Abstract
Osteoporosis is a specific condition which is characterized by low bone mineral density (BMD) and deterioration of bone structure resulting in an increased susceptibility to fractures. It contributes to a great deal of morbidity and mortality, and is a large burden to the healthcare system, especially in the case of the elderly population. In the last four decades, a plethora of studies have reported characteristic oral radiographic findings in the early stages of osteoporosis, suggesting the possible use of oral radiographic signs for the early detection of the condition. Digital orthopantomographs (OPGs) are usually taken for the screening of dental patients during routine dental evaluations. These radiographs and the characteristic changes seen on them may have a significant role in the screening for initial osteoporotic changes. A number of precise radiomorphometric indices of the mandible have also been developed to allow quantification of the mandibular bone mass for identification of the initial signs of osteoporosis. The present review focuses on the possible role of panoramic radiographs in the initial screening for osteoporosis in dental clinics in high-risk groups.
Keywords: Bone density, mass screening, morbidity, mortality, osteoporosis, post-menopausal osteoporosis, senile osteoporosis
Introduction
Osteoporosis is an increasing health burden for both developed and developing countries.[1] In addition, osteoporosis predominantly affects the elderly population and is associated with considerable morbidity and mortality.[2,3] The term osteoporosis is derived from osteos meaning bone and pore meaning porous in Greek and is used to describe the porous nature of the bone which is characteristically seen in this condition. Jean Georges Chrétien Frédéric Martin Lobstein (in German, Johann Friedrich Georg Christian Martin Lobstein), a German-born French pathologist and surgeon (1777–1835), had coined the term osteoporosis to describe the porous bones that he observed in autopsies.[4] By the mid-20th century, the only known type of osteoporosis was age-related or senile osteoporosis; in other cases, the disease was termed idiopathic.[4]
Materials and Methods
The present review was based on a systematic search of all PubMed, Scopus, and Web of Science indexed databases with the keywords osteoporosis, post-menopausal osteoporosis, age-related or senile osteoporosis, mass screening, bone density, morbidity, mortality. Quick reading of abstracts was conducted and significant articles were kept for review. In addition, cross-references which seemed to be clinically relevant were also accessed. All original articles, reviews, and letters to editors in English literature were included for the present review.
Results
The extensive literature search strategy used in the formulation of the present review led to a deep insight into the different aspects of osteoporosis under specific headings like bone physiology in different age groups and osteoporosis, estrogen and osteoporosis, cytokines and osteoporosis, risk factors in osteoporosis, pathologic fractures and osteoporosis, clinical assessment tools to assess risks for pathologic fractures in osteoporosis, novel diagnostic adjuncts in the early detection of osteoporosis, purpose of screening in osteoporosis, screening for osteoporosis in dental clinics using panoramic radiographs, and radiomorphometric indices of the mandible aiding in the initial screening of osteoporosis.
Discussion
Bone physiology in different age groups and osteoporosis: The density of bone decreases with advancing age while this effect becomes more pronounced by around the 3rd decade of life.[5] In females, particularly, this bone loss starts early than in males.[6,7] An increase in skeletal fragility is said to result from failure to produce skeleton of optimal mass and strength during the phases of growth, an excessive bone resorption resulting in decreased bone mass and micro-architectural deterioration of the skeleton and an inadequate formative response to an increased resorption during the bone remodeling phases.[8,9] The process begins with the activation of hematopoietic precursors to become osteoclasts which normally requires an interaction with cells of the osteoblastic lineage. Because the resorption and reversal phases of bone remodeling are short and the period required for osteoblastic replacement of the bone is long, any imbalance in the process results in a net loss of bone mass. Moreover, the larger number of unfilled Howship's lacunae and Haversian canals further weaken the bone structure while excessive resorption also results in complete loss of trabecular structures, so that there is no template for bone formation. Thus, there are multiple ways in which an increase in osteoclastic resorption results in skeletal fragility.[8,9]
