Abstract
Background
Increased physical activity (PA) has positive effects on health and longevity. In Swedish healthcare, the physical activity on prescription (PAP) method reportedly increases patients’ PA levels for up to 12 months, but long-term follow ups are lacking. As it remains difficult to maintain lifestyle changes, our aim was to evaluate adherence and clinical effects at a 5-year follow-up of PAP treatment in primary healthcare.
Methods
This longitudinal, prospective cohort study included 444 patients, (56% female), aged 27–85 years, with at least one metabolic risk factor. Participants were offered PAP by nurses or physiotherapists. The PAP intervention included an individualised dialogue, a PA recommendation by written prescription, and individually adjusted follow-up over 5 years, according to the Swedish PAP model. Patient PA level, metabolic risk factors, and health related quality of life (HRQoL) were measured at baseline and at the 6-month, 1.5-year, 2.5-year, 3.5-year, and 5-year follow-ups. Estimated latent growth curves were used to examine levels and rates of change in the outcomes.
Results
The study dropout rate was 52%, with 215 of 444 patients completing the 5-year follow-up. At follow-up, the mean PA level had increased by 730 MET-minutes per week or 3 hours of moderate-intensity PA/week when compared to baseline. During the 5-year intervention, we observed significant positive changes (p ≤ 0.05) in 9 of 11 metabolic risk factors and HRQoL parameters: body mass index, waist circumference, systolic and diastolic blood pressure, fasting plasma glucose, triglycerides, cholesterol, high-density lipoprotein, and mental component summary.
Conclusion
This first evaluation of a 5-year PAP intervention in primary care demonstrated positive long-term (5 years) effects regarding PA level, metabolic health, and HRQoL. The recorded long-term adherence was ~50%, which is in line with medical treatment. Despite limitations, PAP can have long-term effects in an ordinary primary care setting.
Introduction
Firmly established evidence supports the positive effects of physical activity (PA) on health and longevity [1,2] and regular PA is considered essential for the prevention and treatment of several diseases [3]. Metabolic syndrome (MetS), which includes being overweight, and exhibiting abdominal obesity, insulin resistance, dyslipidaemia, and hypertension in various combinations, increases the risks of cardiovascular disease (CVD), type 2 diabetes (DM), and premature death [4,5], and is positively influenced by regular PA [6,7]. The recently updated recommendations for physical activity among adults include a target of 150–300 min per week of moderate-intensity aerobic PA, or 75–150 min per week of vigorous-intensity aerobic PA [2]. Notably, PA of any duration, even below the recommended threshold, is important for health and being somewhat active is better than doing nothing. Indeed, there is a dose-response relationship between PA and MetS prevalence, with low-intensity PA being associated with a 50% lower MetS prevalence, and moderate- to vigorous-intensity PA with a 67% lower MetS prevalence, compared to no activity [8].
Long-term follow-up of >7 years shows that both metabolically “healthy” (i.e., without any known additional cardiovascular risk factors) obese individuals and metabolically unhealthy (having risk factors) normal weight individuals, carry increased risks of developing CVD and DM [9]. Importantly, positive changes in risk factors included in MetS can yield reduced risks of CVD and all-cause mortality over 7–9 years [10]. The positive metabolic effects of increased PA highlight the importance of identifying interventions in regular healthcare that may have positive long-term effects on increased PA levels in patients.
Several methods to increase patients’ PA levels have been introduced in healthcare, with varying individualization and success [11–14]. In Swedish healthcare, the physical activity on prescription (PAP) method, which has been used during the last 20 years to help patients increase their PA levels [15], includes a patient-centred dialogue; an individually tailored PA recommendation, including a written prescription; and an individualised follow-up. All licensed healthcare professionals can offer PAP treatment for preventive and therapeutic purposes, or as a complementary or first-line treatment [16,17].
A systematic review of Swedish PAP treatment reveals that it effectively increased PA levels among insufficiently active patients [18], for at least 12 months. From the patient’s perspective, tailored PAP including a written prescription and regular follow-up is considered important for increasing and maintaining motivation and PA level [19–22], and may contribute to the efficacy of the PAP method. Long-term adherence is essential, and a proxy of behavioural change. In a recent systematic review and meta-analysis, Arsenijevic et al. indicate that the type and duration of intervention programme may influence both the adherence rate and efficacy [23]. However, earlier clinical studies of lifestyle behavioural change show low levels of long-term adherence [24], while PAP has shown short-term adherence at a similar level to medical care [25,26]. Thus, further studies are needed to evaluate Swedish PAP treatment from a long-term perspective, and for different patient subgroups [23,27]. PAP is still insufficiently implemented in Swedish healthcare [28,29].
In the present part of the Gothenburg PAP study, we aimed to evaluate a 5-year period of PAP treatment in primary healthcare for adult patients who were physically inactive and had metabolic risk factors. The goal was to explore possible long-term changes in PA level, metabolic health, and health-related quality of life (HRQoL), as well as adherence to the PAP intervention over time.
Methods
Study design
A longitudinal, prospective, cohort study of PAP treatment over 5 years was carried out, mainly in the primary care setting at daily clinical healthcare centres (HCCs). The study design has been previously described in detail [30–32]. The study was conducted in accordance with the ethical principles described in the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Gothenburg, Sweden (Dnr 529–09 and Dnr 678–14).
Study population
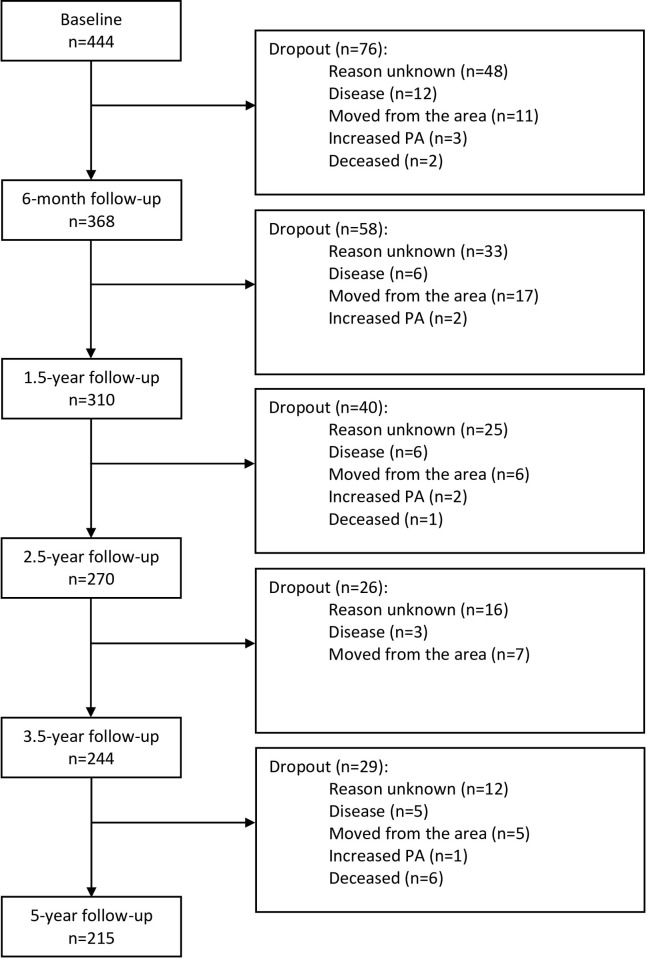
Between 2010–2014, this study included 444 patients, 56% of whom were women, aged 27–85 years (mean age 57 years), with metabolic risk factors and insufficient physical activity (<150 min/week). The patients were recruited from 15 primary healthcare centres in Gothenburg as a convenience sample, and all agreed, both orally and in writing, to participate in the PAP treatment. The 6-month follow-up was completed by 368 patients (dropout rate: 17%), 156 of whom had achieved a sufficient PA level of 150 min/week and continued ordinary PAP treatment at the HCC (>150 HCC group). At this time, the 190 patients reporting a PA level < 150 min/week were randomized to receive either continued ordinary PAP treatment at the HCC (<150 HCC group, n = 92) or PAP treatment supported by a physiotherapist (PT) (<150 PT group, n = 98). The remaining 22 patients in the study either declined the offered randomisation or lacked PA data, and continued ordinary PAP treatment at the HCC, and thus were not included in the current analysis (Fig 1).
