Abstract
Smooth Brucella strains are classified into three serotypes, i.e., A+M−, A−M+, and A+M+, according to slide agglutination with A and M monospecific polyclonal sera. The epitopes involved have been located on the O-polysaccharide (O-PS) moiety of the smooth lipopolysaccharide (S-LPS), which represents the most exposed antigenic structure on the surface of Brucella spp. By use of monoclonal antibodies (MAbs) a number of epitope specificities on the O-PS have been reported: A, M, and epitopes shared by both A and M dominant strains, which have been named common (C) epitopes. The latter have been further subdivided, according to relative MAb binding in enzyme-linked immunosorbent assays (ELISA) to A- and M-dominant Brucella strains and to cross-reacting Yersinia enterocolitica O:9, into five epitopic specificities: C (M>A), C (M=A), C/Y (M>A), C/Y (M=A), and C/Y (A>M). In the present study, we studied the occurrence of these epitopes at the surface of representatives of all Brucella species and biovars including the live vaccine strains by analyzing the levels of MAb binding to whole Brucella cells in ELISA and flow cytometry assays. In ELISA, the level of MAb binding correlated well with the previously defined epitope specificity and the serotype defined by polyclonal sera for each Brucella species, biovar, or strain. However, MAbs to the C (M=A) and C (M>A) epitopes showed insignificant binding to B. suis biovar 2 strains and bound at lower titers to B. suis biovar 3 and B. neotomae than to the other Brucella strains. Some of the flow cytometry results were contradictory to those obtained by ELISA. In fact, it appeared by flow cytometry that all O-PS epitopes, including the A and M epitopes, are shared to different degrees by Brucella spp. which nevertheless show a high degree of O-PS heterogeneity according to MAb binding intensities. The subdivision of MAb specificities and Brucella serotypes was therefore less evident by flow cytometry than by ELISA. Whereas in ELISA the MAb specific for the A epitope showed insignificant binding to Y. enterocolitica O:9, this MAb bound strongly to Y. enterocolitica O:9 in flow cytometry. One of the two MAbs specific to the C (M=A) epitope also bound at a low but significant level to B. suis biovar 2 strains. However, as in ELISA the MAb specific for the C (M>A) epitope did not bind at all to B. suis biovar 2 strains in flow cytometry. Flow cytometry provided new information regarding specificity of the MAbs and may further explain some aspects of the capacity of passive protection of some MAbs against smooth Brucella infection in mice. As shown in the present study the occurrence of Brucella strains apparently completely devoid of one specific C O-PS epitope (e.g., B. suis biovar 2 devoid of the C [M>A] epitope) offers the possibility of obtaining vaccine strains devoid of a diagnostic O-PS epitope, which could further help to resolve the problem of discriminating infected from vaccinated animals that remains a major goal in brucellosis research.
Brucellae are gram-negative, facultative intracellular bacteria that can infect many species of animals and man. Six species are recognized within the genus Brucella: B. abortus, B. melitensis, B. suis, B. ovis, B. canis, and B. neotomae (15). This classification is mainly based on differences in pathogenicity and host preference (15). Brucella species and their different biovars are currently distinguished by differential tests based on serotyping, phage typing, dye sensitivity, CO2 requirement, H2S production, and metabolic properties (2, 31). The main pathogenic species worldwide are B. abortus (responsible for bovine brucellosis), B. melitensis (the main etiologic agent of ovine and caprine brucellosis), and B. suis (responsible for swine brucellosis). These three Brucella species may cause abortion in their hosts, which results in huge economic losses. B. abortus, B. melitensis, and B. suis strains may occur as either smooth (S) or rough (R) strains, expressing smooth lipopolysaccharide (S-LPS) or rough lipopolysaccharide (R-LPS) as major surface antigen, respectively, while B. ovis and B. canis are two naturally rough species, expressing R-LPS as major surface antigen. The latter species are responsible for ram epididymitis and canine brucellosis, respectively (3, 8). For B. neotomae only S strains isolated from desert rats have been reported (2, 31).
