Abstract
In the United States, all blood used for transfusion is tested for the presence of antibodies to the structural components of the human T-cell lymphotropic viruses types 1 and 2 (HTLV-1 and -2). Based on such serologic tests, the prevalence of HTLV-1 infection is estimated to range from 0.016 to 0.1%. As a consequence of studies of patients with mycosis fungoides and some of their healthy relatives who are antibody negative but were found to carry the tax sequence of HTLV-1 in their lymphocytes and who had antibodies to the p40tax protein, a study was undertaken to determine the prevalence of the “tax-only” state in 250 healthy blood donors and other volunteers. Using PCR and Southern analysis for cell lysates and using Western blotting for plasmas, 8.6% of the blood donors proved to be tax sequence positive and antibody positive. Sequence analysis of specimens from 22 individuals proved that 20 of the sequences were homologous with that of HTLV-1 while 2 resembled the HTLV-2 sequence. The latter were obtained from volunteers of Indian origin. The possible clinical significance of the tax-only carrier state is discussed.
The human T-cell lymphotropic virus type 1 (HTLV-1) is the established cause of T-cell leukemia and lymphoma in regions of the world where the virus is endemic (15, 28, 36). HTLV-1 is also causally related to the development of tropical spastic paraparesis (TSP) and HTLV-1-associated myelopathy (HAM), conditions which are inflammatory and autoimmune rather than neoplastic (10, 33). However, the majority of individuals infected with this virus never develop any disease, and if they do, it is usually late in life (2, 52). Nevertheless, since the virus is transmitted by blood transfusion, blood used for this purpose in the United States has been screened routinely for antibodies to HTLV-1 and -2 since 1988 (4). On this basis, the prevalence of HTLV-1 infection among Americans without obvious risk factors, such as origin from a region where the virus is endemic or intravenous drug use, has been estimated to range between 0.016 and 0.1%, depending on locale (24, 50).
There is now reason to question whether the prevalence of HTLV-1 infection in the United States may be greater than detected by currently used serologic tests. This question was first raised by our observation that patients with the cutaneous T-cell lymphoma, mycosis fungoides (MF), harbor the tax sequence of HTLV-1 in their lymphocytes without having antibodies to the structural proteins of the virus (35). For ease of discussion, the 159-bp proviral DNA encoding a portion of the Tax protein is referred to as the tax sequence in this paper. This situation was also found in some healthy relatives of MF patients (55). One of these relatives admitted to having served repeatedly as a volunteer blood donor. In addition, two healthy individuals without risk factors, whose blood specimens were obtained for use as negative controls to be run side by side with samples from MF patients, unexpectedly also turned out to be tax positive.
These observations prompted studies of randomly selected volunteer blood donors who presented at the New York University Medical Center (NYUMC) Blood Bank. Preliminary studies carried out on the first 100 of these donors revealed that more than 8% carried HTLV-1 tax sequences in their peripheral blood mononuclear cells (PBMC) and that the majority of them also had antibodies to the p40tax protein, an antigen currently not included in HTLV-1 and -2 serologic test kits (53). All tax-positive individuals were negative for antibodies to the structural proteins of the virus, which was routinely tested for at the New York Blood Center.
Because the tax sequence and its gene product, p40tax, are the transactivating and transforming components of HTLV-1 (for reviews see references 3, 11, and 52), a larger study of the prevalence of the “tax-only” state and its possible clinical implications seemed imperative.
The present report communicates data derived from 250 specimens, comprising samples obtained from 210 volunteer blood bank donors, who had no risk factors as defined by the United States blood banking community, and 40 additional volunteers recruited from among NYUMC staff.
In order to substantiate the assumption that healthy individuals in regions where the virus is not endemic may carry proviral sequences homologous to that of HTLV-1 tax without having antibodies to the structural proteins of the virus, the tax sequences amplified from PBMC lysates of 22 of these tax-only-positive healthy individuals were subjected to nucleotide sequence analysis.
Furthermore, to explore whether tax-only positivity has any clinical relevance, a study was initiated of specimens obtained from patients with rheumatoid arthritis (RA). This particular autoimmune disease was chosen to begin with, because mice transgenic for HTLV-1 tax develop clinical and pathologic manifestations indistinguishable from those of RA in humans (17, 18). Preliminary data suggest that the tax-only state is more common among patients with RA than among healthy blood donors. The possible implications of these observations are discussed.
MATERIALS AND METHODS
Specimens.
