HAV prevalence reported in 2005–2006 in children confirms that Morocco is an intermediately endemic area for HAV infection and is entering a transitional phase.136 Infection rates in children were high in other countries, such as in Libya,135 Yemen,132 Somalia,121,122 Syria,128 Tunisia111 and in some special populations, such as those living in Palestine.137
Temporal trends in HAV seroprevalence
Five studies reported HAV seroprevalence over time.21,24,42,92,109 These studies reveal that the HAV frequency rate is decreasing over time; this reduced force of infection has significantly increased the average age at infection. One study documented an increase in HAV occurrence in a large Egyptian hospital from 2.1% (1983) to 34% (2002); this is likely caused by delayed initial exposure to HAV resulting in symptomatic cases at older ages.92 Most of these cases occurred in older age groups, with only 20 (29%) of 68 infected patients being younger than five years, compared to 80% in 1983, and 22 (32%) of 68 patients above 9 years of age compared with 1 (20%) of 5 patients in 1983.92
Socioeconomic aspects of HAV seroprevalence
HAV seroprevalence data by area of residence was reported in 10 studies. Overall, a higher seroprevalence of HAV was generally reported among individuals residing in rural areas compared to urban areas, likely due to limited access to improved water sources and to sanitation facilities.23,26,47,52,55,59,60,62,89,90 Four studies reported data on HAV seroprevalence by socioeconomic status;21,23,75,81 collectively the data shows that individuals or families from low-income households (36.8 to 87.7%) had higher HAV seropositivity compared to individuals from middle- or high-income households (5.9 to 50.7%).
Discussion
To our knowledge, this is the first comprehensive review of hepatitis A epidemiology in the EMR. We expect the findings of this review to help raise awareness and inform the development of appropriate interventional strategies to manage the evolving epidemiological situation in the region as well as globally. In recent decades, HAV seroprevalence has been declining in most parts of the world, mainly due to improvement in socioeconomic status, better access to clean water, sanitation, and in some cases, to active immunization. In the EMR, HAV seroprevalence rates are generally high with recent evidence indicating a delay of viral exposure into adulthood in most countries of the region.140 This change leaves older children, adolescents, and adults more likely to develop overt disease. Similar observations have been made in other developing countries in Asia (India, Thailand, and Taiwan),141 Latin America (Argentina, Brazil, Chile, Dominican Republic, Mexico, and Venezuela)142 including a recent comprehensive review on all Latin American countries,143 and Africa (South Africa).144 Given that the severity of HAV symptoms increases with age,3 it may be appropriate for the EMR countries with a high proportion of susceptible older children and adults to consider implementing HAV vaccination programs. These programs could target certain populations such as young children, and simultaneously could foster improvements in access to clean water, sanitation, and hygiene in the region.2
Considering the evolving situation with regard to international trade (specifically food and travel) and rising conflict in the region, the epidemiological context in the EMR is expected to have consequences for global public health. Measures such as immunization of risk groups like travelers and food handlers, and the creation of a common standard for the health, reception, and reporting of asylum seekers and refugees from this region should be considered. Advances in modern transportation and global accessibility have boosted the travel industry in the region. In Europe, travel continues to cause both imported cases and secondary transmission.145 Travel to and from countries with high or intermediate HAV endemicity is a risk factor for infection in residents of countries with low HAV endemicity, such as countries in Europe and North America. Individuals may be exposed to HAV during their travels and thus may transmit the imported infection within their communities, leading to subsequent outbreaks.140 GeoSentinel, the global surveillance network of the International Society of Travel Medicine reported 120 cases of hepatitis A among 737 international travelers to India, Egypt, Morocco and Mexico, between 2007 and 2011.146 Another study reported that 80 cases of HAV infection were diagnosed among European travelers returning from Egypt.147 Two concurrent travel-related HAV clusters were detected in eight European countries after travel to Morocco.148
EMR countries have undergone rapid urbanization and changes in lifestyle and consumer demands. These changes have had a profound effect on the production, supply, availability, and consumption of food.149 In the last few decades, international food trade from the EMR has accelerated but the recent coronavirus disease 2019 (COVID-19) pandemic has, at least temporarily, brought this to a standstill. Notwithstanding the effects of COVID-19 on global travel and trade, risks of HAV contaminated food remain high, with the WHO Foodborne Disease Burden Epidemiology Reference Group estimating that more than 90,000 deaths occurred worldwide due to acute viral hepatitis in 2010. Nearly 30,000 of those deaths could be due to foodborne transmission of HAV.150 The risk is elevated when food products are imported from high and intermediate HAV endemic countries or from countries with poor food processing practices.149 Furthermore, the HAV capsid has a highly stable molecular structure which allows it to persist in certain types of foods for extended periods of time and withstand common food processing practices.151 The European Union has reported two HAV infection outbreaks in 2013 due to frozen strawberries imported from Egypt and Morocco,152 and imported pomegranate seeds from Egypt have been traced as the source of an HAV infection outbreak in British Columbia, Canada, in 2012.153
Some areas in the EMR (i.e., Iraq, Iran, Syria, Palestine, and Yemen) are at the center of turmoil, with conflicts having a significant impact in these countries and beyond the region. The economic and health situation in these countries continues to worsen.154 Regional instability leads to difficulties in addressing public health issues while migratory movements are continuously being reported. One of the ramifications of migration from areas of conflict is the resurgence of infectious diseases such as hepatitis A, especially in low-endemic countries. This could possibly be driven by the influx of refugees and their settlement in underserved camps. Poor sanitation, hygiene, and inadequate supply of clean food and water in refugee camps are likely contributors to the rapid spread of HAV. A HAV outbreak was reported among Syrian refugees residing in hosting camps in Greece in 2016.155 A 45% increase in HAV cases among asylum seekers was reported in Germany in 2015–2016.156 In 2015, asylum applications in Europe amounted to approximately 1.35 million—a record since data collection began in 2008 and more than twice the number of applications than in 2014.157 While the COVID-19 pandemic may have slowed this trend due to restrictions affecting global travel and trade,158 careful monitoring of the situation and timely action to mitigate the risks of hepatitis A outbreaks are warranted.
There are some limitations of this review which are worth noting in the interpretation of the overall findings. A time limit was applied to the searches to identify publications beginning from 1980 onwards. This was considered appropriate by the authors to notice any shift in the burden of disease. More than half of the eligible studies identified in this review are from three countries (Egypt, Iran, and Saudi Arabia). Therefore, generalizability is limited to the countries from which most studies were reported and should not be extended to countries with very poor data representation, i.e., those with a few relevant studies or none at all. There is also a lack of consistency in study designs and age groups reported across the studies which prevents direct comparisons. This is compounded by the fact that the region is diverse with different income levels and healthcare infrastructure. Another factor that limits comparison is the different time periods considered within the studies. Finally, the data reported in this review was collected prior to COVID-19 and as such it does not reflect the travel and trade restrictions imposed on the countries in the EMR during the years 2020 and 2021. Due to these reasons, the overall findings should be interpreted with caution.