Estrogen and osteoporosis: In 1940, the American physician and endocrinologist, Fuller Albright, described postmenopausal osteoporosis and proposed that it was the consequence of impaired bone formation due to estrogen deficiency.[4] The role of estrogen has drawn attention of skeletal researchers since the times Albright introduced the classic concept on postmenopausal osteoporosis, and proposed that menopausal cessation of ovarian function and consequent sharp reduction in circulating estrogen caused bone loss that eventually resulted in the condition he termed as postmenopausal osteoporosis.[4] The concept that estrogen deficiency is critical to the pathogenesis of osteoporosis was based initially on the fact that postmenopausal women, whose estrogen levels declined, were at the highest risk of developing the disease process. Subsequently, deficiency of calcium and aging of the skeleton were proposed to be the major etiological factors for the causation of osteoporosis.[10–13]
This concept, too, was later replaced by the current concept which considers osteoporosis as a continuum of a process in which multiple pathogenic mechanisms converge to cause a loss of bone mass and micro-architectural deterioration of the skeletal structures.[14,15] Morphological studies and measurements of certain biochemical markers have indicated that bone remodeling is accelerated at menopause as both markers of resorption and formation are increased. Hence, contrary to Albright's original hypothesis, an increase in bone resorption and not impaired bone formation was considered to be the driving force for bone loss in the setting of estrogen deficiency.[11,12]
Estrogen deficiency, though, continues to play a role in bone loss in elderly women, as evidenced by the fact that estrogen treatment rapidly reduces bone breakdown while the estimated fracture risk varies inversely with estrogen levels in postmenopausal women.[16] In fact, estrogen has a greater effect than androgen in inhibiting bone resorption in males and is important in the acquisition of peak bone mass in males.[4] This is for similar reasons that because of a higher peak bone mass in men and a lack of a menopause-like process and denser bones, the prevalence of osteoporosis is seen to be lower in men than in women.[4] The concept that osteoporosis is primarily due to calcium deficiency, particularly in the elderly, was initially put forth as a counter-proposal to Albright's estrogen deficiency hypothesis.[8]
Cytokines and osteoporosis: Remodeling imbalance, characterized by an impaired bone formative response to increased activation of bone remodeling is an essential component in the pathogenesis of osteoporosis. This may be due, in part, to an age-related decrease in the capacity of osteoblasts to replicate and differentiate, though it seems likely that specific defects in the production and/or activity of local and systemic growth factors and cytokines released locally contribute to an impaired bone formation.[17] Bone morphogenic proteins (BMPs), members of the family, tumor necrosis factor-alpha (TNF- α), insulin-like growth factor (IGF), which is both a systemic and local regulator, and transforming growth factor-beta (TGF-β) have all been implicated in the pathogenesis of osteoporosis by affecting bone formation. It has also been proposed that locally produced cytokines including interleukin-1 (IL-1) and prostaglandins (PGs), in particular, prostaglandin E2 (PGE2), which is the major prostaglandin produced by bone, can affect bone formation in aged individuals (senile osteoporosis).[18,19]
Risk factors in osteoporosis: Various clinical risk factors which are used to assess the risk of osteoporosis include increasing age, female sex, low body mass defined as <19 kg/m2 by the National Osteoporosis Guideline Group (NOGG) and <18.5 kg/m2 by the National Institute for Health and Care Excellence (NICE), parental history of hip fracture, past history of fragility fracture, especially hip, spine and wrist fractures, corticosteroid therapy, Cushing's syndrome and deleterious habits in the form of smoking and high alcohol intake.[20,21] Secondary causes of osteoporosis include conditions such as rheumatoid arthritis and other systemic/inflammatory arthritides, prolonged immobilization and/or a sedentary lifestyle, primary hypogonadism, treatment history with aromatase inhibitors and/or androgen deprivation therapy, primary hyperparathyroidism, hyperthyroidism, chronic hepatic and/or renal disease, gastrointestinal disease such as Crohn's disease, ulcerative colitis and coeliac disease, untreated premature menopause (<45 years) or prolonged secondary amenorrhea, Type I diabetes mellitus, and chronic obstructive pulmonary disease (COPD).[22–24]
Also, other than aromatase inhibitors and androgen deprivation therapy, other pharmaceutical agents which may increase the risk of fragility fractures include proton pump inhibitors (PPIs), enzyme-inducing anti-convulsants, long-term depot medroxyprogesterone acetate, long-term anti-depressant therapy and thiazolidinediones (anti-diabetic agents).[22–24] For males, androgen deficiency and for females, estrogen deficiency, early menopause (<45 years) including surgical, cessation of menstruation for 6–12 consecutive months (excluding pregnancy, hysterectomy and/or menopause) are also considered as important risk factors. Other significant risk factors include physical characteristics of the bone such as bone density (mass), size, geometry, micro-architecture and composition.[22–24]
