Abstract
The purpose of this study was to determine whether measures of the cell-mediated immune response to influenza virus could be used as markers of influenza virus infection. We studied 23 subjects who developed upper respiratory, lower respiratory, or systemic symptoms during a small outbreak of influenza in a nursing home population. Influenza virus culture from nasopharyngeal swabs yielded influenza virus isolates from 7 of the 23 subjects. Only three of the subjects had a fourfold rise in antibody titer to the influenza virus antigen positivity after the infection. Granzyme B and cytokine levels were measured in peripheral blood mononuclear cells (PBMC) obtained from all subjects and stimulated with live influenza virus. Elevated granzyme B levels in virus-stimulated PBMC in combination with lower respiratory tract or systemic symptoms in study subjects was a significant predictor of culture-confirmed influenza virus infection compared to those from whom influenza virus could not be identified. Cytokine levels did not distinguish between the two groups in a similar type of analysis. Granzyme B in combination with the clinical profile of symptoms may be a useful retrospective marker for influenza virus infection.
Seniors are at high risk for serious complications of influenza virus infections (2, 3, 6). Typical symptoms of influenza, including fever, myalgias, and sore throat, may not be recognized in patients presenting with acute respiratory conditions or exacerbations of underlying chronic conditions. Thus, traditional diagnostic tests, such as virus isolation from nasopharyngeal or throat swabs or determination of acute- and convalescent-phase antibody titers, are impractical in the absence of highly organized influenza surveillance programs (1, 15).
The cell-mediated immune response to influenza virus results in cytokine production and stimulation of cytotoxic T lymphocytes (CTL). Helper T cells (Th cells) produce cytokines that direct the Th type 1 responses, which stimulate virus-specific CTL and antibody production, and Th type 2 responses, which result in antibody production (16, 18). While antibodies protect against mucosal invasion, CTL kill virus-infected cells and are required to clear influenza virus from lung tissue (20, 21). Thus, the activation of CTL during an influenza virus infection would be particularly important in lower respiratory tract illness.
Virus-specific immunological memory is stimulated through vaccination or natural infection. By stimulating peripheral blood mononuclear cells (PBMC) in vitro with live influenza virus after influenza virus vaccination or infection, we can measure Th and CTL responses. Both Th and CTL are activated in these PBMC cultures and produce a variety of cytokines as well as granzyme B. Granzyme B is produced by CTL as part of the cytolytic pathway that leads to apoptotic death of virus-infected cells. We have correlated granzyme B activity in PBMC, stimulated in vitro with live influenza virus, with cytotoxicity as measured by 51Cr release assays (11). In the present study, we showed that increased granzyme B production in PBMC, in combination with lower respiratory tract or systemic symptoms, was highly predictive of influenza virus culture-positive status during an outbreak in institutionalized older adults. These results are in contrast to those of the subject subset who became ill during the outbreak but were culture negative for influenza virus.
MATERIALS AND METHODS
Experimental protocol.
The study was carried out in a veterans’ home as part of a larger study of ∼450 inhabitants of the home. All participants were vaccinated and monitored in an influenza surveillance program which included determination of antibody titers in sera at 6 weekly intervals from October to March of 1994-1995 as previously described (4). A subset of 23 subjects (22 males, 1 female; median age, 68 years; age range, 60 to 86 years) from a larger group became ill during an outbreak of influenza (January 1995). Illness was defined as any acute respiratory, gastrointestinal, or systemic symptoms, not necessarily specific for influenza virus infection. All subjects had been previously vaccinated in the last week of October 1994 with the 1994-1995 licensed influenza virus vaccine which contained A/Shangdong/09/93 (H3N2), A/Texas/36/91 (H1N1), and B/Panama/45/90 (Connaught Laboratories, Inc., Swiftwater, Pa.). Serum samples were obtained from all participants in the larger study prior to vaccination and at 6, 12, and 18 weeks postvaccination; the influenza outbreak occurred just after the 12-week samples were collected. Throat swab specimens were obtained within 24 h of the onset of symptoms to optimize the ability to detect viral shedding. PBMC cultures were prepared from peripheral venous blood samples (20 ml) collected once from each subject between 8 and 14 days after the onset of symptoms. Symptom profiles of study subjects and virus culture and serological results were blinded until all laboratory measures were completed. We have measured the cell-mediated immune responses to influenza virus vaccination in a different subset of members of this veterans’ home. There was no influenza virus activity documented in that study group, and none of that data overlaps with the results reported herein. Protocol and consent form approval were obtained from the University of Wisconsin Human Subjects Committee. All volunteers that were recruited provided informed consent, the only requirement for participation.