Conclusion
In the EMR, hepatitis A remains a significant cause of acute viral hepatitis. While the populations in low-income countries show universal immunity to HAV, the middle- and high-income countries report increasing numbers of susceptible older children, adolescents, and adults which co-exist in rapidly developing societies. Given this shift in endemicity, it is expected that most of the countries in this region would experience a transition in HAV endemicity in the next decades, the consequence of which will be a higher burden of disease as the population ages, and the occurrence of outbreaks. The public health value of childhood vaccination against hepatitis A and of vaccinating only high-risk groups such as those traveling from and to the region should be assessed within this changing epidemiological context in the EMR.
Acknowledgements
The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amandine Radziejwoski coordinated publication development and editorial support. Amrita Ostawal (Arete Communication UG, on behalf of GSK) provided writing support.
Funding Statement
GlaxoSmithKline Biologicals S.A. funded this study and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals S.A. also took in charge and all costs associated with the development and the publishing of this manuscript.
Authors’ contributions
SB, MAG, MK, YL, OO, and KH performed the literature search. All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Disclosure statement
All authors are employed by the GSK group of companies. SB, SÖ, MAG, MK, YL, KH, and DS hold shares in the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
References
- 1.World Health Organization . The immunological basis for immunization series: module 18: hepatitis A; 2011. [accessed 2021 Feb 21]. https://apps.who.int/iris/handle/10665/44570
- 2.World Health Organization . WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87(28/29):261–17.22905367 [Google Scholar]
- 3.Foster MA, Penina Haber P, Nelson NP, Hepatitis A. In: Hamborsky J, Kroger A, Wolfe Seditors, Epidemiology and prevention of vaccine-preventable diseases, 13. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hepa.pdf: Center for Disease Control and Prevention. Washington DC: Public Health Foundation;2015, pp. 125–42 [Google Scholar]
- 4.Wolff MH, Schmidt A.. Hepatitis A infection. In: Weber O, and Protzer U, editors. Comparative hepatitis. Basel: Birkhäuser Basel; 2008. p. 121–34. [Google Scholar]
- 5.Lemon SM, Ott JJ, Van Damme P, Shouval D.. Type A viral hepatitis: a summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J Hepatol. 2018;68(1):167–84. doi: 10.1016/j.jhep.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Lolekha S, Pratuangtham S, Punpanich W, Bowonkiratikachorn P, Chimabutra K, Weber F. Immunogenicity and safety of two doses of a paediatric hepatitisA vaccine in thai children: comparison of three vaccination schedules. J Trop Pediatr. 2003;49(6):333–39. doi: 10.1093/tropej/49.6.333. [DOI] [PubMed] [Google Scholar]
- 7.Raczniak GA, Bulkow LR, Bruce MG, Zanis CL, Baum RL, Snowball MM, Byrd KK, Sharapov UM, Hennessy TW, McMahon BJ, et al. Long-Term immunogenicity of hepatitis A virus vaccine in Alaska 17 years after initial childhood series. J Infect Dis. 2013;207(3):493–96. doi: 10.1093/infdis/jis710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharapov UM, Bulkow LR, Negus SE, Spradling PR, Homan C, Drobeniuc J, Bruce M, Kamili S, Hu DJ, McMahon BJ, et al. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology. 2012;56(2):516–22. doi: 10.1002/hep.25687. [DOI] [PubMed] [Google Scholar]
- 9.Itani T, Jacobsen KH, Nguyen T, Wiktor SZ. A new method for imputing country-level estimates of hepatitis A virus endemicity levels in the Eastern Mediterranean region. Vaccine. 2014;32(46):6067–74. doi: 10.1016/j.vaccine.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Koroglu M, Jacobsen, KH, Demiray T, Ozbek A, Erkorkzam U, Altindis M. Socioeconomic indicators are strong predictors of hepatitis A seroprevalence rates in the Middle East and North Africa. J Infect Public Health. 2017;10(5):513–17. doi: 10.1016/j.jiph.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10(1):57. doi: 10.1186/1476-072X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . The global prevalence of hepatitis A virus infection and susceptibility : a systematic review. WHO/IVB/10.012.010; 2010. [accessed 2020 Nov 4]. https://apps.who.int/iris/handle/10665/70180
- 13.World Health Organization . Regional office for the Eastern Mediterranean. [accessed 2020 Nov 4]. http://www.emro.who.int/countries.html
- 14.The World Bank . World Bank country and lending groups. [accessed 2020 Nov 4]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519
- 15.Aljouni K, Al-Mazrou YY, Ammar WS, Daar AS, Daulaire N, Ezzati M, Fathalla M, Houssin DP, Kickbusch I, Mechbal A, et al. Refugees in the eastern Mediterranean region. Lancet. 2015;386(10012):2476–77. doi: 10.1016/S0140-6736(15)01242-8. [DOI] [PubMed] [Google Scholar]
- 16.Safiabadi M, Rezaee-Zavareh MS, Moayed Alavian S. Estimation of hepatitis A virus infection prevalence among Eastern Mediterranean and Middle Eastern countries: a systematic review and pooled analysis. Hepat Mon. 2017;17(2):e44695. doi: 10.5812/hepatmon.44695. [DOI] [Google Scholar]