Heparinized blood was collected with informed consent and approval by the Institutional Review Board from 250 healthy adults who had no risk factors which would have disqualified them from donating blood for transfusion. All of the subjects tested negative for antibodies to HTLV-1 and -2 by enzyme-linked immunosorbent assay and Western blot tests performed at the New York Blood Center and/or in our own laboratory. Among the 250 were 210 who, independently of this study, donated a unit of blood at the NYUMC Blood Bank and 40 healthy volunteers who were recruited by advertisement from among the personnel at NYUMC. Twenty-six of the volunteers who were not blood donors were Caucasian Americans, three were African Americans, and three were African Caribbeans. The remaining eight consisted of one black Nigerian, two Koreans, one Japanese from Taiwan, and four Indians and Pakistanis.
Blood samples were also obtained from 57 patients diagnosed to have RA according to criteria established by the American Rheumatism Association (1). The patients with RA were referred by rheumatologists affiliated with our institution.
Blood samples were fractionated into plasma and PBMC by Ficoll-Hypaque gradient centrifugation as routinely carried out in this laboratory (35). Sterile techniques were strictly adhered to during all procedures, and each specimen was handled at a different site and usually on a different day than the others.
Detection of HTLV-1 and -2 proviral DNA sequences in PBMC. (i) Preparation of cell lysates and PCR amplification.
Whole-cell lysates were prepared from ∼105 PBMC as described previously (35). Briefly, the cells were lysed in autoclave-sterilized distilled water by sonication and boiling, followed by incubation for 1 h at 55°C in the presence of 2 μg of proteinase K per sample. The samples were then boiled to inactivate the protease and subjected to 30 cycles of PCR amplification (1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C per cycle, followed by a final incubation for 10 min at 72°C in the buffer and concentrations of deoxynucleoside triphosphates described previously (35). The final sample reaction mixture volume of 80 μl included 40 pmol of primers (see below) and 4 U of Taq polymerase (Perkin-Elmer, Foster City, Calif.) (12, 22, 35, 55). PCR was carried out in the Perkin-Elmer model 480 thermal cycler. Positive and negative control cells for the PCR consisted of lysates of the prototypic HTLV-1 and -2-infected cell lines, C91PL (HTLV-1 (37) and MoT (HTLV-2) (39), respectively, in addition to PBMC from HTLV-1 and -2 gag, pol, env, and tax PCR sequence-negative healthy volunteers.
(ii) Southern analysis.
PCR products were resolved through 4% agarose gels in the presence of ethidium bromide, followed by denaturation, neutralization, and overnight transfer of DNA to nylon membranes, as described before (35). DNA was cross-linked to the membranes by incubation for 1 h at 80°C followed by prehybridization and hybridization at 43°C for 2 h and overnight, respectively, using the probes listed below, 3′ tailed with digoxigenin. Detection of bound probe entailed use of Fab′ fragments of antibodies to digoxigenin, conjugated with alkaline phosphatase and the alkaline phosphatase substrates 4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP). The reagents for 3′ tailing the probes with digoxigenin and for the detection of bound probe were obtained from Boehringer Mannheim (Indianapolis, Ind.).
(iii) Primers and probes.
The study employed the HTLV gag-1 and env-1 primers and probes (12); the pol-1 and -2 primers SK110 and SK111 and probes SK112 (pol-1) and SK188 (pol-2); and the tax-1 and -2 primers SK43 and SK44 and probe SK45 (22). We recently published the sequences and HTLV genome locations of the following additional gag primers and probes (55): gag-1 and -2 sense and antisense primers BP4 (5′-CCCATCTTACGTTCCCTAGC-3′) (HTLV-1 1690–1709; HTLV-2 1713–1732), BP5 (5′-GGATCTTGACATAGGGGGCA-3′) (HTLV-1 1939–1958; HTLV-2 1962–1981), and probe BP6 (5′-AGGACATGGAGTCGGGACTGCACCCAGCC-3′) (HTLV-1 1890–1919; HTLV-2 1913–1942). The gag-2 primers included BP1 (5′-TCACGGGTTTCCCAACT-3′) (HTLV-2 817–833) and BP2 (5′-GGGCAGATAGGTGTCGGAAC-3′) (HTLV-2 1107–1126), and the probe was BP3 (5′-TGTCAAAAATCAAGTCTCCCCTAGCC-3′) (HTLV-2 1067–1092). The genome sequences and locations reported are those of Seiki et al. for HTLV-1 (40) and Shimotohno et al. for HTLV-2 (41). The conditions and temperatures for PCR amplification and hybridization with these primers and probes were the same as those for HTLV tax (SK43, SK44, and SK45) and pol (SK110, SK111, and SK112) described previously (35).
Detection of antibodies to HTLV p40tax. (i) Preparation of Tax antigen.
The proviral DNA sequences encoding the full-length tax-1 open reading frame were amplified by PCR from the prototypic HTLV-1-infected cell line, C91PL (37), as described in detail elsewhere (34). The amplified sequences were cloned into the glutathione S-transferase (GST) fusion protein expression vector, pGEX-2T, and p40tax antigens were prepared by expression of recombinant GST-p40tax proteins in Escherichia coli BL21 cells, followed by isolation of the recombinant GST-p40tax protein by chromatography with glutathione cross-linked to Sepharose 4B (Pharmacia Biotech, Piscataway, N.J.) and release of the p40tax protein by thrombin cleavage.