Fig 1. Flow of patients involved in the study.
The patients were recruited from 15 health care centres.
Intervention
At the HCC, PAP treatment was offered by nurses educated on the health effects of PA and on PAP treatment. The intervention included an individualised dialogue concerning PA, an individually dosed PA recommendation with a written prescription, and an individually adjusted follow-up [30], following the concept of the Swedish PAP model [16] and the Physical Activity in the Prevention and Treatment of Disease (FYSS) reference handbook [15].
The PAP intervention offered by PTs, who were also educated in PAP treatment, included the individualised dialogue and the individual PA recommendation [31]. However, the third part (follow-up) was arranged via a fixed follow-up schedule comprising a total of eleven follow-up sessions during the intervention period: six during the first year of intervention, three during the second year, and one each at the 3-year, and 4.5-year follow-ups, respectively. Additionally, the PT group received six aerobic physical fitness tests using an ergometer bicycle during the intervention period. The results from these tests formed the basis for a continuing dialogue with the patient concerning the choice and individual dosage of PA, which was recorded in a written prescription.
Measurements
All measurements, except the physical fitness test, were performed at the sampling unit and by the ordinary nurses at the HCCs at baseline and at the 6-month, 1.5-year, 2.5-year, 3.5-year, and 5-year follow-ups.
PA level
PA was assessed using two questionnaires. First, the American College of Sports Medicine (ACSM)/American Heart Association (AHA) questionnaire was used to assess whether the patient had reached the recommended PA level of 150 min/week. Patients received 1 point if they were physically active at a moderate intensity level for 30 min per day, and 1.7 points if they were physically active at a more vigorous intensity level for 20 min per day. A weekly score of <5 points indicated an inadequate PA level, according to public health recommendations of the ACSM and AHA [33]. Secondly, the International Physical Activity Questionnaire (IPAQ) was used to score the reported duration (min) and frequency (days) of three specific types of PA performed during the past 7 days: walking, moderate-intensity activities, and vigorous-intensity activities. The results are presented as either a score from three categories—low, moderate, and high PA level—or as energy expenditure estimated as median metabolic equivalent (MET)-minutes per week, with a total MET-minutes/week (TotalMET) summarized from the three types of PA: walking, moderate-intensity activity, and vigorous-intensity activity. A summary score of <600 MET-minutes/week was considered an inadequate (low) PA level [34,35] according to the previously mentioned public health recommendations [33].
Anthropometrics
To determine BMI (kg/m2), body weight was measured with the patient wearing light clothing and no shoes, estimated to the nearest 0.1 kg (electric scale; Carl Lidén AFW D300, Jönköping, Sweden). Body height was measured with the patient in an upright position, without shoes, estimated to the nearest 0.5 cm (scale fixed to the wall; PEM 136, Hultafors, Sweden). Waist circumference (WC) was measured with the patient standing, after exhaling air from the lungs. A measuring tape (Kirchner Wilhelm, Aspberg, Germany) was placed on the patient´s skin, between the lower rib and the iliac crest, and the WC was estimated to the nearest 0.5 cm.
Blood pressure
Systolic and diastolic blood pressure (SBP, DBP) were measured (in mmHg) with the patient seated, after 5 min of rest [36]. The blood pressure sphygmomanometer (Omron HEM-907, Kyoto, Japan) was attached to the right upper arm at the level of the heart.
Blood samples
Blood samples were analysed to determine the levels of fasting plasma glucose (FPG) after an overnight fast, triglycerides (TG), total cholesterol (Chol), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), all expressed in mmol/L. The blood samples were analysed according to the European Accreditation system [37].
The cut-off values of MetS components
Cut-off values for MetS components were selected based on the National Cholesterol Education Program (NCEP) classification—WC: >88 cm for women, >102 cm for men; BP: ≥130/85 mm Hg; FPG: ≥6.1 mmol/L; TG: ≥1.7 mmol/L; and HDL: <1.3 mmol/L for women, <1.0 mmol/L for men [38].
Health-related quality of life
The Swedish version of the Short Form 36 (SF-36 Standard Swedish Version 1.0) was used to measure HRQoL [39]. The 36 questions covered eight health concepts, which were grouped to express the physical component summary (PCS) and the mental component summary (MCS). These scores were converted to a range of 0–100 points, where higher values represented a better HRQoL.
Statistical analysis
Baseline data were presented as the mean (standard deviation; SD), median (minimum-maximum; min-max), or number (percentage; %). Baseline differences between the follow-up group vs. dropout group, and between the >150 HCC group vs. <150 PT/HCC group were analysed using the independent samples t-test or Mann-Whitney U-test, based on the data level and the data distribution. For the whole group, characteristics regarding physical activity level, anthropometrics, metabolic risk factors, and health-related quality of life were presented as mean (SD) at each measurement time-point.
According to the 5-year data analysis, we estimated individual differences in the levels and rates of linear and quadratic changes in measurements of health, using latent growth curve (LGC) models with repeated measures (i.e., time) nested within individuals. Models were estimated with structural equation modelling techniques in RStudio version 1.4.1106, using the latent variable analysis (lavaan) package [40]; for more information about LGC models, see [41,42]. All models used full information maximum likelihood (FIML) estimation [43], which is robust against a missing at random missing data assumption. The FIML procedure uses all available information to compute parameters (i.e., both partially complete and fully complete cases are used in the estimation), such that cases with partially missing data on the study variables can still be used in the analysis. The time factors were specified as 1-year linear effects over the study period. Due to non-normal data distribution, TG, FPG, and TotalMet were log-transformed.
Results
Study population
Of the 444 included patients, 215 completed the 5-year follow-up, amounting to a 52% dropout rate (Fig 1).
Baseline characteristics
The baseline characteristics of the whole group have been previously described (30). In summary, the participants’ mean age was 57 years, 56% were female, and a majority had at least two components of MetS (72% WC and BP, 53%WC and TG). A majority (61%) were taking medications for metabolic risk factors, including 54% for arterial hypertension (Table 1). A majority of the patients estimated that they had a low PA level, corresponding to an average of 1–2 brisk 30-minute walks per week (Table 2).