- 17.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–57. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 19.Abdo AA, Sanai FM. Viral hepatitis in Saudi Arabia. An unfinished story. Saudi Med J. 2015;36(7):785–86. doi: 10.15537/smj.2015.7.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: are we off the hook? Saudi J Gastroenterol. 2012;18(6):349–57. doi: 10.4103/1319-3767.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Faleh F, Al Shehri S, Al Ansari S, Jeffri MA, Mazrou YA, Shaffi A, Abdo AA. Changing patterns of hepatitis A prevalence within the Saudi population over the last 18 years. World J Gastroenterol. 2008;14(48):7371–75. doi: 10.3748/wjg.14.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Faleh FZ, Ali S, Aljebreen AM, Alhammad E, Abdo AA. Seroprevalence rates of Helicobacter pylori and viral hepatitis A among adolescents in three regions of the Kingdom of Saudi Arabia: is there any correlation? Helicobacter. 2010;15(6):532–37. doi: 10.1111/j.1523-5378.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 23.Al Rashed RS. Prevalence of hepatitis A virus among Saudi Arabian children: a community-based study. Ann Saudi Med. 1997;17(2):200–03. doi: 10.5144/0256-4947.1997.200. [DOI] [PubMed] [Google Scholar]
- 24.Al-Faleh F, Al-Jeffri M, Ramia S, Al-Rashed RS, Arif MA, Mohammed OM, Bakhsh MH, Al-Freihi HM, Aljumah AA, Rezeig MA, et al. Hepatitis A in Saudi Arabia: a comparative sero-epidemiological study. Saudi Med J. 1999;20(9):678–81. [PubMed] [Google Scholar]
- 25.Al-Faleh FZ. Changing pattern of hepatitis viral infection in Saudi Arabia in the last two decades. Ann Saudi Med. 2003;23:367–71. [DOI] [PubMed] [Google Scholar]
- 26.Alghamdi A, Khalil M, Alyahia O. Serological approach to need assessment for hepatitis A vaccination in primary schools in Al-Qassim. King AbdulAziz City for Science and Technology; 2004. [accessed 2020 Nov 4]. https://www.researchgate.net/publication/266949043_Serological_approach_to_need_assessment_for_hepatitis_A_vaccination_in_primary_schools_in_Al-Qassim
- 27.Al-Knawy B, El-Mekki AA, Yarbough PO. The Role of Hepatitis E Virus Infection among patients with acute viral hepatitis in Southern Saudi Arabia. Ann Saudi Med. 1997;17(1):32–34. doi: 10.5144/0256-4947.1997.32. [DOI] [PubMed] [Google Scholar]
- 28.Al-Majed SA, Bakir TM, Al-Aska A, Ayoola B. An outbreak of acute hepatitis A infection in rural Saudi Arabia. Trop Gastroenterol. 1990;11:202–05. [PubMed] [Google Scholar]
- 29.Almuneef MA, Memish ZA, Balkhy HH, Otaibi B, Helmi M. Seroprevalence survey of varicella, measles, rubella, and hepatitis A and B viruses in a multinational healthcare workforce in Saudi Arabia. Infect Control Hosp Epidemiol. 2006;27(11):1178–83. doi: 10.1086/508826. [DOI] [PubMed] [Google Scholar]
- 30.Almuneef MA, Memish ZA, Balkhy HH, Qahtani M, AlOtaibi B, Hajeer A, Qasim L, Al Knawy B. Epidemiologic shift in the prevalence of hepatitis A virus in Saudi Arabia: a case for routine Hepatitis A vaccination. Vaccine. 2006;24(27–28):5599–603. doi: 10.1016/j.vaccine.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Alshabanat A, Albacker R, Basalamah A, Salamah A, Al-Frayh A. Profile of viral hepatitis in Saudi Arabia. Biomed Res. 2013;24:396–99. [Google Scholar]
- 32.Al-Tawfiq JA, Anani A. Profile of viral hepatitis A, B, and C in a Saudi Arabian hospital. Med Sci Monit. 2008;14:Cr52–6. [PubMed] [Google Scholar]
- 33.Arif M. Enterically transmitted hepatitis in Saudi Arabia: an epidemiological study. Ann Trop Med Parasitol. 1996;90(2):197–201. doi: 10.1080/00034983.1996.11813044. [DOI] [PubMed] [Google Scholar]
- 34.Arif M, Al-Faleh FZ, Al-Frayh AR, Ramia S. Reduction in the prevalence of antibody to hepatitis A virus among young Saudi adults: implications for hepatitis A vaccine. Saudi J Gastroenterol. 1995;1:93–96. [PubMed] [Google Scholar]
- 35.Ashraf SJ, Arya SC, Parande CM. Viral hepatitis markers in patients on haemodialysis in a hyperendemic area. J Med Virol. 1986;19(1):41–46. doi: 10.1002/jmv.1890190107. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf SJ, Arya SC, Parande CM, Kristensen E. Hepatitis A virus among natives and expatriates in Saudia Arabia. J Med Virol. 1986;19(2):151–53. doi: 10.1002/jmv.1890190207. [DOI] [PubMed] [Google Scholar]
- 37.Ayoola A, Aderoju A, Gadour MO, Al-Hazmi M, Hamza MK, Ene D, Hafeez M, Anderson D, Riddell M. Serological profile of sporadic acute viral hepatitis in an area of hyper-endemic hepatitis B virus infection. Saudi J Gastroenterol. 2001;7:95–102. [PubMed] [Google Scholar]
- 38.Babaeer M, Awfi MSH. Prevalence study of hepatitis A virus (HAV) on Jeddah population. Biosci Biotechnol Res Asia. 2011;8(2):585–90. doi: 10.13005/bbra/903. [DOI] [Google Scholar]
- 39.El-Gilany A-H, Hammad S, Refaat K, Al-Enazi R. Seroprevalence of hepatitis A antibodies among children in a Saudi community. Asian Pac J Trop Med. 2010;3:278–82. [Google Scholar]
- 40.Fathalla SE, Al-Jama AA, Al-Sheikh IH, Islam SI. Seroprevalence of hepatitis A virus markers in Eastern Saudi Arabia. Saudi Med J. 2000;21:945–49. [PubMed] [Google Scholar]
- 41.Jaber SM. Prevalence of anti-hepatitis B and anti-hepatitis A antibodies among school aged children in Western Saudi Arabia. Saudi Med J. 2006;27:1515–22. [PubMed] [Google Scholar]
- 42.Khalil M, Al-Mazrou Y, Al-Jeffri M, Al-Howasi M. Childhood epidemiology of hepatitis A virus in Riyadh, Saudi Arabia. Ann Saudi Med. 1998;18(1):18–21. doi: 10.5144/0256-4947.1998.18. [DOI] [PubMed] [Google Scholar]
- 43.Memish Z, Qasim L, Abed E, AlBasheer A, Aldraihim A, Knawy B, Hajeer AH. Pattern of viral hepatitis infection in a selected population from Saudi Arabia. Mil Med. 2003;168(7):565–68. doi: 10.1093/milmed/168.7.565. [DOI] [PubMed] [Google Scholar]
- 44.Memish ZA, Knawy BA, El-Saed A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis. 2010;14(2):e115–20. doi: 10.1016/j.ijid.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Ramia S. Antibody against hepatitis A in Saudi Arabians and in expatriates from various parts of the world working in Saudi Arabia. J Infect. 1986;12(2):153–55. doi: 10.1016/S0163-4453(86)93633-9. [DOI] [PubMed] [Google Scholar]