(ii) Western blot assay.
Thrombin-cleaved full-length C91PL p40tax antigen was resolved through preparative sodium dodecyl sulfate–8.5% polyacrylamide gels, followed by electrophoretic transfer to nitrocellulose. After overnight blocking in 100 mM Tris-HCl (pH 7.5)–150 mM NaCl containing 2% blocking reagent (Boehringer Mannheim), the blots were exposed to test and control plasmas, using the Bio-Rad Mini-Protean II multiscreen apparatus as a template. As reported elsewhere (34), the majority of plasmas were diluted 1:10, as we had shown that higher dilutions often failed to detect the antibody. After incubation in the presence of the test plasmas for 1 h at room temperature, the blots were washed extensively, followed by incubation with secondary antibody (goat anti-human immunoglobulin A [IgA] plus IgG plus IgM, H and L chains, conjugated with alkaline phosphatase; Pierce Chemical Co., Rockford, Ill.) and the alkaline phosphatase substrates 4-nitroblue tetrazolium and BCIP (Boehringer Mannheim). HTLV-positive and -negative human sera were included as controls in each assay.
Sequence analysis.
tax sequences amplified from specimens from 22 individuals were subjected to oligonucleotide sequence analysis. All specimens chosen for sequence analysis were derived from individuals who also had antibodies to the Tax protein.
Whole-cell lysates prepared from Ficoll-Hypaque gradient-fractionated PBMC as described above were subjected to two rounds of PCR amplification, each consisting of 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C, followed by a final 10-min incubation at 72°C under the PCR conditions described above. In PCR 1, primers SK43 and SK44 were utilized. The second PCR was initiated by the transfer of 2 μl of the PCR products generated in PCR 1 to a final PCR 2 volume of 80 μl per sample. The primers used in PCR 2 consisted of the sense and antisense primers 9376 (5′-CGTGTTTGGAGACTGTGTAC-3′) and 9377 (5′-CATCGATGGGGTCCCAGGTG-3′). The underlined sequences correspond to the 5-base 3′ ends of SK43 and SK44, respectively. The oligonucleotide HTLV gag, pol, env, and tax primers and probes were synthesized in the Oligonucleotide Synthesis and Sequencing Facility at our institution.
Positive and negative controls for PCRs included lysates of the HTLV-1-infected cell line, C91PL (37), and the HTLV-2-infected cell line, MoT (39), and lysates of known HTLV-1 and -2 PCR-negative PBMC, respectively. The products from PCRs 1 and 2 were resolved through ethidium bromide-stained 4% agarose gels. The remaining DNA amplification products that gave rise to visible bands in gels for the PCR 2 samples were subsequently isolated with reagents and columns supplied in the QIAquick Spin PCR purification kit obtained from Qiagen, Inc. (Chatsworth, Calif.) and were sequenced directly by the NYUMC Sequencing Facility, using primers 9376 and 9377. Sequences detected in the samples were compared with those published for HTLV-1 and -2 (8, 14, 20, 22, 40, 41) for the 128-bp tax proviral DNA sequence being analyzed. HTLV-1 and -2 differ in this region by 16 bp.
RESULTS
Detection of HTLV proviral DNA sequences and antibodies to p40tax.
Because of the questionable nature of data based solely on PCR, we report here as positive only those individuals who had both tax proviral sequences and antibodies to the Tax protein.
Among the 210 blood bank donors whose blood was found acceptable for transfusion purposes, 18 (8.6%) were tax sequence positive as well as p40tax antibody positive, which is consistent with our published preliminary studies (53). As for the 40 subjects who were not blood bank donors, 5 were shown to have tax-1 sequences and 2 had tax-2 sequences. These seven all had antibodies to p40tax. The apparent difference between the two groups of healthy subjects is not statistically significant. PBMC from neither the blood bank donors nor the other subjects were found to contain HTLV gag-1, gag-2, gag-1 and -2, pol-1, pol-2, or env-1 sequences. A representative Southern blot of tax proviral sequences detected in six different blood donors is shown in Fig. 1, and representative Western blot data for the same tax sequence-positive donors are presented in Fig. 2. None of the 250 individuals were seropositive for antibodies to the viral structural proteins Gag and Env, which was tested for routinely at the New York Blood Center.
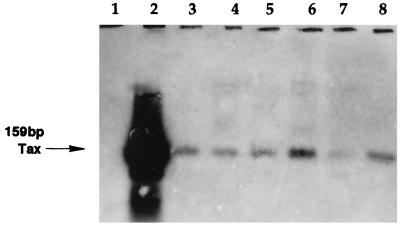
FIG. 1.
