Abstract
A hallmark of adaptive behavior is the ability to flexibly respond to sensory cues, depending on context and experience. To understand how neural circuits implement this flexibility, it is critical to resolve how a static anatomical connectome can be modulated such that functional connectivity in the network can be dynamically regulated. Here, we review recent work in the roundworm Caenorhabditis elegans that is clarifying how a defined connectome can be modulated in such a manner. Recent EM studies have mapped the anatomical connectomes of many C. elegans animals, highlighting the level of stereotypy in the anatomical network. Brain-wide calcium imaging and focused studies of specified neural circuits in C. elegans have uncovered a high degree of flexibility in the functional coupling of neurons within circuits. The coupling between neurons is controlled in large part by neuromodulatory systems that act over long time scales. This then gives rise to persistent behavioral states that animals switch between, allowing them to generate adaptive behavioral responses across a range of environmental conditions. Thus, the dynamic coupling of neurons enables multiple behavioral states to be encoded in a physically stereotyped connectome.
Introduction
As animals navigate their environments, they are bombarded by a constant stream of diverse sensory cues. It is therefore essential that their nervous systems extract behaviorally meaningful information from the environment and generate adaptive responses to these stimuli. Understanding how sensorimotor circuits are able to flexibly process sensory cues, depending on context and experience, has been the subject of intense investigation in recent decades. A key issue in understanding these circuits will be to resolve how neuronal activity in a defined anatomical connectome can be modulated over short and long timescales to allow for dynamic sensorimotor integration.
Studies of mammalian internal states and cognitive control have revealed the broad organization of neural circuits that underlie flexible sensorimotor processing. Behavioral responses to sensory cues are profoundly influenced by an animal’s internal state – for example, whether it is awake, asleep, hungry, or thirsty. The ascending neuromodulatory systems, including the noradrenergic and cholinergic systems, play critical roles in generating these states. Correspondingly, many of these neuromodulators have been shown to impact sensory responses in higher brain regions that receive modulatory afferents1–3. Sensory processing is also flexible over faster timescales, for example in the context of top-down (or “executive”) control over decision-making. Studies of these higher cognitive functions have shown that neurons in specific cortical areas, most prominently areas in prefrontal cortex, often represent task-relevant variables, rather than faithfully encoding sensory stimuli, like neurons in primary sensory cortical areas4,5. These representations can rapidly change depending on the demands of the task and provide a way to link behaviorally relevant inputs to sensorimotor transformations elsewhere. These studies continue to provide important insights into the flexible neural dynamics that underlie sensory processing, but due to the complexity of mammalian circuits, they have not yet established clear links between the physical connectivity of neural circuits and flexible changes in neural circuit function and behavior.
In recent years, the nematode Caenorhabditis elegans has emerged as a popular system for the investigation of flexible sensory processing and behavior. Studies of C. elegans benefit from its experimental tractability: its nervous system contains exactly 302* neurons connected through a fully-defined connectome6. Moreover, a robust genetic toolset enables neural circuit analysis with single-cell precision in this simple nervous system. While early studies of C. elegans behavior focused on the neural circuits underlying hard-wired, innate behaviors, subsequent work revealed a surprising degree of flexibility in how this animal responds to sensory cues in its environment. Modern systems neuroscience tools applied in this simple system have now begun to reveal the basis of this flexibility. Here, we review this recent progress and provide an outlook on what remains to be discovered in this small, flexible nervous system.
Dynamic Neuronal Activity Occurs in a Largely Invariant Physical Network
While the invariance of neuron identity in C. elegans is well-established, evidence that this extends to synaptic stereotypy has only recently been addressed. Of the 302* neurons identified in the hermaphrodite, 294 are shared between hermaphrodites and males, with males having an additional 91 neurons and 39 muscles, largely devoted to male mating behaviors7. Circuits devoted to shared behaviors, like locomotion and chemotaxis, are largely conserved, although there are differences in how male-specific neurons synapse onto these shared networks.
The architecture of the C. elegans network has been carefully analyzed. The distributions of the neurons’ degrees of connectivity in the electrical and chemical synaptic networks are fairly scale-free, with tails that follow power-law distributions8. This means that both networks have “small world” properties like other scale-free networks, such as social networks and the worldwide web, where a small number of nodes serve as “hubs” for the majority of information traffic. The neurons themselves can be roughly divided into three categories: sensory neurons, interneurons, and motor neurons. On average, the minimum number of chemical synapses crossed from sensory to motor neurons is roughly 3. This means that, on average, the shortest path from sensory neuron to motor neuron will pass through two interneurons. Within the interneuron class are a small number of hub interneurons called the “command neurons” that possess the most synapses in the network, and that form their own, highly interconnected network that controls forward and reverse movement9–11. These neurons are pre-synaptic to body motor neurons, and post-synaptic to a large class of interneurons that are themselves post-synaptic to sensory neurons (Figure 1A).
Figure 1: A physically invariant network with dynamic activity.