Vaccination against Brucella infections in animals is presently done by administration of live attenuated Brucella strains: S B. abortus B19 (for cattle); S B. melitensis Rev.1 (for sheep and goats); and S B. suis S2 (for pigs and sheep) (34, 44, 53). The S vaccine strains, however, are antigenically similar to virulent strains, and serodiagnosis by conventional tests (29), which principally measure antibody to S-LPS and, in particular, to its O-polysaccharide (O-PS) moiety, does not permit precise differentiation of vaccinated from infected animals.
The S-LPS, and more precisely its O-PS moiety, represents the most exposed antigenic structure of the Brucella cell surface (5, 10) and has been shown to be an important protective antigen against S Brucella infection in the mouse model, both in active immunization experiments (24, 50) and by passive immunization with monoclonal antibodies (MAbs) (11, 19, 26, 28, 32, 36, 46, 49). The antigenic determinants involved in serotyping with polyclonal sera are also borne by the O-PS. At present, S Brucella strains are classified into three serotypes, i.e., A+M−, A−M+, and A+M+, according to slide agglutination with A and M monospecific polyclonal sera (2). These agglutination results correspond to strains expressing mainly the A (A dominant) or M (M dominant) antigen or both antigens in nearly equivalent amounts. Additionally, S Brucella strains share common epitopes on the O-PS with cross-reacting strains, of which the most important is Yersinia enterocolitica O:9 (7, 9, 14, 16, 29, 47, 52). By using MAbs a number of epitope specificities on the O-PS have been reported: A, M, and epitopes shared by both A- and M-dominant strains, which have been named common (C) epitopes (6, 7, 11, 13, 17, 18, 20, 21, 26, 28, 35, 37, 38, 45–47). The latter have recently been further subdivided, according to relative preferential MAb binding in enzyme-linked immunosorbent assays (ELISA) to A- and M-dominant biovar 1 strains of B. abortus or B. melitensis and to cross-reacting Y. enterocolitica O:9, into five epitopic specificities: C (M>A), C (M=A), C/Y (M>A), C/Y (M=A), and C/Y (A>M) (48). MAbs indicated as C are specific for Brucella while those indicated as C/Y cross-react with Y. enterocolitica O:9. The preferential binding to A- or M-dominant strains and equal binding to both strains are indicated by A>M, M>A, and A=M, respectively. It has been recently suggested from data of competitions between MAbs that the different O-PS epitopes are probably overlapping structures (48).
In the present study, we studied the occurrence of the different O-PS epitopes at the surface of representatives of all Brucella species and biovars including the live S vaccine strains by analyzing MAb binding to whole Brucella cells in ELISA and flow cytometry. Our purpose was to determine if some of these epitopes are variably expressed in relation to the serotype and at the Brucella species, biovar, or strain level and in particular if Brucella strains exist that are deficient in the expression of one or more O-PS epitopes. Flow cytometry has recently been used to study the surface exposure of outer membrane protein epitopes in Brucella spp. (5). It has an advantage over ELISA in allowing study of binding intensity and of the homogeneity or heterogeneity of binding distribution in a cell population.
MATERIALS AND METHODS
Bacteria and cultures.
The strains used in this study are listed in Table 1. They were from the culture collection of the brucellosis laboratory, Institut National de la Recherche Agronomique, Nouzilly, France. Brucella spp. and Yersinia enterocolitica O:9 cultures were grown for 24 h at 37°C in Roux flasks on tryptic soy agar (Gibco BRL) supplemented with 0.1% (wt/vol) yeast extract (Difco) (TSAYE). For fastidious strains (B. abortus biovar 2 and B. ovis), sterile horse serum (Gibco BRL) was added to TSAYE to a final concentration of 5% (vol/vol) (TSAYES). The Brucella strains were checked for purity, colony phase, and species and biovar characterization by standard procedures (2). Cells were harvested by flooding the culture surface with phosphate-buffered saline (PBS) (pH 7.4) and were killed by a 1-h incubation at 65°C.
TABLE 1.