Table 1. Baseline characteristics of the follow-up and dropout group.
| Variable | Follow-up (n = 215) |
Dropout (n = 229) |
p value |
|---|---|---|---|
| Agea–years | 56.9 (10.6) | 58.0 (11.9) | 0.328c |
| Sex b | 0.498d | ||
| Female | 118 (54.9) | 133 (58.1) | |
| Male | 97 (45.1) | 96 (41.9) | |
| Social situation b | 0.001 d | ||
| Single | 63 (30.6) | 107 (48.2) | |
| Married/cohabit | 133 (64.6) | 105 (47.3) | |
| Other | 10 (4.9) | 10 (4.5) | |
| Economic statusb–perceived | 0.063d | ||
| Good | 134 (63.8) | 115 (52.3) | |
| Neither nor | 59 (28.1) | 67 (30.5) | |
| Bad | 17 (8.1) | 38 (17.3) | |
| Education b | 0.060d | ||
| Elementary grade | 35 (16.7) | 48 (21.6) | |
| Upper secondary school | 78 (37.1) | 89 (40.1) | |
| University college | 97 (46.2) | 85 (38.3) | |
| Tobacco b | 0.857d | ||
| Smokers | 14 (6.7) | 30 (11.6) | |
| Non-smokers | 144 (68.6) | 126 (62.1) | |
| Ex-smokers | 52 (24.8) | 65 (26.3) | |
| Part of metabolic syndrome b | |||
| Overweight/Obesity | 196 (91.2) | 208 (91.2) | 0.981d |
| Hyperglycaemia | 76 (35.8) | 98 (43.9) | 0.085d |
| Hypertension | 171 (79.5) | 175 (77.4) | 0.592d |
| Hyperlipidaemia | 124 (57.9) | 129 (57.1) | 0.855d |
| Other diagnosis | |||
| Mental health, depression | 26 (12.2) | 39 (17.5) | 0.122d |
| Musculoskeletal disorders | 28 (13.1) | 49 (22.0) | 0.016 d |
| Other | 84 (39.4) | 109 (48.7) | 0.053d |
| Drug treatment b | |||
| Overweight/Obesity | 1 (0.5) | 1 (0.4) | 0.974d |
| Hyperglycemia | 20 (9.4) | 39 (17.5) | 0.014 d |
| Hypertension | 115 (54.0) | 121 (54.3) | 0.955d |
| Hyperlipidemia | 39 (18.3) | 55 (24.7) | 0.107d |
| Other drug treatment | |||
| Mental health, depression | 26 (12.2) | 38 (17.0) | 0.154d |
| Musculoskeletal disorders | 24 (11.3) | 36 (16.1) | 0.140d |
| Other | 70 (32.9) | 97 (43.5) | 0.023 d |
PT, physiotherapist; HCC, health care centre.
Data are given as a mean (standard deviation), as b number (percentage).
Difference between follow-up group and dropout group. P-value was determined by c an independent samples t-test or by d a Mann-Whitney U-test. Statistical significance was set at p ≤ 0.05.
Table 2. Baseline characteristics in physical activity, anthropometrics, metabolic risk factors, and health related quality of life for the follow-up and dropout group.
| Variable | Follow-up (n = 215) |
Dropout (n = 229) |
p value |
|---|---|---|---|
| Physical activity level | |||
| ACSM/AHA questionnairea, score | 1.8 (1.5) | 1.6 (1.5) | 0.158d |
| IPAQ 1-3b, score | 1 (1–2) | 1 (1–2) | 0.702e |
| IPAQ 1-3c, category | |||
| • Low | 108 (61.4) | 114 (63.3) | |
| • Moderate | 68 (38.6) | 66 (36.7) | |
| • High | 0 | 0 | |
| BMIa, kg/m2 | 31.7 (5.0) | 32.6 (5.5) | 0.065d |
| Waist circumferencea, cm | 107.0 (13.1) | 109.1 (13.2) | 0.109d |
| Blood pressurea, mm/Hg | |||
| Systolic | 137.6 (17.5) | 136.5 (17.9) | 0.487d |
| Diastolic | 83.4 (11.0) | 81.0 (9.2) | 0.016d |
| Metabolic componentsa, mmol/l | |||
| Fasting plasma glucose | 6.1 (1.5) | 6.4 (2.2) | 0.086d |
| Triglycerides | 1.7 (1.1) | 1.7 (0.9) | 0.588d |
| Cholesterol | 5.5 (1.1) | 5.6 (1.3) | 0.398d |
| HDL | 1.4 (0.4) | 1.4 (0.5) | 0.537d |
| LDL | 3.6 (1.0) | 3.7 (1.1) | 0.481d |
| HRQOL SF-36a, score | |||
| Physical component summary | 46.5 (9.4) | 43.4 (10.6) | 0.002d |
| Mental component summary | 43.9 (13.6) | 43.5 (13.4) | 0.726d |
PT, physiotherapist; HCC, health care centre; ACSM, American College of Sports Medicine; AHA, American Heart Association; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; HRQOL SF-36, health related quality of life 36-Item Short Form Health Survey.
Data are given as a mean (standard deviation), as b median (min-max), or as c number (percentage).
Difference between follow-up group and dropout group. P-value was determined by d an independent samples t-test or by e a Mann-Whitney U-test. Statistical significance was set at p ≤ 0.05.
In the dropout group, participants were more commonly single, and higher proportions had musculoskeletal disorders and received drug treatment for hyperglycaemia and other diagnoses (e.g., respiratory, neurological, rheumatological, and endocrine diseases) (Table 1). Compared to participants who attended the 5-year follow-up, those in the dropout group also had a lower DBP, and lower quality of life PCS-score at baseline (Table 2).
We also performed a subgroup analysis of baseline values between the non-randomised group (>150 HCC) and the randomised groups (<150 PT and <150 HCC). The results revealed few differences, except that the >150 HCC group had a higher PA level at baseline, and lower BMI and WC, as shown in the (S1 and S2 Tables).
Outcomes: 5-year intervention
Whole group analysis
The results revealed a statistically significant positive change, when analysing the whole study group, over the study period in ten of the twelve measured outcomes: TotalMET, BMI, WC, SBP, DBP, FPG, TG, Chol, HDL, and MCS (Tables 3 and 4). BMI, WC, SBP, TG, and Chol showed similar patterns of change, in which the levels significantly decreased over the study period; these decreases waned over time (Fig 2), but did not return to baseline values. DBP and FPG both exhibited significant linear declines over the study period. HDL and TotalMET showed linear increases, which also waned over time. The LDL and MCS levels significantly increased over the study period, while PCS levels decreased over the study period, a trend that accelerated over the course of the study.
Table 3. Characteristics in physical activity level, anthropometrics, metabolic risk factors and health related quality of life, for the whole study group, at each time of measurement a.
| T1–Baseline | T2–6-month | T3–1.5-year | T4–2.5-year | T5–3.5-year | T6–5-year | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | |
| TotalMET*** | 287 | 1121.6 (1348.3) | 264 | 1794.6 (1962.8) | 226 | 2190.2 (2302.3) | 191 | 1851.6 (1879.4) | 188 | 2028.9 (2242.4) | 159 | 1898.4 (1810.7) |
| BMI*** | 341 | 31.9 (5.2) | 338 | 31.6 (5.3) | 285 | 31.2 (5.3) | 250 | 31.3 (5.2) | 226 | 31.5 (5.4) | 199 | 31.5 (5.6) |
| WC*** | 339 | 107.9 (13.4) | 342 | 106.1 (14.0) | 286 | 104.7 (14.0) | 253 | 105.3 (13.4) | 227 | 105.8 (13.4) | 195 | 106.1 (14.1) |
| SBP*** | 343 | 137.3 (17.7) | 340 | 133.8 (16.3) | 288 | 131.6 (15.4) | 254 | 131.8 (15.9) | 228 | 133.5 (16.1) | 202 | 133.0 (15.5) |
| DBP** | 342 | 83.0 (10.2) | 340 | 82.6 (9.2) | 288 | 81.2 (9.3) | 254 | 81.0 (9.3) | 228 | 81.8 (9.0) | 201 | 81.1 (8.7) |
| FPG** | 341 | 6.2 (1.9) | 340 | 6.0 (1.5) | 284 | 6.1 (1.8) | 248 | 6.0 (1.7) | 224 | 5.4 (1.6) | 199 | 6.0 (1.5) |
| TG* | 343 | 1.7 (1.0) | 340 | 1.6 (0.9) | 287 | 1.5 (0.9) | 252 | 1.6 (1.1) | 227 | 1.6 (1.2) | 198 | 1.6 (0.8) |
| Chol** | 344 | 5.5 (1.2) | 341 | 5.4 (1.2) | 287 | 5.3 (1.1) | 253 | 5.2 (1.1) | 227 | 5.3 (1.3) | 199 | 6.0 (1.5) |
| HDL*** | 345 | 1.4 (0.4) | 341 | 1.4 (0.6) | 287 | 1.5 (0.5) | 252 | 1.5 (0.5) | 227 | 1.5 (0.5) | 199 | 1.5 (0.4) |
| LDL | 340 | 3.6 (1.1) | 339 | 3.5 (1.0) | 286 | 3.4 (1.0) | 252 | 3.4 (1.0) | 226 | 3.9 (1.5) | 200 | 3.4 (1.1) |
| PCS | 328 | 45.8 (9.9) | 315 | 47.0 (9.8) | 252 | 46.4 (10.2) | 230 | 45.7 (10.7) | 208 | 46.1 (10.1) | 189 | 44.4 (11.0) |
| MCS** | 328 | 44.5 (13.2) | 315 | 46.3 (11.7) | 252 | 46.8 (11.7) | 230 | 46.3 (12.3) | 208 | 47.5 (12.0) | 189 | 48.3 (11.4) |
T, time; M, mean; SD, standard deviation; MET, metabolic equivalent (minutes/week); BMI, body mass index (kg/m2); WC, waist circumference (cm); SBP, systolic blood pressure (mm/Hg); DBP, diastolic blood pressure (mm/Hg); FPG, fasting plasma glucose (mmol/L); TG, triglycerides (mmol/L); Chol, cholesterol (mmol/L); HDL, high-density lipoprotein (mmol/L); LDL, low-density lipoprotein (mmol/L); PCS, physical component summary (score); MCS, mental component summary (score).
