Abstract
Background and Objectives
To evaluate the predictive value of serum neurofilament light chain (sNfL) and CSF NfL (cNfL) in patients with radiologically isolated syndrome (RIS) for evidence of disease activity (EDA) and clinical conversion (CC).
Methods
sNfL and cNfL were measured at RIS diagnosis by single-molecule array (Simoa). The risk of EDA and CC according to sNfL and cNfL was evaluated using the Kaplan-Meier analysis and multivariate Cox regression models including age, spinal cord (SC) or infratentorial lesions, oligoclonal bands, CSF chitinase 3–like protein 1, and CSF white blood cells.
Results
Sixty-one patients with RIS were included. At diagnosis, sNfL and cNfL were correlated (Spearman r = 0.78, p < 0.001). During follow-up, 47 patients with RIS showed EDA and 36 patients showed CC (median time 12.6 months, 1–86). When compared with low levels, medium and high cNfL (>260 pg/mL) and sNfL (>5.0 pg/mL) levels were predictive of EDA (log rank, p < 0.01 and p = 0.02, respectively). Medium-high cNfL levels were predictive of CC (log rank, p < 0.01). In Cox regression models, cNfL and sNfL were independent factors of EDA, while SC lesions, cNfL, and sNfL were independent factors of CC.
Discussion
cNfL >260 pg/mL and sNfL >5.0 pg/mL at diagnosis are independent predictive factors of EDA and CC in RIS. Although cNfL predicts disease activity better, sNfL is more accessible than cNfL and can be considered when a lumbar puncture is not performed.
Classification of Evidence
This study provides Class II evidence that in people with radiologic isolated syndrome (RIS), initial serum and CSF NfL levels are associated with subsequent evidence of disease activity or clinical conversion.
Radiologically isolated syndrome (RIS) is a preclinical stage of multiple sclerosis (MS) characterized by MRI brain lesions typical of MS detected in patients with clinical conditions not suggestive of MS.1 Given the absence of clinical features suggestive of MS, the gold standard for defining RIS is the specific dissemination in space (DIS) criteria (3 or 4 2005 DIS criteria for diagnosis of MS), which have not been updated since 2009 despite changes in MS criteria.1-3 Natural history studies suggest that the clinical course for patients with RIS is heterogeneous.4,5 Not all individuals will experience definable clinical symptoms attributable to inflammatory CNS demyelination and axonal damage. Identifying patients at high risk of clinical conversion and disability over time is mandatory for optimal clinical care. The risk for a future event may be stratified by key risk factors, but no biological biomarkers are used in clinical practice.4-6 Retrospective studies performed by an international working group, the Radiologically Isolated Syndrome Consortium (RISC), have demonstrated that one-third of patients with RIS converted to MS after 5 years and that another one-third experienced new brain lesions on follow-up scans.4 After 10 years, most patients with RIS convert to MS.5 Several factors have been associated with the risk of clinical conversion (CC) to MS in RIS: male sex, younger age, positive CSF for oligoclonal bands (OCBs), infratentorial (IT) lesions, spinal cord (SC) lesions on the index scan, and evidence of disease activity (EDA) defined by gadolinium-enhancing (Gd+) lesions on MRI follow-up.1,4,5,7,8 These prognostic factors have been confirmed on a large prospective cohort who had Gd+ lesions on the index scan.6
Several additional biomarkers have been explored for their specificity for MS. These include MRI markers such as the central vein sign that reflects perivenous inflammatory demyelination and can help differentiate MS from other white matter disorders.9 Another is the paramagnetic rim sign, which can be detected in most patients with RIS, suggesting the presence of subclinical chronically active demyelination at an early stage of the disease.10 Visual evoked potential anomalies and peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography have also been associated with the risk of conversion to MS.7,11 Biological markers of MS in patients with RIS include CSF OCBs, high CSF neurofilament light chain (cNfL) levels, and IL-8 levels. By contrast, high CSF chitinase 3–like protein 1 (CHI3L1) and high CSF IL-17 levels are not associated with the risk of a clinical event.12-15 However, no peripheral biological marker providing additional prognostic value to epidemiologic, MRI, and CSF markers has so far been identified.16
CSF is a fluid of choice for characterizing biomarkers of brain disorders and has been widely investigated in MS at all stages of the disease.17,18 cNfL is a useful biomarker in several inflammatory and neurodegenerative CNS diseases.19 In MS, cNfL was mainly associated with EDA in relapsing-remitting MS (RRMS), clinically isolated syndromes (CIS), and RIS.12,20,21 Using an ultrasensitive single-molecule array (Simoa) technology, NfL levels can now be accurately determined in serum obtained from a blood sampling, avoiding invasive lumbar puncture. Serum NfL (sNfL) levels, investigated in MS at different stages of the disease, are associated with disease activity, treatment response, and long-term outcomes.22,23 They were also slightly increased in patients compared with those in controls many years before MS onset, suggesting that neuroaxonal damage already occurs during MS prodromal phase.24
In this study, we explored the value of sNfL as a predictive biomarker of disease activity in patients with RIS. We evaluated the predictive value of sNfL and cNfL levels for EDA and CC in patients with RIS in the context of other prognostic factors.
Methods
This study followed the STARD 2015 (Standards for Reporting Diagnostic accuracy studies) reporting guideline.