Binding of the anti-O-PS MAbs in ELISA to Brucella spp. and Y. enterocolitica O:9
| Species | Biovar | Strain | Agglutination with monospecific serum
|
Binding titer (max. abs.) by ELISA of the following MAba:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 2C8C4 (A) | 2 E11 (M) | 04F03 (M) | 12G12 (C [A=M]) | 07F09 (C [A=M]) | 12B12 (C [M>A]) | 18H08 (C/Y [A=M]) | 04F9 (C/Y [A>M]) | 16C10 (C/Y [M>A]) | |||
| B. abortus | 1 | 544 | + | − | 90 (2.921) | <10 (0.103) | <10 (0.122) | 810 (3.000) | 270 (3.000) | 30 (2.748) | 2,430 (3.000) | 21,870 (3.000) | 30 (3.000) |
| 1 | B19 | + | − | 270 (3.000) | <10 (0.136) | <10 (0.088) | 2,430 (2.867) | 810 (3.000) | 270 (2.917) | 2,430 (3.000) | 21,870 (3.000) | 270 (3.000) | |
| 2 | 86/8/59 | + | − | 270 (2.826) | <10 (0.733) | <10 (0.719) | 7,290 (2.880) | 2,430 (3.000) | 90 (3.000) | 2,430 (3.000) | 21,870 (3.000) | 90 (3.000) | |
| 3 | Tulya | + | − | 90 (3.000) | 10 (1.067) | 10 (2.870) | 2,430 (3.000) | 810 (3.000) | 90 (3.000) | 810 (3.000) | 21,870 (3.000) | 30 (3.000) | |
| 4 | 292 | − | + | <10 (0.267) | 30 (2.469) | 270 (2.876) | 7,290 (3.000) | 810 (3.000) | 810 (3.000) | 810 (3.000) | 10 (1.505) | 810 (3.000) | |
| 5 | B3196 | − | + | <10 (0.265) | 30 (3.000) | 90 (3.000) | 7,290 (3.000) | 2,430 (3.000) | 810 (3.000) | 810 (3.000) | 30 (2.201) | 2,430 (3.000) | |
| 6 | 870 | + | − | 270 (3.000) | <10 (0.095) | <10 (0.225) | 2,430 (3.000) | 810 (3.000) | 90 (3.000) | 810 (3.000) | 21,870 (3.000) | 30 (3.000) | |
| 9 | C68 | − | + | <10 (0.501) | 30 (3.000) | 90 (3.000) | 21,870 (3.000) | 2,430 (3.000) | 810 (3.000) | 810 (3.000) | 30 (2.523) | 2,430 (3.000) | |
| B. melitensis | 1 | 16M | − | + | <10 (0.158) | 90 (3.000) | 270 (3.000) | 7,290 (3.000) | 2,430 (3.000) | 810 (3.000) | 810 (3.000) | 90 (3.000) | 7,290 (3.000) |
| 1 | Rev.1 | − | + | <10 (0.931) | 30 (2.233) | 270 (3.000) | 7,290 (3.000) | 2,430 (3.000) | 810 (3.000) | 810 (3.000) | 30 (1.901) | 810 (3.000) | |
| 2 | 63/9 | + | − | 90 (3.000) | <10 (0.240) | 90 (2.597) | 7,290 (3.000) | 2,430 (3.000) | 270 (3.000) | 2,430 (3.000) | 21,870 (3.000) | 90 (3.000) | |
| 3 | Ether | + | + | 30 (1.933) | <10 (0.442) | 270 (3.000) | 2,430 (3.000) | 810 (3.000) | 270 (3.000) | 2,430 (3.000) | 7,290 (3.000) | 270 (3.000) | |
| B. suis | 1 | 1330 | + | − | 90 (2.888) | <10 (0.100) | 30 (2.009) | 810 (3.000) | 270 (3.000) | 30 (2.871) | 810 (3.000) | 7,290 (3.000) | 30 (3.000) |
| 1 | S2 | + | − | 90 (3.000) | <10 (0.185) | 10 (1.957) | 2,430 (3.000) | 810 (3.000) | 90 (3.000) | 810 (3.000) | 21,870 (3.000) | 30 (3.000) | |