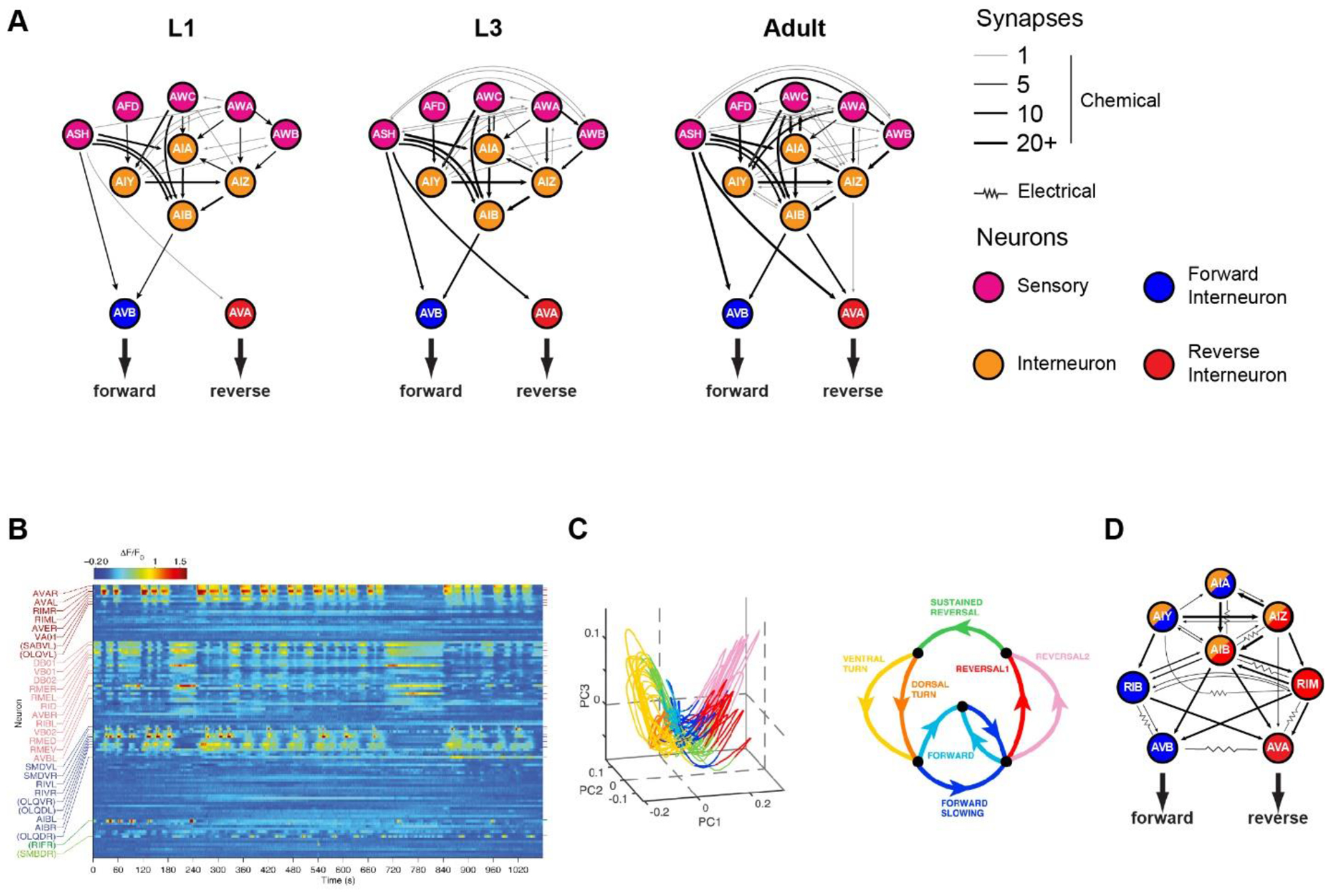
A. Sensory circuit at 3 different developmental stages. Circles represent neurons, while lines represent chemical synapses. Only chemical synapses were mapped in Witvliet et al. B. Heatmap of neuronal activity measured by NLS-GCaMP5k fluorescence. Image is reproduced from Kato et al. C. A large fraction of neuronal activity from B represents the motor program. Most dynamics lie along a manifold in the first 3 principal components from PCA dimension reduction of neuronal activity (left panel). Manifold is colored according to the motor phase (right panel). Images are reproduced from Kato et al. D. Interneuron circuit. Certain neurons like AVB and RIB strongly correlate with forward behavior while neurons like AVA and RIM strongly correlate with reversal behavior. However, other interneurons like AIA, AIB, AIY and AIZ transiently correlate with motor state.
An analysis of volumetric reconstructions across distinct hermaphrodite animals found that half of the membrane contacts are largely conserved across several length scales of organization12,13. The brain’s physical structure follows a conserved developmental program that imposes physical constraints on synaptic placement, restricting the potential connectivity of neurons to local neighborhoods. The shape and relative position of neurites are largely conserved at birth, and synapse number grows in proportion to the body14. Most membrane contacts are stable during development, with most additional synapses strengthening existing connections, and new connections largely occurring at existing large contact areas (Figure 1A). Motor networks in particular are highly stereotyped, likely reflecting the importance of motor fidelity. However, modulatory neurons, which rely less on synaptic signaling, are the least precise in synaptic loci and number. Like the stereotypy of cellular identity, the physical geometry and contacts of neurons across the nervous system are largely conserved, with most individual variability occurring at sparse synapse loci.
Two important features of brain organization are absent from physical connectivity maps. First, the signs and strengths of the synaptic connections cannot be inferred from EM images. Second, the physical network provides no information about extra-synaptic communication. Neuromodulatory networks can be built by pairing known cellular expression of neuromodulators with that of their target receptors15. While the identities of neuromodulatory neurons and their targets are known in some cases, the receptors for most neuropeptides remain unknown and the list of C. elegans neuromodulators keeps expanding, including the recent addition of the IL-17 cytokine16. Of the neuromodulatory networks that have been mapped, these networks do not overlap well with the chemical and electrical synaptic networks. For example, most neurons that express serotonin receptors do not share synapses with serotonin-producing neurons. “Hub” neurons in the chemical and synaptic networks are not necessarily hub neurons in neuromodulatory networks. However, a small set of neurons like RIM appear to be “hub” neurons in all three networks15.
It is not possible to determine the flow of information through a nervous system based solely on physical synaptic connectivity. However, large-scale calcium imaging approaches are beginning to reveal the flow of information in the C. elegans network. These studies have utilized a nuclear-localized GCaMP that allows densely-packed neurons to be easily segmented. These recordings cannot recover local calcium signals in neurites17 and lack the temporal resolution of voltage recordings, but have still provided important insights into C. elegans brain-wide dynamics. Brain-wide imaging of most anterior neurons in restrained worms has revealed a high degree of spontaneous correlated activity across the brain that is altered in response to sensory stimuli18. Based on comparisons to neural activity in moving animals, most of this ongoing activity seems to correlate with motor patterns of the worm, which can largely be represented as a two- lobed manifold by three principal components of neuron population activities (Figure 1B–D)19. Different regions of this manifold represent different action sequences. Indeed, brain-wide imaging in freely-moving worms has similarly revealed large subsets of neurons associated with different action sequences20,21.