a Data are given as mean (standard deviation).
Statistically significant positive change over the study period in ten of the twelve measured outcomes was marked in the table as: *p < .05, ** p < .01, *** p < .001.
Table 4. Changes in physical activity level, anthropometric-, metabolic characteristics and health related quality of life over the study period of 5 years a.
| A | ||||||
| TotalMET | BMI | WC | SBP | DBP | FPG | |
| β (S.E) | β (S.E) | β (S.E) | β (S.E) | β (S.E) | β (S.E) | |
| Fixed Effects | ||||||
| Level | 6.93*** (0.08) | 31.20*** (0.42) | 105.65*** (1.07) | 136.74*** (1.36) | 83.26*** (0.71) | 1.78*** (0.02) |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | -0.45*** (0.13) | 1.25 (0.67) | 3.48* (1.72) | -0.06 (2.10) | -0.31 (1.14) | 0.02 (0.03) |
| < 150 HCC | -0.46*** (0.13) | 1.31 (0.68) | 4.14* (1.76) | 1.34 (2.23) | -1.23 (1.16) | 0.03 (0.03) |
| Rate of change | 0.39*** (0.07) | -0.59*** (0.13) | -2.01*** (0.38) | -3.35*** (0.92) | -0.52** (0.18) | -0.02** (0.01) |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | -0.09 (0.12) | 0.31 (0.21) | 0.81 (0.60) | 0.06 (1.48) | -0.17 (0.29) | 0.01 (0.01) |
| < 150 HCC | -0.07 (0.02) | 0.16 (0.21) | 0.25 (0.62) | -0.66 (1.51) | 0.39 (0.30) | 0.01 (0.01) |
| Change in rate of change | -0.07*** (0.02) | 0.12*** (0.03) | 0.38*** (0.08) | 0.60*** (0.18) | - | - |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | 0.03 (0.02) | -0.09* (0.05) | -0.22 (0.13) | -0.17 (0.29) | - | - |
| < 150 HCC | 0.02 (0.02) | -0.04 (0.05) | -0.01 (0.14) | 0.08 (0.30) | - | - |
| Random Effects | ||||||
| Level | 0.26** (0.08) | 25.44*** (2.05) | 166.14*** (13.69) | 188.07*** (20.63) | 50.72*** (6.00) | 0.04*** (0.01) |
| Rate of change | 0.06 (0.07) | 0.91*** (0.21) | 7.23*** (1.76) | 23.71* (10.91) | 1.08** (0.37) | 0.01* (0.01) |
| Change in rate of change | 0.01 (0.01) | 0.06*** (0.05) | 0.35*** (0.08) | 0.67 (0.41) | - | - |
| Residuals | 0.62*** (0.04) | 1.68*** (0.09) | 14.60*** (0.77) | 118.06*** (5.99) | 47.08*** (2.06) | 0.03*** (0.01) |
| B | ||||||
| TG | Chol | HDL | LDL | PCS | MCS | |
| β (S.E) | β (S.E) | β (S.E) | β (S.E) | β (S.E) | β (S.E) | |
| Fixed Effects | ||||||
| Level | 0.34*** (0.04) | 5.57*** (0.10) | 1.42*** (0.04) | 3.59*** (0.09) | 46.75*** (0.80) | 46.17*** (0.96) |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | 0.03 (0.06) | -0.01 (0.16) | -0.02 (0.06) | 0.02 (0.13) | -2.14 (1.27) | -3.29* (1.53) |
| < 150 HCC | 0.15* (0.06) | -0.13 (0.16) | -0.09 (0.06) | -0.09 (0.14) | -0.45 (1.30) | -1.16 (1.56) |
| Rate of change | -0.05* (0.02) | -0.17** (0.06) | 0.05*** (0.02) | 0.02 (0.02) | 0.84 (0.52) | 0.80** (0.22) |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | 0.02 (0.03) | 0.05 (0.09) | 0.01 (0.03) | -0.03 (0.04) | 0.07 (0.82) | -0.21 (0.35) |
| < 150 HCC | -0.01 (0.03) | 0.04 (0.09) | 0.01 (0.03) | -0.03 (0.04) | -0.62 (0.85) | -0.21 (0.36) |
| Change in rate of change | 0.01* (0.01) | 0.02* (0.01) | -0.01** (0.01) | - | -0.30** (0.11) | - |
| Group (> 150 HCC ref.) | ||||||
| < 150 PT | -0.01 (0.01) | -0.01 (0.02) | 0.01 (0.01) | - | 0.11 (0.17) | - |
| < 150 HCC | -0.01 (0.01) | -0.01 (0.02) | -0.01 (0.01) | - | 0.17 (0.17) | - |
| Random Effects | ||||||
| Level | 0.17*** (0.02) | 1.09*** (0.12) | 0.16*** (0.01) | 0.69*** (0.09) | 64.54*** (7.60) | 104.17*** (10.79) |
| Rate of change | 0.01 (0.01) | 0.09* (0.04) | 0.01*** (0.01) | 0.02** (0.01) | 4.62 (3.41) | 1.76*** (0.52) |
| Change in rate of change | 0.01 (0.01) | 0.01 (0.01) | - | - | 0.18 (0.13) | - |
| Residuals | 0.07*** (0.01) | 0.45*** (0.02) | 0.04*** (0.01) | 0.63*** (0.03) | 35.89*** (1.96) | 58.22*** (2.69) |
MET, metabolic equivalent (minutes/week); BMI, body mass index (kg/m2); WC, waist circumference (cm); SBP, systolic blood pressure (mm/Hg); DBP, diastolic blood pressure (mm/Hg); FPG, fasting plasma glucose (mmol/L); TG, triglycerides (mmol/L); Chol, cholesterol (mmol/L); HDL, high-density lipoprotein (mmol/L); LDL, low-density lipoprotein (mmol/L); PCS, physical component summary (score); MCS, mental component summary (score).
a Univariate latent growth curve models were used as statistical method, separately for each of the measurement. Statistical significance was set at p ≤ .05: *p < .05, ** p < .01, *** p < .001.