| 2 | Thomsen | + | − | 30 (2.921) | <10 (0.134) | <10 (0.172) | <10 (0.645) | <10 (0.346) | <10 (0.182) | 2,430 (3.000) | 21,870 (3.000) | 10 (2.425) | |
| 2 | 93-63 | + | − | 30 (2.647) | <10 (0.125) | <10 (0.140) | <10 (0.361) | <10 (0.231) | <10 (0.111) | 2,430 (3.000) | 21,870 (3.000) | 10 (2.482) | |
| 2 | 94-9 | + | − | 30 (2.766) | <10 (0.121) | <10 (0.105) | <10 (0.895) | <10 (0.356) | <10 (0.145) | 810 (3.000) | 7,290 (3.000) | 10 (1.754) | |
| 2 | 94-10 | + | − | 90 (3.000) | <10 (0.230) | <10 (0.437) | <10 (0.864) | <10 (0.277) | <10 (0.412) | 810 (3.000) | 21,870 (3.000) | 10 (1.641) | |
| 2 | 94-11 | + | − | 90 (3.000) | <10 (0.150) | <10 (0.152) | 10 (1.302) | <10 (0.839) | <10 (0.407) | 810 (3.000) | 21,870 (3.000) | 10 (1.247) | |
| 3 | 686 | + | − | 90 (3.000) | <10 (0.107) | <10 (0.121) | 270 (3.000) | 90 (2.871) | <10 (0.832) | 810 (3.000) | 7,290 (3.000) | 30 (3.000) | |
| 4 | 40 | + | + | 10 (2.611) | 10 (1.936) | 90 (2.680) | 7,290 (3.000) | 2,430 (3.000) | 810 (3.000) | 810 (3.000) | 2,430 (3.000) | 810 (3.000) | |
| 5 | 513 | − | + | <10 (0.514) | 30 (3.000) | 270 (3.000) | 21,870 (3.000) | 7,290 (3.000) | 810 (3.000) | 810 (3.000) | 270 (3.000) | 2,430 (3.000) | |
| B. neotomae | 5K33 | + | − | 30 (2.554) | <10 (0.098) | <10 (0.172) | 90 (2.573) | 30 (2.339) | <10 (0.984) | 810 (2.862) | 7,290 (3.000) | 30 (3.000) | |
| B. ovis | 63/290 | − | − | <10 (0.101) | <10 (0.121) | <10 (0.099) | <10 (0.111) | <10 (0.102) | <10 (0.076) | <10 (0.089) | <10 (0.093) | <10 (0.073) | |
| B. canis | RM6/66 | − | − | <10 (0.087) | <10 (0.067) | <10 (0.093) | <10 (0.094) | <10 (0.096) | <10 (0.085) | <10 (0.086) | <10 (0.102) | <10 (0.067) | |
| Y. enterocolitica O:9 | <10 (0.450) | <10 (0.053) | <10 (0.023) | <10 (0.111) | <10 (0.095) | <10 (0.055) | 2,430 (2.868) | 7,290 (3.000) | 90 (3.000) | ||||
Results are expressed as titers of the MAbs, i.e., the highest dilutions of the MAbs giving an absorbance value above 1.0. Maximal absorbance (max. abs.) was observed mostly at a 1/10 dilution of the MAb. The epitope specificities of the MAbs are identified in parentheses.
MAbs.
MAbs to the Brucella O-PS epitopes were produced and characterized as described previously (10, 13, 26, 28, 47, 48). The anti-O-PS MAbs used were MAb 2C8C4 (immunoglobulin G3 [IgG3]) (A epitope), MAb 2E11 (IgG3) (M epitope), MAb 04F03 (IgM) (M epitope), MAb 12G12 (IgG1) (C [M=A] epitope), MAb 07F09 (IgG1) (C [M=A] epitope), MAb 12B12 (IgG3) (C [M>A] epitope), MAb 18H08 (IgA) (C/Y [M=A] epitope), MAb 04F9 (IgG2a) (C/Y [A>M] epitope), and MAb 16C10 (IgG3) (C/Y [M>A] epitope). MAbs were used as hybridoma culture supernatants.