However, even though large numbers of neurons correlate with the motor circuit, the neuronal composition of these correlated states is not conserved in different contexts, even within the same worm22. Neurons such as AVA which directly synapse onto motor neurons consistently correlate with motor patterns, while other neurons only occasionally correlate with specific instances of these features. Thus, motor features are more reliably encoded by a more distributive linear combination of sparse neuronal activities. Comparisons between worms have been hampered by the challenge of reliable neuron identification. Recent combinatorial fluorophore labelling of neurons has greatly improved this annotation, and improved the comparison of brain-wide dynamics across worms23. Brain-wide imaging combined with neural identification revealed that different sensory contexts in restrained worms also produce different combinations of correlated network states (Figure 2A). These datasets also allowed for a direct comparison between physical connectivity and functional connectivity. Strikingly, when compared to the chemical and electrical network, functional relationships between neurons have very little correlation with physical connectivity (Figure 2B). This is also observed in neuronal circuits devoted to male mating, where functional correlations between neurons are not fixed, but dependent on sensory inputs and motor state24.
Figure 2: Sensory and neuromodulatory context influence interneuron correlation with motor program.
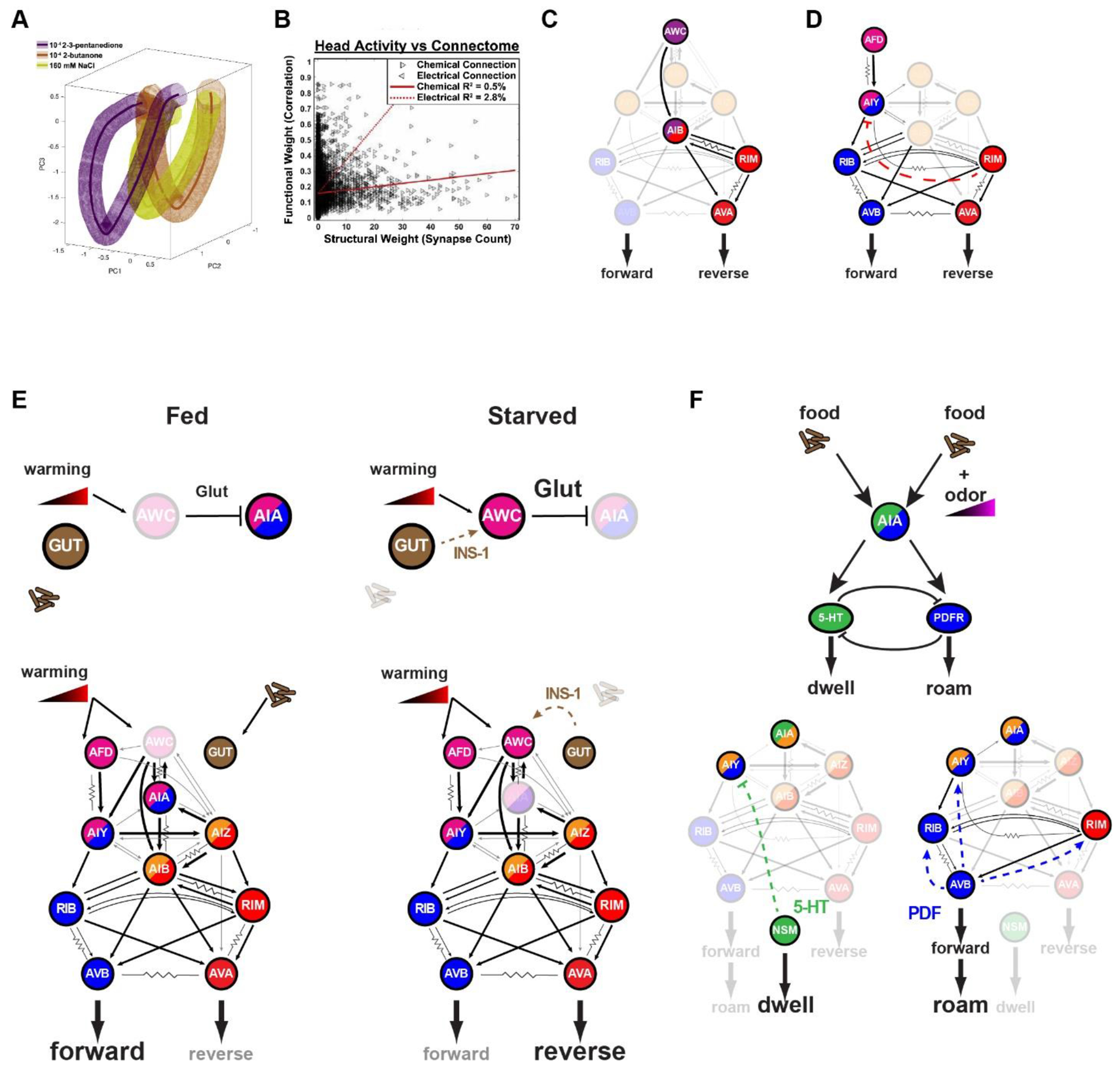
A. The path global neuronal dynamics take through PCA-reduced space is strongly influenced by sensory context. Each colored volume represents the average phase trajectory of whole-brain neuronal activity during sensory stimulation with 2-3 pentanedione (purple), 2-butanone (orange), or NaCl (yellow). Image is reproduced from Yemini et al. B. Neuronal activity correlates poorly with chemical and electrical synapse connectivity. Image is reproduced from Yemini et al. C. The AIB interneuron exhibits activity that correlates with sensory-induced activity from AWC, and/or reverse motor activity mediated by synaptic input from RIM. D. The AIY interneuron exhibits activity that correlates with sensory-induced activity from AFD, and/or forward motor activity mediated by neuromodulatory inhibition from RIM. E. AIA drives dwelling in the presence of food and the absence of odor gradients. Its activity is tightly coupled to the serotonergic neuron NSM which inhibits AIY and other MOD-1 expressing neurons. However, in the presence of odor gradients while on food, AIA drives roaming behavior. In this context, AIA activity is tightly coupled to forward interneuron activity. F. AIA activity is strongly influenced by both sensory and food context. Under well-fed conditions, AWC activity does not correlate with warming gradients, and in turn does not inhibit AIA activity via glutamate. Under starved conditions, AWC is sensitized to warm gradients via INS-1 signaling from the gut. Increased AWC activity silences AIA, which in turn decreases forward drive.