Southern blot detection of HTLV tax proviral DNA sequences amplified by PCR in lysates of PBMC from HTLV-seronegative blood donors. Primers SK43 and SK44 were used in the PCR, and digoxigenin-tailed probe SK45 was used for hybridization. The sources of PCR targets were PBMC from an HTLV tax-negative volunteer (lane 1), an HTLV-1-infected cell line, C91PL (lane 2), and PBMC from six different healthy blood donors (lanes 3 to 8).
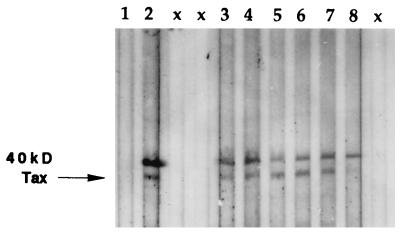
FIG. 2.
Detection of HTLV antibodies to p40tax in sera from the same individuals whose amplified tax sequences are shown in the Southern blot in Fig. 1. Western blot detection of Tax antibodies was done with recombinant full-length p40tax antigen. Bound antibody was detected with goat anti-human IgA plus IgG plus IgM, heavy and light chains, conjugated with alkaline phosphatase. The upper band is uncleaved GST-Tax, and the lower band is p40tax only. The sources of plasmas were an HTLV tax sequence-negative and antibody-negative volunteer (lane 1), an HTLV-1-infected patient with TSP and HAM (lane 2), and six different healthy blood donors and volunteers (lanes 3 to 8). No sample was added in lane x.
tax sequence analysis.
The tax proviral DNA sequences amplified in PBMC lysates obtained from 22 tax-positive healthy individuals are shown in Fig. 3. The HTLV tax sequence detected in 20 of these subjects proved to be homologous to prototypic HTLV-1 tax (Fig. 3A), whereas sequences from 2 individuals had several bases in common with HTLV-2 (Fig. 3B). It should be pointed out that in this region the prototypic tax-1 and -2 sequences differ by 16 bp (8, 14, 20, 22, 40, 41). Both of the individuals whose cells harbored HTLV-2-like tax sequences rather than HTLV-1-like tax sequences were of Indian or Pakistani origin.
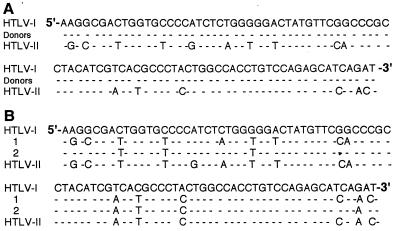
FIG. 3.
HTLV-1- and HTLV-2-like tax proviral sequences detected in PBMC lysates. (A) Representative HTLV-1 tax sequence (Donors) detected in PBMC of 14 blood donors and 6 other healthy volunteers. (B) HTLV-2-like tax proviral sequences (1 and 2) detected in PBMC of two volunteers. The tax sequences for prototypic HTLV-1 and -2 are those published by Seiki et al. and Shimotohno et al., respectively (40, 41). The dashes indicate sequence identity with the prototypic HTLV-1 sequence.
Observations of specimens from patients with RA.
To date, paired PBMC and plasmas have been obtained from only 57 patients with RA. Fifteen of the 57 (26.3%) were positive for both tax sequences and antibodies to p40tax (data not shown). None of the RA patients harbored gag, pol, or env sequences, and all were HTLV-1 and -2 seronegative when tested by routine analyses. On the basis of these preliminary data, it appears that the tax-only state is three times more prevalent among patients with RA than among the healthy blood donor population (P < 0.0001).