- 46.Yohannan MD, Arif M, Ramia S. Aetiology of icteric hepatitis and fulminant hepatic failure in children and the possible predisposition to hepatic failure by sickle cell disease. Acta Paediatr Scand. 1990;79(2):201–05. doi: 10.1111/j.1651-2227.1990.tb11439.x. [DOI] [PubMed] [Google Scholar]
- 47.Alian S, Ajami A, Ghasemian R, Yadegarinia D. Age-Specific seroprevalence of hepatitis A in Sari, northern Islamic Republic of Iran. East Med Health J. 2011;17(10):754–58. doi: 10.26719/2011.17.10.754. [DOI] [PubMed] [Google Scholar]
- 48.Asaei S, Ziyaeyan M, Moeini M, Jamalidoust M, Behzadi MA. Seroprevalence of hepatitis A and E virus infections among healthy population in Shiraz, Southern Iran. Jundishapur J Microbiol. 2015;8(7):e19311. doi: 10.5812/jjm.19311v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ataei B, Javadi AA, Nokhodian Z, Kassaeian N, Shoaei P, Farajzadegan Z, Adibi P. HAV in Isfahan province: a population-based study. Trop Gastroenterol. 2008;29:160–62. [PubMed] [Google Scholar]
- 50.Bayani M, Sadeghi M, Kalantari N, Sayadmanesh A. Hepatitis A virus seropositivity in nurses and paramedical personnel at a university hospital in north Iran. Iran Red Crescent Med J. 2013;15(5):409–13. doi: 10.5812/ircmj.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davoudi S, Rasoolinejad M, Jafari S, Erfanzadeh M, Foroughi M, Hajiabdolbaghi M, Mohraz M. Prevalence of hepatitis A virus infection in a HIV positive community. Acta Med Iran. 2010;48:192–95. [PubMed] [Google Scholar]
- 52.Farajzadegan Z, Hoseini SG, Kelishadi R, Jamshidi F, Nokhodian Z, Noori R, Mirmoghtadaee P, Hovsepian S, Mostafavi S-N. Systematic review and meta-analysis on the age-specific seroprevalence of hepatitis A in Iran. J Res Med Sci. 2014;19:S56–63. [PMC free article] [PubMed] [Google Scholar]
- 53.Hesamizadeh K, Sharafi H, Keyvani H, Alavian SM, Najafi-Tireh Shabankareh A, Sharifi Olyaie R, Keshvari M. Hepatitis A virus and hepatitis E virus seroprevalence among blood donors in Tehran, Iran. Hepat Mon. 2016;16(1):e32215. doi: 10.5812/hepatmon.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoseini SG, Kelishadi R, Ataei B, Yaran M, Motlagh ME, Ardalan G, Tajadini MH, Mostafavi SN. Seroprevalence of hepatitis A in Iranian adolescents: is it time to introduce a vaccine? Epidemiol Infect. 2016;144(2):291–96. doi: 10.1017/S0950268815001302. [DOI] [PubMed] [Google Scholar]
- 55.Izadi M, Esfahani AA, Hassannia H, Jonaidi Jafari N, Rahmati Najarkolaei F, Rezaee-Zavareh MS. Seroprevalence of hepatitis A virus among Iranian soldiers. Gastroenterol Hepatol Bed Bench. 2016;9:100–04. [PMC free article] [PubMed] [Google Scholar]
- 56.Jahanbakhsh F, Bagheri Amiri F, Sedaghat A, Fahimfar N, Mostafavi E. Prevalence of HAV Ab, HEV (IgG), HSV2 IgG, and Syphilis among sheltered homeless adults in Tehran, 2012. Int J Health Policy Manag. 2018;7(3):225–30. doi: 10.15171/ijhpm.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karimi A, Mortazaei S, Moradi MT. High prevalence of symptomatic hepatitis A infection in rural area of Chaharmahal VA Bakhtiari Province, Iran. J Clin Diagn Res. 2015;9(2):Dc01–3. doi: 10.7860/JCDR/2015/9798.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehr AJ, Ardakani MJ, Hedayati M, Shahraz S, Mehr EJ, Zali MR. Age-specific seroprevalence of hepatitis A infection among children visited in pediatric hospitals of Tehran, Iran. Eur J Epidemiol. 2004;19(3):275–78. doi: 10.1023/B:EJEP.0000020345.37091.cd. [DOI] [PubMed] [Google Scholar]
- 59.Merat S, Rezvan H, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, Maghsudlu M, Pourshams A, Malekzadeh R. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Arch Iran Med. 2010;13:99–104. [PubMed] [Google Scholar]
- 60.Mirzaei J, Ziaee M, Farsad SA, Fereydooni M, Anani Sarab G, Rezvani Khorashad MR. Vaccination against hepatitis A for hemophilic patients: is it necessary? Hepat Mon. 2016;16(4):e37447. doi: 10.5812/hepatmon.37447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohebbi SR, Rostami Nejad M, Tahaei SM, Pourhoseingholi MA, Habibi M, Azimzadeh P, Naghoosi H, Karayiannis P, Zali MR. Seroepidemiology of hepatitis A and E virus infections in Tehran, Iran: a population based study. Trans R Soc Trop Med Hyg. 2012;106(9):528–31. doi: 10.1016/j.trstmh.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Mostafavi N, Kelishadi R, Kazemi E, Ataei B, Yaran M, Motlagh ME, Qorbani M, Heshmat R, Tajadini MH, Ghaffari Hoseini S, et al. Comparison of the prevalence and risk factors of hepatitis A in 10 to 18-year-old adolescents of sixteen Iranian Provinces: the CASPIAN-III study. Hepat Mon. 2016;16(9):e36437. doi: 10.5812/hepatmon.36437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabiee A, Nikayin S, Hashemi SR, Mohaghegh M, Amini M, Rabiee R, Merat S. Seroprevalence of hepatitis A among students enrolled in Tehran University of Medical Sciences during 2011. Middle East J Dig Dis. 2013;5:137–40. [PMC free article] [PubMed] [Google Scholar]
- 64.Ramezani H, Bozorgi SH, Nooranipour M, Mostajeri A, Kargar-Fard H, Molaverdikhani S, Mazdaki A, Alavian SM. Prevalence and risk factors of hepatitis A among blood donors in Qazvin, Central Iran. Singapore Med J. 2011;52:107–12. [PubMed] [Google Scholar]
- 65.Roushan MRH, Bijani A, Sagheb R, Jazayeri O. Prevalence of hepatitis A IgG in individuals with chronic hepatitis B infection in Babol. East Mediterr Health J. 2007;13(5):1108–13. doi: 10.26719/2007.13.5.1108. [DOI] [PubMed] [Google Scholar]
- 66.Saberifiroozi M, Serati AR, Taghvaee T, Marooofi GR, Shirazi KM. Prevalence of hepatitis A virus antibodies in patients with chronic liver disease in Shiraz, Iran. Indian J Gastroenterol. 2005;24:33–34. [PubMed] [Google Scholar]