Even though a detailed map of the physical neuronal network has revealed a structure that is largely invariant over time and between individuals, whole-brain imaging has revealed a dynamically evolving functional network that is highly divergent in different sensory and motor contexts. Like changing traffic patterns on a static road-network, how information flows through the network largely depends on constantly evolving functional relationships between neurons.
Interneuron Coupling is Influenced by Neuromodulatory Signaling
Interneurons in particular appear to be more plastic in their functional coupling with other neurons. While sensory neurons have fairly stereotyped responses to sensory cues, and motor neurons reliably produce behavioral responses, interneurons integrate information from a diversity of presynaptic partners with varying degrees of correlation. AIB is an interneuron important for relaying sensory information from the olfactory neuron AWC to the motor circuit (Figure 2C). On average, the activity of AIB correlates with sensory input, but most of its activity is driven by the motor circuit through its synapses with the interneuron RIM. In the absence of RIM activity, AIB activity is more strongly correlated with sensory input and, in turn, causes the motor circuit to be more strongly coupled to AWC25. The role of RIM in maintaining motor states that influence sensory perception also occurs in AIY, another interneuron which encodes both thermosensory information from AFD and the motor state of the animal (Figure 2D). Here too, RIM maintains the motor drive by providing motor feedback to AIY, which in turn influences sensory perception. This feedback maintains the motor state of the animal, and makes it less susceptible to transient temperature fluctuations26. However, unlike AIB, RIM inhibits AIY through neuromodulatory signaling. In addition to being a hub of the physical synaptic network, RIM is also a hub of the neuromodulatory network15, and likely plays a dual role in coordinating the activity of numerous neurons synaptically (like AIB) and extra-synaptically (like AIY). Thus, RIM may play a pivotal role in coordinating dynamics across the network of forward- and reverse-active neurons.
A more explicit role of the influence of neuromodulatory state on interneuron coupling can be observed with the interneuron AIA. Most of its presynaptic partners are sensory neurons, and its activity is strongly driven by coincident activities of these neurons27. However, the functional coupling of AIA to these neurons is highly dynamic. Feeding state does not alter the AFD-AIY thermotaxis circuit, but it does alter temperature-mediated behavior (Figure 2E)28. Under fed conditions, AIA has tonic activity that is permissive for AFD-AIY driven thermotaxis. Even though AFD is the primary thermosensor, other chemosensory neurons like AWC can become temperature-responsive under starved conditions due to INS-1 insulin-like peptide signaling from the gut. The increase in temperature-mediated AWC activity increases glutamate-mediated inhibition of AIA, which in turn influences behavior by causing a decrease in forward runs. Thus, non-neuronal tissues like the gut can strongly influence neuronal couplings, and in turn, behavior. This likely evolved to alter the relative saliency of sensory cues based on food availability, a critical contextual cue for the animal29. Under starved conditions, chemotaxis toward food takes precedence over thermotaxis toward optimal temperatures. Given the widespread neuronal expression of the DAF-2 insulin receptor, it is possible that there may be similar state-dependent changes in other components of the sensory circuits.
In addition to neuromodulatory signaling from the gut to the brain that influences behavior, olfactory perception by the brain also regulates gut metabolism. Odor detection by sensory neurons leads to decreased octopamine release by the neuron RIC, which in turn leads to a decrease in AMPK activity in the gut30. Octopamine is the invertebrate analog of norepinephrine, and AMPK is a key metabolic regulator which activates glycolysis and fatty-acid metabolism. This study and others31,32 highlight how communication along the gut-brain axis is bidirectional: neuromodulatory signaling from the gut influences behavior, but neuromodulators released by the brain influence gut metabolism.
The dual roles of chemical synapses and neuromodulation on functional connectivity can also be seen in the timescales of neuronal coupling. When animals are actively moving in the absence of food, AIA has transient activity that is positively correlated with the forward state, which is consistent with its role in driving forward behavior in response to attractive sensory input. However, during dwelling states on food, AIA activity is more sustained and correlated with the dwelling-promoting serotonergic neuron NSM (Figure 2F)33. Alternatively, odor gradients in the presence of food can trigger AIA to promote roaming, and return to being highly correlated with the forward motor circuit. The ability of AIA to exert opposing effects on behavior is enabled by its dual excitatory outputs to serotonin and PDF neurons that mutually inhibit one another to promote dwelling and roaming states, respectively (see below for further details). Thus, even though AIA is activated by food odors in both states, its influence on speed (fast timescale) is influenced by the roaming/dwelling state of the animal (slow timescale). This change in neuronal coupling reflects the changing priorities of the animals in different behavioral states: food odors during roaming provide a cue for navigation to a distal target, whereas food odors during dwelling and food ingestion indicate that the animal is already in a plentiful food resource. These studies reveal that despite a largely invariant physical network, the functional network is dynamic, and influenced by extra-synaptic signaling that alters how interneurons couple to both sensory and motor circuits.
The Internal States of the C. elegans Nervous System
The internal state of an animal’s nervous system can robustly alter sensorimotor processing and behavior. Although “internal state” is a somewhat loosely-defined term, here we define an internal state to be a persistent change in the function of the nervous system whose effects span multiple sensory modalities and/or motor systems. C. elegans exhibits a range of distinct internal states that influence functional circuit connectivity and sensorimotor transformations, such as sleep, hunger, and exploration34. We now review the neural mechanisms that generate these states.