DISCUSSION
The pX region of the HTLV-1 genome encodes at least four proteins, Tax, Rex, p12, and p21 (3, 9, 11, 52). Interestingly, even in regions of the world where HTLV-1 is endemic, it is not unusual to find instances among carriers of the virus, as well as among patients with HTLV-1-related diseases, where all viral DNA has been deleted with the exception of the region encoding Tax (21, 31, 51). The Tax protein is a transcriptional transactivator of its own long terminal repeat (43). The antigen used to detect Tax antibodies consisted of the entire p40tax protein. In regions where HTLV-1 is endemic, the presence of antibodies to the Tax protein has been correlated strongly with both vertical (38) and sexual (5) transmission of the virus. Equally intriguing are reports from Japan that 1.5% of healthy HTLV-1 carriers and 8.5% of patients with adult T-cell leukemia have antibodies to Tax without having detectable antibodies to the structural proteins of the virus (42). This phenomenon does not seem to be unusual in regions where the virus is endemic (19, 21, 32) and pertains to our own studies of patients with MF (34, 35). Also relevant is a recently studied 9-year-old black patient with MF whose skin biopsy and PBMC harbored HTLV-1 tax and who had antibodies to p40tax while being negative for coding sequences and antibodies to the HTLV-1 structural proteins. The same was true of his healthy younger brother, whereas the mother was a carrier, serologically positive for HTLV-1, with no deleted sequences (54). Unfortunately, many of the early reports on the subject of HTLV-1 transmission did not include biomolecular analyses, making it difficult to deduce whether individuals with antibodies only to p40tax also harbored proviral DNA. Be that as it may, the present observations suggest that a substantial number of healthy American blood donors have DNA sequences homologous to the HTLV-1 tax sequence in their PBMC and that practically all of these individuals also have antibodies to p40tax without being serologically positive for antibodies to the structural proteins of the virus. Since, as of this writing, tax has not been found in the human genome, the conclusions that the tax-only state reflects an infection with HTLV-1 or a very closely related virus and that this may be more common in the United States than previously believed seem warranted. Proof of the possibility that yet another variant of HTLV is involved must await analysis of the complete tax sequence found in the specimens under consideration. Meanwhile, the presence of antibodies to the p40tax in the same individuals who have tax sequences in their PBMC may help to alleviate concern about the possibility of PCR contamination. The latter possibility was also all but ruled out by repeated collection and analysis of specimens obtained from the same donors at different times and handled by different laboratory personnel in a blind manner.
While the tax sequence found in the PBMC of 20 of 22 subjects was characteristic of HTLV-1, the cells of 2 subjects harbored tax sequences resembling that of HTLV-2. Look-back investigation revealed that both of these donors were Pakistani. Although the prevalence of HTLV-1 and -2 infection in India and Pakistan has not been established, it is known that HTLV-2 is endemic in Southeast Asia as well as among American Indian populations (13). The relationship of HTLV-2 to the development of disease remains ill defined. It seems appropriate, therefore, to limit this discussion to tax of HTLV-1.
The obvious questions raised by our observations are (i) whether the tax-only state is clinically significant and (ii) whether it can be transmitted by transfusion.
While the transforming ability of HTLV-1 tax is common knowledge, its role in the development of autoimmunity is less well recognized. In regions of the world where the virus is not endemic, such as the United States, few clinicians encounter patients with TSP and HAM. Therefore, as a rule, little thought is given to the fact that the latter condition is not neoplastic. It is also not widely known that Tax transactivates numerous cellular genes, that it is involved in the activation of many cytokines, and that, via NF-κB, it dysregulates the cellular as well as humoral immune response (reviewed in references 3, 11, and 52). In regions where HTLV-1 is endemic, many autoimmune conditions, such as Sjögren’s syndrome (44, 45), HTLV-related arthropathy (27, 30), and idiopathic uveitis (16, 29), are considerably more prevalent among carriers of HTLV-1 than among cohorts that are serologically negative for antibodies to the virus. For instance, on the Japanese island of Kyushu, idiopathic uveitis is three times more prevalent among HTLV-1 carriers than in the uninfected population (29). Another Japanese study showed a 20% incidence of rheumatoid arthropathy in otherwise healthy HTLV-1 carriers, whereas this condition was only found in 4% of 19,796 HTLV-1-negative blood donors tested in the same geographic region (7).
Because mice transgenic for tax happen to serve as good animal models for RA (17, 18), we elected to begin our investigation of a possible association of tax positivity with autoimmune diseases by studying patients with RA. On the basis of preliminary observations of the first 57 patients, it appears that the prevalence of the tax-only state is three times higher among RA patients than among healthy blood donors. tax sequences have also been identified by in situ hybridization in labial epithelial cells of some patients with Sjögren’s syndrome who were seronegative for HTLV-1 and -2 (25, 26, 44).
It is now generally recognized that autoimmune diseases like RA may have multiple etiologies. Concepts of antigenic cross-reactivity, “molecular mimicry,” and “epitope spreading” have been reviewed elsewhere (46, 47). A large variety of foreign antigens can elicit cellular and humoral immune responses, which affect self antigens—particularly in the context of a conducive histocompatibility setting. HTLV-1 tax may have to be added to the list of such agents.
The second question raised by these studies, i.e., whether the tax-only state is transmitted by transfusion, cannot be answered at the present time. It should be realized, however, that even the intact virus is transmitted only by cells containing it and not by free particles (52). Such cells would have to survive in the recipient and may even have to proliferate, a possibility considered by others (6). Clonal expansion of cells harboring HTLV-1 appears to be the rule in individuals carrying the complete virus (48, 49). Whether this also holds true for cells harboring only the tax sequence cannot be tested directly. For ethical reasons, it will have to be deduced from epidemiologic studies of multiply transfused patients.
ACKNOWLEDGMENTS
These studies were supported by grant R01-CA-58519 from the National Cancer Institute of the National Institutes of Health and by an award from the National Blood Foundation to D. Zucker-Franklin.
