Abstract
Sterile α motif domain–containing 9 (SAMD9) and SAMD9-like (SAMD9L) syndromes are inherited bone marrow failure syndromes known for their frequent development of myelodysplastic syndrome with monosomy 7. In this issue of the JCI, Abdelhamed, Thomas, et al. report a mouse model with a hematopoietic cell–specific heterozygous Samd9l mutation knockin. This mouse model resembles human disease in many ways, including bone marrow failure and the nonrandom loss of the mutant allele. Samd9l-mutant hematopoietic stem progenitor cells showed reduced fitness at baseline, which was further exacerbated by inflammation. TGF-β hyperactivation was found to underlie reduced fitness, which was partially rescued by a TGF-β inhibitor. These findings illustrate the potential role of TGF-β inhibitors in the treatment of SAMD9/SAMD9L syndromes.
SAMD9/SAMD9L syndromes
Sterile α motif domain–containing 9 (SAMD9) and SAMD9-like (SAMD9L) syndromes are inherited bone marrow (BM) failure syndromes due to mutations in SAMD9 or SAMD9L. The genes are paralogs located on chromosome 7q21 and their function remains elusive but has a growth inhibitory effect. Patients with SAMD9/SAMD9L syndromes have diverse clinical manifestations, including cytopenias, myelodysplastic syndrome (MDS), growth restriction, and immune dysregulation. Previously, SAMD9 and SAMD9L mutations were thought to cause MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, and enteropathy) syndrome and ataxia pancytopenia syndrome, respectively (1, 2). But, more recent studies suggest those syndromes can be observed in patients with either gene mutations (3).
SAMD9/SAMD9L syndromes are notorious for their frequent association with monosomy 7, which carries one of the worst prognoses in myeloid malignancies as a single cytogenetic abnormality (4). Because the gain-of-function mutants are severely growth inhibitory, cells that lost the mutant allele (i.e., monosomy 7, uniparental isodisomy 7q, or loss-of-function second-site mutation) have a growth advantage leading to an expansion of clones that lost SAMD9/SAMD9L-mutation-containing chromosome 7 (5). In fact, patients carrying germline SAMD9/SAMD9L mutations comprise 8% to 17% of childhood MDS with monosomy 7 (3, 6) and it has been described as familial monosomy 7 syndrome (7). Selection pressure for monosomy 7 underlies a predisposition to MDS and acute myeloid leukemia.
In this issue of the JCI, Abdelhamed, Thomas, and colleagues established a mouse model of SAMD9/SAMD9L syndromes. The mice (referred to as Samd9l-Mut) possessed a heterozygous Samd9l p.W1171R mutation, which is equivalent to the human SAMD9L p.W1180R mutation noted in patients with impaired hematopoietic cell function (8).
Lessons learned from Samd9l mouse models
The mouse genome lacks Samd9 and only has Samd9l on chromosome 6, which opens the opportunity to study the function of Samd9l without redundancy of Samd9.
We have learned from prior Samd9l mouse models that (a) Samd9l haploinsufficiency (heterozygous knockout) predisposes mice to myeloid malignancies spontaneously, which is even more accelerated by viral infections, and it increases the repopulating capacity of hematopoietic stem progenitor cells (HSPCs) (2); (b) Samd9l plays an important role in the degradation of cytokine receptors by endocytosis and endosome fusion with lysosomes, thereby its deficiency increases the availability of cytokine receptors on the cell surface and promotes growth (2, 9); and (c) a gain-of-function Samd9l homozygous mutant mouse model develops BM failure, growth retardation, and both homozygous and heterozygous mutant mice show reduced repopulating capacity (9).
In their study, Abdelhamed, Thomas, et al. generated conditional knockin mice that had a skewed myeloid commitment at the expense of decreased lymphoid commitment (8). B cell lymphopenia was the most notable finding in the peripheral blood. Single-cell RNA sequencing (RNA-Seq) further showed a differentiation block in the B cell lineage. Samd9l-Mut HSPCs also showed decreased colony formation upon replating and were outcompeted in competitive transplantation assays, suggesting decreased overall fitness of Samd9l-Mut HSPCs, similar to a prior study (2). Interestingly, 4 out of 26 Samd9l-Mut mice had a partial loss of chromosome 6 on which mouse Samd9l is located, phenocopying nonrandom loss of a mutant allele on chromosome 7 in patients with SAMD9/SAMD9L syndromes. This finding suggests that loss of the Samd9l-mutant allele confers a growth advantage in mice, as in humans (8).
Inflammation and BM failure
Inflammation caused by infection or autoimmune conditions triggers HSCs to transition rapidly from quiescence to active cell cycling with the eventual loss of self-renewal capacity (10–13). Proinflammatory cytokines, including IFN-α and IFN-β, in response to viral infections can cause BM suppression or even aplastic anemia (14–16). This pathology may be due to a direct effect on HSCs or an indirect effect via the niche.