Pathologic fractures and osteoporosis: Osteoporotic fractures are recognized as low-trauma, pathologic fractures resulting from low bone mineral density (BMD). The best characterized are, by order of the related disability burden, hip, spine, and wrist fractures.[25] There are other peripheral fractures related to low BMD or poor quality of bone mass such as proximal humeral, pelvic, rib, proximal tibial and/or ankle fractures.[26] Pain and disability become worse with each new fracture as does the mortality rates. In addition, lumbar fractures have the worst impact with the spinal mobility getting impaired even in the absence of significant pain. Comorbidity is common (kyphosis, obstructive and restrictive pulmonary diseases, etc.) in particular at advanced stages and contributes to the burden on the quality of life (QoL) and increased mortality, while the fracture risk of a patient can be estimated as low (<10%), moderate (10%–20%), or high (>20%) risk in the next 10 years using known risk factors and clinical assessment tools.[26,27]
Clinical assessment tools to assess risks for pathologic fractures in osteoporosis: There are two tools available to calculate 10-year fracture risk rates and these include FRAX®, developed by the World Health Organization (WHO), and The Q Fracture®, an alternative risk calculator based on the UK population. Amongst these, NOGG advises the use of FRAX® while the Scottish Intercollegiate Guidelines Network (SIGN) advocates the use of Q Fracture®. Also, there is another index suggested by the Canadian Association of Radiologists and Osteoporosis, Canada (CAROC).[28–30]
The FRAX® tool was developed in 2008 by the WHO to calculate risk of fractures in males and females from several clinical risk factors with or without measurement of femoral neck BMD. The clinical risk factors included in the FRAX® algorithm were age and sex, and also weight and height of the individual, previous history of fractures, parental history of hip fracture, glucocorticoid therapy, rheumatoid arthritis, secondary osteoporosis and smoking and high alcohol intake. The outputs are a 10-year probability of hip fracture and a 10-year probability of a major osteoporotic fracture (clinical spine, forearm, hip or humerus fracture). FRAX® was developed using baseline and follow-up data from nine prospective population-based cohorts (including Europe, Australia, Canada and Japan) and validated in 11 prospective population-based cohorts. The FRAX® tool can be used either with or without BMD results and is applicable to people aged between 40 and 90 years.[28,29]
The Q Fracture® algorithm, on the other hand, was developed in 2009 and has been internally and externally validated based on large primary care populations in the UK. The algorithm is based on variables that are readily available in the electronic healthcare records estimating a 10-year probability of developing both hip and major osteoporotic fractures (including hip, spine and wrist fractures) without BMD measurement. The clinical risk factors included in the Q Fracture® algorithm in males and females were age and sex and body mass index (BMI) of the individual, smoking and high alcohol intake, glucocorticoid therapy, asthma and cardiovascular disease, previous history of fractures, chronic hepatic and/or renal disease, rheumatoid arthritis, type II diabetes mellitus, and treatment history with tricyclic antidepressants (TCAs). Additional risk factors assessed exclusively in females include hormone replacement therapy (HRT), parental history of hip fracture, menopausal symptoms, gastrointestinal and/or malabsorption syndromes, and history of endocrine disorders. The Q Fracture® algorithm can as well be used with or without BMD results like the FRAX® tool and is applicable to people aged between 30 and 85 years.[28–30]
The CAROC paper-based risk table takes into account age, sex, past history of fractures and glucocorticoid use secondary to any reason to determine a 10-year absolute risk of all osteoporotic fractures; however, BMD is required to calculate this increased risk, a parameter which is optional for use in the previous two algorithms.[30]
Novel diagnostic adjuncts in the early detection of osteoporosis: Various diagnostic adjuncts helpful in the early detection of osteoporosis include quantitative computed tomography (QCT), magnetic resonance imaging (MRI), quantitative ultrasonography (Q-USG), single- and dual-photon absorptiometry, single- and dual-energyX-ray absorptiometry (DEXA). Amongst these, QCT provides volumetric acquisitions from which BMD can be estimated. QCT of lumbar spine (central QCT) has advantages in terms of an ability to differentiate cortical and trabecular bone masses, assess geometry of the vertebrae and estimate the BMD volumetrically expressed in g/cm3, though, the major disadvantages of central QCT include high radiation dose and a lack of validated diagnostic criteria.[31,32] Q-USG is used for measuring BMD in the peripheral skeleton, generally, at the calcaneus. Photonic absorptiometry with iodine-125 (I-125) was initially used to study peripheral skeleton (radius and calcaneus) but was subsequently replaced by dual photonic absorptiometry that uses gadolinium-153 (Gd-153).[32] High resolution MRI including advanced MRI techniques based on diffusion, perfusion and spectroscopy are used for assessment of trabecular structure of peripheral bones (calcaneus, distal radius and phalanx). The bone architecture studied using CT or MRI quantified in terms of scale, shape, anisotropy, and connectivity allows for the assessment of bone strength without considering BMD.[31,32]