Fig 2. Physical activity level and health outcomes over time for the whole group, and >150 HCC, <150 PT, and <150 HCC subgroups respectively a.
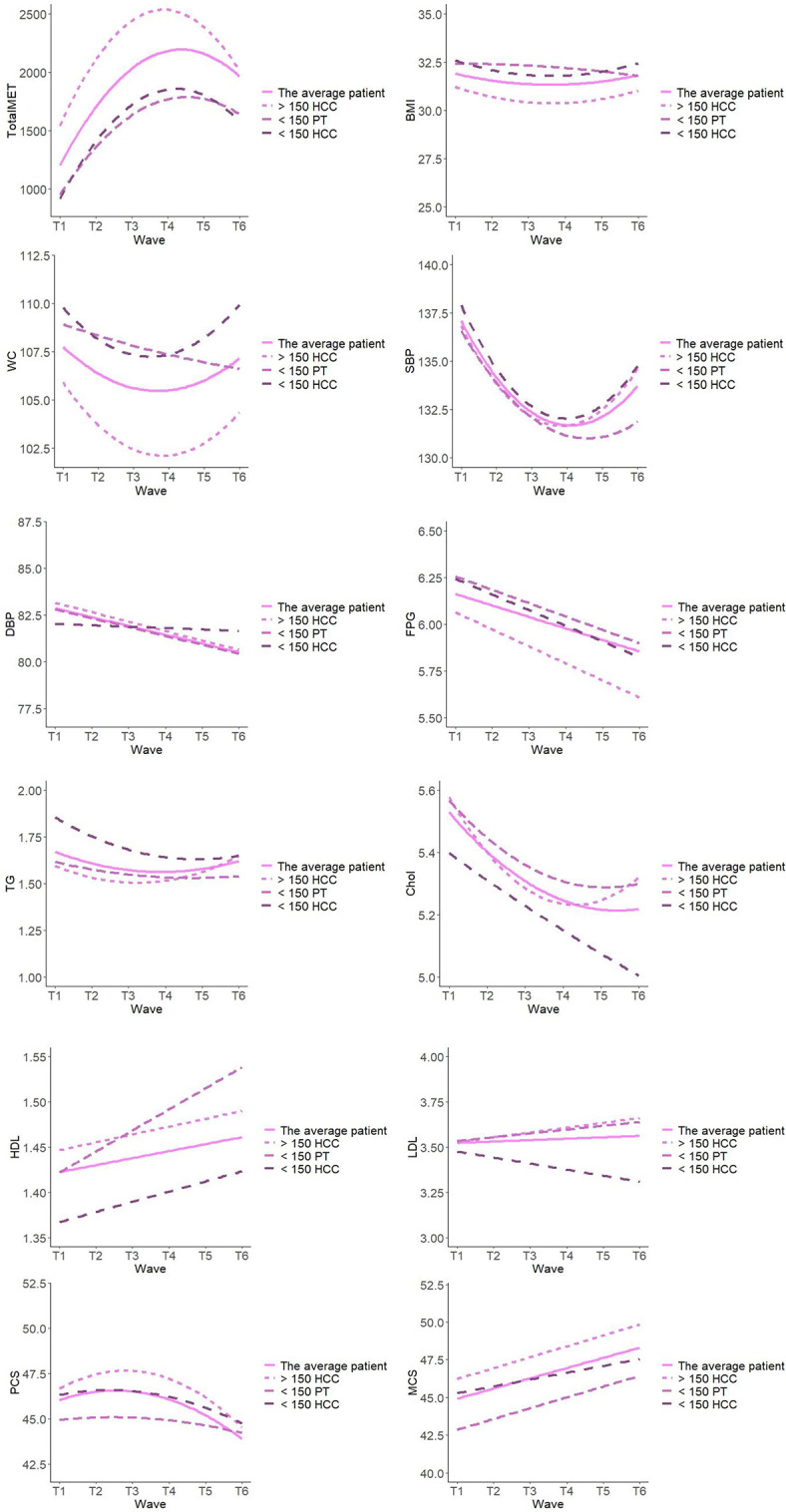
MET, metabolic equivalent (minutes/week); BMI, body mass index (kg/m2); WC, waist circumference (cm); SBP, blood pressure (mm/Hg); DBP, diastolic blood pressure (mm/Hg); FPG, fasting plasma glucose (mmol/L); TG, triglycerides (mmol/L); Chol, cholesterol (mmol/L); HDL, high-density lipoprotein (mmol/L); LDL, low-density lipoprotein (mmol/L); PCS, physical component summary (score); MCS mental component summary (score). a Analysed with univariate latent growth curve models separately for each of the measurement.
Table 3 presents the participants’ characteristics regarding physical activity level, anthropometrics, metabolic risk factors, and health-related quality of life at each follow-up time-point, for the whole study group. Table 4 presents the changes in physical activity level, anthropometric-, metabolic characteristics and health related quality of life over the study period of 5 years for the whole group and >150 HCC, <150 PT, and <150 HCC subgroups respectively. To illustrate the changes, Fig 2 shows these characteristics for the whole group and for the three subgroups respectively.
Subgroup analysis
We identified few differences among the three subgroups (>150 HCC, <150 PT, and <150 HCC), with the groups showing similar overall patterns of change. Notably, compared to the <150 PT group, the >150 HCC group had significantly lower WC levels, and higher levels of TotalMET and MCS (Table 4). Additionally, compared to the <150 HCC, the >150 HCC group exhibited significantly lower levels of WC and TG, and higher levels of TotalMET. The groups did not significantly differ in the rates of change over the study period, although it was an overall trend that the <150 PT group showed stronger increases in the level of health in several outcomes (Fig 2).
Adjustment for confounders, sex, age, civil status, economy, education, and smoking revealed few and small differences, see (S1 File). Compared to men, women had significantly lower levels of WC, TG, and FPG, and significantly higher levels of Chol and HDL. Higher age was associated with lower BMI and PCS, and with higher levels of SBP, HDL, and MCS. Gender and age had no effect on the rates of change.
Compared to participants who were in a relationship, those who were single had higher levels of FPG, but showed a greater decline during the study period. Compared to participants who had never smoked, those who had smoked but quit showed higher levels of BMI, WC, and FPG, and lower SBP. Those who smoked at the study baseline showed a steeper decline in TG, while smoking was related to a steeper decline but opposite quadratic effect in Chol (S1 File).
Discussion
The main findings of the present study were that PAP treatment was associated with long-term adherence and positive long-term effects on PA level, metabolic health, and HRQoL, at the 5-year follow-up in patients with metabolic risk factors.
Of the twelve tested outcome measures, ten showed a statistically significant positive change during the 5-year intervention, with the maximal effect seen after 3–4 years. The degree of improvement of PA was 730 MET-minutes per week (median value), corresponding to approximately 3 hours of moderate-intensity PA per week. This amount of PA is highly clinically important, in light of the global recommendations of 150–300 minutes/week (2.5–5 hours/week) of moderate-intensity PA. There is a known dose-response association between PA and outcome parameters, such as cardiovascular mortality and all-cause mortality [44,45]. A previous study showed that even light-intensity PA for 3 hours per week was associated with a 40% reduction of mortality risk in elderly men [44]. In another study, 15 min per day of moderate intensity PA was associated with a 14% reduced risk of all-cause mortality, and physically active adult men and women showed a 3-year longer life expectancy compared to inactive individuals [45]. The increased PA level in our present study corresponds to, or is greater than, the levels of change achieved in these previous studies, suggesting that the presently observed changes would have a positive effect on life expectancy.