Virus culture.
Nasopharyngeal and throat swab specimens were placed in transport medium (veal broth with gentamycin, penicillin, streptomycin, and amphotericin), stored at 4°C within 1 h, transported to the Wisconsin State Laboratory of Hygiene, and cultured for influenza A and B viruses, rhinovirus, adenovirus, and parainfluenza virus types 1 to 4 within 24 h of specimen collection.
Serum antibody titers.
Hemagglutination assays were performed in the laboratory of one of the authors (S.G.), using hemagglutinin antigens (supplied by Connaught Laboratories, Inc.) representing the strains of virus contained in the vaccine. Hemagglutination inhibition was performed as previously described (19), using twofold dilutions of serum from 1/10 to 1/1,024. Titers of less than 1/10 were calculated as 1/5. Median antibody titers were calculated for each subset of the data. Seroconversion was defined as a fourfold rise in antibody titers, and a seroprotective antibody titer was ≥1/40.
In vitro stimulation.
PBMC cultures were prepared by Ficoll gradient purification and stimulated with live virus preparations of A/Shangdong/09/93 (H3N2) and A/Texas/36/91 (H1N1) (11) (generous gifts from IAF Biovac, Laval, Quebec, Canada) as previously described. PBMC supernatants and lysates were harvested for analysis of cytokine and granzyme B production, respectively.
Cytokine assays.
Cytokine levels were measured as previously described (8, 10). Briefly, interleukin-2 (IL-2) levels were determined by measuring biological activity in culture supernatants (day 2.5) via quantification of [3H]thymidine incorporation in the IL-2-dependent cell line MTL 2.8.2. IL-4, IL-10, and gamma interferon (IFN-γ) levels were measured in day 6 supernatants by the use of commercially available enzyme-linked immunosorbent assay substrates (13). The minimum level of detection was as previously reported for our laboratory; undetectable cytokine levels were arbitrarily assigned a concentration value of zero.
Assay of granzyme B activity.
Granzyme B activity was measured in PBMC lysates by enzymatic cleavage of the substrate t-butyloxycarbonyl-Ala-Ala-Asp-thiobenzyl ester (BAADT; Enzyme Systems Products, Dublin, Calif.) as previously described (12). Enzymatic (aspase equals cleavage at the Asp residue) activity was calculated as A405 units per milligram of protein in the PBMC lysate.
Statistical analysis.
Differences in the levels of the different cytokines and granzyme B between PBMC from subjects who were culture positive and those from subjects who were culture negative for influenza virus were analyzed by the Mann-Whitney U test for unpaired data; statistical significance was established at P < 0.05 (two tailed). Fisher’s exact test was used in 2-by-2 analyses to determine the significance of clinical features and laboratory measures in distinguishing between the culture-positive and culture-negative groups; statistical significance was set at P < 0.05. Differences in paired data were analyzed for statistical significance (P < 0.05) by the Wilcoxon signed rank test. The statistical package Statview 4.1 (Abacus Concepts, Berkeley, Calif.) was used for the analysis. The 95% confidence intervals were calculated by a nonparametric method according to the program RESAMPLING STATS 4.03 (Resampling Stats, Inc., Arlington, Va.).
RESULTS
Influenza virus (A/Shangdong/09/93) was isolated from the upper respiratory tracts of 7 of the 23 study participants (influenza group). The median age of the influenza virus-positive group was 74 years (range, 63 to 81 years), compared to 69.5 years (range, 60 to 86 years) for subjects who were the influenza virus negative (noninfluenza group). Clinical data collected on all study participants showed that all seven influenza virus-positive subjects had evidence of systemic or lower respiratory tract illness, with five of them developing fever. One of the two subjects who did not develop fever exhibited the highest mean fold increase in antibody titer, and the other had the highest granzyme B level in A/Shangdong-stimulated PBMC after the outbreak. Only 2 of 16 subjects in the influenza virus-negative group developed lower respiratory tract illness and fever; there were no changes in antibody titers, and granzyme B levels were less than the median granzyme B level for this group, consistent with a non-influenza virus cause of illness in these two cases.