We thank John G. Gorman, Director of the NYUMC Blood Bank, for his continued support of this project and his staff for supplying samples from blood donors. The blood samples from RA patients were kindly provided by S. Lee, H. Mitnick, and M. Mundheim. We are also indebted to Susan Dittmar for technical assistance.
REFERENCES
- 1.Arnett F C. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 2.Blattner W A. Human T-lymphotropic viruses and diseases of long latency. Ann Intern Med. 1989;111:4–6. doi: 10.7326/0003-4819-111-1-4. [DOI] [PubMed] [Google Scholar]
- 3.Buckle, G. J., D. A. Hafler, and P. Höllsberg. 1996. HTLV-I-induced T cell activation. J. Acquired Immune Defic. Syndr. Hum. Retroviral. 13(Suppl. 1):S107–S113. [DOI] [PubMed]
- 4.Centers for Disease Control and Prevention. Licensure of screening tests for antibody to human T lymphotropic virus type I. Morbid Mortal Weekly Rep. 1988;37:736–740. and 745–747. [PubMed] [Google Scholar]
- 5.Chen Y-M A, Okayama A, Lee T H, Tachibana N, Mueller N, Essex M. Sexual transmission of human T-cell leukemia virus type I associated with the presence of anti-Tax antibody. Proc Natl Acad Sci USA. 1991;88:1182–1186. doi: 10.1073/pnas.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzik W W. Mononuclear cell microchimerism and the immunomodulatory effect of transfusion. Transfusion. 1994;34:1007–1012. doi: 10.1046/j.1537-2995.1994.341195065024.x. [DOI] [PubMed] [Google Scholar]
- 7.Eguchi K, Iwata K, Katamine S, Nagataki S. High seroprevalence of anti-HTLV-I antibodies in rheumatoid arthritis. Arthritis Rheum. 1996;39:463–466. doi: 10.1002/art.1780390314. [DOI] [PubMed] [Google Scholar]
- 8.Eiraku N, Novoa P, da Costa Ferreira M, Monken C, Ishak R, da Costa Ferreira O, Zhu S W, Lorenco R, Ishak M, Azvedo V, Guerreiro J, de Oliveira M P, Luoreiro P, Hammerschlak N, Ijichi S, Hall W W. Identification and characterization of a new and distinct molecular subtype of HTLV-II. J Virol. 1996;70:1481–1492. doi: 10.1128/jvi.70.3.1481-1492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphoma virus type-I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 10.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calendar A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin S D, Dittmer J, Reid R L, Brady J N. The molecular biology of human T-cell leukemia virus. In: Cullen B R, editor. Human retroviruses. New York, N.Y: IRL Press; 1993. pp. 159–192. [Google Scholar]
- 12.Hall W W, Liu C R, Schneewind O, Takahashi H, Kaplan M H, Roupe G, Vahlne A. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science. 1991;253:317–320. doi: 10.1126/science.1857968. [DOI] [PubMed] [Google Scholar]
- 13.Hall, W. W., R. Ishak, S. W. Zhu, P. Novoa, N. Eiraku, H. Takahashi, M. da Costa Ferreira, V. Azevedo, M. O. Ishak, O. da Costa Ferreira, C. Monken, and T. Kurata. 1996. Human T lymphotropic virus type II (HTLV-II): epidemiology, molecular properties, and clinical features of infection. J. Acquir. Immune Defic. Syndr. Hum. Retroviral. 13(Suppl. 1):S204–S214. [DOI] [PubMed]
- 14.Hall W W, Takahashi H, Kaplan M H, Schneewind O, Ijichi S, Nagashima K, Gallo R C. Multiple isolates and characteristics of human T-cell leukemia virus type II. J Virol. 1992;66:2456–2463. doi: 10.1128/jvi.66.4.2456-2463.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda E, Wada K, Yoshimura K, Mochizuki M. Statistics of uveitis of northern and southern districts of Kyushu. Jpn J Clin Ophthalmol. 1993;47:1267–1270. [Google Scholar]
- 17.Iwakura Y, Saijo S, Kioka Y, Nakayama-Yamada J, Itagaki K, Tosu M, Asano M, Kanai Y, Kakimoto K. Autoimmunity induction by human T cell leukemia virus type I in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. J Immunol. 1995;155:1588–1598. [PubMed] [Google Scholar]