- 67.Saffar MJ, Abedian O, Ajami A, Abedian F, Mirabi AM, Khalilian A-R, Saffar H. Age-specific seroprevalence of anti-hepatitis A antibody among 1-30 years old population of Savadkuh, Mazandaran, Iran with literature review. Hepat Mon. 2012;12(5):326–32. doi: 10.5812/hepatmon.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saneian H, Rahimi H, Shoaei P. Hepatitis A seropositivity among first-year students of the Medical University in Isfahan, Iran. Int J Prev Med. 2014;5:S208–12. [PMC free article] [PubMed] [Google Scholar]
- 69.Shoaei P, Zeidabadinejad L, Hassannejad R, Ataei B, Yaran M, Kassaian N, Nokhodian Z, Foroughifar M. Seroprevalence of hepatitis A in patients with chronic hepatitis C in isfahan province. Int J Prev Med. 2012;3:S102–6. [PMC free article] [PubMed] [Google Scholar]
- 70.Sofian M, Aghakhani A, Farazi AA, Banifazl M, Etemadi G, Ziazarifi A, Abhari Z, Eslamifar A, Khadem-Sadegh A, et al. Seroepidemiology of hepatitis A virus in children of different age groups in Tehran, Iran: implications for health policy. Travel Med Infect Dis. 2010;8(3):176–79. doi: 10.1016/j.tmaid.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Taghavi SA, Hosseini Asl MK, Talebzadeh M, Eshraghian A. Seroprevalence study of hepatitis A virus in Fars province, southern Iran. Hepat Mon. 2011;11:285–88. [PMC free article] [PubMed] [Google Scholar]
- 72.Vakili B, Rahimi H, Ataei B, Janghorbani M, Khorvash F, Shoaei P, Yaran M. Hepatitis A seropositivity among newly admitted medical students of Isfahan, Kermanshah, and Hamedan: a seroprevalence study. J Res Med Sci. 2014;19:S9–s12. [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmadi Vasmehjani A, Javeshghani D, Baharlou R, Shayestehpour M, Mousavinasab SD, Joharinia N, Enderami SE. Hepatitis A infection in patients with chronic viral liver disease: a cross-sectional study in Jahrom, Iran. Epidemiol Infect. 2015;143(3):534–39. doi: 10.1017/S0950268814000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosseini Shokouh SJ, Dadashi A, Abiri M, Zohrevand I, Eshraghian A, Khoshdel A, Heidari B, Khoshkish S. HAV immunity in Iranian Medical Students. Hepat Mon. 2015;15(3):e26219. doi: 10.5812/hepatmon.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Aziz AM, Awad MA. Seroprevalence of hepatitis A virus antibodies among a sample of Egyptian children. East Mediterr Health J. 2008;14:1028–35. [PubMed] [Google Scholar]
- 76.Darwish MA, Faris R, Clemens JD, Rao MR, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in the Nile Delta: a pilot study. Am J Trop Med Hyg. 1996;54(6):554–58. doi: 10.4269/ajtmh.1996.54.554. [DOI] [PubMed] [Google Scholar]
- 77.El-Karaksy H, El-Sayed R, El-Raziky M, El-Koofy N, Mansour S. Cost-Effectiveness of prescreening versus empirical vaccination for hepatitis A in Egyptian children with chronic liver disease. East Mediterr Health J. 2008;14:804–09. [PubMed] [Google Scholar]
- 78.El-Karaksy HM, El-Hawary MI, El-Koofy NM, El-Sayed R, El-Raziky MAS, Mansour SA, Taha GM, El-Mougy F. Safety and efficacy of hepatitis A vaccine in children with chronic liver disease. World J Gastroenterol. 2006;12(45):7337–40. doi: 10.3748/wjg.v12.i45.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamel MA, Troonen H, Kapprell HP, el-Ayady A, Miller FD. Seroepidemiology of hepatitis E virus in the Egyptian Nile Delta. J Med Virol. 1995;47(4):399–403. doi: 10.1002/jmv.1890470417. [DOI] [PubMed] [Google Scholar]
- 80.Omar AA, Hashish MH. Screening for hepatitis A virus antibodies among a disadvantaged group of preschool children in Alexandria. J Egypt Public Health Assoc. 2000;75:529–39. [PubMed] [Google Scholar]
- 81.Salama II, Samy SM, Shaaban FA, Hassanin AI, Abou Ismail LA. Seroprevalence of hepatitis A among children of different socioeconomic status in Cairo. East Mediterr Health J. 2007;13(6):1256–64. doi: 10.26719/2007.13.6.1256. [DOI] [PubMed] [Google Scholar]
- 82.Darwish MA, Shaker M, Al-Kady AM. Non-A, non-B viral hepatitis in Egypt. J Egypt Public Health Assoc. 1992;67:171–79. [PubMed] [Google Scholar]
- 83.Divizia M, Gabrieli R, Stefanoni ML, Ghazzawi EE, Kader OA, Gamil F, Sawaf GE, Sherbini EE, Saleh E, Degener AM, et al. HAV and HEV infection in hospitalised hepatitis patients in Alexandria, Egypt. Eur J Epidemiol. 1999;15(7):603–09. doi: 10.1023/A:1007514030062. [DOI] [PubMed] [Google Scholar]
- 84.Eldin SS, Seddik I, Daef EA, Shata MT, Raafat M, Abdel Baky L, Nafeh MA. Risk factors and immune response to hepatitis E viral infection among acute hepatitis patients in Assiut, Egypt. Egypt J Immunol. 2010;17:73–86. [PMC free article] [PubMed] [Google Scholar]
- 85.Fouad HM, Reyad EM, El-Din AG. Acute hepatitis A is the chief etiology of acute hepatitis in Egyptian children: a single-center study. Eur J Clin Microbiol Infect Dis. 2018;37(10):1941–47. doi: 10.1007/s10096-018-3329-0. [DOI] [PubMed] [Google Scholar]
- 86.Hasan G, Assiri A, Marzuuk N, Daef E, Abdelwahab S, Ahmed A, Mohamad I, Al-Eyadhy A, Alhaboob A, Temsah MH. Incidence and characteristics of hepatitis E virus infection in children in Assiut, Upper Egypt. J Int Med Res. 2016;44(5):1115–22. doi: 10.1177/0300060516659575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyams KC, McCarthy MC, Kaur M, Purdyt MA, Bradley DW, Mansour MM, Gray S, Watts DM, Carl M. Acute sporadic hepatitis E in children living in Cairo, Egypt. J Med Virol. 1992;37(4):274–77. doi: 10.1002/jmv.1890370407. [DOI] [PubMed] [Google Scholar]
- 88.Meky FA, Stoszek SK, Abdel-Hamid M, Selim S, Wahab AA, Mikhail N, El-Kafrawy S, El-Daly M, Abdel-Aziz F, Sharaf S, Mohamed MK. Active surveillance for acute viral hepatitis in rural villages in the Nile Delta. Clin Infect Dis. 2006;42(5):628–33. doi: 10.1086/500133. [DOI] [PubMed] [Google Scholar]