The clearest example of an internal state that alters sensorimotor behavior in C. elegans is the sleep state. Similar to mammals, sleep in C. elegans can be defined based on behavioral criteria: a cessation of movement and an increased arousal threshold, which is rapidly reversible and exhibits homeostatic buildup35,36 Unlike mammalian systems, there is not yet a widely-established neurophysiological definition of C. elegans sleep, though recent brain-wide recordings suggest that sleep is correlated with a broad inhibition of the brain-wide neural dynamics described above37. The increased arousal threshold appears to be due to both reduced responsiveness of sensory neurons and alterations in downstream sensory processing circuits38. C. elegans display sleep states primarily in two contexts (Figure 3A). First, they display developmentally-timed sleep (DTS) during each of their four larval molts35. Second, they display sickness-induced sleep (SIS) after exposure to robust stressors, such as potentially lethal levels of heat or infection39,40. The neural control of sleep involves command neurons that act through neuropeptide release to modulate downstream circuits. DTS is associated with activation of the RIS neuron, whose release of FLP-11 neuropeptides induces behavioral quiescence41. In addition, PDF-1/2 and FLP-2 neuropeptides produced by sensory neurons modulate DTS42. On the other hand, SIS is controlled primarily by the ALA neuron that releases several neuropeptides43,44. The ALA-secreted neuropeptides have unique but overlapping functions in inhibiting specific motor programs of the worm, suggesting combinatorial control43. The control of C. elegans sleep by neuropeptides is reflective of the broadly important role that neuropeptide signaling plays in organizing C. elegans neural dynamics and behavior.
Figure 3: Neural Circuits for Behavioral State Control in C. elegans.
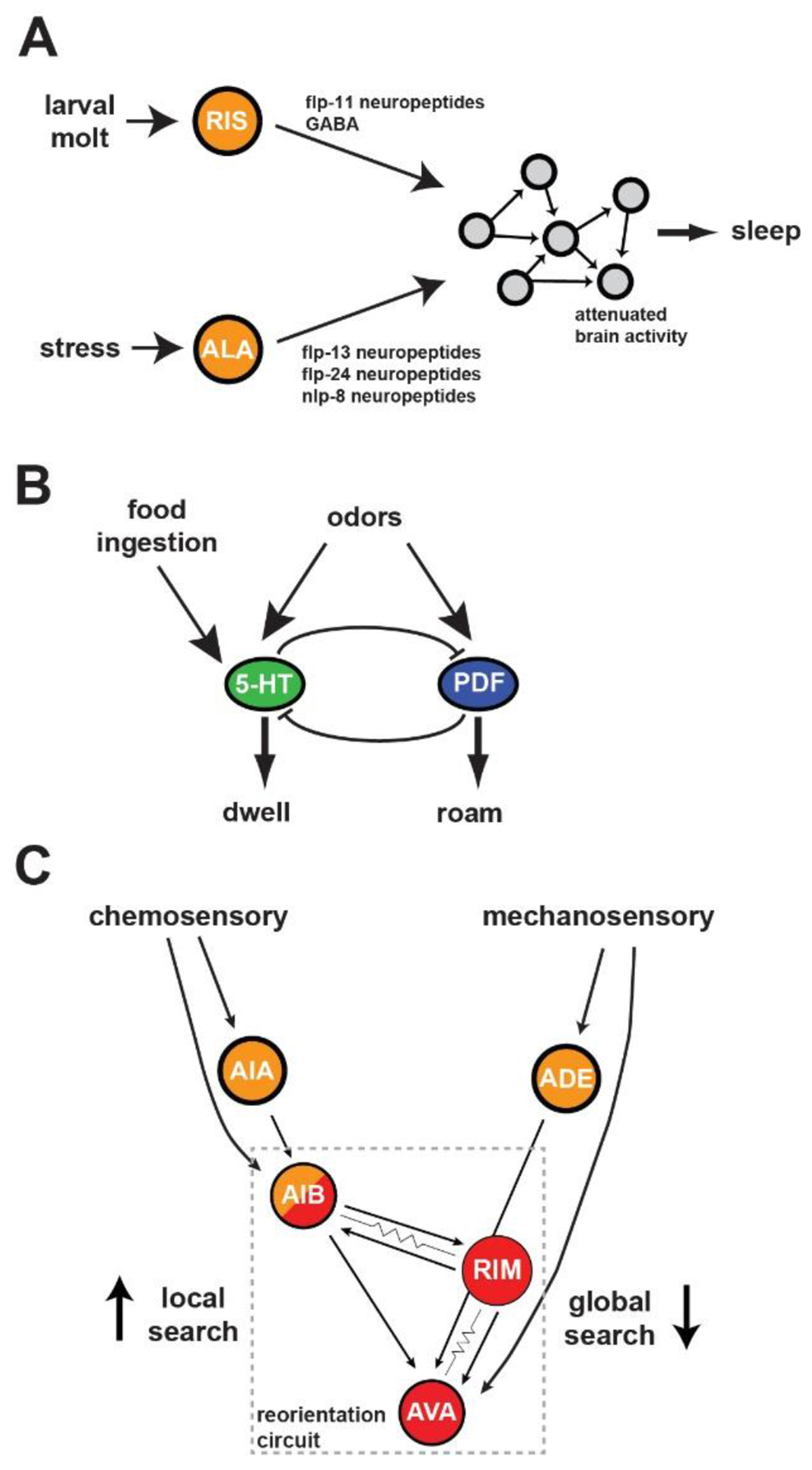
A. The RIS interneuron acts as a command neuron to control developmentally timed sleep, whereas the ALA interneuron controls stress-induced sleep. Each releases distinct neuropeptides that have causal roles in inhibiting the main C. elegans motor programs and thereby inducing sleep. B. Mutual inhibition between the neurons that produce the opposing 5-HT and PDF neuromodulators underlies bi-stable switching between roaming and dwelling behavioral states. Food ingestion activates the serotonergic system to increase dwelling, while both neuromodulatory systems receive feedforward inputs from the chemosensory system. C. Chemosensory and mechanosensory cues are detected by different sets of sensory neurons that feed into a reorientation circuit. Immediately after food removal, the sensory neurons are spontaneously active and strongly coupled to the reorientation circuit. As animal continue to explore without successfully finding food, sensory neurons display reduced activity and reduced coupling to the reorientation circuit.