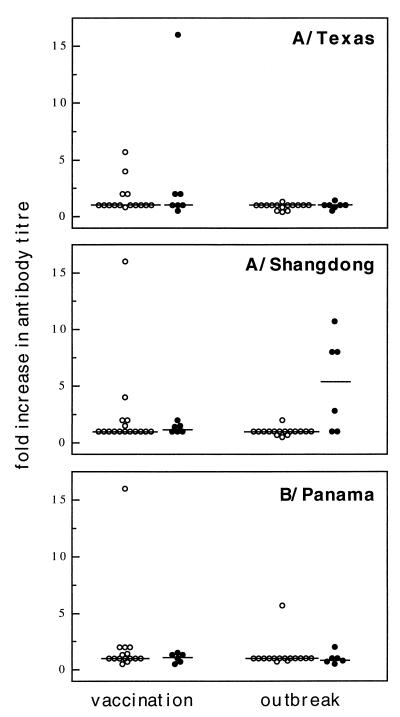
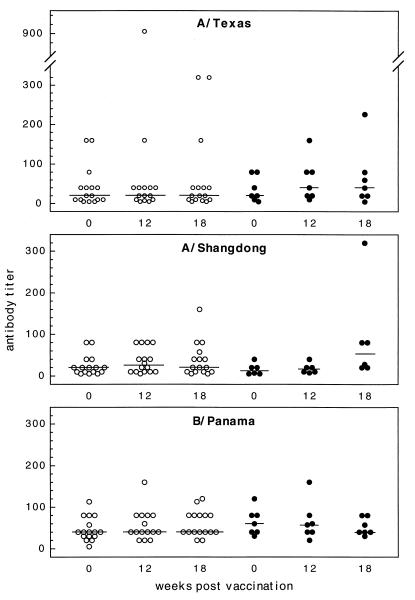
The results of serological testing prevaccination and at 12 weeks (acute-phase sera) and 18 weeks (convalescent-phase sera) postvaccination are shown in terms of the mean fold increase in antibody titer after vaccination and after the influenza outbreak (Fig. 1). In two cases (one in the influenza group and one in the noninfluenza group), 6 weeks postvaccination results were used for the acute-phase antibody titers in the study. Analysis of the humoral response to vaccination revealed that only 2 of 22 participants (serological data were not available for one influenza virus-positive case) had a fourfold rise in antibody titers to A/Shangdong/03/93. Both were in the noninfluenza group, but this result was not statistically different from that of the influenza group (Fisher’s exact test). To consider the effect of previous vaccinations administered to this population, we also determined the number of study participants with antibody titers of ≥40 at each time point (Fig. 2). One of 6 influenza virus-positive and 4 of 16 influenza virus-negative subjects had antibody titers of ≥1/40 prevaccination, and this number increased to 7 of 16 subjects by 12 weeks postvaccination. Again, the results for the two groups were not statistically different. Similar pre- and postvaccination results were obtained for A/Texas/36/91 and B/Panama/45/90, with the exception of the median antibody titer, which was twofold higher for B/Panama than for A/Texas (1/40 versus 1/20) prior to vaccination.
FIG. 1.
Changes in titers of antibodies to each of the three strains of virus contained in the 1994-1995 influenza virus vaccine are shown. Represented are the individual fold increases in antibody titers for the influenza virus culture-negative (○) and culture-positive (•) groups. Shown are results for the response to influenza virus vaccination and the acute- to convalescent-phase period of the influenza outbreak.
FIG. 2.
Individual antibody titers at each of the time points in the study are shown. Influenza virus culture-negative (○) and culture-positive (•) results were used to calculate the fold increases in antibody titers shown in Fig. 1.