In addition to these, BMD testing using DEXA is recognized, till date, as the best available technique for in-vivo bone measurements. DEXA is based on the variable absorption of high- and low-energy X-ray photons. Depending on the equipment used, these photons can be obtained using two mechanisms. In some cases, the generator emits alternating radiation of high (140kVp) and low (70–100kVp) kilovoltage peaks while moving across the surface of the body to be examined whereas in others, the generator emits a constant beam while a rare-earth filter separates high energy (70keV) from low energy (40keV) photons.[33–36] DEXA uses low radiation doses and generally, most of these devices do not require lead shielding of the room and/or special protective measures for the operators. The use of two beam energies in DEXA allows the thickness of the overlying soft tissues to be removed and the density of the bone alone to be measured.[37] The major limitations of DEXA include concerns over its cost effectiveness, limited number of facilities providing DEXA, and trained personnel for the same.[38,39]
Purpose of screening in osteoporosis: Low BMD is one of the major risk factors for fractures from osteoporosis. The American College of Preventive Medicine (ACPM) has stated that screening with BMD testing for osteoporosis is recommended in females, aged 65 years and above, and in males, aged 70 years and above.[40] The ACPM also recommends that younger post-menopausal females and males, aged 50–69 years, should undergo BMD testing if they have at least one major or two minor risk factors present for osteoporosis.[40]
Screening for osteoporosis in dental clinics using panoramic radiographs: In the last four decades, a plethora of studies have reported characteristic oral radiographic findings in the early stages of osteoporosis, suggesting the possible use of oral radiographic signs for the early detection of the condition. The goal of such screening is not to diagnose osteoporosis but rather to identify individuals at risk for osteoporosis.[41] It is well-known that osteoporosis results in reduced bone mass, as well as, alterations of the associated structures. The mandibular cortex is a wide area which gets influenced by various developmental, physiological, pathological, and age-related alterations. Various studies have confirmed such radiographic changes associated with jaw bones including changes in their morphology.[42,43] It has been shown that mandibular BMD can be directly correlated with skeletal BMD. Studies have also confirmed that a continuous age- and sex-related bone loss in the mandible, as in the rest of the skeleton, causes increased cortical porosity and thinning of the mandibular structures, and that these criteria can be used for the initial screening of osteoporosis.[44,45] In addition, digital orthopantomographs (OPGs) are usually taken for the screening of dental patients during routine dental evaluations. While any exposure to X-rays is believed to carry a risk of inducing some radiation hazard, dental radiography is generally associated with low radiation exposure and the risks associated with it. OPGs, therefore, serve as an effective tool in the initial screening of osteoporosis. These radiographs and the characteristic changes seen on them may have a significant role in the screening for initial osteoporotic changes.[46]
Radiomorphometric indices of the mandible aiding in the initial screening of osteoporosis: The most commonly studied measures of mandibular morphology in relation to osteoporosis on OPGs include the thickness and integrity of the inferior border of the mandible. Mandibular cortical porosity, buccal cortical width, inferior cortical thickness and radiographic density have all been reported to be affected with age.[47,48] Numerous qualitative as well as quantitative indices have also been developed over the years and these include the morphology of mandibular inferior cortex (MIC), mental index (MI) or mandibular cortical width (MCW), panoramic mandibular index (PMI), panoramic analysis (PA) or gonial index (GI), antegonial index (AI), gonial angle (GA), antegonial angle (AA) and antegonial depth (AD), etc., for the assessment of initial signs of osteoporosis.[49–54]
-
Qualitative indices:
-
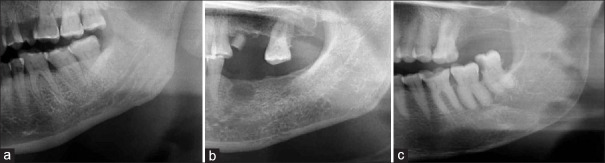
Morphology of mandibular inferior cortex (MIC): As devised by Klemetti et al.,[50] morphology of mandibular inferior cortex (MIC) is classified as: [Figure 1abc]
- Class I: with smooth endosteal margin of the inferior cortex [Figure 1a];
- Class II: endosteal margin presents with semilunar defects (lacunar resorption) and dense endosteal residues, one to three layers thick [Figure b]; and
- Class III: cortex is obviously porous with dense endosteal residues [Figure 1c].