ELISA.
Binding of MAbs at the surface of Brucella or Y. enterocolitica O:9 cells was determined by ELISA as described previously (5). Briefly, heat-killed cells were adsorbed on microtiter plates (Greiner, Stuttgart, Germany) at a cell suspension A600 of 1.0 in PBS by overnight incubation at room temperature. Hybridoma supernatants were serially diluted (serial threefold dilutions of 1/10 to 1/21,870) in PBS containing 0.05% Tween 20 (PBS-T) and incubated on the plates for 1 h at 37°C. Binding of the MAbs was detected by incubation for 1 h at room temperature with peroxidase-conjugated goat anti-mouse immunoglobulins (Bio-Rad, France) diluted 1/3,000 in PBS-T. Excess reagents between the different incubations were removed by five washings with 0.15 M NaCl containing 0.05% Tween 20. The substrate solution for detecting peroxidase activity was 4 mM H2O2 and 1 mM ABTS [2,2-azino-di-(3-ethylbenzthiazoline-sulfonic acid)] in 50 mM sodium citrate, pH 4.2. A414s were recorded with an automatic ELISA reader (Bio-Tek EL 312; Packard Instruments, Rungis, France).
Flow cytometry.
For flow cytometry analysis, 200 μl of a suspension (A600 of 0.4) of heat-killed Brucella or Y. enterocolitica O:9 cells was incubated (1 h, 37°C) with MAbs (hybridoma supernatants diluted twofold in PBS-T) in Eppendorf polystyrene conical vials. After two 0.5-ml PBS-T washes, the cells were incubated (1 h, 37°C) with 200 μl of a 1/150 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (heavy plus light chains) serum (Sigma) and then washed twice and resuspended in 0.5 ml of PBS. The suspensions were then filtered through a nylon mesh. Negative controls were bacteria incubated with only the conjugate, and these cells were used to set the acquisition parameters. For data acquisition, the flow cytometer (FACScan; Becton Dickinson, Mountain View, Calif.) was set as follows: detector threshold (FSC-H) = 32; FSC = E00; SSC = 368; parameters, FSC = 5.35 (linear) and SSC-H = 5.55 (linear) and FL1-H 588 (logarithmic scale).
Twenty-thousand Brucella or Yersinia cells were analyzed in each sample. For determination of the mean and median fluorescence values, analyses were performed on ungated (i.e., all) cells. Percentages of cells labeled by each MAb were determined after exclusion of background and nonspecific fluorescence signals by establishing appropriate region markers. Sample analyses were performed with LYSIS II software (Becton Dickinson).
RESULTS
ELISA. (i) A and M epitopes.
In ELISA, anti-O-PS MAb binding levels correlated well with the previously defined epitope specificities and the serotypes defined by polyclonal sera for each Brucella species, biovar, or strain. Indeed, MAb 2C8C4 specific for the A epitope bound preferentially to the A-dominant strains and to strains defined as A+M+, i.e., B. melitensis Ether (biovar 3) and B. suis 40 (biovar 4) (Table 1). However, binding of MAb 2C8C4 to the latter strains was, according to the titer, lower than that to the A-dominant strains. Binding to M-dominant strains was not significant or the level was very low (absorbance values around 0.5 at the first 1/10 dilution). Alternatively, MAbs 2E11 and 04F03, specific for the M epitope, showed preferential binding to the M-dominant strains. However, differential levels of binding between the two anti-M MAbs were observed for some strains. In contrast to MAb 2E11, MAb 04F03 showed a significant level of binding to the A-dominant B. melitensis strain 63/9 (biovar 2) and to the A+M+ B. melitensis strain Ether (biovar 3) and in a more limited but still significant level of binding to the A-dominant B. suis strains 1330 and S2. Both MAbs also showed a low but significant level of binding to the A-dominant B. abortus strain Tulya (biovar 3). The MAbs specific for the A and M epitopes appeared to be specific for Brucella, as no significant binding or a very low level of binding (for MAb 2C8C4) was observed to Y. enterocolitica O:9 cells. Thus, it appears according to the ELISA results that the distribution of the A and M epitopes defined by MAbs on the different Brucella species, biovars, and strains follows that previously defined by polyclonal sera, but the results also suggest that some differences in the occurrence and abundance of these epitopes between strains may be observed.