Standard Protocol Approvals, Registrations, and Patient Consents
This study used biological samples previously withdrawn in a prospective study, which was approved by a local ethic committee with patient informed consent. Because this study retrospectively analyzed data from a cohort of patients, it was approved by the Institutional Review Board of Nîmes (IRB no. 22.01.09), and no participant consent was required, according to the French Law.25
Patients
We selected a multicenter retrospective cohort of patients with untreated RIS from University Hospital Centers of Nice, Istanbul, and Nîmes on the basis of availability of CSF and/or serum samples. CSF samples from 50 patients and serum samples from 57 patients had been prospectively collected (biobank registered under no. 914066V2) between 2001 and 2018 after discovering unexpected demyelinating lesions fulfilling Okuda criteria,1 processed locally and centralized for this study. Epidemiologic, MRI, and biological data had been collected from patients with RIS, and CC occurrence prospectively followed. Patients treated with disease-modifying drugs during follow-up were not included in the cohort. Clinical conversion (CC) was defined as a first clinical event typical of MS (according to the 2017 revision of the McDonald criteria) in patients with RIS during follow-up.26 Nonconversion (NC) was defined as the absence of a first clinical event specific to MS in patients with RIS during follow-up. Evidence of disease activity (EDA) was determined by CC and/or the presence of new T2 lesion(s) and/or new Gd+ lesion(s) on follow-up scans. Nonevidence of disease activity (NEDA) was defined as the absence of EDA during follow-up. In all cases, MRI data were retrieved from prospectively acquired information noted in the database of each center. There was no standardized MRI protocol, but all patients had 1.5T or 3.0T brain and spinal cord MRI studies. For all brain MRI studies, 3D FLAIR and 3D MPRAGE sequences were acquired. Standard CSF analyses from local laboratories were collected (red cell count, white blood cell [WBC] count, and CSF protein concentration). The presence of OCBs was also determined during biobanking as a routine test in each center by parallel isoelectric focusing of serum and CSF in combination with immunoblotting.
CSF and Serum Sample Collection
CSF samples were collected using polypropylene tubes at the end of lumbar punctures and centrifuged within 2 hours at 1,500 ✕ g for 10 minutes according to the guidelines of the BioMS-eu consortium, except that sample transport and centrifugation were performed at 4°C instead of room temperature.27 Aliquots (500 µL) were stored at −80°C in 1.5 mL tubes (Protein LoBind 0030108.116, Eppendorf) until use. Patients with traumatic lumbar punctures (>500 red cells/mm3) were excluded.
Serum samples were spun at the latest 2 hours after blood sampling at 1,500 × g for 10 minutes at room temperature and aliquots (500 µL) stored at −80°C in 1.5 mL tubes (Protein LoBind 0030108.116, Eppendorf) until use.
Measure of CSF and Serum Biomarkers
All serum and CSF samples were centralized at the Clinical Proteomics Platform of the Laboratory of Biochemistry—Clinical Proteomic in Montpellier. sNfL and cNfL concentrations were determined using commercial NF-Light assay (Quanterix, Billerica, MA) based on ultrasensitive Simoa technology, as previously described.28 CSF CHI3L1 levels were measured by ELISA using the MicroVue YKL-40 EIA kit (Quidel Corporation, San Diego, CA), as already described.29 All experiments were performed with a single batch of reagents by researchers blinded from any clinical information. There were no missing values for NfL or CHI3L1 measures.
Statistical Analysis
Statistical analyses were performed using the R software (v3.0.2) and SAS software, version 9.4 (SAS Institute, Cary, NC). The type I error rate was 0.05, and no correction for multiple testing was performed. Median NfL values were compared using the nonparametric Mann-Whitney test. Area under the curve (AUC) values were estimated from time-dependent ROC curves for censored data using the inverse probability of censoring weighting technique of Uno method30 for the occurrence of events at 2, 3, and 5 years. The time from RIS diagnosis to CC or EDA was analyzed using the Kaplan-Meier estimator and compared between groups using the log-rank test. Because log hazard of CC or EDA and the continuous value of cNFL and sNfL did not have a linear relationship, we chose to perform an analysis based on the tertiles of the distribution of these variables. These analyses did not show a risk gradient between the medium and high tertiles of cNFL and sNfL (see Results section Figure 2), indicating a possible threshold effect. We therefore continued our analyses by dichotomizing these variables on the value of the first tertile and thus grouping the medium and high tertiles. Age, sNfL (low vs medium-high), cNfL (low vs medium-high), CSF CHI3L1, CSF WBCs, OCBs, 3 or 4 2005 DIS criteria, and the presence Gd+ lesions, IT lesions, and SC lesions were tested in a univariate Cox model analysis, followed by a multivariate analysis when significant at a threshold of 0.2 in univariate analyses. Factors with more than 10% of missing data were not included in the multivariate models. Finally, we analyzed the additive value of sNfL and cNfL by comparing the predictive value of several predictive models based on the SC lesions and OCBs, with and without the addition of sNfL (low vs medium-high) or cNfL (low vs medium-high), analyzing the differences in Uno concordance statistic (for censored data) between the different models.31
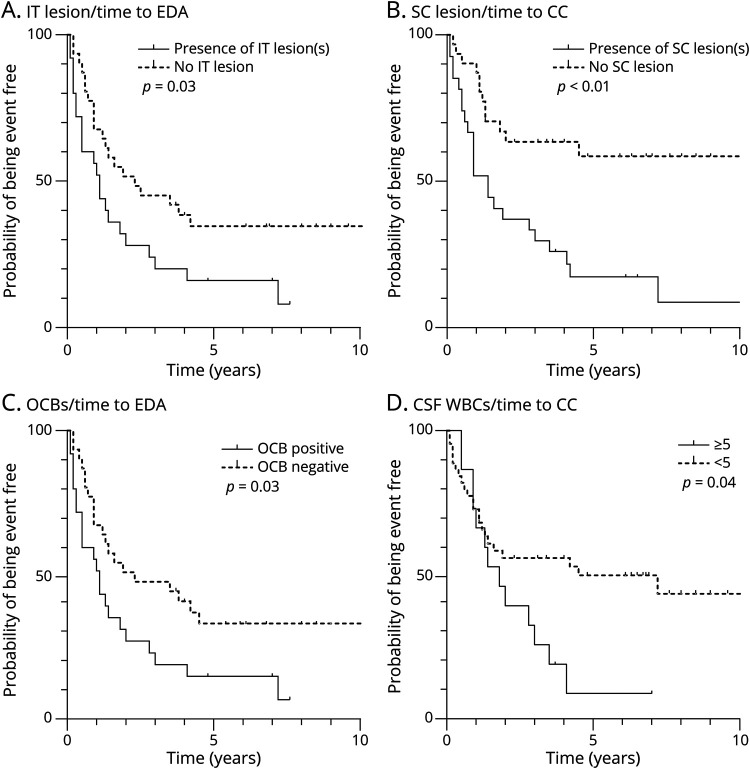
Figure 2. Survival Curves for Evidence of Clinical Activity and Clinical Conversion According to Brain MRI and OCBs.