SAMD9 and SAMD9L are IFN-responsive genes and they play an important role in innate immunity against viral infections (17–19). The authors show that one way to directly affect HSCs in the setting of inflammation is through the type I IFN signaling–induced expression of SAMD9L in HSCs. Increased SAMD9L expression favors the elimination of inflamed (or infected) cells by inhibiting their growth. In vitro IFN-α or in vivo polyinosinic/polycytidylic acid (pI:pC) injections, which induce type I IFNs (IFN-α and IFN-β) in vivo, increased the expression of both WT and mutant SAMD9L proteins. However, the treatment substantially increased apoptosis and decreased colony-forming capacity only in Samd9l-Mut BM cells.
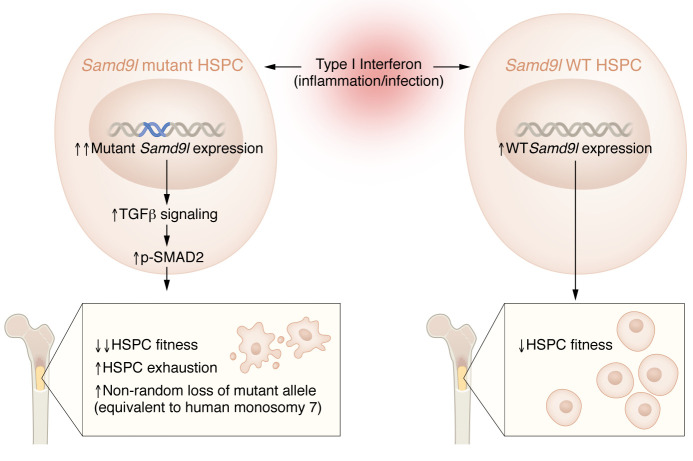
Interestingly, inflammation induced by pI:pC reduced the engraftment potential of both Samd9l-WT and Samd9l-Mut, but it is noteworthy that inflammation further decreased the already reduced engraftment potential and increased apoptosis of Samd9l-Mut BM cells. Similarly, both Samd9l-WT and Samd9l-Mut mice decreased their lymphocyte counts upon pI:pC challenge, but a greater degree of reduction along with myeloid hyperplasia was observed in Samd9l-Mut mice. Subsequent RNA-Seq analysis of lineage-negative cKit-positive HSPCs showed that the TGF-β pathway was upregulated in pI:pC-treated Samd9l-Mut HSCs, which was confirmed by flow cytometry as increased p-SMAD2/3 signaling (Figure 1). This upregulation of p-SMAD2/3 was most notable in B cells, which may explain B cell lymphopenia in Samd9l-Mut mice. The TGF-β small molecule inhibitor, SD-208, partially rescued in vitro colony-forming capacity of mutant Samd9l, albeit not to the WT levels (8). These findings suggest that TGF-β inhibitors may boost the colony-forming capacity of the Samd9l-Mut mouse and patient BM; however, whether the inhibitors can actually reverse BM failure in mice and humans requires future studies.
Figure 1. Model for bone marrow exhaustion and monosomy 7 exacerbated by inflammation in SAMD9/SAMD9L syndromes.
In a mouse model of SAMD9/SAMD9L syndrome, exposure to type I IFNs increases the expression of both WT and mutant Samd9l expression in HSPCs. However, only mutant Samd9l leads to upregulation of TGF-β and subsequent p-SMAD2/3 signaling upon inflammatory insults, which further exacerbate already severely decreased HSPC fitness, BM exhaustion, and promote nonrandom loss of mutant allele (equivalent to human monosomy 7) in mutant mice.
Interestingly, TGF-β hyperactivation is not specific to SAMD9/SAMD9L syndromes but is also observed in other inherited BM failure syndromes, such as Fanconi anemia (20), Diamond-Blackfan anemia (21, 22), and Shwachman-Diamond syndrome (23). These observations suggest that TGF-β hyperactivation may be a common pathway leading to HSC exhaustion due to various underlying mechanisms.
Summary and future directions
Abdelhamed, Thomas, et al. generated a Samd9l mouse model with a heterozygous human SAMD9L mutation equivalent at its endogenous locus. This mouse model phenocopies human disease in that it develops BM failure and nonrandom loss of the mutant allele. The authors further investigated the effects of inflammation using in vitro and in vivo models and showed that inflammation exacerbates apoptosis of lymphocytes, and reduces colony-forming capacity and engraftment potential, particularly in Samd9l-Mut HSPCs. They discovered that TGF-β hyperactivation was one of the mechanisms underlying reduced HSPC fitness in the setting of inflammation, and a TGF-β inhibitor rescued the colony-forming capacity of mouse and human mutant HSPCs at least partially. It will be critically important to confirm whether TGF-β hyperactivation is present also in patient HSPCs and whether it is only induced with inflammation or also present at a steady state, before moving TGF-β inhibitors to clinical trials. It would also be interesting if TGF-β inhibitors could reverse growth restriction and prolong survival in their mouse model. The work by Abdelhamed, Thomas, et al. opens the possibility of TGF-β inhibitors as therapeutics in this rare disease with no current treatment options other than BM transplant (8).