- 89.Talaat M, Afifi S, Reaves EJ, Abu Elsood H, El-Gohary A, Refaey S, Hammad R, Abdel Fadeel M, Kandeel A. Evidence of sustained reductions in the relative risk of acute hepatitis B and C virus infections, and the increasing burden of hepatitis A virus infection in Egypt: comparison of sentinel acute viral hepatitis surveillance results, 2001–17. BMC Infect Dis. 2019;19(1):159. doi: 10.1186/s12879-019-3806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Talaat M, El-Sayed N, Kandeel A, Azab MA, Afifi S, Youssef FG, Ismael T, Hajjeh R, Mahoney FJ. Sentinel surveillance for patients with acute hepatitis in Egypt, 2001-04. East Mediterr Health J. 2010;16(2):134–40. doi: 10.26719/2010.16.2.134. [DOI] [PubMed] [Google Scholar]
- 91.Youssef A, Yano Y, El-Sayed Zaki M, Utsumi T, Hayashi Y. Characteristics of hepatitis viruses among Egyptian children with acute hepatitis. Int J Oncol. 2013;42(4):1459–65. doi: 10.3892/ijo.2013.1822. [DOI] [PubMed] [Google Scholar]
- 92.Zakaria S, Fouad R, Shaker O, Zaki S, Hashem A, Kamary SSE, Esmat G, Zakaria S. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis. 2007;44(4):e30–6. doi: 10.1086/511074. [DOI] [PubMed] [Google Scholar]
- 93.Zaki MS, Salama OS, Mansour FA, Hossein S. Hepatitis E virus coinfection with hepatotropic viruses in Egyptian children. J Microbiol Immunol Infect. 2008;41:254–58. [PubMed] [Google Scholar]
- 94.Agboatwalla M, Isomura S, Miyake K, Yamashita T, Morishita T, Akram DS. Hepatitis A, B and C seroprevalence in Pakistan. Indian J Pediatr. 1994;61(5):545–49. doi: 10.1007/BF02751716. [DOI] [PubMed] [Google Scholar]
- 95.Aziz S, Muzaffar R, Hafiz S, Abbas Z, Zafar MN, Naqvi SAA, Rizvi SAUH. Helicobacter pylori, hepatitis viruses A, C, E, antibodies and HBsAg - prevalence and associated risk factors in pediatric communities of Karachi. J Coll Physicians Surg Pak. 2007;17(4):195–98. [PubMed] [Google Scholar]
- 96.Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, Siddiqui A, Jafri W. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36(2):474–78. doi: 10.1053/jhep.2002.34856. [DOI] [PubMed] [Google Scholar]
- 97.Ahmed W, Qureshi H, Arif A, Alam SE. Changing trend of viral hepatitis–“a twenty one year report from Pakistan Medical Research Council Research Centre, Jinnah Postgraduate Medical Centre, Karachi”. J Pak Med Assoc. 2010;60:86–89. [PubMed] [Google Scholar]
- 98.Haider Z, Khan AA, Rehman K, Janjua MI, Iqbal J, Chishti MA, Qayyum A, Hasnain S, Shahzad A. Sero-Diagnosis for viral hepatitis in 93 patients admitted with acute hepatitis in three different teaching hospitals in Lahore. J Pak Med Assoc. 1994;44:182–84. [PubMed] [Google Scholar]
- 99.Khan A, Tanaka Y, Kurbanov F, Elkady A, Abbas Z, Azam Z, Subhan A, Razza S, Hamid S, Jafri W, et al. Investigating an outbreak of acute viral hepatitis caused by hepatitis E virus variants in Karachi, South Pakistan. J Med Virol. 2011;83(4):622–29. doi: 10.1002/jmv.22036. [DOI] [PubMed] [Google Scholar]
- 100.Syed SA, Sarwari AR, Smego RA, Hamid S, Nissa T. Does infection with hepatitis A virus provide protection against hepatitis E virus? Med Hypotheses. 2003;60(3):337–39. doi: 10.1016/S0306-9877(02)00399-7. [DOI] [PubMed] [Google Scholar]
- 101.Waheed-Uz-Zaman T, Hussain AB, Hussain T, Anwar M, Ghani E, Asad U. Hepatitis A virus infection – shifting epidemiology. J Coll Physicians Surg Pak. 2006;16(1):15–18. [PubMed] [Google Scholar]
- 102.Bizri AR, Nuwayhid IA, Hamadeh GN, Steitieh SW, Choukair AM, Musharrafieh UM. Association between hepatitis A virus and Helicobacter pylori in a developing country: the saga continues. J Gastroenterol Hepatol. 2006;21(10):1615–21. doi: 10.1111/j.1440-1746.2006.04268.x. [DOI] [PubMed] [Google Scholar]
- 103.Kalaajieh W, Rima A, Dennaoui M, Khodayry RA. Seroprevalence of hepatitis A antibodies in Lebanese children. Médecine Et Maladies Infectieuses. 2000;30(12):757–61. doi: 10.1016/S0399-077X(01)80031-7. [DOI] [Google Scholar]
- 104.Melhem NM, Jaffa M, Zaatari M, Awada H, Salibi NE, Ramia S. The changing pattern of hepatitis A in Lebanese adults. Int J Infect Dis. 2015;30:87–90. doi: 10.1016/j.ijid.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 105.Sacy RG, Haddad M, Baasiri G, Khoriati A, Gerbaka BJ, Abu-Elyazeed R. Hepatitis A in Lebanon: a changing epidemiological pattern. Am J Trop Med Hyg. 2005;73(2):453–56. doi: 10.4269/ajtmh.2005.73.453. [DOI] [PubMed] [Google Scholar]
- 106.Shamma’a MH. Acute viral hepatitis in Lebanon: evidence for a HAV-like non-A non-B hepatitis. Liver. 1984;4(1):39–44. doi: 10.1111/j.1600-0676.1984.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 107.Shamma’-a MH, Abu-Samra S, Salameh V, Nassar NT. The significance of anti-HAV in different population sectors in Lebanon: a comparative seroepidemiologic study. Int J Epidemiol. 1982;11(4):406–09. doi: 10.1093/ije/11.4.406. [DOI] [PubMed] [Google Scholar]
- 108.Letaief A, Kaabia N, Gaha R, Letaief A, Bousaadia A, Gaha R, Ghannem H, Jemni L. Age-Specific seroprevalence of hepatitis A among school children in central Tunisia. Am J Trop Med Hyg. 2005;73(1):40–43. doi: 10.4269/ajtmh.2005.73.40. [DOI] [PubMed] [Google Scholar]
- 109.Louati N, Feki L, Rekik H, Feki H, Chaabouni M, Hammami A, Gargouri J, Karray-Hakim H. Comparison of hepatitis A seroprevalence in blood donors in South Tunisia between 2000 and 2007. Arch Inst Pasteur Tunis. 2009;86:69–74. [PubMed] [Google Scholar]
- 110.Neffatti H, Lebraud P, Hottelet C, Gharbi J, Challouf T, Roque-Afonso A-M. Southern Tunisia: a still high endemicity area for hepatitis A. PLoS ONE. 2017;12(4):e0175887. doi: 10.1371/journal.pone.0175887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rezig D, Ouneissa R, Mhiri L, Mejri S, Haddad-Boubaker S, Ben Alaya N, Triki H. Séroprévalences des infections à hépatite A et E en Tunisie. Pathol Biol. 2008;56(3):148–53. doi: 10.1016/j.patbio.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 112.Gharbi-Khelifi H, Abid NB, Beji A, Bhiri L, Harrath R, Sdiri K, Billaudel S, Ferre V, Aouni M. Seroprevalence and molecular characterisation of human hepatitis A virus in serum samples of Tunisian patients with clinical symptoms of viral hepatitis. Indian J Virol. 2012;23(1):29–35. doi: 10.1007/s13337-012-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hellara O, Melki W, Mastouri M, Ben Chaabène N, Loghmari H, Ben Mansour W, Bdioui F, Safer L, Saffar H. Serodiagnosis of acute hepatitis in adults patients: results of a prospective study in a central region of Tunisia. Tunis Med. 2014;92:201–07. [PubMed] [Google Scholar]