Kaplan-Meier estimates of the probability of EDA and CC during follow-up are depicted as a function of the presence of IT lesions (A), SC lesions (B), the presence of OCBs (C), high levels of WBCs (D). CC = clinical conversion; EDA = evidence of disease activity; IT = infratentorial; OCBs = oligoclonal bands; SC = spinal cord; WBCs = white blood cells.
Classification of Evidence
This study is based on an RIS cohort study with prospective data collection in local databases using EDMUS. RIS status was determined centrally according to the presence of 2009 RIS criteria without neurologic symptoms of MS, without knowledge of the diagnostic test results for OCBs, CSF CHI3L1 levels, CSF WBC count, sNfL levels, and cNfL levels. These markers were determined in more than 80% of the participants. Patients were prospectively followed up from RIS discovery with regular MRI and clinical evaluation.
Data Availability
Anonymized participant data that underlie the results reported in this article will be shared beginning 6 months and ending 12 months after article publication on reasonable request by qualified investigators.
Results
Patient Characteristics at Diagnosis
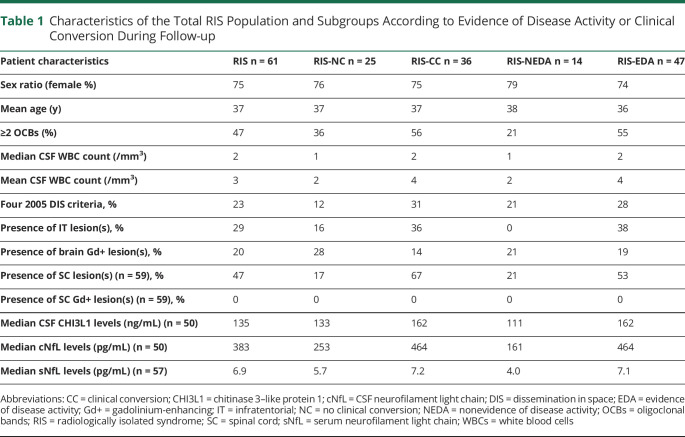
Sixty-one patients with RIS (75% females, mean age 37 years; SD 12) with a biocollection of serum (n = 57) and/or CSF (n = 50) samples were included in the study (Table 1). Of the processed samples, 47% had CSF OCBs, median WBC count was 2/mm3 (0–25), 23% had 4 2005 DIS criteria, 20% had at least 1 brain Gd+ lesion, and 29% had at least 1 infratentorial (IT) lesion. In a subgroup of 59 patients with spinal cord MRI, 47% had at least 1 SC lesion, none of which was enhanced after administration of gadolinium. Median (95% CI) cNfL and sNfL levels were 383 (276; 569) pg/mL and 6.9 (5.3; 7.6) pg/mL, respectively, and median (95% CI) CSF CHI3L1 level was 135 (108; 183) ng/mL (Table 1).
Table 1.
Characteristics of the Total RIS Population and Subgroups According to Evidence of Disease Activity or Clinical Conversion During Follow-up
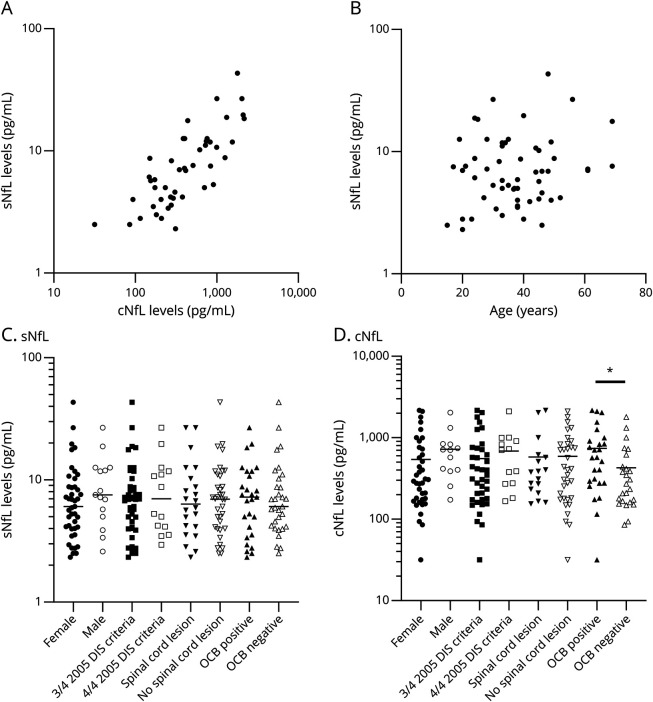
In a subgroup of 46 patients (75%) with both sNfL and cNfL levels available, sNfL and cNfL levels were correlated (Spearman correlation coefficient r = 0.780, p < 0.01, Figure 1A). Neither sNfL (r = 0.098, p = 0.47) (Figure 1B) nor cNfL levels (r = 0.020, p = 0.89) were correlated with age (data not shown). sNfL levels were not significantly different between male and female patients, RIS patients with 3 or 4 2005 DIS criteria, and those with or without SC lesion (Figure 1, C and D). cNfL levels, but not sNfL levels, were significantly higher in RIS patients with positive OCBs than in those with negative OCBs (p = 0.02, Mann Whitney test, Figure 1, C and D).