Awake C. elegans transition between a wide range of additional internal states. While foraging on a bacterial food source, animals abruptly switch between stable (5-60 min) “roaming” and “dwelling” behavioral states in which they display either active exploration or slow exploitation of their food source, respectively45–47. Roaming and dwelling animals also exhibit behavioral differences beyond locomotion, for example different levels of egg-laying and olfactory-driven behavioral responses33,48. Animals favor dwelling in environments with dense, nutritive food, whereas they favor roaming when there is less food, aversive sensory stimuli, or a food odor gradient from a better nearby food source33,46,49. Transitions between roaming and dwelling are controlled by a neural circuit consisting of a mutual inhibitory loop between neurons that make two opposing neuromodulators: serotonin, which promotes dwelling, and PDF neuropeptides, which promote roaming (Figure 3B)33. Optogenetic activation of the serotonergic or PDF systems can flip the behavioral state of the animal and loss of these neuromodulatory systems causes fragmented, short states47. Diverse sensory signals feed into this core mutual inhibitory loop. The ingestion of bacterial food activates the serotonergic neuron NSM, which extends a sensory dendrite into the alimentary canal to directly sense bacterial food50. Olfactory processing neurons can couple to neurons in the roaming or dwelling circuit, depending on which state the animal is in33. Convergence of diverse sensory signals onto the core mutual inhibitory circuit allows these states to be influenced by the environment. State-dependent coupling of sensory neurons to either roaming or dwelling neurons allows for state-dependent sensory processing.
In the absence of bacterial food, C. elegans display a different set of behavioral states: immediately after food removal, they exhibit Local Search (LS) in which they display a high incidence of turns that allows them to potentially re-encounter food. After several minutes, they transition to a Global Search (GS) in which they reduce their turning (Figure 3C)51–53. The LS state is triggered by food-detecting olfactory and mechanosensory neurons that act in a redundant fashion on downstream processing circuits54. Onset of the GS state is associated with reduced activity in the sensory neurons and reduced coupling of the sensory neurons to downstream motor circuits.
The metabolism and satiety of C. elegans exerts a strong influence over its behavioral states. Starvation followed by re-feeding can trigger a quiescence state that is reminiscent of sleep55. Entry into this state is influenced by the level of fat stores in the animal56, as well as neurohumoral signaling via insulin/DAF-2 signaling and TGF- beta/DAF-7 signaling. Alterations in TGF-beta/DAF-7 signaling during pathogenic infection further modulates behavior57,58. Roaming and dwelling states are also influenced by satiety: animals favor dwelling after periods of fasting46. This appears to involve detection of peripheral fat stores, as well as enhanced activation of the serotonergic neuron NSM by food ingestion in fasted animals50,59–61. Starvation also impacts other complex C. elegans behaviors. For example, the drive to cross an aversive sensory barrier to reach an appetitive food cue is increased by starvation due to inhibition of the tyraminergic neuron RIM, which controls the gain of the response to the aversive cue62. Starvation can also change the valence of individual sensory cues, like carbon dioxide63. The profound effect of satiety levels on C. elegans behavior illustrates the strong influence that an animal’s metabolic state can exert on neural circuit function.
Finally, C. elegans behavioral states are also influenced by developmental age and developmental history. The fraction of time that an animal roams and dwells changes in a characteristic manner as it passes through its four larval stages into adulthood64. These changes are influenced by a wide range of neuromodulatory pathways. Related to these changes, the reproductive status of the animal has a causal role in altering roaming and dwelling behaviors as adult animals gain the ability to lay eggs65,66. Early life events also shape adult behavioral states days later. Animals that are food-deprived as young larvae and pass through the dauer diapause stage display more cautious foraging strategies as adults, consisting of reduced exploration67. These behavioral changes involve altered neural dynamics in a sensory processing circuit important for navigation, rather than changes at the sensory periphery. These studies add to an emerging view that the internal states of C. elegans represent highly integrative responses to sensory and physiological cues that endow the animal with flexible behavioral control over long time scales.
Concluding Remarks
While the neuronal network itself is physically static, the functional connections between neurons are dynamic over time and across different sensory contexts. Evolving neuromodulatory signaling alters the functional state of the neuronal network, which is manifested as different behavioral states. The brain-wide dynamics that accompany these states are the subject of active investigation and initial studies are revealing different correlative structure and dynamics across states. Knowing these correlative dynamics will be critically helpful to parameterize computational models of network function. A variety of computational approaches exist, from biophysical models that model each neuron and synapse68, to Bayesian models that capture the correlative relationships between neurons69, to models that combine neurons into state spaces based on the largest principle components of neuronal dynamics70. All of these models provide helpful insights into network function at different resolutions of network simplification. However, these models fit their parameters based on observed dynamics and functional relationships, and these empirical measurements remain very limited in the field. Further studies of brain-wide activity states will provide useful output solutions to constrain models and should reveal which underlying parameters of network function are critical to producing observed changes in functional connectivity and brain-wide dynamics.
The diversity of possible solutions encoded within the physical synaptic network provides the worm with a multitude of behaviors to engage with a constantly evolving environment. By encoding multiple solutions in a single network, the worm is better able to adapt to a changing environment on both individual and evolutionary timescales. New behavioral responses can occur by altering the functional relationships of neurons that already exist. This strategy is ancient. The roles of neuromodulators such as serotonin and dopamine in controlling states like satiety and sensory valence are conserved amongst invertebrates and vertebrates71–73. However, with the single-neuron resolution of the C. elegans network, it should be feasible understand how these signaling pathways explicitly alter network properties that may be difficult to define in larger systems.