-
-
Quantitative indices:
- Mental index (MI): Mental index (MI) or mandibular cortical width (MCW) is a measure of the thickness of the cortex in the region of mental foramen [Figure 2];
- Panoramic analysis (PA) or gonial index (GI): Panoramic analysis (PA) or gonial index (GI) is used to measure the thickness of the cortex at the gonial angle and is measured at the point that bisects the angle formed by tangent to the inferior and posterior border of the mandible [Figure 3];
- Antegonial index (AI): As devised by Ledgerton et al.,[51] antegonial index (AI) is a measure of the cortical width in the region anterior to the gonion at a point identified by extending a line of “best fit” on the anterior border of the ascending ramus down to the inferior border of the mandible. For this, a tangent to the inferior border is drawn and a perpendicular to the tangent is plotted. The measurement of the cortical width in the region anterior to the gonion (antegonion) is then made along this perpendicular (antegonial index) [Figure 3];
- Gonial angle (GA): Gonial angle (GA) is assessed by tracing a line tangent to the inferior and another to the posterior border of the mandible with the intersection of these two lines identified as the mandibular or gonial angle [Figure 3];
- Antegonial depth (AD): Antegonial depth (AD) is the distance measured along the perpendicular drawn from the deepest point of the antegonial notch to a line parallel to the inferior border of the mandible [Figure 4].
Figure 1.
(a-c) Cropped orthopantomograph (OPG) showing C1, C2, C3 cortex as per Klemetti et al.,[50] proposed classification of the morphology of mandibular inferior cortex (MIC): (a) Cropped OPG showing C1 cortex as per Klemetti et al.,[50] proposed classification of the morphology of MIC; (b) Cropped OPG showing C2 cortex as per Klemetti et al.,[50] proposed classification of the morphology of mandibular inferior cortex (MIC); (c) Cropped OPG showing C3 cortex as per Klemetti et al.,[50] proposed classification of the morphology of MIC
Figure 2.
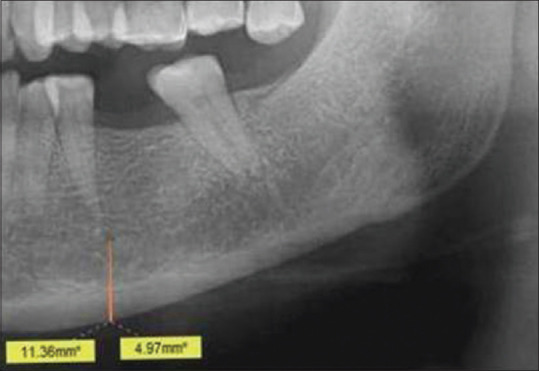
Cropped OPG showing mental index (MI) and panoramic mandibular index (PMI) calculation
Figure 3.
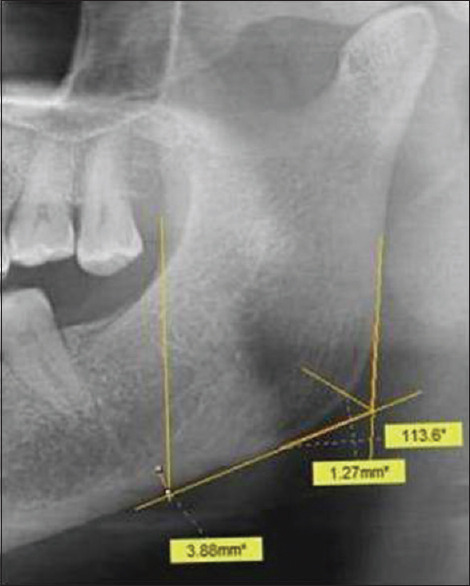
Cropped OPG showing panoramic analysis (PA) or gonial index (GI), antegonial index (AI) and gonial angle (GA) calculation
Figure 4.