Figure 1. Association of sNfL and cNfL Levels With Different Clinical, MRI, and CSF Parameters.
(A) Correlation between sNfL and cNfL levels. (B) sNfL and the age of patients with RIS are not correlated. (C) sNfL levels are not significantly different in subgroups determined by sex, number of 2005 DIS criteria, spinal cord lesion, or OCB positive. (D) cNfL levels are not significantly different in subgroups determined by sex, 2005 DIS criteria, or spinal cord lesion. They are significantly elevated in RIS patients with OCB compared with those in others (*p = 0.02, Mann-Whitney test). cNfL = CSF neurofilament light chain; DIS = dissemination in space; NfL = neurofilament light chain; OCBs = oligoclonal bands; RIS = radiologically isolated syndrome; sNfL = serum neurofilament light chain.
Evolution of Patients With RIS During Follow-up
Patients were prospectively followed up from diagnosis of RIS for a median time of 23 months (2–228 months). During follow-up, 36 patients with RIS (59%) evolved to a CC with a median conversion time of 12.6 months (1–86 months), while 25 patients remained NC after a median follow-up of 78 months (7–228 months). Forty-seven patients with RIS (76%) showed EDA in a median delay of 11.1 months (1–86 months). The characteristics of the subgroups of patients regarding EDA vs NEDA and CC vs NC are summarized in Table 1. cNFL levels were higher in RIS patients with CC vs NC and EDA vs NEDA (464 pg/mL vs 253 pg/mL, p = 0.5, and 464 pg/mL vs 161 pg/mL, p < 0.01, respectively). sNfL levels were also higher in RIS patients with EDA vs NEDA (7.1 pg/mL vs and 4.0 pg/mL, p < 0.01), but not in RIS patients with CC compared with NC (7.2 vs 5.7 pg/mL, p = 0.13).
Association of cNfL and sNfL With Clinical Conversion and Evidence of Disease Activity
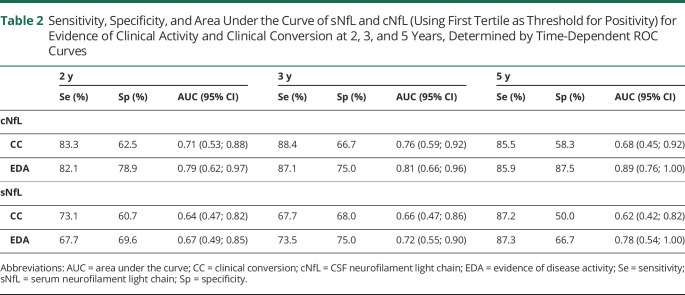
Using ROC curves, we determined time-dependent AUC [95% CI] values of sNfL and cNfL that best discriminate EDA vs NEDA and CC vs NC at 2, 3, and 5 years of follow-up (Table 2).
Table 2.
Sensitivity, Specificity, and Area Under the Curve of sNfL and cNfL (Using First Tertile as Threshold for Positivity) for Evidence of Clinical Activity and Clinical Conversion at 2, 3, and 5 Years, Determined by Time-Dependent ROC Curves
Best AUC values for CC vs NC were provided at 3 years (0.76 [0.59–0.92] for cNfL and 0.66 [0.47–0.86] for sNfL). Best AUC values for EDA vs NEDA were provided at 5 years (0.89 [0.76–1.00] for cNfL and 0.78 [0.54–1.00] for sNfL).
Comparison of cNfL and sNfL AUC [95% CI] values for CC and EDA at 3 years in a subgroup of 46 patients with both cNfL and sNfL levels available showed that cNfL has a more robust association profile with CC (0.77 [0.61; 0.92] and 0.62 [0.41; 0.84], respectively) and EDA (0.82 [0.65; 0.98] and 0.69 [0.49; 0.88], respectively) than sNfL, although not significantly.