In addition to neuromodulators like serotonin and dopamine, there are over 200 neuropeptides encoded in the C. elegans genome74. Future studies that further define the neuromodulatory connectome and reveal brain-wide dynamics during defined neuromodulatory states should provide new insights into the context dependence of C. elegans brain-wide activities. Studies of specific subsets of neurons, described above, are beginning to reveal mechanisms for context-dependent behavior, but this work should be contextualized in a broader framework of network-wide dynamics. The number of possible internal states encoded by neuromodulators, and their behavioral consequences, are likely to be vast, and a rich topic for future research.
Highlights.
The connectomes of multiple C. elegans animals have now been characterized
Anatomical connections between neurons are largely invariant, but functional connectivity between neurons is highly dynamic
Functional connectivity between neurons is influenced by neuromodulation
Long timescale modulation of functional connectivity gives rise to stable behavioral states
Acknowledgments
We thank members of the Flavell and Gordus labs for critical comments on the manuscript. S.W.F. acknowledges funding from NIH (NS104892 and GM135413), NSF (1845663), the JPB Foundation, the Alfred P. Sloan Foundation, the Brain Research Foundation, and the McKnight Scholars Program. A.G. acknowledges funding from NIH (GM124883).
Footnotes
While critical for development, the CANL/R neurons lack synapses with any neurons.
Declaration of Interests
The authors have no competing interests to declare.
References
- 1.Niell CM & Stryker MP Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goard M & Dan Y Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci 12, 1444–1449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack P-O, Friedman J & Golshani P Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci 16, 1331–1339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brincat SL, Siegel M, von Nicolai C & Miller EK Gradual progression from sensory to task-related processing in cerebral cortex. Proc. Natl. Acad. Sci. U. S. A 115, E7202–E7211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore T & Zirnsak M Neural Mechanisms of Selective Visual Attention. Annu. Rev. Psychol 68, 47–72 (2017). [DOI] [PubMed] [Google Scholar]
- 6.White JG, Southgate E, Thomson JN & Brenner S The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B. Biol. Sci 314, 1–340 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Cook SJ et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571, 63–71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varshney LR, Chen BL, Paniagua E, Hall DH & Chklovskii DB Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol 7, e1001066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towlson EK, Vértes PE, Ahnert SE, Schafer WR & Bullmore ET The rich club of the C. elegans neuronal connectome. J. Neurosci. Off. J. Soc. Neurosci 33, 6380–6387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfie M et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. Off. J. Soc. Neurosci 5, 956–964 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts WM et al. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. eLife 5, e12572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brittin CA, Cook SJ, Hall DH, Emmons SW & Cohen N A multi-scale brain map derived from whole-brain volumetric reconstructions. Nature 591, 105–110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study used detailed EM annotation to create a volumetric model of neuronal membrane contacts to characterize how the connectome is spatially embedded.
- 13.Moyle MW et al. Structural and developmental principles of neuropil assembly in C. elegans. Nature 591, 99–104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witvliet D et al. Connectomes across development reveal principles of brain maturation. Nature 596, 257–261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study used EM data to build connectomes for different larval stages, and characterized how the connectome evolves during development.
- 15.Bentley B et al. The Multilayer Connectome of Caenorhabditis elegans. PLoS Comput. Biol 12, e1005283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C et al. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendricks M, Ha H, Maffey N & Zhang Y Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature 487, 99–103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrödel T, Prevedel R, Aumayr K, Zimmer M & Vaziri A Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 10, 1013–1020 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Kato S et al. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Nguyen JP et al. Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 113, E1074–1081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatachalam V et al. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 113, E1082–1088 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallinen KM et al. Decoding locomotion from population neural activity in moving C. elegans. eLife 10, e66135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yemini E et al. NeuroPAL: A Multicolor Atlas for Whole-Brain Neuronal Identification in C. elegans. Cell 184, 272–288.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study developed a combinatorial fluorophore approach to differentially label neurons to enable reliable cellular identification for developmental and fluorescent calcium activity assays.
- 24.Susoy V et al. Natural sensory context drives diverse brain-wide activity during C. elegans mating. Cell 184, 5122–5137.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordus A, Pokala N, Levy S, Flavell SW & Bargmann CI Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161, 215–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji N et al. Corollary discharge promotes a sustained motor state in a neural circuit for navigation. eLife 10, e68848 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobosiewicz M, Liu Q & Bargmann CI Reliability of an interneuron response depends on an integrated sensory state. eLife 8, e50566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeishi A, Yeon J, Harris N, Yang W & Sengupta P Feeding state functionally reconfigures a sensory circuit to drive thermosensory behavioral plasticity. eLife 9, e61167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study revealed how signaling from the gut can modify thermosensory integration by modulating both sensory neuron potentiation to thermal inputs as well as the strength of coupling with postsynaptic interneurons.
- 29.Kim DH & Flavell SW Host-microbe interactions and the behavior of Caenorhabditis elegans. J. Neurogenet 34, 500–509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Jun H, Wu J, Liu J & Xu XZS Olfactory perception of food abundance regulates dietary restriction-mediated longevity via a brain-to-gut signal. Nat. Aging 1, 255–268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussey R et al. Pheromone-sensing neurons regulate peripheral lipid metabolism in Caenorhabditis elegans. PLoS Genet. 13, e1006806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussey R et al. Oxygen-sensing neurons reciprocally regulate peripheral lipid metabolism via neuropeptide signaling in Caenorhabditis elegans. PLoS Genet. 14, e1007305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji N et al. A neural circuit for flexible control of persistent behavioral states. eLife 10, e62889 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study utilized large-scale calcium imaging in defined populations of neurons, together with neural circuit perturbations, to uncover the functional logic of a neural circuit controlling opposing roaming and dwelling states.
