Abstract
Normal-tension glaucoma is a form of optic nerve degeneration that is characterized by loss of retinal ganglion cells independent of eye pressure elevation. In this issue of the JCI, Pan et al. report the discovery in a Japanese family of a mutation in the METTL23 gene, which encodes a DNA methyltransferase that causes normal-pressure glaucoma in haploinsufficiency. Inherited as an autosomal dominant condition, METTL23 deficiency revealed an important function in the regulation of pS2 and the downstream NF-κB signaling pathway, which has previously been linked to glaucomatous optic nerve degeneration. These findings are the first direct link between defective epigenetic regulatory machinery and genetic forms of optic nerve degeneration.
Black cataract
Ancient Indian medical culture described glaucoma as “black cataract”— the loss of vision that leads to blindness in the absence of clouding of the lens. The term glaucoma has its origin in the Greek word glaukos, meaning “shiny” or “grayish blue.” This term is thought to describe the bluish pupillary hue found in some affected eyes (1). In the mid-19th century, improved methods for measuring intraocular pressure (IOP) and the invention of the ophthalmoscope allowed for better descriptions of glaucoma as a clinical entity. Soon after, glaucoma was proposed to be a pressure excavation, as elevated IOP and excavation of the optic nerve were discovered as key features that distinguished glaucoma from other ophthalmologic conditions (2). Today, glaucoma is the leading cause of irreversible blindness, affecting approximately 80 million people worldwide. IOP is a major risk factor for most types of glaucoma. Elevated IOP is thought to be the mechanical force that drives the damage of retinal ganglion cells, leading to the clinical features of optic nerve excavation and visual field loss. For the past century, lowering the IOP has been the mainstay of glaucoma treatment, with much research effort directed at understanding how IOP affects retinal ganglion cell (RGC) survival (3).
Glaucoma with normal pressure
Although the term glaucoma is historically associated with high IOP, a substantial proportion of patients with glaucoma do not have elevated IOP. They have features of optic nerve excavation and visual field loss characteristic of glaucoma, but their IOP is within the normal range. The underlying pathophysiology of this kind of glaucoma is not well understood and is referred to as “normal-tension glaucoma” (NTG) (4). IOP has been shown to be a contributing factor for NTG, and reducing IOP slows the rate of glaucoma progression in patients with NTG (5). In addition, vascular dysfunction and poor optic nerve head perfusion have been proposed as important pathogenic factors. In Japan, the vast majority of patients with glaucoma fall into the NTG category (6). In the United States, the prevalence of NTG is thought to be as high as 30% to 39%. This observation raises interesting questions. If mechanical forces from high IOP lead to ganglion cell loss in glaucoma, why do patients with normal IOP also develop optic nerve excavation and field loss? In addition to IOP, what other factors and cellular pathways are involved in glaucoma pathogenesis?
The increasing recognition that eye pressure–independent forms of glaucoma are contributing to blindness worldwide has broadened the search for genes that are intrinsic to RGC survival. In recent years, genetic and genomic studies have uncovered many genes contributing to glaucoma (7). Mendelian inheritance is common for rare, early-onset forms of the disease, while adult-onset forms that are more prevalent typically have complex inheritance. Discovery of these genes and genetic loci provides insight into the molecular mechanisms of the disease and forms the basis for developing gene-based therapies and diagnostics. Genes that have been implicated in glaucoma include those involved in diverse cellular processes, such as inflammatory responses, lipid metabolism, ocular development, and mitochondrial function. To date, rare mutations in two genes have been found to cause early-onset familial NTG with autosomal dominant inheritance: optineurin (OPTN) and Tank-binding protein 1 (TBK1). OPTN and TBK1 are known to interact and have critical roles in autophagy and the NF-κB signaling pathways. These genes account for approximately 2% to 3% of NTG cases. Hence, additional genes involved in the pathogenesis of NTG have yet to be discovered. The discovery of these genes will not only shed light on the pathogenesis of glaucoma, but may also inform our understanding of other neurodegenerative diseases.
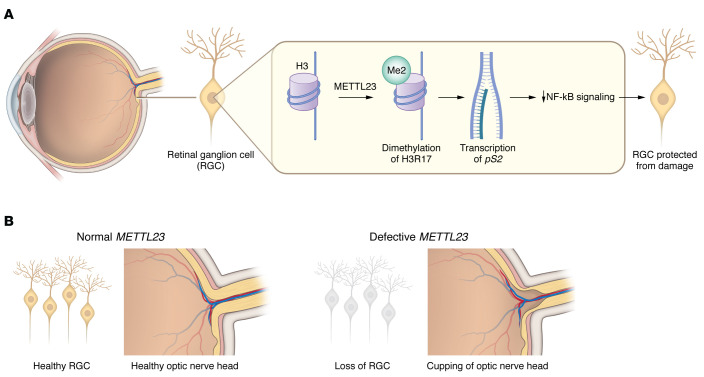
Epigenetics and neurodegeneration
The elegant study by Pan et al. in this issue of the JCI describes a genetic cause of NTG and reports a mutant histone methyltransferase leading to NTG (8). The investigators identified a family of three generations of Japanese patients with NTG and a splicing mutation in the methyltransferase-like 23 (METTL23) gene, which encodes a histone arginine methyltransferase. The autosomal dominant pattern of inheritance supporting the METTL23 c.A83G mutation was found in all six affected members of the family. This mutation resulted in METTL23 mRNA aberrant splicing. The haploinsufficiency of this gene resulted in decreased levels and abnormal subcellular localization of protein. Mechanistically, METTL23 catalyzes dimethylation of H3R17 in the retina. The estrogen receptor pS2 was identified as a target effector of this methylation activity and negatively regulated NF-κB signaling (Figure 1). Using multiple approaches, including Mettl23-knockin and -knockout mice and NTG patient–derived induced pluripotent stem cells (iPSCs), the investigators showed a critical function of METTL23 in RGC soma survival and optic nerve neuroprotection. Nonetheless, there may be other factors that modulate the phenotype of METTL23 heterozygosity. A METTL23 c.84+60delAT variant that promotes exon 2 skipping was enriched in patients with NTG but could also be found in controls (1.4% of patients with NTG and 0.6% of controls), suggesting that METTL23 splice variants may have varying pathogenicity.