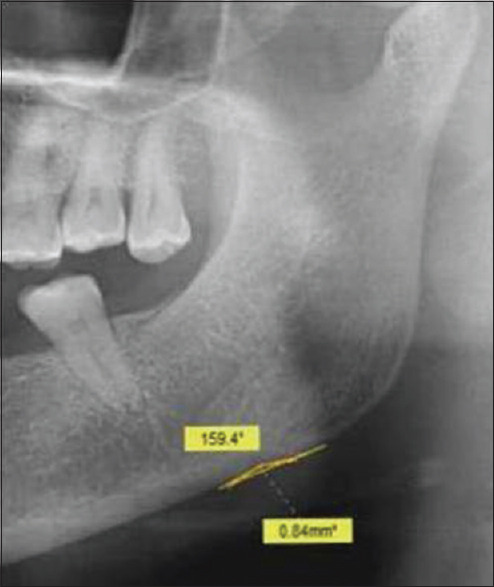
Cropped OPG showing antegonial angle (AA) and antegonial depth (AD) calculation
Conclusions
Based on an extensive literature search, the present review provides an in-depth analysis of the possible or proposed etiopathogenesis of osteoporosis in addition to highlighting various aspects including the several risk factors which often coexist to increase the risk of development of osteoporosis. Also, based on the present review, it could be concluded that clinicians trying to improve early detection and prevent fragility fractures in osteoporosis often have conflicting guidelines to follow. In such a setting, OPGs serve as an effective tool in the initial screening for the signs of osteoporosis. The various qualitative and quantitative indices developed offer a high degree of sensitivity and can help the affected individuals as well as individuals at risk by detecting the condition in its early stages so that such individuals can be referred for an appropriate care to stop further progression as well as to prevent the sequels which are associated with an increased morbidity and considerable decrease in the quality of life of the affected individuals. This, however, mandates further studies in this regard to validate results and/or findings in the perspective of individuals from different age groups and genders and from different geographic locales and ethnicity to come to valid conclusions. Also, results, findings, or impressions obtained from various diagnostic modalities can be correlated to further emphasize the utility of panoramic radiographs in the screening of patients in the high-risk group for osteoporosis in dental clinics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
To all the scholars who have worked on this ignored, yet, significant topic with practical application in improving the quality of life (QoL) of the affected individuals.
References
- 1.Lane NE. Epidemiology, etiology and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins CH, Birge SJ. Prevention of osteoporotic fractures in the elderly. Am J Med. 2005;118:1190–5. doi: 10.1016/j.amjmed.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 3.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: A geriatric perspective. Best Pract Res Clin Endocrinol Metab. 2008;22:765–85. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Manring MM, Calhoun JH. Biographical sketch: Fuller Albright. Clin Orthop Relat Res. 2011;469:2092–5. doi: 10.1007/s11999-011-1831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettinger MP. Aging bone and osteoporosis: Strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237–46. doi: 10.1001/archinte.163.18.2237. [DOI] [PubMed] [Google Scholar]
- 6.Martens MG. Risk of fracture and treatment to prevent osteoporosis-related fracture in post-menopausal women: A review. J Reprod Med. 2003;48:425–34. [PubMed] [Google Scholar]
- 7.Vondracek SF, Hansen LB, McDermott MT. Osteoporosis risk in pre-menopausal women. Pharmacotherap. 2009;29:305–17. doi: 10.1592/phco.29.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts and prospects. J Clin Invest. 2005;115:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamdy RC. Osteoporosis: An update. J Clin Densitom. 2017;20:131. doi: 10.1016/j.jocd.2017.04.001. doi: 10.1016/j.jocd. 2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Dargent-Molina P, Douchin MN, Cormier C, Meunier PJ, Bréart G EPIDOS Study Group. Use of clinical risk factors in elderly women with low bone mineral density to identify women at higher risk of hip fracture: The EPIDOS prospective study. Osteoporos Int. 2002;13:593–9. doi: 10.1007/s001980200078. [DOI] [PubMed] [Google Scholar]
- 11.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in post-menopausal women. Can Med Assoc J. 2007;177:575–80. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewiecki EM. Prevention and treatment of post-menopausal osteoporosis. Obstet Gynecol Clin North Am. 2008;35:301–15. doi: 10.1016/j.ogc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Honig S. Low bone mass: Assessing absolute fracture risk and the need to treat younger post-menopausal women. Bull NYU Hosp Jt Dis. 2009;67:281–4. [PubMed] [Google Scholar]