(ii) C epitopes.
The MAbs specific for the C epitopes could be classified into two groups according to whether they bound to Y. enterocolitica O:9, i.e., C and C/Y. MAbs 12G12 and 07F09, specific for the C (A=M) epitope, bound at high titers to most A- and M-dominant strains and also to the A+M+ Brucella strains (Table 1). Their binding levels were generally equal relative to A or M dominance and confirmed their (A=M) specificity. However, these MAbs bound insignificantly or showed very low levels of binding to B. suis Thomsen, representative of biovar 2 of B. suis. To determine if this observation was specific to B. suis biovar 2, field strains of B. suis biovar 2 were included. The binding levels of MAbs 12G12 and 07F09 to these strains were similar to that for B. suis Thomsen. Both MAbs also bound at lower titers to B. suis 686 (biovar 3) and B. neotomae reference strain 5K33. MAb 12B12, specific for the C (M>A) epitope, bound significantly to A- and M-dominant strains but bound at higher titers to M-dominant strains. This MAb, similar to MAbs 12G12 and 07F09, showed insignificant or a very low level of binding to B. suis biovar 2 strains and in addition showed very low levels of binding to B. suis 686 and B. neotomae 5K33. Thus, these results indicated that both the C (A=M) and C (M>A) epitopes, although widely distributed on most Brucella species and biovars, are poorly or not represented in B. suis biovar 2 strains. In addition, they seem also to be less expressed at the surface of B. suis 686 (representative of biovar 3 of B. suis) and B. neotomae reference strain 5K33.
(iii) C/Y epitopes.
MAbs that cross-react with Y. enterocolitica O:9 have been further subdivided into three epitope specificities, i.e., C/Y (A=M), C/Y (A>M), and C/Y (M>A) (Table 1) (48). MAb 18H08, specific for the C/Y (A=M) epitope, showed similar levels of binding to all S Brucella species, biovars, and strains studied and thus appeared to recognize an epitope which is the most invariable or most uniformly distributed among S Brucella strains. MAb 04F9, specific for the C/Y (A>M), epitope, bound significantly to all S Brucella strains studied but at much higher titers to A-dominant and A+M+ strains than to M-dominant strains. In contrast, MAb 16C10, specific for the C/Y (M>A) epitope, bound generally better to M-dominant than to A-dominant strains. Interestingly, the latter MAb showed lower levels of binding to B. suis biovar 2 strains than to the other A-dominant strains studied.
Aside from the observations reported above, it must be noted that the currently used live attenuated S vaccine strains B. abortus B19 (A dominant), B. melitensis Rev.1 (M dominant), and B. suis S2 (A dominant) showed no specific characteristics related to the distribution of the presently defined O-PS epitopes. Indeed, all these strains appear to carry the same epitopes as their representative Brucella biovar reference strain and thus cannot be distinguished from field strains on the basis of their O-PS epitope distribution.
Flow cytometry. (i) A and M epitopes.