Overall, there was a significant difference in the mean fold increases in the convalescent/acute anti-A/Shangdong/03/93 antibody titer ratios for the influenza virus-positive and the influenza virus-negative groups (P = 0.026, Mann-Whitney U test); 3 of 6 and 0 of 16 subjects, respectively, showed a fourfold increase in antibody titer after the outbreak, and this result was also statistically different in a 2-by-2 analysis (P = 0.013, Fisher’s exact test). One participant in the influenza virus-negative group seroconverted to B/Panama positivity between 12 and 18 weeks postvaccination. A/Shangdong/03/93 was identified as the only infectious strain during the outbreak, and no other influenza virus strains were identified during continued influenza surveillance throughout the study period. Therefore, the seroconversion to B/Panama positivity could not be explained, but the rest of the results are consistent with a serotype-specific response to documented infection with A/Shangdong/03/93.
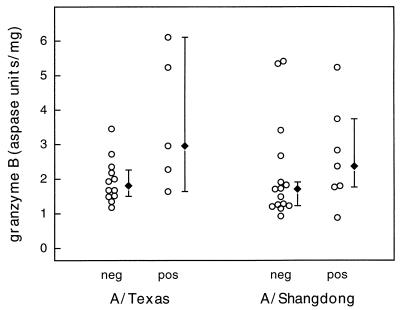
Granzyme B levels were measured in PBMC obtained from the study participants, stimulated with live influenza virus in culture, and prepared as cell lysates for the assay. The levels of granzyme B, which correlate with CTL-mediated cytotoxic activity in virus-stimulated PBMC cultures, were increased in PBMC obtained from the influenza group subjects compared to those obtained from the noninfluenza group subjects (Fig. 3). In A/Texas-stimulated (H1N1) cultures, this difference approached clinical significance (P = 0.058). The results for A/Shangdong-stimulated PBMC showed a similar trend, but the results for the two groups as represented were not statistically significantly different. Because the data did not show a normal distribution, 95% confidence intervals were calculated by a nonparametric method. The overlapping, wide confidence intervals demonstrate the limited precision of the granzyme B assay with the influenza virus-positive group PBMC. Within the influenza and noninfluenza groups, the granzyme B levels to A/Shangdong/03/93 and A/Texas/36/91 were not statistically significantly different (Wilcoxon signed rank test), consistent with the known cross-reactivity of CTL responses to H3N2 and H1N1 strains, but the numbers were too small to exclude a type II error.
FIG. 3.
Granzyme B levels in individual PBMC lysates prepared after stimulation of the cultures with A/Texas/36/91 or A/Shangdong/09/93 are represented by the open circles (○). Aspase units reflect the colorimetric change as a result of granzyme B substrate cleavage. Results for influenza virus culture-positive (pos) and culture-negative (neg) subjects are shown. The median granzyme B levels (⧫) are shown, and error bars represent 95% confidence intervals. The difference between the two groups in A/Texas-stimulated cultures was just below statistical significance (P = 0.058).
Since granzyme B levels did not conclusively distinguish between influenza virus-positive and -negative subjects, we also analyzed the potential of the granzyme B assay, in combination with the clinical profile of symptoms in the study subjects, to predict culture-positive versus culture-negative status from nasopharyngeal swab specimens. The presence or absence of lower respiratory tract or systemic symptoms (LRTSS), in the context of a flu-like illness, was used in combination with high or low granzyme B levels, respectively, in the analysis. The median level of granzyme B activity in A/Shangdong-stimulated PBMC from the influenza virus-negative group was used as the cutoff (>1.71 U of granzyme B/mg of protein) for a potential positive case. Subjects were divided according to whether their granzyme B levels were >1.71 U/mg of protein and whether LRTSS were present and then compared for influenza virus-positive versus influenza virus-negative status (Table 1). There was a statistically significant difference between the subsets defined in this analysis (P = 0.0005, Fisher’s exact test). The sensitivity and specificity of this test, which combines the results of granzyme B and evidence of LRTSS, were calculated as 83 and 100%, respectively. The accuracy of a predicted diagnostic level of granzyme B alone is limited, as reflected in the statistical analysis of the difference between groups. By combining granzyme B levels and clinical symptoms, the ability to distinguish between a culture-positive and a culture-negative result is significantly enhanced. The results of a similar analysis, combining convalescent antibody titers that were above the median titer for the culture-negative group with the presence of LRTSS, were not statistically different.
TABLE 1.