Individual Factors Predicting Clinical Conversion and Evidence of Disease Activity
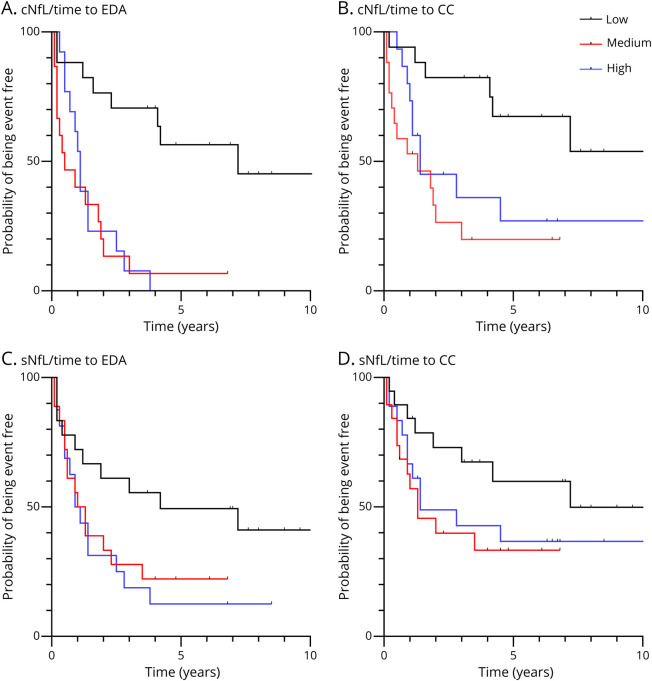
Using cutoff values defined as the first tertile of distribution as cutoff values (cNfL >260 and sNfL >5.0 pg/mL) and taking into account the time of follow-up, the Kaplan-Meier analysis revealed that the presence of IT lesion(s) and OCBs, medium-high cNfL, and medium-high sNfL are predictive of EDA (log rank, p = 0.03, p = 0.03, p < 0.01, and p = 0.02, respectively, Figure 2, A and C, Figure 3, A and C). It also indicated that the presence of SC lesion(s), medium-high cNfL, and elevated WBCs (≥5/mm3) are predictive of CC (log rank, p < 0.01, p < 0.01, and p = 0.04, respectively, Figure 2, B and D, Figure 3B), while medium-high sNfL was not (log rank, p = 0.08, Figure 3D). Age, CSF CHI3L1, number of 2005 DIS criteria, and the presence of Gd+ lesions on the index MRI were not significantly associated with EDA nor CC (data not shown).
Figure 3. Survival Curves for Evidence of Clinical Activity and Clinical Conversion According to cNfL and sNfL.
Kaplan-Meier estimates of the probability of EDA and CC during follow-up are depicted as a function of the presence of low (≤260 pg/mL), medium (260–710 pg/mL), or high (>710 pg/mL) cNfL levels (A, B) and low (≤5.0 pg/mL), medium (5.0–8.5 pg/mL), or high (>8.5 pg/mL) sNfL levels (C, D). CC = clinical conversion; cNfL = CSF neurofilament light chain; EDA = evidence of disease activity; sNfL = serum neurofilament light chain.
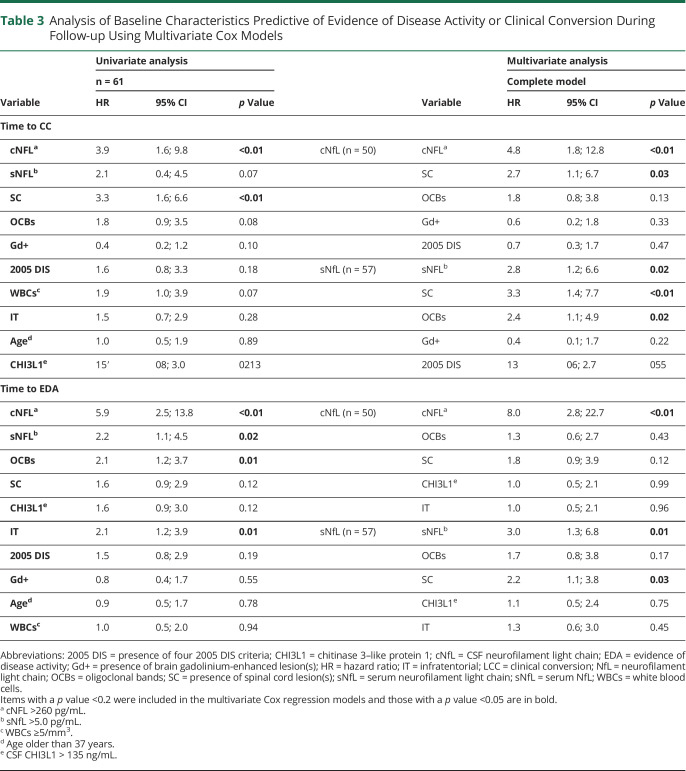
Cox univariate analysis of age, sNfL, cNfL, CSF CHI3L1, CSF WBCs, OCBs, number of 2005 DIS criteria, Gd+ lesions, IT lesions, and SC lesions on the index MRI revealed that medium-high cNfL and the presence of SC lesions were predictive of CC, while medium-high cNfL, medium-high sNfL, the presence of OCBs, and IT lesions were predictive of EDA (Table 3).
Table 3.
Analysis of Baseline Characteristics Predictive of Evidence of Disease Activity or Clinical Conversion During Follow-up Using Multivariate Cox Models
Multivariate Analysis of Prognostic Factors in RIS
cNfL and sNfL being highly correlated, we built 2 multivariate Cox regression models to identify EDA and CC prognostic factors in patients with RIS (Table 3).
In the multivariate model analyzing cNfL, medium-high cNfL was an independent predictive factor of EDA (HR = 8.0, p < 0.01). By contrast, medium-high cNfL and the presence of SC lesion(s) were independent factors of CC (HR = 4.8, p < 0.01 and HR = 2.7, p = 0.03, respectively, Table 3).
In the multivariate model analyzing sNfL, medium-high sNfL and SC lesions were independent predictive factors of EDA (HR = 3.0, p = 0.01 and HR = 2.2, p = 0.03, respectively), while medium-high sNfL, the presence of SC lesion(s), and OCBs were independent factors of CC (HR = 2.8, p = 0.02, HR = 3.3, p < 0.01, and HR = 2.36, p = 0.02, respectively) (Table 3). Especially, 84% of patients with RIS with medium-high sNfL levels, SC lesions, and OCBs had a CC at 3 years, compared with 34% in other patients with RIS.