Based on the magnitude of the observed long-term effects on metabolic risk factors, these changes are clinically significant. For example, the 5-year decrease in SBP exceeded 3 mmHg, and a meta-analysis involving one million individuals [46] demonstrated that each 2 mmHg decrease of blood pressure is associated with a 10% reduction in stroke mortality and a 7% reduction in mortality from ischaemic heart disease (IHD) or other vascular diseases. Therefore, the blood pressure decrease in our study would, hypothetically, be associated with an approximately 15% lower risk of mortality from stroke and 10% lower mortality from IHD. In addition, other significant positive changes were observed in BMI, WC, DBP, FPG, TG, Chol, and HDL (except LDL). While, small in effect sizes, they may also be important findings because the normal clinical course for this patient group involves continued deterioration of the metabolic risk profile [47–49]. Thus, the 5-year PAP intervention period yielded both a treatment effect—with improved metabolic risk factors and attenuation of expected worsening of risk factors—and a potential preventive effect against future diseases and premature death. The improved PA level for this large patient group could potentially reduce their future healthcare needs [5,50].
The blood lipid outcome measures showed positive changes in TG, Chol, and HDL at follow-up, but, somewhat paradoxically, negative changes (increased values) in LDL. Previous research has shown that HDL is the lipid fraction most sensitive to increased PA, while PA of increased intensity is typically required to reduce LDL and TG levels [7,51,52]. However, the findings of PA-induced effects on LDL are inconsistent, and are considered to be linked to variations in human weight [51]. It has also been suggested that total LDL should be analysed according to LDL subfractions. Increased PA is reportedly followed by a decrease of atherogenic small LDL particles, in combination with an increased average size of LDL particles, which may conceal positive effects. Our present study did not include subfraction analysis of LDL, and the most commonly prescribed PA was walking at a moderate intensity level. It is possible that PA of a more vigorous intensity may have further positively affected the metabolic risk factors, particularly LDL.
In terms of HRQoL, mental health (MCS) increased while physical health (PCS) decreased over the study period. A minimal clinically important difference [53] of 3–5 points has been suggested for the SF-36 assessment [54,55]. Thus, the MCS increase of 4.2 points (p < 0.001) seems clinically relevant, while the 2.4-point decrease in PCS (p < 0.001) may not be. It is possible that PCS did not increase during the 5-year intervention due to the age distribution in the group, with a mean age of 57 years at baseline, and 26% being over 65 years of age. Among older individuals, the incidence and prevalence rates of illness and disease would normally increase during a 5-year period [4,56], possibly influencing physical function and the estimated PCS.
To our knowledge, this is the first evaluation of a PAP intervention with a 5-year follow-up period. The 48% adherence rate at 5 years obviously increased the risk of selection bias. However, similar PAP studies with shorter follow-up times have shown dropout rates of between 30–38% at 6–12 months of follow-up [57–59], and 41–48% at 2 years of follow-up [60,61]. Additionally, the large LOOK-ahead study reported very low compliance even after only 2 years of the 10-year follow-up, as indicated by the fitness values and metabolic risk factors returning to normal [24]. Swedish PAP treatment is associated with a compliance rate of around 65% at 6 months [25], which is comparable to that of regular medical interventions. Given that behavioural change is very difficult to achieve, a drop-out of about 50% at 5 years can be considered quite good. It has been suggested that the individualization of the method is important for minimizing the drop-out rate. Notably, adherence may have been influenced by logistical issues during the course of our study. At the start of the study intervention, a care choice reform [62] was implemented in the region—which clearly led to increased stress among the personnel, decreased time for working with PAP, and increased staff turnover among nurses responsible for PAP. Additionally, the earmarked financial compensation to the healthcare provider for PAP treatment was removed (but still fully funded within the general compensation), which clearly decreased PAP use at the HCC´s, during the course of the study. These changes resulted in decreased expertise in handling PAP among co-workers, and disturbed follow-up routines for the patients. Both factors probably affected the patient dropout rate.
The present findings have important clinical implications. We demonstrated that PAP has long-term effects, and these results strengthen the clinical role of PAP in healthcare. The development of major risk factor outcomes followed similar patterns over time for the three intervention groups (>150 HCC, <150 PT, and <150 HCC) and for different subgroups based on sex, age, civil status, economy, education, and smoking. Although for many outcomes, the effects waned during the last two years of intervention, we observed virtually no deterioration in the general metabolic risk profile, compared to baseline. Importantly, in previous studies, patients have emphasized that long-term increases and maintenance of PA levels require individually customized PAP treatment, with support from skilled healthcare providers [19,20,63]. At the same time, healthcare providers have requested organizational support—including more interested, clear, and supportive management; and the prioritization of more resources, particularly ear-marked time for PAP treatment [64,65]. There is presently both organizational and logistic challenges to the implementation of PAP as part of regular healthcare. Importantly, interventions promoting PA for patients in health care have been shown to be cost-effective both internationally [66–68] and in Sweden [69,70] with the possibility to save costs for the health care system. The PAP intervention used in the Gothenburg PAP-study have been considered as a low budget intervention [30]. However, no cost-effectiveness analysis of the Swedish PAP has yet been carried out, but is ongoing within the framework of the Gothenburg PAP-study. Further cost-effectiveness analyses are probably of most importance to reach a full-scale implementation of Swedish PAP.
Strengths and limitations
One strength of the present study is that PAP treatment was conducted within the regular primary healthcare system, making the results more externally valid and applicable. Another strength, which reduced the risk of type I and type II errors, was the use of linear and quadratic mixed-effects models in the statistical analysis [71,72] estimated in latent growth curve models with repeated measure nested within individuals. All models used the FIML procedure, which includes cases with partially missing data for the study variables, and all available information was used to compute parameters.
This study also has some limitations. The selection bias was increased by the convenience sample recruitment of the study population, with patients more willing to change their PA level, and without data regarding how many patients meeting the same inclusion criteria were not included in the study. The drop-out rate of around 50% would also have affected the risk of selection bias, as discussed above. This study was based on regular daily clinical practice, in which the personnel offering PAP treatment to the patients had no extra resources, hampering consecutive inclusion in the study. Additionally, the person-centred PAP treatment method is determined by the patient’s attitude towards changing PA, and is considered most appropriate for patients in the contemplation or preparation stages [73].