Comparison of PBMC granzyme B levels and LRTSS status with influenza virus culture resultsc
| Granzyme B level and LRTSS status | No. of subjects who were influenza virus culture:
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| grz B >1.71a plus LRTSS | 6 | 0 | 6 |
| grz B ≤1.71b or no LRTSS | 1 | 16 | 17 |
| Total | 7 | 16 | 23 |
Granzyme B level of >1.71 U/mg in A/Shangdong-stimulated PBMC.
Granzyme B level of ≤1.71 U/mg in A/Shangdong-stimulated PBMC.
P = 0.005; Fisher’s exact test of the 2-by-2 analysis.
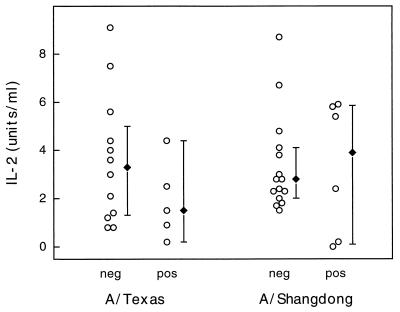
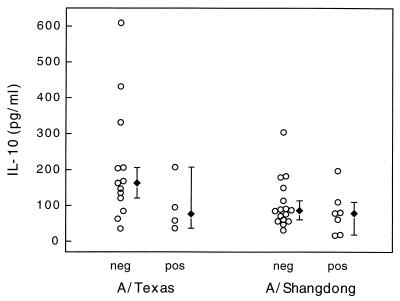
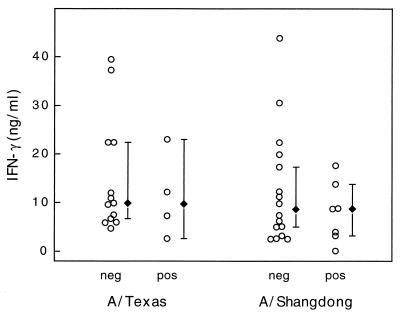
The analysis of IL-2, IL-10, and IFN-γ production in virus-stimulated PBMC showed that there was no significant difference in the cytokine levels of the influenza virus culture-positive and culture-negative groups (Fig. 4 to 6). Again, combining clinical symptoms and cytokine levels did not distinguish between the culture-positive and culture-negative groups. Thus, granzyme B was the only T-cell product measured that was shown to be of potential diagnostic value during this influenza outbreak.
FIG. 4.
IL-2 levels measured in individual PBMC supernatants (○) after stimulation with either A/Texas/36/91 or A/Shangdong/09/93. Results for culture-positive (pos) and culture-negative (neg) subjects are shown. The median IL-2 levels (⧫) for each subset are shown, and error bars represent 95% confidence intervals.
FIG. 6.
IL-10 levels measured in individual PBMC supernatants (○) after stimulation with either A/Texas/36/91 or A/Shangdong/09/93. Results for culture-positive (pos) and culture-negative (neg) subjects are shown. The median IL-10 levels (⧫) for each subset are shown, and error bars represent 95% confidence intervals.
DISCUSSION
This study of a small outbreak of influenza tested the use of granzyme B levels in virus-stimulated PBMC to distinguish between influenza virus culture-positive and culture-negative subjects. The increased levels of granzyme B in the influenza group compared to those in the noninfluenza group did not achieve statistical significance. However, when granzyme B results were used in combination with the presence or absence of LRTSS, the ability to distinguish between the two study groups of frail older adults was highly significant. Since CTL memory lasts for approximately 12 weeks after influenza virus infection in young adults (5, 14), elevated granzyme B levels, as a marker of influenza virus activity, should persist for a prolonged period of time after the outbreak. This assay may prove useful when virus detection is not possible due to delays in recognizing an influenza outbreak or individual illness. In these cases, the granzyme B levels could be retrospectively linked to a history of LRTSS to document influenza.
Increased granzyme B activity in virus-stimulated PBMC was associated with isolation of influenza virus from throat swabs specimens, seroconversion to A/Shangdong/03/93, and clinical evidence of influenza illness. The potential of the granzyme B assay, in combination with the presence of a lower respiratory tract infection during the outbreak, was highly sensitive and specific for the diagnosis of influenza virus infection based on the virus culture results. The accuracy, as determined by the P value of the difference between groups, and the precision of the results, based on the 95% confidence intervals in the study, reflect the considerable overlap between the two groups and are probably due to the small numbers of study subjects. Much larger study groups will be required to define the granzyme B level that can be used as a sole determinant of influenza virus infection. Preliminary results from a study of a more recent, larger outbreak of influenza in this population again showed a granzyme B response to infection (unpublished data); the response appears to be type specific for influenza A or B virus, although influenza virus type specificity was not tested in this smaller study.