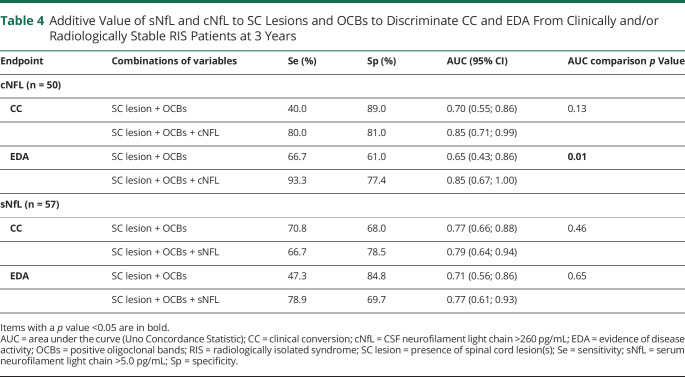
To analyze the additive value of NfL to SC lesions and OCBs, we further compared the AUC values of SC lesions + OCBs and SC lesions + OCBs + NfL for EDA and CC at 3 years (Table 4). All AUCs values were improved when adding sNfL or cNfL to SC lesions and OCBs for the prediction of EDA and CC, although it was statistically significant only for cNfL and EDA at 3 years (AUC = 0.65 [0.43; 0.86] for SC lesions + OCBs, vs AUC = 0.85 [0.67; 1.00] for SC lesions + OCBs + cNfL, p = 0.01 for AUC comparison).
Table 4.
Additive Value of sNfL and cNfL to SC Lesions and OCBs to Discriminate CC and EDA From Clinically and/or Radiologically Stable RIS Patients at 3 Years
Classification of Evidence
This study provides Class II evidence that in people with RIS, initial serum and CSF NfL levels are associated with subsequent evidence of disease activity or clinical conversion.
Discussion
We compared the value of NfL levels in serum and CSF samples from patients with RIS in the context of clinical risk. We demonstrated that sNfL and cNfL levels were highly correlated and constituted independent predictors of CC. Our results align with the most extensive RIS studies that described the importance of SC lesions for RIS prognosis, also identified here as an independent predictor of CC in the multivariate Cox model.4-6 These factors reflect the risk of CC during follow-up. They could be considered to counsel patients with RIS during diagnosis and to organize the clinical and MRI surveillance to detect disease activity. Indeed, EDA is a classical endpoint in the follow-up of patients with MS, but predictive factors of EDA have never been investigated in patients with RIS. Identifying OCBs and SC lesions as predictors of EDA also suggests that these factors provide complementary information to sNfL and cNfL that remain independent predictive factors in both cases (EDA and CC). Moreover, sNfL or cNfL provide additive value to SC lesions and OCBs to discriminate RIS patients with CC or EDA from clinically and/or radiologically stable RIS patients, especially for cNfL and EDA (Table 4). As a matter of fact, NfL are released from axons and reflect neuroaxonal injury in MS. NfL seems to be more specifically associated with focal inflammation (active plaques and relapses) than to the neurodegenerative process associated with disease progression.32
Recently, improved detection of sNfL based on ultrasensitive ELISA kits or Simoa allowed to reliably measure sNfL concentrations that are well correlated to that of CSF in patients with MS.33 The high sensitivity of this test, illustrated by the absence of missing values, allows accurate measures of sNfL in serum. This study provides the first measurement of sNfL in patients with RIS using the NF-light assay. Using this assay, the median sNFL (6.9 pg/mL) in patients with RIS was lower than that observed by others in patients with presymptomatic MS (16.7 pg/mL) and patients with CIS (17.0 pg/mL) using the same antibody adapted to Simoa homebrew kits.24,34 However, our results corroborate sNfL median levels measured in our previous study using the NF-light assay in patients with MS (9.4 pg/mL)35 and those recently found in patients with early RRMS (10.1 pg/mL) using the same assay.36
Compared with the electrochemiluminescence-based method or ELISA, the high accuracy of the Simoa assay, even at lower values, allows sNfL to strongly correlate with cNfL.37 This probably contributed to the performance of sNfL as a prognostic biomarker of clinical evolution in patients with RIS in our study. However, cNfL showed an overall more robust association profile with CC and EDA, suggesting that cNfL better reflects disease activity and predicts the evolution of patients with RIS than sNfL.34 This might be the consequence of differences among individuals in the kinetics of neurofilament protein release from neurons and trafficking between the brain and blood compartments.32 Nevertheless, blood sampling is less invasive, making sNfL an excellent alternative to cNfL for RIS prognosis when a lumbar puncture cannot be performed. More extensive validation studies are required before sNfL may replace cNfL.
The high accuracy of the Simoa assay probably enabled sNFL to overcome OCBs to predict CC. Even if this suggests that OCBs might no longer be required for the prognosis of CC in RIS patients with SC lesions, their detection provides a reasonable specificity for the differential diagnosis of RIS vs other conditions.