- 114.Al-Naaimi AS, Turky AM, Khaleel HA, Jalil RW, Mekhlef OA, Kareem SA, Hasan NY, Dhadain AA. Predicting acute viral hepatitis serum markers (A and E) in patients with suspected acute viral hepatitis attending primary health care centers in Baghdad: a one year cross-sectional study. Glob J Health Sci. 2012;4(5):172–83. doi: 10.5539/gjhs.v4n5p172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marcus S, Al-Moslih M, Al-Tawil NG, Kassir ZA. Virological and immunological studies in patients with acute viral hepatitis. Scand J Immunol. 1993;37(2):265–70. doi: 10.1111/j.1365-3083.1993.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 116.Rassam SW, Goudeau AM, Dubois F, Al-Khoury BN, Al-Deen JA, Sadik AM. Acute viral hepatitis: an aetiological study of 253 patients. J Gastroenterol Hepatol. 1989;4(2):151–54. doi: 10.1111/j.1440-1746.1989.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 117.Turky A, Akram W, Al-Naaimi A, Omer A, Al-Rawi J. Analysis of acute viral hepatitis (A and E) in Iraq. Glob J Health Sci. 2011;3(1):70–76. doi: 10.5539/gjhs.v3n1p70. [DOI] [Google Scholar]
- 118.Alkhalidi J, Alenezi B, Al-Mufti S, Hussain E, Askar H, Kemmer N, Neff GW. Seroepidemiology of hepatitis A virus in Kuwait. World J Gastroenterol. 2009;15(1):102–05. doi: 10.3748/wjg.15.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al-Kandari S, Nordenfelt E, Al-Nakib B, Grover S, Al-Nakib W. Viral hepatitis and pregnancy in Kuwait. Trans R Soc Trop Med Hyg. 1987;81(3):395–97. doi: 10.1016/0035-9203(87)90146-5. [DOI] [PubMed] [Google Scholar]
- 120.Al-Kandari S, Nordenfelt E, Al-Nakib B, Al-Nakib W. Hepatitis A and B virus infections in children in Kuwait. Ann Trop Paediatr. 1986;6(2):135–39. doi: 10.1080/02724936.1986.11748424. [DOI] [PubMed] [Google Scholar]
- 121.Hassan-Kadle MA, Osman MS, Ogurtsov PP. Epidemiology of viral hepatitis in Somalia: Systematic review and meta-analysis study. World J Gastroenterol. 2018;24(34):3927–57. doi: 10.3748/wjg.v24.i34.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bile K, Mohamud O, Aden C, Norder H, Mohamud O, Aden C, Nilsson L. The risk for hepatitis A, B, and C at two institutions for children in Somalia with different socioeconomic conditions. Am J Trop Med Hyg. 1992;47(3):357–64. doi: 10.4269/ajtmh.1992.47.357. [DOI] [PubMed] [Google Scholar]
- 123.Mohamud KB, Aceti A, Mohamed OM, Pennica A, Maalin KA, Biondi R, Hagi MA, Quaranta G, Paparo SB, Celestino D, et al. [The circulation of the hepatitis A and B viruses in the Somali population]. Ann Ital Med Int. 1992;7(2):78–83. [PubMed] [Google Scholar]
- 124.Fox E, Abbatte EA, Said S, Constantine NT, Wassef HH, Woody JN. Viral hepatitis markers in Djibouti: an epidemiological survey. Trans R Soc Trop Med Hyg. 1988;82(5):750–52. doi: 10.1016/0035-9203(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 125.Coursaget P, Buisson Y, Enogat N, Bercion R, Baudet J-M, Delmaire P, Prigent D, Desramé J. Outbreak of enterically-transmitted hepatitis due to hepatitis A and hepatitis E viruses. J Hepatol. 1998;28(5):745–50. doi: 10.1016/S0168-8278(98)80222-5. [DOI] [PubMed] [Google Scholar]
- 126.Battikhi MN, Battikhi EG. The seroepidemiology of Hepatitis A virus in Amman, Jordan. New Microbiol. 2004;27:215–20. [PubMed] [Google Scholar]
- 127.Hayajneh WA, Balbeesi A, Faouri S. Hepatitis A virus age-specific sero-prevalence and risk factors among Jordanian children. J Med Virol. 2015;87(4):569–74. doi: 10.1002/jmv.24137. [DOI] [PubMed] [Google Scholar]
- 128.Antaki N, Kebbewar MK. Hepatitis A seroprevalence rate in Syria. Trop Doct. 2000;30(2):99–101. doi: 10.1177/004947550003000215. [DOI] [PubMed] [Google Scholar]
- 129.Al-Azmeh J, Frösner G, Darwish Z, Bashour H, Monem F. Hepatitis E in Damascus, Syria. Infection. 1999;27(3):221–23. doi: 10.1007/BF02561535. [DOI] [PubMed] [Google Scholar]
- 130.Sheek-Hussein M, Hashmey R, Alsuwaidi AR, Al Maskari F, Amiri L, Souid A-K. Seroprevalence of measles, mumps, rubella, varicella–zoster and hepatitis A–C in Emirati medical students. BMC Public Health. 2012;12(1):1047. doi: 10.1186/1471-2458-12-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharar ZA, Rajah J, Parsons H. Childhood seroprevalence of hepatitis A in the United Arab Emirates. Trop Doct. 2008;38(1):65–66. doi: 10.1258/td.2007.053390. [DOI] [PubMed] [Google Scholar]
- 132.Bawazir AA, Hart CA, Sallam TA, Parry CM, Beeching NJ, Cuevas LE. Seroepidemiology of hepatitis A and hepatitis E viruses in Aden, Yemen. Trans R Soc Trop Med Hyg. 2010;104(12):801–05. doi: 10.1016/j.trstmh.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 133.Gunaid AA, Nasher TM, El-Guneid AM, Hill M, Dayton R, Pal A, Skidmore SJ, Coleman JC, Murray-Lyon IM. Acute sporadic hepatitis in the Republic of Yemen. J Med Virol. 1997;51(1):64–66. doi:. [DOI] [PubMed] [Google Scholar]
- 134.Carmoi T, Safiullah S, Nicand E. Risk of enterically transmitted hepatitis A, hepatitis E, and Plasmodium falciparum malaria in Afghanistan. Clin Infect Dis. 2009;48(12):1800. doi: 10.1086/599231. [DOI] [PubMed] [Google Scholar]
- 135.Gebreel AO, Christie AB. Viral hepatitis in children: a study in Libya. Ann Trop Paediatr. 1983;3(1):9–11. doi: 10.1080/02724936.1983.11748260. [DOI] [PubMed] [Google Scholar]