We have previously measured the granzyme B response to the 1994-1995 influenza virus vaccine administered to a different subset of older adults in this veterans’ home (13). The response was significant for the H3N2 strain (A/Shangdong/03/93) contained in the vaccine but not for the H1N1 strain (A/Texas/36/91), indicating a less-cross-reactive response to intramuscularly administered killed virus. Virus-specific CTL are recruited to the lungs to clear influenza virus and would thus be removed from the peripheral circulation during a lower respiratory tract infection. Although the granzyme B activities in A/Shangdong/03/93- and A/Texas/36/91-stimulated PBMC were not statistically significantly different, this may be related to the small sample size. Potential differences in granzyme B responses to these two viral strains in the vaccine may have resulted in a larger proportion of the H3N2-specific CTL being removed from the peripheral circulation during the influenza virus infection. It has been shown that T-lymphocyte proliferation may be decreased for a period of up to 2 weeks after influenza virus vaccination, presumably due to the removal of influenza virus-specific T cells from the circulating blood pool (9). Other studies suggest that the peak CTL response occurs as early as 2 weeks postvaccination (7, 17). Because our blood samples were collected between 8 and 14 days after the onset of symptoms, some of the virus-specific CTL may have remained in the lung tissue, with a relative depletion occurring in the peripheral blood. This explanation may be particularly relevant to the A/Shangdong results for two of the six influenza virus-positive individuals for whom granzyme B levels were close to the cutoff level used in the analysis. Again, larger groups, studied over a longer period after infection, will be required to more precisely define this effect on different subsets of circulating T cells and the resulting changes in cytokine and granzyme B levels.
It is important to note that the results of this study are limited to frail older adults residing in an institutional setting. Since these individuals represent a very-high-risk population with regard to influenza-related morbidity and mortality, a significant proportion of the influenza virus culture-positive group would be predicted to develop LRTSS. It is possible that in vitro granzyme B levels are not elevated in PBMC after an influenza virus infection if the individual did not experience LRTSS. Thus, granzyme B may be a marker of serious illness, as opposed to a mild case of influenza. A larger study of young and older adults living in the community and experiencing a range of symptoms related to influenza illness will be required to further elucidate the potential role of granzyme B in predicting the cause of viral illness during an influenza outbreak.
In summary, the documentation of influenza virus infections is difficult due to the short duration of virus shedding from the nasopharynx. Seroconversion in terms of virus-specific antibodies is another method of documenting influenza virus infections, but it may lack sensitivity in a vaccinated population and is dependent on the collection of serum during both the acute and convalescent phases of the illness. The measurement of elevated granzyme B levels, in conjunction with LRTSS, may be an important method for retrospectively confirming an influenza outbreak or identifying the infectious agent during an outbreak of respiratory illness.
FIG. 5.
IFN-γ levels measured in individual PBMC supernatants (○) after stimulation with either A/Texas/36/91 or A/Shangdong/09/93. Results for culture-positive (pos) and culture-negative (neg) subjects are shown. The median IFN-γ levels (⧫) for each subset are shown, and error bars represent 95% confidence intervals.
ACKNOWLEDGMENTS
Financial support for this work was provided by the Medical Research Council of Canada (J.M.); Connaught Laboratories Inc., Canada (J.M.); and the National Institutes of Health (S.G.; R01 AG 09632 and K08 AG 0548).
We are grateful to the staff of the Wisconsin Veterans Home who assisted with volunteer recruitment and blood collection. We thank Lynne Nadon for excellent assistance in the preparation of the manuscript.