- 14.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–8. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins CH. Osteoporosis screening and risk management. Clin Interv Aging. 2007;2:389–94. [PMC free article] [PubMed] [Google Scholar]
- 16.Trémollieres FA, Pouillès JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P. Fracture risk prediction using BMD and clinical risk factors in early post-menopausal women: Sensitivity of the WHO FRAX tool. J Bone Miner Res. 2010;25:1002–9. doi: 10.1002/jbmr.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis and therapy. J Am Med Assoc. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 18.Mauck KF, Clarke BL. Diagnosis, screening, prevention and treatment of osteoporosis. Mayo Clin Proc. 2006;81:662–72. doi: 10.4065/81.5.662. [DOI] [PubMed] [Google Scholar]
- 19.Sweet MG, Sweet JM, Jeremiah MP, Galazka SS. Diagnosis and treatment of osteoporosis. Am Fam Physician. 2009;79:193–200. [PubMed] [Google Scholar]
- 20.Golob AL, Laya MB. Osteoporosis: Screening, prevention and management. Med Clin North Am. 2015;99:587–606. doi: 10.1016/j.mcna.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. US Preventive Services Task Force. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. J Am Med Assoc. 2018;319:2521–31. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor KM. Evaluation and treatment of osteoporosis. Med Clin North Am. 2016;100:807–26. doi: 10.1016/j.mcna.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Jin J. Screening for osteoporosis to prevent fractures. J Am Med Assoc. 2018;319:2566. doi: 10.1001/jama.2018.8361. doi: 10.1001/jama. 2018.8361. [DOI] [PubMed] [Google Scholar]
- 24.Cauley JA. Screening for osteoporosis. J Am Med Assoc. 2018;319:2483–5. doi: 10.1001/jama.2018.5722. [DOI] [PubMed] [Google Scholar]
- 25.SànchezRiera L, Wilson N, Kamalaraj N, Nolla JM, Kok C, Li Y, et al. Osteoporosis and fragility fractures. Best Pract Res Clin Rheumatol. 2010;24:793–810. doi: 10.1016/j.berh.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Lewiecki EM. Clinical applications of bone density testing for osteoporosis. Minerva Med. 2005;96:317–30. [PubMed] [Google Scholar]
- 27.McCloskey E. Preventing osteoporotic fractures in older people. Practitioner. 2011;255:19–22. [PubMed] [Google Scholar]
- 28.Siris ES, Baim S, Nattiv A. Primary care use of FRAX: Absolute fracture risk assessment in post-menopausal women and older men. Postgrad Med. 2010;122:82–90. doi: 10.3810/pgm.2010.01.2102. [DOI] [PubMed] [Google Scholar]
- 29.Baró F, Cano A, Sánchez Borrego R, Ferrer J, González Rodríguez SP, Neyro JL, et al. Frequency of FRAX risk factors in osteopenic post-menopausal women with and without history of fragility fracture. Menopause. 2012;19:1193–9. doi: 10.1097/gme.0b013e31825d65c5. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan M, Reddy S, Berkman N, Cullen K, Middleton JC, Nicholson WK, et al. Screening to prevent osteoporotic fractures: Updated evidence report and systematic review for the US preventive services task force. J Am Med Assoc. 2018;319:2532–51. doi: 10.1001/jama.2018.6537. [DOI] [PubMed] [Google Scholar]
- 31.Formica CA, Nieves JW, Cosman F, Garrett P, Lindsay R. Comparative assessment of bone mineral measurements using dual X-ray absorptiometry and peripheral quantitative computed tomography. Osteoporos Int. 1998;8:460–7. doi: 10.1007/s001980050092. [DOI] [PubMed] [Google Scholar]
- 32.El-Desouki MI, Sherafzal MS, Othman SA. Comparison of bone mineral density with dual energy x-ray absorptiometry, quantitative ultrasound and single energy x-ray absorptiometry. Saudi Med J. 2005;26:1346–50. [PubMed] [Google Scholar]
- 33.Blake GM, Fogelman I. Dual energy x-ray absorptiometry and its clinical applications. Semin Musculoskelet Radiol. 2002;6:207–18. doi: 10.1055/s-2002-36718. [DOI] [PubMed] [Google Scholar]