- 136.Bouskraoui M, Bourrous M, Amine M. Prevalence of anti-hepatitis a virus antibodies in children in Marrakech. Arch Pediatr. 2009;16(Suppl 2):S132–6. doi: 10.1016/S0929-693X(09)75317-5. [DOI] [PubMed] [Google Scholar]
- 137.Yassin K, Awad R, Tebi A, Queder A, Laaser U. The epidemiology of hepatitis A infection in Palestine: a universal vaccination programme is not yet needed. Epidemiol Infect. 2001;127(2):335–39. doi: 10.1017/S0950268801005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Glynn MJ, Rashid A, Antao AJ, Coleman JC, Howard CR, Zuckerman AJ, Murray-Lyon IM. Imported epidemic non-A, non-B hepatitis in Qatar. J Med Virol. 1985;17(4):371–75. doi: 10.1002/jmv.1890170409. [DOI] [PubMed] [Google Scholar]
- 139.Hyams KC, Hussain MA, Al-Arabi MA, Al-Huda Atallah N, el-Tigani A, McCarthy MC. Acute sporadic hepatitis in Sudanese children. J Med Virol. 1991;33(2):73–76. doi: 10.1002/jmv.1890330202. [DOI] [PubMed] [Google Scholar]
- 140.Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med. 2018;8(10):a031716. doi: 10.1101/cshperspect.a031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gripenberg M, Aloysia D’Cor N, L’Azou M, Marsh G, Druelles S, Nealon J. Changing sero-epidemiology of hepatitis A in Asia Pacific countries: a systematic review. Intl J Infect Dis. 2018;68:13–17. doi: 10.1016/j.ijid.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka J. Hepatitis a shifting epidemiology in Latin America. Vaccine. 2000;18(Suppl 1):S57–60. doi: 10.1016/S0264-410X(99)00466-1. [DOI] [PubMed] [Google Scholar]
- 143.Andani A, van Elten TM, Bunge EM, Marano C, Salgado F, Jacobsen KH. Hepatitis A epidemiology in Latin American countries: a 2020 view from a systematic literature review. Expert Rev Vaccines. 2020;19(9):795–805. doi: 10.1080/14760584.2020.1813575. [DOI] [PubMed] [Google Scholar]
- 144.Patterson J, Abdullahi L, Hussey GD, Muloiwa R, Kagina BM. A systematic review of the epidemiology of hepatitis A in Africa. BMC Infect Dis. 2019;19(1):651. doi: 10.1186/s12879-019-4235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gossner C, Severi E, Danielsson N, Hutin Y, Coulombier D. Changing hepatitis A epidemiology in the European Union: new challenges and opportunities. Euro Surveill. 2015;20(16):21101. doi: 10.2807/1560-7917.ES2015.20.16.21101. [DOI] [PubMed] [Google Scholar]
- 146.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, Wilder-Smith A, Wilson ME, Keystone JS, Schwartz E, et al. GeoSentinel surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158(6):456–68. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.MacDonald E, Steens A, Stene-Johansen K, Gillesberg Lassen S, Midgley SE, Lawrence J, Crofts J, Ngui SL, Balogun K, Frank C, et al. Increase in hepatitis A in tourists from Denmark, England, Germany, the Netherlands, Norway and Sweden returning from Egypt, November 2012 to March 2013. Euro Surveill. 2013;18(17) pii=20468 doi: 10.2807/ese.18.17.20468-en. [DOI] [PubMed] [Google Scholar]
- 148.Gassowski M, Michaelis K, Wenzel JJ, Faber M, Figoni J, Mouna L, Friesema IH, Vennema H, Avellon A, Varela C, et al. Two concurrent outbreaks of hepatitis A highlight the risk of infection for non-immune travellers to Morocco, January to June 2018. Euro Surveill. 2018;23(27) pii=1800329 doi: 10.2807/1560-7917.ES.2018.23.27.1800329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alwan A, Elmi M. Food safety in the Eastern Mediterranean Region: time to act. East Mediterr Health J. 2015;21(3):153–54. doi: 10.26719/2015.21.3.153. [DOI] [PubMed] [Google Scholar]
- 150.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12(12):e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sánchez G. Processing strategies to inactivate hepatitis A virus in food products: a critical review. Compr Rev Food Sci Food Saf. 2015;14(6):771–84. doi: 10.1111/1541-4337.12154. [DOI] [Google Scholar]
- 152.Gossner CM, Severi E. Three simultaneous, food-borne, multi-country outbreaks of hepatitis A virus infection reported in EPIS-FWD in 2013: what does it mean for the European Union? Euro Surveill. 2014;19(43):20941. doi: 10.2807/1560-7917.ES2014.19.43.20941. [DOI] [PubMed] [Google Scholar]
- 153.Swinkels HM, Kuo M, Embree G, Fraser Health Environmental Health Investigation Team C, Andonov A, Henry B, Buxton JA. Hepatitis A outbreak in British Columbia, Canada: the roles of established surveillance, consumer loyalty cards and collaboration, February to May 2012. Euro Surveill. 2014;19(18):20792. doi: 10.2807/1560-7917.ES2014.19.18.20792. [DOI] [PubMed] [Google Scholar]
- 154.World Health Organization (WHO) Health emergencies. Countries in crisis; 2016. [accessed 2021 Feb 5]. http://www.emro.who.int/eha/countries-in-crisis/index.html
- 155.Mellou K, Chrisostomou A, Sideroglou T, Georgakopoulou T, Kyritsi M, Hadjichristodoulou C, Tsiodras S. Hepatitis A among refugees, asylum seekers and migrants living in hosting facilities, Greece, April to December 2016. Euro Surveill. 2017;22(4):30448. doi: 10.2807/1560-7917.ES.2017.22.4.30448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michaelis K, Wenzel JJ, Stark K, Faber M. Hepatitis A virus infections and outbreaks in asylum seekers arriving to Germany, September 2015 to March 2016. Emerg Microbes Infect. 2017;6(4):e26. doi: 10.1038/emi.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.European Asylum Support Office . Latest asylum trends – 2015 overview; 2015. [accessed 2021 Feb 5]. https://www.easo.europa.eu/sites/default/files/public/LatestAsylumTrends20151.pdf
- 158.Lee K, Worsnop CZ, Grépin KA, Kamradt-Scott A. Global coordination on cross-border travel and trade measures crucial to COVID-19 response. Lancet. 2020;395(10237):1593–95. doi: 10.1016/S0140-6736(20)31032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