Conclusions
To our knowledge, this study is the first to evaluate the long-term (5 years) clinical effects of a PAP intervention in primary care, in physically inactive patients with metabolic risk factors. PAP was associated with positive long-term effects regarding PA level, metabolic health outcomes, and HRQoL. The long-term adherence rate was around 50% at 5 years, which is only slightly lower than in previous 2-year follow-ups, showing that lifestyle behavioural change interventions may be effective in the long-term. Despite limitations in terms of selection, the clinical implications of the findings are important, and strengthen the clinical role of PAP in healthcare. There remains a need for further research to study how to increase adherence to individualized long-term PAP treatment.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
The authors gratefully acknowledge the PAP-responsible co-workers at Närhälsan Askim HCC, Capio HCC Axess, Carlanderska HCC, Närhälsan Frölunda HCC, Närhälsan Gibraltargatan HCC, Närhälsan Högsbo HCC, Johannesvården HCC, Närhälsan Kungshöjd HCC, Närhälsan Kungssten HCC, Närhälsan Majorna HCC, Närhälsan Masthugget HCC, Närhälsan Opaltorget HCC, Närhälsan Slottsskogen HCC, Närhälsan Styrsö HCC, Omtanken HCC, and the physiotherapists at Center for Physical Activity Gothenburg, Region Västra Götaland for skilful assistance in this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 2.World Health Organization. WHO guidelines on physical activity and sedentary behaviour 2020. [PubMed]
- 3.King AC, Whitt-Glover MC, Marquez DX, Buman MP, Napolitano MA, Jakicic J, et al. Physical Activity Promotion: Highlights from the 2018 Physical Activity Guidelines Advisory Committee Systematic Review. Medicine and science in sports and exercise. 2019;51(6):1340–53. doi: 10.1249/MSS.0000000000001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovascular diabetology. 2017;16(1):110. doi: 10.1186/s12933-017-0590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood G, Taylor E, Ng V, Murrell A, Patil A, van der Touw T, et al. Determining the effect size of aerobic exercise training on the standard lipid profile in sedentary adults with three or more metabolic syndrome factors: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2021. doi: 10.1136/bjsports-2021-103999 [DOI] [PubMed] [Google Scholar]
- 8.Ekblom Ö, Ekblom-Bak E, Rosengren A, Hallsten M, Bergström G, Börjesson M. Cardiorespiratory Fitness, Sedentary Behaviour and Physical Activity Are Independently Associated with the Metabolic Syndrome, Results from the SCAPIS Pilot Study. PLoS One. 2015;10(6). doi: 10.1371/journal.pone.0131586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. The Journal of clinical endocrinology and metabolism. 2014;99(2):462–8. doi: 10.1210/jc.2013-2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He D, Zhang X, Chen S, Dai C, Wu Q, Zhou Y, et al. Dynamic Changes of Metabolic Syndrome Alter the Risks of Cardiovascular Diseases and All-Cause Mortality: Evidence From a Prospective Cohort Study. Frontiers in cardiovascular medicine. 2021;8:706999. doi: 10.3389/fcvm.2021.706999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagliardi AR, Abdallah F, Faulkner G, Ciliska D, Hicks A. Factors contributing to the effectiveness of physical activity counselling in primary care: a realist systematic review. Patient Educ Couns. 2015;98(4):412–9. doi: 10.1016/j.pec.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 12.Love R, Adams J, van Sluijs EMF, Foster C, Humphreys D. A cumulative meta-analysis of the effects of individual physical activity interventions targeting healthy adults. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2018;19(8):1164–72. doi: 10.1111/obr.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shore CB, Hubbard G, Gorely T, Polson R, Hunter A, Galloway SD. Insufficient Reporting of Factors Associated With Exercise Referral Scheme Uptake, Attendance, and Adherence: A Systematic Review of Reviews. Journal of physical activity & health. 2019;16(8):667–76. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen J, Sørensen JB, Skovgaard T, Bredahl T, Puggaard L. Exercise on prescription: changes in physical activity and health-related quality of life in five Danish programmes. Eur J Public Health. 2011;21(1):56–62. doi: 10.1093/eurpub/ckq003 [DOI] [PubMed] [Google Scholar]
- 15.Yrkesföreningar för fysisk aktivitet (YFA). FYSS 2021: fysisk aktivitet i sjukdomsprevention och sjukdomsbehandling. [4., rev. uppl.]. Stockholm: Läkartidningen förlag AB; 2021.; 2021.
- 16.Kallings LV. The Swedish approach on physical activity on prescription. (“Implementation of physical activity in health care—facilitators and barriers” Supplement by the HPH Task Force on Health Enhancing Physical Activity in Hospitals and Health Services). Clinical Health Promotion. 2016;6(Supplement 2):31–3. [Google Scholar]
- 17.Raustorp A, Sundberg CJ. The evolution of physical activity on prescription (FaR) in Sweden. Schweizerische Zeitschrift für Sportmedizin Sporttraumatologie. 2014;62:23–5. [Google Scholar]
- 18.Onerup A, Arvidsson D, Blomqvist A, Daxberg EL, Jivegard L, Jonsdottir IH, et al. Physical activity on prescription in accordance with the Swedish model increases physical activity: a systematic review. Br J Sports Med. 2018. doi: 10.1136/bjsports-2018-099598 [DOI] [PubMed] [Google Scholar]
- 19.Andersen P, Lendahls L, Holmberg S, Nilsen P. Patients’ experiences of physical activity on prescription with access to counsellors in routine care: a qualitative study in Sweden. BMC public health. 2019;19(1):210. doi: 10.1186/s12889-019-6535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joelsson M, Lundqvist S, Larsson MEH. Tailored physical activity on prescription with follow-ups improved motivation and physical activity levels. A qualitative study of a 5-year Swedish primary care intervention. Scandinavian journal of primary health care. 2020;38(4):399–410. doi: 10.1080/02813432.2020.1842965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert FA, Malau-Aduli AEO, Crowe MJ, Malau-Aduli BS. Australian patients’ perception of the efficacy of the physical activity referral scheme (PARS). Patient Educ Couns. 2021;104(11):2803–13. doi: 10.1016/j.pec.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Hanson CL, Neubeck L, Kyle RG, Brown N, Gallagher R, Clark RA, et al. Gender Differences in Uptake, Adherence and Experiences: A Longitudinal, Mixed-Methods Study of a Physical Activity Referral Scheme in Scotland, UK. International journal of environmental research and public health. 2021;18(4). doi: 10.3390/ijerph18041700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arsenijevic J, Groot W. Physical activity on prescription schemes (PARS): do programme characteristics influence effectiveness? Results of a systematic review and meta-analyses. BMJ open. 2017;7(2):e012156. doi: 10.1136/bmjopen-2016-012156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallings LV, Leijon ME, Kowalski J, Hellenius ML, Stahle A. Self-reported adherence: a method for evaluating prescribed physical activity in primary health care patients. Journal of physical activity & health. 2009;6(4):483–92. doi: 10.1123/jpah.6.4.483 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization; 2003. [cited 2020 Sep 11]. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. [Google Scholar]
- 27.Sallis R, Franklin B, Joy L, Ross R, Sabgir D, Stone J. Strategies for promoting physical activity in clinical practice. Progress in cardiovascular diseases. 2015;57(4):375–86. doi: 10.1016/j.pcad.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Borjesson M. [Health care services can boost physical activity on prescription—more people need prescriptions]. Lakartidningen. 2012;109(51–52):2340. [PubMed] [Google Scholar]
- 29.Kallings LV. [Physical activity on prescription—an underutilized resource. Statistics on prescription shows large variations between counties]. Lakartidningen. 2012;109(51–52):2348–50. [PubMed] [Google Scholar]
- 30.Lundqvist S, Borjesson M, Larsson ME, Hagberg L, Cider A. Physical Activity on Prescription (PAP), in patients with metabolic risk factors. A 6-month follow-up study in primary health care. PLoS One. 2017;12(4):e0175190. doi: 10.1371/journal.pone.0175190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundqvist S, Börjesson M, Cider Å, Hagberg L, Ottehall CB, Sjöström J, et al. Long-term physical activity on prescription intervention for patients with insufficient physical activity level-a randomized controlled trial. Trials. 2020;21(1):793. doi: 10.1186/s13063-020-04727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundqvist S, Börjesson M, Larsson MEH, Cider Å, Hagberg L. Which patients benefit from physical activity on prescription (PAP)? A prospective observational analysis of factors that predict increased physical activity. BMC public health. 2019;19(1):482-. doi: 10.1186/s12889-019-6830-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and science in sports and exercise. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- 34.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 35.Ekelund U, Sepp H, Brage S, Becker W, Jakes R, Hennings M, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public health nutrition. 2006;9(2):258–65. doi: 10.1079/phn2005840 [DOI] [PubMed] [Google Scholar]
- 36.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21(5):821–48. doi: 10.1097/00004872-200305000-00001 [DOI] [PubMed] [Google Scholar]
- 37.European co-operation for Accreditation (E A). 2020 [cited 2020 Sep 11]. Available from: http://www.european-accreditation.org/.