- 34.Flavell SW, Raizen DM & You Y-J. Behavioral States. Genetics 216, 315–332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raizen DM et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 451, 569–572 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Van Buskirk C & Sternberg PW Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat. Neurosci 10, 1300–1307 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Nichols ALA, Eichler T, Latham R & Zimmer M A global brain state underlies C. elegans sleep behavior. Science 356, eaam6851 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Cho JY & Sternberg PW Multilevel modulation of a sensory motor circuit during C. elegans sleep and arousal. Cell 156, 249–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill AJ, Mansfield R, Lopez JMNG, Raizen DM & Van Buskirk C Cellular stress induces a protective sleep-like state in C. elegans. Curr. Biol. CB 24, 2399–2405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson MD et al. FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr. Biol. CB 24, 2406–2410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turek M, Besseling J, Spies J-P, König S & Bringmann H Sleep-active neuron specification and sleep induction require FLP-11 neuropeptides to systemically induce sleep. eLife 5, e12499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, Taylor KP, Hall Q & Kaplan JM The Neuropeptides FLP-2 and PDF- 1 Act in Concert To Arouse Caenorhabditis elegans Locomotion. Genetics 204, 1151–1159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nath RD, Chow ES, Wang H, Schwarz EM & Sternberg PWC elegans Stress-Induced Sleep Emerges from the Collective Action of Multiple Neuropeptides. Curr. Biol. CB 26, 2446–2455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.lannacone MJ et al. The RFamide receptor DMSR-1 regulates stress-induced sleep in C. elegans. eLife 6, e19837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara M, Sengupta P & Mclntire SL Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron 36, 1091–1102 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Ben Arous J, Laffont S & Chatenay D Molecular and sensory basis of a food related two-state behavior in C. elegans. PloS One 4, e7584 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flavell SW et al. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cermak N et al. Whole-organism behavioral profiling reveals a role for dopamine in state-dependent motor program coupling in C. elegans. eLife 9, e57093 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chew YL et al. An Afferent Neuropeptide System Transmits Mechanosensory Signals Triggering Sensitization and Arousal in C. elegans. Neuron 99, 1233–1246.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhoades JL et al. ASICs Mediate Food Responses in an Enteric Serotonergic Neuron that Controls Foraging Behaviors. Cell 176, 85–97.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study clarified how the ingestion of food activates a key serotonergic neuron, NSM, which elicits a behavioral state change to allow for exploitation of a food patch.
- 51.Gray JM, Hill JJ & Bargmann CI A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 102, 3184–3191 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakabayashi T, Kitagawa I & Shingai R Neurons regulating the duration of forward locomotion in Caenorhabditis elegans. Neurosci. Res 50, 103–111 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Hills T, Brockie PJ & Maricq AV Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. Off. J. Soc. Neurosci 24, 1217–1225 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Cruz A et al. Parallel Multimodal Circuits Control an Innate Foraging Behavior. Neuron 102, 407–419.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study mapped out the neural circuit that allows recent food availability to alter the behavioral state of the animal from a local to global search as time off food progresses.
- 55.You Y, Kim J, Raizen DM & Avery L Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7, 249–257 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyun M et al. Fat Metabolism Regulates Satiety Behavior in C. elegans. Sci. Rep 6, 24841 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meisel JD, Panda O, Mahanti P, Schroeder FC, & Kim DH, Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 159, 267–280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilbert ZA & Kim DH Sexually dimorphic control of gene expression in sensory neurons regulates decision-making behavior in C. elegans. eLife 6, e21166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawin ER, Ranganathan R & Horvitz HRC elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Juozaityte V et al. The ETS-5 transcription factor regulates activity states in Caenorhabditis elegans by controlling satiety. Proc. Natl. Acad. Sci. U. S. A 114, E1651–E1658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Churgin MA, McCloskey RJ, Peters E & Fang-Yen C Antagonistic Serotonergic and Octopaminergic Neural Circuits Mediate Food-Dependent Locomotory Behavior in Caenorhabditis elegans. J. Neurosci. Off. J. Soc. Neurosci 37, 7811–7823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh DD et al. Neural Architecture of Hunger-Dependent Multisensory Decision Making in C. elegans. Neuron 92, 1049–1062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rengarajan S, Yankura KA, Guillermin ML, Fung W & Hallem EA Feeding state sculpts a circuit for sensory valence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 116, 1776–1781 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern S, Kirst C & Bargmann CI Neuromodulatory Control of Long-Term Behavioral Patterns and Individuality across Development. Cell 171, 1649–1662.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Aprison EZ & Ruvinsky I Dynamic Regulation of Adult-Specific Functions of the Nervous System by Signaling from the Reproductive System. Curr. Biol CB 29, 4116–4123.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aprison EZ & Ruvinsky I Coordinated Behavioral and Physiological Responses to a Social Signal Are Regulated by a Shared Neuronal Circuit. Curr. Biol. CB 29, 4108–4115.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pradhan S, Quilez S, Homer K & Hendricks M Environmental Programming of Adult Foraging Behavior in C. elegans. Curr. Biol. CB 29, 2867–2879.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Kunert J, Shlizerman E & Kutz JN Low-dimensional functionality of complex network dynamics: neurosensory integration in the Caenorhabditis Elegans connectome. Phys. Rev. E Stat. Nonlin. Soft Matter Phys 89, 052805 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Linderman S, Nichols A, Blei D, Zimmer M & Paninski L Hierarchical recurrent state space models reveal discrete and continuous dynamics of neural activity in C. elegans. 621540 10.1101/621540v1 (2019) doi:. [DOI]
- 70.Morrison M, Fieseler C & Kutz JN Nonlinear Control in the Nematode C. elegans. Front. Comput. Neurosci. 14, 616639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tecott LH Serotonin and the orchestration of energy balance. Cell Metab. 6, 352–361 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Scaplen KM & Kaun KR Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J. Neurogenet 30, 133–148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marques JC, Li M, Schaak D, Robson DN & Li JM Internal state dynamics shape brainwide activity and foraging behaviour. Nature 577, 239–243 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Van Bael S et al. A Caenorhabditis elegans Mass Spectrometric Resource for Neuropeptidomics. J. Am. Soc. Mass Spectrom 29, 879–889 (2018). [DOI] [PubMed] [Google Scholar]