- 18.Iwakura Y, Tosu M, Yoshida E, Takiguchi M, Sato K, Kitajima I, Nishioka K, Yamamoto K, Takeda T, Hatanaka M, Yamamoto H, Sekiguchi T. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science. 1991;253:1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- 19.Kamihira S, Kazuhiro T, Amagasaki T, Momita S, Ikeda S, Yamada Y, Tomonaga M, Ichimaru M, Kinoshita K, Sawada T. Antibodies against p40tax gene product of human T-lymphotropic virus type-I (HTLV-I) under various conditions of HTLV-I infection. Jpn J Cancer Res. 1989;80:1066–1071. doi: 10.1111/j.1349-7006.1989.tb02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitajima I, Yamamoto K, Sato K, Nakajima Y, Nakajima T, Maruyama I, Osame M, Nishioka K. Detection of human T cell lymphotropic virus type I proviral DNA and its gene expression in synovial cells in chronic inflammatory arthropathy. J Clin Investig. 1991;88:1315–1322. doi: 10.1172/JCI115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korber B, Okayama A, Donnelly R, Tachibana N, Essex M. Polymerase chain reaction analysis of defective human T-cell leukemia virus type I proviral genomes in leukemic cells of patients with adult T-cell leukemia. J Virol. 1991;65:5471–5476. doi: 10.1128/jvi.65.10.5471-5476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwok S, Kellogg D, Ehrlich G, Poiesz B, Bhagavati S, Sninsky J J. Characterization of a sequence of the human T cell leukemia virus type I from a patient with chronic progressive myelopathy. J Infect Dis. 1988;158:1193–1197. doi: 10.1093/infdis/158.6.1193. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Idler K B, Swanson P, Aparicio J J, Chin K K, Lax J P, Nguyen M, Mann T, Leckie G, Zanetti A, Marinucci G, Chen I S, Rosenblatt J D. Complete nucleotide sequence of HTLV-II isolate NRA. Comparison of envelope sequence variation of HTLV-II isolates from U.S. blood donors and U.S. and Italian IV drug users. Virology. 1993;196:57–69. doi: 10.1006/viro.1993.1454. [DOI] [PubMed] [Google Scholar]
- 24.Lee H H, Swanson P, Rosenblatt J D, Chen I S, Sherwood W C, Smith D E, Tegtmeier G E, Fernando L P, Fang C T, Osame M, Kleinman S H. Relative prevalence and risk factors of HTLV-I and HTLV-II infection in US blood donors. Lancet. 1991;337:1435–1439. doi: 10.1016/0140-6736(91)93126-t. [DOI] [PubMed] [Google Scholar]
- 25.Mariette X, Agbalika F, Daniel M T, Bisson M, Lagrange P, Brouet J C, Morinet F. Detection of T lymphotropic virus type I in salivary epithelium from 2 patients with Sjögren’s syndrome. Arthritis Rheum. 1993;36:1423–1428. doi: 10.1002/art.1780361015. [DOI] [PubMed] [Google Scholar]
- 26.Mariette, X., F. Agbalika, D. Zucker-Franklin, D. Clerc, J. D. Lelievre, C. Brocheriou, P. Cherot, and J.-C. Brouet. Detection of the pX gene of HTLV-I in labial salivary glands from patients with Sjögren’s syndrome and with other diseases of the oral cavity. Submitted for publication. [PubMed]
- 27.McCallum R M, Dhavalkumar D P, Moore J O, Haynes B F. Arthritis syndromes associated with human T-cell lymphotropic virus type I infection. Adv Rheumatol. 1997;81:261–276. doi: 10.1016/s0025-7125(05)70514-9. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type-C virus particles in a cord T-cell line derived by cocultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki M, Wanatabe T, Yamaguchi K, Yoshimura K, Nakashima S, Shirao M, Araki S, Takatsuki K, Mori S, Miyata N. Uveitis associated with human T-cell lymphotropic virus type I. Am J Ophthalmol. 1992;114:123–129. doi: 10.1016/s0002-9394(14)73974-1. [DOI] [PubMed] [Google Scholar]
- 30.Nishioka, K. 1996. HTLV-I arthropathy and Sjögren’s syndrome. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S57–S62. [DOI] [PubMed]
- 31.Ohshima K, Kikuchi M, Masuda Y, Kobari S, Sumiyoshi Y, Eguchi F, Mohtai H, Yoshida T, Takashita M, Kimura N. Defective provirus form of human T cell leukemia virus type I in adult T cell leukemia/lymphoma: clinicopathological features. Cancer Res. 1991;51:4639–4642. [PubMed] [Google Scholar]
- 32.Okayama A, Korber B, Chen Y-M A, Allan J, Lee T H, Shioiri S, Tachibana N, Tsuda K, Mueller N, McLane M F, Maayan S, Orgad S, Ernst J, Marlink R, Essex M. Unusual pattern of antibodies to human T-cell leukemia virus type-I in family members of adult T cell leukemia patients. Blood. 1991;78:3323–3329. [PubMed] [Google Scholar]
- 33.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 34.Pancake B A, Wassef E H, Zucker-Franklin D. Demonstration of antibodies to HTLV-I tax in patients with the cutaneous T cell lymphoma, mycosis fungoides, who are seronegative for antibodies to the structural proteins of the virus. Blood. 1996;88:3004–3009. [PubMed] [Google Scholar]