Figure 1. A model for the role of METTL23 in RGC survival and optic nerve neuroprotection.
METTL23 catalyzes the dimethylation of H3R17 in the retina. Its activity ensures transcription of the estrogen receptor pS2, which negatively regulates NF-κB signaling and protects RGCs from glaucomatous damage. METTL23 loss of function results in RGC death and normal-pressure glaucoma.
A notable contribution of Pan et al. (8) is the link between histone methylation and glaucoma, which may have implications for other forms of neurodegenerative disorders. There has been increasing evidence that alterations in DNA methylation play an important role in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyloid lateral sclerosis (ALS) (9). Interestingly, both OPTN and TBK1 mutations have been identified in patients with ALS (10). PRMT1-mediated arginine methylation of RNA-binding protein fused in sarcoma (FUS), also implicated in ALS, causes aggregates in the cytoplasm that may inhibit FUS function in RNA splicing, DNA repair, and transcriptional regulation that are essential for neuronal homeostasis (11).
Another major epigenetic regulatory mechanism revolves around histone acetylation and methylation. The enzymes involved in these processes have also been linked to RGC survival in animal models of optic nerve injury and neurodegenerative diseases (12, 13). RGC-specific knockout of histone deacetylase (Hdac3) resulted in reduced heterochromatic formation and increased RGC survival in the optic nerve crush model of optic neuropathy (12). However, human variants in HDACs have not been associated with glaucoma.
This study by Pan et al. highlights the role of histone arginine methylation in neurodegenerative processes and raises important questions for the field (8). Are there other targets of METTL23 that play critical roles in RGC survival? Do other pathways downstream of METTL23 mediate its protective effect in RGCs? Are METTL23 mutations observed in other neurodegenerative conditions? NF-κB–mediated inflammation has been suggested to play a role in NTG and other neurodegenerations. However, its pleiotropic effects in the body may make it a challenging target in the design of neuroprotective strategies. Will METTL23 be a more viable target? The study by Pan and colleagues (8) opens new areas of epigenetics as therapeutic targets for glaucomatous neurodegeneration and provides important evidence for the role of histone arginine methylation in neuronal survival and function.
Acknowledgments
This work was supported by NIH grants (R01-EY025295 and R01 EY032159, to YS), a Veteran Affairs Merit Award (CX001481, to YS), a Stanford Maternal and Children’s Health Research Institute Lacob Faculty Scholar Award (to YS), and the Stanford Optic Disc Drusen Center Fund (to YS). This work was also supported by a KL2 Mentored Career Development Award (KL2TR003143, to WWL), an American Glaucoma Society Young Clinician Scientist Award (to WWL), an American Glaucoma Society Mentoring for the Advancement of Physician Scientists Award (to WWL), a Research to Prevent Blindness Career Development Award (to WWL), the SPARK Program at the Stanford University School of Medicine (to WWL), the NIH NEI P30 Vision Research Core (EY026877, Stanford Ophthalmology), and by an unrestricted grant from Research to Prevent Blindness (Stanford Ophthalmology).
Version 1. 11/01/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Liu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(21):e163670. https://doi.org/10.1172/JCI163670.
See the related article at METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma.
Contributor Information
Wendy W. Liu, Email: wendywliu@stanford.edu.
Yang Sun, Email: yangsun@stanford.edu.
References
- 1.Parihar JK. Glaucoma: the ‘black hole’ of irreversible blindness. Med J Armed Forces India. 2016;72(1):3–4. doi: 10.1016/j.mjafi.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leffler CT, et al. The early history of glaucoma: the glaucous eye (800 BC to 1050 AD) Clin Ophthalmol. 2015;9:207–215. doi: 10.2147/OPTH.S77471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland V, et al. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Int Med. 2013;158(4):271–279. doi: 10.7326/0003-4819-158-4-201302190-00008. [DOI] [PubMed] [Google Scholar]
- 4.Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond) 2018;32(5):924–930. doi: 10.1038/s41433-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. doi: 10.1016/S0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 6.Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59(4):434–447. doi: 10.1016/j.survophthal.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017;26(r1):21–27. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, et al. METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma. J Clin Invest. 2022;132(21):e153589. doi: 10.1172/JCI153589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur G, et al. DNA Methylation: a promising approach in management of Alzheimer’s disease and other neurodegenerative disorders. Biology. 2022;11(1):90. doi: 10.3390/biology11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirulli ET, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasperini L, et al. The hnRNP RALY regulates PRMT1 expression and interacts with the ALS-linked protein FUS: implication for reciprocal cellular localization. Mol Biol Cell. 2018;29(26):3067–3081. doi: 10.1091/mbc.E18-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt HM, et al. Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury. Mol Neurodegener. 2014;9(1):39. doi: 10.1186/1750-1326-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla S, Tekwani BL. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol. 2020;11:537. doi: 10.3389/fphar.2020.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]