- 34.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. 2007;83:509–17. doi: 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blake GM, Fogelman I. Role of dual-energy X-ray absorptiometry in the diagnosis and treatment of osteoporosis. J Clin Densitom. 2007;10:102–10. doi: 10.1016/j.jocd.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Blake GM, Fogelman I. The clinical role of dual energy X-ray absorptiometry. Eur J Radiol. 2009;71:406–14. doi: 10.1016/j.ejrad.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 37.Blake GM, Fogelman I. An update on dual-energy x-ray absorptiometry. Semin Nucl Med. 2010;40:62–73. doi: 10.1053/j.semnuclmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Tanner SB. Dual-energy X-ray absorptiometry in clinical practice: New guidelines and concerns. Curr Opin Rheumatol. 2011;23:385–8. doi: 10.1097/BOR.0b013e328347d90c. [DOI] [PubMed] [Google Scholar]
- 39.Lorente Ramos RM, Azpeitia Armán J, Arévalo Galeano N, Muñoz Hernández A, García Gómez JM, Gredilla Molinero J. Dual energy xray absorptimetry: Fundamentals, methodology and clinical applications. Radiologia. 2012;54:410–23. doi: 10.1016/j.rx.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Lim LS, Hoeksema LJ, Sherin K. ACPM Prevention Practice Committee. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med. 2009;36:366–75. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–56. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Dutra V, Devlin H, Susin C, Yang J, Horner K, Fernandes AR. Mandibular morphological changes in low bone mass edentulous females: Evaluation of panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:663–8. doi: 10.1016/j.tripleo.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Taguchi A, Ishii K, Suei Y, Fujita M, Nakamoto T, et al. Diagnostic efficacy of alveolar bone loss of the mandible for identifying post-menopausal females with femoral osteoporosis. Dentomaxillofac Radiol. 2007;36:28–33. doi: 10.1259/dmfr/28366679. [DOI] [PubMed] [Google Scholar]
- 44.White SC. Oral radiographic predictors of osteoporosis. Dentomaxillofac Radiol. 2002;31:84–92. doi: 10.1038/sj.dmfr.4600674. [DOI] [PubMed] [Google Scholar]
- 45.Taguchi A. Triage screening for osteoporosis in dental clinics using panoramic radiographs. Oral Dis. 2010;16:316–27. doi: 10.1111/j.1601-0825.2009.01615.x. [DOI] [PubMed] [Google Scholar]
- 46.Božic M, Ihan Hren N. A novel method of dental panoramic tomogram analysis: A perspective tool for a screening test for osteoporosis. J Craniomaxillofac Surg. 2013;41:808–15. doi: 10.1016/j.jcms.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Marandi S, Bagherpour A, Imanimoghaddam M, Hatef M, Haghighi A. Panoramicbased mandibular indices and bone mineral density of femoral neck and lumbar vertebrae in women. J Dent (Tehran) 2010;7:98–106. [PMC free article] [PubMed] [Google Scholar]
- 48.Leite AF, Figueiredo PT, Barra FR, Melo NS, de Paula AP. Relationships between mandibular cortical indexes, bone mineral density and osteoporotic fractures in Brazilian men over 60 years old. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:648–56. doi: 10.1016/j.tripleo.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Benson BW, Prihoda TJ, Glass BJ. Variations in adult cortical bone mass as measured by a panoramic mandibular index. Oral Surg Oral Med Oral Pathol. 1991;71:349–56. doi: 10.1016/0030-4220(91)90314-3. [DOI] [PubMed] [Google Scholar]
- 50.Klemetti E, Kolmakov S, Kröger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994;102:68–72. doi: 10.1111/j.1600-0722.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 51.Ledgerton D, Horner K, Devlin H, Worthington H. Radiomorphometric indices of the mandible in a British female population. Dentomaxillofac Radiol. 1999;28:173–81. doi: 10.1038/sj/dmfr/4600435. [DOI] [PubMed] [Google Scholar]
- 52.Gulsahi A, Yüzügüllü B, Imirzalioglu P, Genç Y. Assessment of panoramic radiomorphometric indices in Turkish patients of different age groups, gender and dental status. Dentomaxillofac Radiol. 2010;39:284–9. doi: 10.1259/dmfr/19491030. [DOI] [PubMed] [Google Scholar]
- 53.Leite AF, Figueiredo PT, Guia CM, Melo NS, de Paula AP. Correlations between seven panoramic radiomorphometric indices and bone mineral density in post-menopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:449–56. doi: 10.1016/j.tripleo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh S, Vengal M, Pai KM, K Abhishek. Remodeling of the antegonial angle region in the human mandible: A panoramic radiographic cross-sectional study. Med Oral Patol Oral Cir Bucal. 2010;15:e802–7. doi: 10.4317/medoral.15.e802. [DOI] [PubMed] [Google Scholar]