- 35.Pancake B A, Zucker-Franklin D, Coutavas E E. The cutaneous T cell lymphoma, mycosis fungoides, is a human T cell lymphotropic virus-associated disease. A study of 50 patients. J Clin Investig. 1995;95:545–554. doi: 10.1172/JCI117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popovic M, Sarin P S, Robert-Guroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 38.Sawada T, Tohmatsu J, Obara T, Koide A, Kamihira S, Ichimaru M, Kashiwagi S, Kajiyama W, Matsumura N, Kinoshita K, Yano M, Yamaguchi K, Kiyokawa T, Takatsuki K, Taguchi H, Miyoshi I. High risk of mother-to-child transmission of HTLV-I in p40 tax antibody-positive mothers. Jpn J Cancer Res. 1989;80:506–508. doi: 10.1111/j.1349-7006.1989.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxon A, Stevens R H, Golde D W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978;88:323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- 40.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimotohno K, Takahashi Y, Shimizu N, Gojobori T, Golde D W, Chen I S, Miwa M, Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II. Proc Natl Acad Sci USA. 1985;82:3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shioiri S, Tachibana N, Okayama A, Tsuda K, Essex M, Stuver S O, Mueller N. Analysis of anti-tax antibody of HTLV-I carriers in an endemic area in Japan. Int J Cancer. 1993;53:1–4. doi: 10.1002/ijc.2910530102. [DOI] [PubMed] [Google Scholar]
- 43.Sodroski J G, Rosen C A, Haseltine W A. Trans-activating transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984;225:381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- 44.Sumida T, Yonaha F, Maeda T, Kita Y, Iwamoto I, Koike T, Yoshida S. Expression of sequences homologous to HTLV-I Tax gene in the labial salivary glands of Japanese patients with Sjögren’s syndrome. Arthritis Rheum. 1994;37:545–550. doi: 10.1002/art.1780370415. [DOI] [PubMed] [Google Scholar]
- 45.Terada K, Katmine S, Eguchi K, Moriuchi R, Kita M, Shimida H, Yamashita I, Iwata K, Tsuji Y, Nagataki S, Miyamoto T. Prevalence of serum and salivary antibodies to HTLV-I in Sjögren’s syndrome. Lancet. 1994;344:1116–1119. doi: 10.1016/s0140-6736(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 46.Vanderlugt C J, Miller S D. Epitope spreading. Curr Opin Immunol. 1996;8:831–839. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Herrath M G, Oldstone M B A. Virus-induced autoimmune disease. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wattel E, Vartanian J P, Pannetier C, Wain-Hobson S. Clonal expansion of human leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wattel, E., M. Cavrois, A. Gessain, and S. Wain-Hobson. 1996. Clonal expansion of infected cells: a way of life for HTLV-I. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S92–S99. [DOI] [PubMed]
- 50.Williams A E, Fang C T, Slamon D J, Poiesz B J, Sandler S G, Darr W F, Shulman G, McGowan E I, Douglas D K, Bowman R J, Peetoom F, Kleinman S H, Lenes B, Dodd R Y. Seroprevalence and epidemiological correlates of HTLV-I infection in U.S. blood donors. Science. 1988;240:643–646. doi: 10.1126/science.2896386. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida M, Hattori S, Seiki M. Molecular biology of human T-cell leukemia virus associated with adult T-cell leukemia. Curr Top Microbiol Immunol. 1985;115:157–175. doi: 10.1007/978-3-642-70113-9_11. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida M. Retroviruses (HTLVs) In: Stamatoyannopoulos G, Nienhuis A, Majerus P W, Varmus H, editors. The molecular basis of blood diseases. W. B. Philadelphia, Pa: Saunders; 1994. pp. 929–941. [Google Scholar]
- 53.Zucker-Franklin D, Gorman J G. HTLV-I provirus in seronegative healthy blood donors. Lancet. 1997;349:999. doi: 10.1016/S0140-6736(05)62896-6. [DOI] [PubMed] [Google Scholar]
- 54.Zucker-Franklin, D., M. Klein Kosann, B. A. Pancake, D. L. Ramsay, and N. A. Soter. Hypopigmented mycosis fungoides associated with human T cell lymphotropic virus type I tax in a pediatric patient. Submitted for publication. [DOI] [PubMed]
- 55.Zucker-Franklin D, Pancake B A, Marmor M, Legler P. Reexamination of human T cell lymphotropic virus (HTLV-I/II) prevalence. Proc Natl Acad Sci USA. 1997;94:6403–6407. doi: 10.1073/pnas.94.12.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]