Acknowledgments
MJ is supported by NIH grant K99 HL150628 and a Maryland Stem Cell Research Fund Launch Award.
Version 1. 11/01/2022
Electronic publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2022, Jung et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(21):e164136. https://doi.org/10.1172/JCI164136.
See the related article at Mutant Samd9l expression impairs hematopoiesis and induces bone marrow failure in mice.
References
- 1.Narumi S, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48(7):792–797. doi: 10.1038/ng.3569. [DOI] [PubMed] [Google Scholar]
- 2.Nagamachi A, et al. Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell. 2013;24(3):305–317. doi: 10.1016/j.ccr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Sahoo SS, et al. Clinical evolution, genetic landscape and trajectories of clonal hematopoiesis in SAMD9/SAMD9L syndromes. Nat Med. 2021;27(10):1806–1017. doi: 10.1038/s41591-021-01511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inaba T, et al. The enigma of monosomy 7. Blood. 2018;131(26):2891–2898. doi: 10.1182/blood-2017-12-822262. [DOI] [PubMed] [Google Scholar]
- 5.Tesi B, et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood. 2017;129(16):2266–2279. doi: 10.1182/blood-2016-10-743302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz JR, et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat Commun. 2017;8(1):1557. doi: 10.1038/s41467-017-01590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastor VB, et al. Constitutional SAMD9L mutations cause familial myelodysplastic syndrome and transient monosomy 7. Haematologica. 2018;103(3):427–437. doi: 10.3324/haematol.2017.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelhamed S, et al. Mutant Samd9l expression impairs hematopoiesis and induces bone marrow failure in mice. J Clin Invest. 2022;132(21):e158869. doi: 10.1172/JCI158869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamachi A, et al. Multiorgan failure with abnormal receptor metabolism in mice mimicking Samd9/9L syndromes. J Clin Invest. 2021;131(4):e140147. doi: 10.1172/JCI140147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 11.Baldridge MT, et al. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465(7299):793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hormaechea-Agulla D, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell. 2021;28(8):1428–1442.e6. doi: 10.1016/j.stem.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogeska R, et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell. 2022;29(8):1273–1284. doi: 10.1016/j.stem.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zauli G, Capitani S. HIV-1-related mechanisms of suppression of CD34+ hematopoietic progenitors. Pathobiology. 1996;64(1):53–58. doi: 10.1159/000164006. [DOI] [PubMed] [Google Scholar]
- 15.Brown KE, et al. Hepatitis-associated aplastic anemia. N Engl J Med. 1997;336(15):1059–1064. doi: 10.1056/NEJM199704103361504. [DOI] [PubMed] [Google Scholar]
- 16.Binder D, et al. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J Exp Med. 1998;187(11):1903–1920. doi: 10.1084/jem.187.11.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang LK, et al. Identification of host proteins involved in Japanese encephalitis virus infection by quantitative proteomics analysis. J Proteome Res. 2013;12(6):2666–2678. doi: 10.1021/pr400011k. [DOI] [PubMed] [Google Scholar]
- 18.Nounamo B, et al. An interaction domain in human SAMD9 is essential for myxoma virus host-range determinant M062 antagonism of host anti-viral function. Virology. 2017;503:94–102. doi: 10.1016/j.virol.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, et al. A paralogous pair of mammalian host restriction factors form a critical host barrier against poxvirus infection. PLoS Pathog. 2018;14(2):e1006884. doi: 10.1371/journal.ppat.1006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, et al. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016;18(5):668–681. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge J, et al. Dysregulation of the transforming growth factor β pathway in induced pluripotent stem cells generated from patients with Diamond Blackfan anemia. PLoS One. 2015;10(8):e0134878. doi: 10.1371/journal.pone.0134878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danilova N, et al. Innate immune system activation in zebrafish and cellular models of Diamond Blackfan anemia. Sci Rep. 2018;8(1):5165. doi: 10.1038/s41598-018-23561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce CE, et al. TGFβ signaling underlies hematopoietic dysfunction and bone marrow failure in Shwachman-Diamond syndrome. J Clin Invest. 2019;129(9):3821–3826. doi: 10.1172/JCI125375. [DOI] [PMC free article] [PubMed] [Google Scholar]