Contrasting with the observations of a previous study12 showing that OCBs and cNfL were independent predictive factors of CC in patients with RIS, OCBs and sNFL were independent predictors of CC in our study. Including the presence of SC lesions, one of the most robust predictors of CC in our Cox regression analysis might explain why cNfL and SC lesions, but not OCBs, were independent predictors of CC in our study. This discrepancy might also reflect the different populations, time of follow-up, and assays used to measure NfL (ELISA vs Simoa) or the association between the presence of OCBs and cNfL levels that potentially minimize their prognostic value (Figure 1D). Nevertheless, we confirm that CSF CHI3L1 is not a predictor of EDA and CC in patients with RIS.12,14
This study has some limitations given its retrospective nature. Incomplete availability of CSF and serum samples to determine cNfL, CSF CHI3L1, and sNfL led to missing data, lowering the power of the regression models, limited to 50 and 57 patients for the cNfL and the sNfL models, respectively (Table 3). Moreover, the small size and the small number of centers limit knowledge regarding how these data are translatable to other races and ethnicities with RIS. In addition, although our population was comparable with cohorts from previous published RIS studies (age, sex ratio, MRI, and CSF findings), the proportion of patients with RIS converting to MS was higher (50% converters at 36 months) in our cohort.4,5 This might be explained by the selection of patients with untreated RIS only. This also probably enhanced the statistical power of the study for identifying predictors of CC and constitutes a potential caveat for future confirmation studies with less active cohorts. As illustrated in the Kaplan-Meier analyses, cNfL and sNfL values did not appear as proportionally correlated with CC and EDA risk in a linear relationship, with similar risks of conversion for medium values and high values of each marker, when compared with low values (Figure 2). This suggests that NfL increase above a threshold value of 260 pg/mL for cNFL and 5.0 pg/mL for sNfL (corresponding to the first tertile of cNfL and sNfL in our study) indicates active neuroaxonal injury predictive of EDA and CC, especially for cNfL (Table 3). Intriguingly, the cutoff value for cNfL to predict CC was lower using Simoa (260 pg/mL) than that previously determined by ELISA in patients with RIS (619 pg/mL).12 It can be the consequence of different assays used to measure NfL (ELISA vs Simoa) and of diverse populations investigated, as already observed in previous studies dedicated to MS biomarkers, such as those examining CHI3L1 in patients with CIS.29,38 A threshold value determined using Simoa of 5.0 pg/mL could be chosen for prospective studies aiming at validating the performance of sNfL alone or in combination with SC lesions and OCBs to predict CC and EDA and at establishing an RIS conversion score combining these factors.
In conclusion, we showed that elevated cNfL and sNfL levels at diagnosis are both predictive factors of EDA and CC in RIS. Our study provides the first analysis of sNfL in an RIS cohort and offers Class I evidence that sNfL >5.0 pg/mL is an independent predictor of CC and EDA in patients with RIS. Although cNfL predicts disease activity better, sNfL is more accessible and can be considered to identify patients at higher risk of conversion to help clinicians for counseling and monitoring patients when a lumbar puncture is not performed.
Acknowledgment
The authors are most grateful to Sarah Kabani, Medical Writer at the B.E.S.P.I.M, Nimes University Hospital, for revising and editing this article.
Glossary
- AUC
area under the curve
- CC
clinical conversion
- CHI3L1
CSF chitinase 3–like protein 1
- DIS
dissemination in space
- EDA
evidence of disease activity
- MS
multiple sclerosis
- NEDA
nonevidence of disease activity
- OCBs
oligoclonal bands
- RIS
radiologically isolated syndrome
- RRMS
relapsing-remitting MS
- sNfL
serum neurofilament light chain
- SC
spinal cord
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The study was funded by CHU de Nîmes and has also been supported by the University of Montpellier, the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Occitanie Region, the fondation pour l'aide à la recherche sur la Sclérose en Plaques (ARSEP Fondation), the Radiologically Isolated Syndrome Consortium (RISC), and the Société Francophone de la Sclérose en Plaques (SFSEP).
Disclosure
M. Rival has nothing to disclose. E. Thouvenot has received honoraria, travel grants, or research grants from the following pharmaceutical companies: Actelion, Biogen, Genzyme, Merck Serono, Novartis, Roche, and Teva pharma. L. Du Trieu de Terdonck has nothing to disclose. S. Laurent-Chabalier has nothing to disclose. C. Demattei has nothing to disclose. U. Uygunoglu has nothing to disclose. G. Castelnovo has nothing to disclose. M. Cohen received personal fees from Biogen, Merck, Novartis, Roche, Alexion, Ad Scientiam, Teva, and Sanofi. D.T. Okuda received personal compensation for consulting and advisory services from Biogen, Celgene/Bristol Myers Squibb, EMD Serono, Genentech, Genzyme, Janssen Pharmaceuticals, Novartis, Osmotica Pharmaceuticals, RVL Pharmaceuticals, Inc., TG Therapeutics, and Viela Bio, Inc. and research support from Biogen and EMD Serono/Merck. Royalties received for intellectual property licensed by The Board of Regents of The University of Texas System. O.H. Kantarci has nothing to disclose. Daniel Pelletier has nothing to disclose. C. Azevedo has nothing to disclose. S. Lehmann has nothing to disclose. P. Marin has nothing to disclose. A. Siva has received honoraria for educational presentations internationally and at national meetings and symposia sponsored by Bayer-Schering AG; Merck-Serono; Teva-Turkey; Biogen Idec/Gen Pharma of Turkey and Genzyme and consultation fees or travel and registration coverage for attending several national or international congresses or symposia from Merck Serono, Novartis, Genzyme, and Roche. T. Mura has nothing to disclose. C. Lebrun-Frenay has participated in expert boards for Biogen, Novartis, Roche, and Genzyme in the last 5 years. Expert and Speaker honoraria are either declined or donated to the URRIS research unit, University Cote d’Azur, Nice, France. She did not receive any financial compensation for her participation in the scientific committee of the French MS Society, ARSEP, and ECTRIMS apart from travel expenses. Go to Neurology.org/NN for full disclosures.