REFERENCES
- 1.Arroyo J C, Jordan W, Milligan L. Upper respiratory tract infection and serum antibody responses in nursing home patients. Am J Infect Control. 1988;16:152–158. doi: 10.1016/0196-6553(88)90026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker W, Mullooly J. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- 3.Barker W, Mullooly P. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142:85–89. [PubMed] [Google Scholar]
- 4.Drinka P J, Krause P, Schilling M, Miller B A, Shult P, Gravenstein S. Report of an outbreak: nursing home architecture and influenza-A attack rates. J Am Geriatr Soc. 1996;44:910–913. doi: 10.1111/j.1532-5415.1996.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 5.Ennis F A, Rook A H, Qi Y L, et al. HLA-restricted virus-specific cytotoxic T lymphocyte responses to live and inactivated influenza viruses. Lancet. 1981;ii:887–891. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- 6.Falsey A R, Treanor J J, Betts R F, et al. Viral respiratory infection in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc. 1992;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorse G J, Belshe R B. Enhancement of anti-influenza A virus cytotoxicity following influenza A virus vaccination in older, chronically ill adults. J Clin Microbiol. 1990;28:2539–2550. doi: 10.1128/jcm.28.11.2539-2550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooton J W L, Gibbs C, Paetkau V. Interaction of interleukin 2 with cells: quantitative analysis of effects. J Immunol. 1985;135:2464–2473. [PubMed] [Google Scholar]
- 9.Huang V P, Pechere J C, Michel M, Guathey L, Loreto M, Curran J A, Michel J-P. In vivo T-cell activation, in vitro defective IL-2 secretion, and response to influenza vaccination in elderly women. J Immunol. 1992;148:715–722. [PubMed] [Google Scholar]
- 10.McElhaney J E, Meneilly G S, Lechelt K E, Bleackley R C. Split-virus influenza vaccines: do they provide adequate immunity in the elderly? J Gerontol. 1994;49:M37–M43. doi: 10.1093/geronj/49.2.m37. [DOI] [PubMed] [Google Scholar]
- 11.McElhaney J E, Pinkoski M J, Upshaw C M, Bleackley R C. The cell-mediated cytotoxic response to influenza vaccination using an assay for granzyme B activity. J Immunol Methods. 1996;190:11–26. doi: 10.1016/0022-1759(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 12.McElhaney J E, Pinkoski M J, Au D, Lechelt K E, Bleackley R C, Meneilly G S. Helper and cytotoxic T lymphocyte responses to influenza vaccination in healthy compared to diabetic elderly. Vaccine. 1996;14:539–544. doi: 10.1016/0264-410x(95)00219-q. [DOI] [PubMed] [Google Scholar]
- 13.McElhaney J E, Gravenstein S, Upshaw C M, Hooton J W, Krause P, Drinka P. Immune response to influenza vaccination in institutionalized elderly: effect on different T-cell subsets. Vaccine. 1998;16:403–409. doi: 10.1016/s0264-410x(97)80918-8. [DOI] [PubMed] [Google Scholar]
- 14.McMichael A J, Askonas B A. Influenza virus-specific cytotoxic T-cells in man: induction and properties of the cytotoxic cell. Eur J Immunol. 1978;8:705–711. doi: 10.1002/eji.1830081007. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson K G, Baker D J, Farquhar A, et al. Acute upper respiratory tract viral illness and influenza immunization in homes for the elderly. Epidemiol Infect. 1990;105:609–618. doi: 10.1017/s0950268800048251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paladino G, Scherle P A, Gerhard W. Activity of CD4+ T-cell clones of type 1 and type 2 in generation of influenza virus-specific cytotoxic responses in vitro. J Virol. 1991;65:6071–6076. doi: 10.1128/jvi.65.11.6071-6076.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers D C, Belshe R B. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167:584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- 18.Scherle P A, Gerhard W. Differential ability of B-cells specific for external vs. internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc Natl Acad Sci USA. 1988;85:4446–4450. doi: 10.1073/pnas.85.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Collaborating Center for Influenza, Biological Products Division. The hemagglutination inhibition test for influenza viruses, version 31. Atlanta, Ga: Center for Infectious Diseases, Centers for Disease Control, U. S. Public Health Service; 1981. pp. 1–21. [Google Scholar]
- 20.Yap K L, Ada G L, McKenzie F C. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with live influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 21.Yap K L, Ada G L. Cytotoxic T-cells in the lungs of mice infected with influenza A virus. Scand J Immunol. 1978;7:73–80. doi: 10.1111/j.1365-3083.1978.tb00428.x. [DOI] [PubMed] [Google Scholar]