- 38.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 39.Sullivan M, Karlsson J, Ware JE, Jr. The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–58. doi: 10.1016/0277-9536(95)00125-q [DOI] [PubMed] [Google Scholar]
- 40.Rossel Y. Lavaan: na R package for structural equation modeling. Jounal of Statistical Sofware, 48 (2), 1–36. 2012. [Google Scholar]
- 41.Curran PJ, Muthén BO. The application of latent curve analysis to testing developmental theories in intervention research. American journal of community psychology. 1999;27(4):567–95. doi: 10.1023/A:1022137429115 [DOI] [PubMed] [Google Scholar]
- 42.McArdle JJ, Epstein D. Latent growth curves within developmental structural equation models. Child development. 1987;58(1):110–33. [PubMed] [Google Scholar]
- 43.Enders CK. Analyzing structural equation models with missing data. Structural equation modeling: A second course. 2006;2:493–519. [Google Scholar]
- 44.Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015;49(11):743–8. doi: 10.1136/bjsports-2014-094522 [DOI] [PubMed] [Google Scholar]
- 45.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. doi: 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 46.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 47.Eshtiaghi R, Keihani S, Hosseinpanah F, Barzin M, Azizi F. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: the Tehran Lipid and Glucose Study. International journal of obesity (2005). 2015;39(3):514–9. doi: 10.1038/ijo.2014.176 [DOI] [PubMed] [Google Scholar]
- 48.Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, Toledo E, Moreno-Iribas C. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovascular diabetology. 2020;19(1):195. doi: 10.1186/s12933-020-01166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalsen VL, Wild SH, Kvaløy K, Svartberg J, Melhus M, Broderstad AR. Obesity measures, metabolic health and their association with 15-year all-cause and cardiovascular mortality in the SAMINOR 1 Survey: a population-based cohort study. BMC cardiovascular disorders. 2021;21(1):510. doi: 10.1186/s12872-021-02288-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Global Health Estimates 2016: Death by Cause, Age, Sex, by Country and by Region, 2000–2016 Geneva: World Health Organization; 2018 [cited 2020 July 20]. Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/.
- 51.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids in health and disease. 2017;16(1):132. doi: 10.1186/s12944-017-0515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albarrati AM, Alghamdi MSM, Nazer RI, Alkorashy MM, Alshowier N, Gale N. Effectiveness of Low to Moderate Physical Exercise Training on the Level of Low-Density Lipoproteins: A Systematic Review. BioMed research international. 2018;2018:5982980. doi: 10.1155/2018/5982980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook CE. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. The Journal of manual & manipulative therapy. 2008;16(4):E82–3. doi: 10.1179/jmt.2008.16.4.82E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18(5):419–23. doi: 10.2165/00019053-200018050-00001 [DOI] [PubMed] [Google Scholar]
- 55.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures. Pharmacoeconomics. 1999;15(2):141–55. [DOI] [PubMed] [Google Scholar]
- 56.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen P, Holmberg S, Årestedt K, Lendahls L, Nilsen P. Physical Activity on Prescription in Routine Health Care: 1-Year Follow-Up of Patients with and without Counsellor Support. International journal of environmental research and public health. 2020;17(16). doi: 10.3390/ijerph17165679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kallings LV, Leijon M, Hellenius ML, Stahle A. Physical activity on prescription in primary health care: a follow-up of physical activity level and quality of life. Scand J Med Sci Sports. 2008;18(2):154–61. doi: 10.1111/j.1600-0838.2007.00678.x [DOI] [PubMed] [Google Scholar]
- 59.Morén C, Welmer A-K, Hagströmer M, Karlsson E, Sommerfeld DK. The Effects of "Physical Activity on Prescription" in Persons With Transient Ischemic Attack: A Randomized Controlled Study. J Neurol Phys Ther. 2016;40(3):176–83. doi: 10.1097/NPT.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 60.Anderson RT, King A, Stewart AL, Camacho F, Rejeski WJ. Physical activity counseling in primary care and patient well-being: Do patients benefit? Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2005;30(2):146–54. doi: 10.1207/s15324796abm3002_7 [DOI] [PubMed] [Google Scholar]
- 61.Rodjer L, I HJ, Borjesson M. Physical activity on prescription (PAP): self-reported physical activity and quality of life in a Swedish primary care population, 2-year follow-up. Scandinavian journal of primary health care. 2016;34(4):443–52. doi: 10.1080/02813432.2016.1253820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Västra Götalandsregionen. Vårdval Vårdcentral. Vårdgivarwebben: Västra Götalandsregionen; 2020 [cited 2020 Aug 16]. Available from: https://www.vgregion.se/halsa-och-vard/vardgivarwebben/uppdrag-och-avtal/vardval-vardcentral/.
- 63.Joelsson M, Bernhardsson S, Larsson ME. Patients with chronic pain may need extra support when prescribed physical activity in primary care: a qualitative study. Scandinavian journal of primary health care. 2017;35(1):64–74. doi: 10.1080/02813432.2017.1288815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bohman DM, Mattsson L, Borglin G. Primary healthcare nurses’ experiences of physical activity referrals: an interview study. Prim Health Care Res Dev. 2015;16(3):270–80. doi: 10.1017/S1463423614000267 [DOI] [PubMed] [Google Scholar]
- 65.Gustavsson C, Nordqvist M, Broms K, Jerden L, Kallings LV, Wallin L. What is required to facilitate implementation of Swedish physical activity on prescription?—interview study with primary healthcare staff and management. BMC health services research. 2018;18(1):196. doi: 10.1186/s12913-018-3021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrett S, Elley CR, Rose SB, O’Dea D, Lawton BA, Dowell AC. Are physical activity interventions in primary care and the community cost-effective? A systematic review of the evidence. The British journal of general practice: the journal of the Royal College of General Practitioners. 2011;61(584):e125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gc V, Wilson ECF, Suhrcke M, Hardeman W, Sutton S, Team VBIP. Are brief interventions to increase physical activity cost-effective? A systematic review. British journal of sports medicine. 2016;50(7):408–17. doi: 10.1136/bjsports-2015-094655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oldridge N, Taylor RS. Cost-effectiveness of exercise therapy in patients with coronary heart disease, chronic heart failure and associated risk factors: A systematic review of economic evaluations of randomized clinical trials. European journal of preventive cardiology. 2020;27(10):1045–55. doi: 10.1177/2047487319881839 [DOI] [PubMed] [Google Scholar]
- 69.Eriksson MK, Hagberg L, Lindholm L, Malmgren-Olsson EB, Osterlind J, Eliasson M. Quality of life and cost-effectiveness of a 3-year trial of lifestyle intervention in primary health care. Arch Intern Med. 2010;170(16):1470–9. doi: 10.1001/archinternmed.2010.301 [DOI] [PubMed] [Google Scholar]
- 70.Feldman I, Hellstrom L, Johansson P. Heterogeneity in cost-effectiveness of lifestyle counseling for metabolic syndrome risk groups -primary care patients in Sweden. Cost effectiveness and resource allocation: C/E. 2013;11(1):19. doi: 10.1186/1478-7547-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brauer M, Curtin JJ. Linear mixed-effects models and the analysis of nonindependent data: A unified framework to analyze categorical and continuous independent variables that vary within-subjects and/or within-items. Psychol Methods. 2018;23(3):389–411. doi: 10.1037/met0000159 [DOI] [PubMed] [Google Scholar]
- 72.Maurissen JP, Vidmar TJ. Repeated-measure analyses: Which one? A survey of statistical models and recommendations for reporting. Neurotoxicol Teratol. 2017;59:78–84. doi: 10.1016/j.ntt.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 73.Prochaska JO. Decision making in the transtheoretical model of behavior change. Medical decision making: an international journal of the Society for Medical Decision Making. 2008;28(6):845–9. doi: 10.1177/0272989X08327068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.