References
- 1.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800-805. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005;58(6):840-846. [DOI] [PubMed] [Google Scholar]
- 4.Okuda DT, Siva A, Kantarci O, et al. ; Radiologically Isolated Syndrome Consortium RISC; Club Francophone de la Sclerose en Plaques CFSEP. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebrun-Frenay C, Kantarci O, Siva A, Sormani MP, Pelletier D, Okuda DT; 10-Year RISC Study Group on behalf of SFSEP, OFSEP. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann Neurol. 2020;88(2):407-417. [DOI] [PubMed] [Google Scholar]
- 6.Lebrun-Frénay C, Rollot F, Mondot L, et al. ; RISC, SFSEP, and OFSEP Investigators. Risk factors and time to clinical symptoms of multiple sclerosis among patients with radiologically isolated syndrome. JAMA Netw Open. 2021;4(10):e2128271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebrun C, Bensa C, Debouverie M, et al. ; Club Francophone de la Sclerose en Plaques. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol. 2009;66(7):841-846. [DOI] [PubMed] [Google Scholar]
- 8.Lebrun C, Bensa C, Debouverie M, et al. ; CFSEP. Unexpected multiple sclerosis: follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. J Neurol Neurosurg Psychiatry. 2008;79(2):195-198. [DOI] [PubMed] [Google Scholar]
- 9.Suthiphosuwan S, Sati P, Guenette M, et al. The central vein sign in radiologically isolated syndrome. AJNR Am J Neuroradiol. 2019;40(5):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthiphosuwan S, Sati P, Absinta M, et al. Paramagnetic rim sign in radiologically isolated syndrome. JAMA Neurol. 2020;77(5):653-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aly L, Havla J, Lepennetier G, et al. Inner retinal layer thinning in radiologically isolated syndrome predicts conversion to multiple sclerosis. Eur J Neurol. 2020;27(11):2217-2224. [DOI] [PubMed] [Google Scholar]
- 12.Matute-Blanch C, Villar LM, Álvarez-Cermeño JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain. 2018;141(4):1085-1093. [DOI] [PubMed] [Google Scholar]
- 13.Rossi S, Motta C, Studer V, et al. Subclinical central inflammation is risk for RIS and CIS conversion to MS. Mult Scler. 2015;21(11):1443-1452. [DOI] [PubMed] [Google Scholar]
- 14.Thouvenot E, Hinsinger G, Demattei C, et al. Cerebrospinal fluid chitinase-3-like protein 1 level is not an independent predictive factor for the risk of clinical conversion in radiologically isolated syndrome. Mult Scler. 2019;25(5):669-677. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun C, Cohen M, Pignolet B, et al. ; on behalf SFSEP, BIONAT Network; RISC. Interleukin 17 alone is not a discriminant biomarker in early demyelinating spectrum disorders. J Neurol Sci. 2016;368:334-336. [DOI] [PubMed] [Google Scholar]
- 16.Rival M, Galoppin M, Thouvenot E. Biological markers in early multiple sclerosis: the paved way for radiologically isolated syndrome. Front Immunol. 2022. Apr 27;13:866092. doi: 10.3389/fimmu.2022.866092. eCollection 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thouvenot E. Multiple sclerosis biomarkers: helping the diagnosis? Rev Neurol (Paris). 2018;174(6):364-371. [DOI] [PubMed] [Google Scholar]
- 18.Teunissen CE, Malekzadeh A, Leurs C, Bridel C, Killestein J. Body fluid biomarkers for multiple sclerosis—the long road to clinical application. Nat Rev Neurol. 2015;11(10):585-596. [DOI] [PubMed] [Google Scholar]
- 19.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. [DOI] [PubMed] [Google Scholar]
- 20.Sellebjerg F, Royen L, Soelberg Sørensen P, Oturai AB, Jensen PEH. Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase-3-like-1 in newly diagnosed patients with multiple sclerosis. Mult Scler. 2019;25(11):1444-1451. [DOI] [PubMed] [Google Scholar]
- 21.Håkansson I, Tisell A, Cassel P, et al. Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol. 2017;24(5):703-712. [DOI] [PubMed] [Google Scholar]
- 22.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhle J, Plavina T, Barro C, et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult Scler. 2020;26(13):1691-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2020;77(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toulouse E, Lafont B, Granier S, Mcgurk G, Bazin JE. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;39(6):883-885. [DOI] [PubMed] [Google Scholar]
- 26.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 27.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thouvenot E, Demattei C, Lehmann S, et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(2):251-257. [DOI] [PubMed] [Google Scholar]
- 29.Hinsinger G, Galéotti N, Nabholz N, et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler. 2015;21(10):1251-1261. [DOI] [PubMed] [Google Scholar]
- 30.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t -year survivors with censored regression models. J Am Stat Assoc. 2007;102(478):527-537. [Google Scholar]
- 31.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gafson AR, Barthélemy NR, Bomont P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143(7):1975-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550-1559. [DOI] [PubMed] [Google Scholar]
- 34.Håkansson I, Tisell A, Cassel P, et al. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflamm. 2018;15(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier A, Viel S, Perret M, et al. Comparison of Simoa and Ella to assess serum neurofilament-light chain in multiple sclerosis. Ann Clin Transl Neurol;8(5):1141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thebault S, Abdoli M, Fereshtehnejad SM, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655-1661. [DOI] [PubMed] [Google Scholar]
- 38.Comabella M, Fernández M, Martin R, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain. 2010;133(pt 4):1082-1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized participant data that underlie the results reported in this article will be shared beginning 6 months and ending 12 months after article publication on reasonable request by qualified investigators.