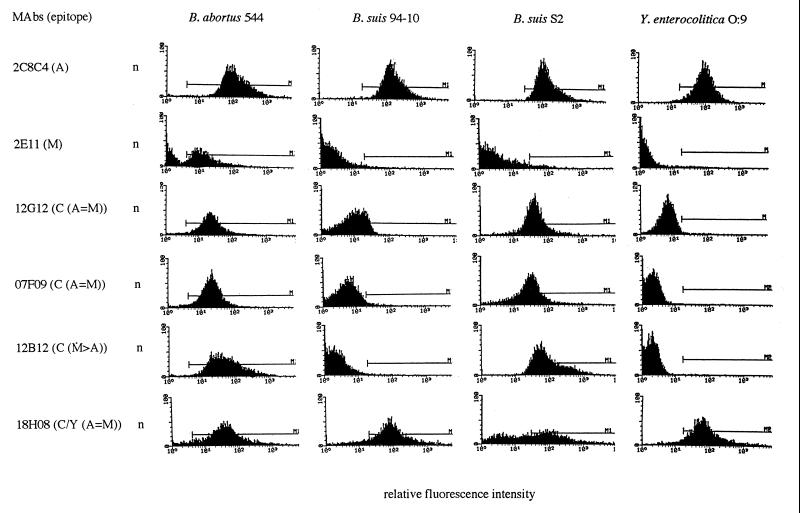
Flow cytometry showed some results which were contradictory to those obtained by ELISA. In fact, it appeared by flow cytometry that all O-PS epitopes, including the A and M epitopes, are shared to different degrees by S Brucella spp. which nevertheless show a great degree of O-PS heterogeneity among Brucella strains, according to MAb binding intensities and percentages of cells labeled (Fig. 1) (Table 2). The subdivision of MAb specificities and Brucella serotypes was therefore less evident by flow cytometry than by ELISA. Indeed, MAb 2C8C4, specific for the A epitope, labeled in most cases more than 90% of cells of A-dominant and A+M+ strains (Table 2). However, it also labeled a low but significant percentage (between 30 and 60%) of cells of most M-dominant strains with similar or lower fluorescence intensities. Among the M-dominant strains only B. melitensis Rev.1 (vaccine) was not specifically labeled by MAb 2C8C4. Whereas in ELISA MAb 2C8C4 showed insignificant binding to Y. enterocolitica O:9, this MAb bound strongly to Y. enterocolitica O:9 in flow cytometry (Fig. 1). Indeed, MAb 2C8C4 labeled 98.9% of Y. enterocolitica O:9 cells with a mean fluorescence intensity similar to that observed for A-dominant Brucella strains. MAbs 2E11 and 04F03, specific for the M epitope, bound to more than 90% of cells of most M-dominant and A+M+ strains and to between 9.5 and 89.0% (although with low intensity) of cells of most A-dominant strains. Among the A-dominant strains only B. abortus B19 (vaccine) and B. suis biovar 2 strains were not specifically labeled by either MAb 2E11 or MAb 04F03. However, as in ELISA, differential levels of binding between the two anti-M MAbs were observed for some strains. In contrast to MAb 2E11, MAb 04F03 labeled a significant percentage of cells of the A-dominant B. melitensis strain 63/9 (35.4%), the A-dominant B. suis strain S2 (60.3%), and the A+M+ B. melitensis strain Ether (95.3%). Both MAbs 2E11 and 04F03 did not bind significantly to Y. enterocolitica O:9 cells in flow cytometry. Thus, it appears from the flow cytometry results that the distribution of the A and M epitopes among S Brucella strains, although following the A or M dominance defined by polyclonal sera, may be rather heterogeneous.
FIG. 1.
O-PS epitopic heterogeneity studied by flow cytometry. Flow cytometry analysis of binding of anti-O-PS MAbs to A-dominant strains B. abortus 544 (biovar 1 reference strain), B. suis 94-10 (biovar 2 field strain), B. suis S2 (biovar 1 vaccine strain), and Y. enterocolitica O:9. n, number of bacteria.
TABLE 2.
Binding of the anti-O-PS MAbs in flow cytometry to Brucella spp. and Y. enterocolitica O:9
| Species | Biovar | Strain | Agglutination with monospecific serum
|
% Cells labeled (f.i.) in flow cytometry by the following MAba:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 2C8C4 (A) | 2 E11 (M) | 04F03 (M) | 12G12 (C [A=M]) | 07F09 (C [A=M]) | 12B12 (C [M>A]) | 18H08 (C/Y [A=M]) | 04F9 (C/Y [A>M]) | 16C10 (C/Y [M>A]) | |||
| B. abortus | 1 | 544 | + | − | 98.5 (105.5) | 50.4 (12.0) | 10.8 (10.2) | 95.9 (22.7) | 94.4 (20.3) | 97.4 (44.5) | 91.4 (38.5) | 99.2 (99.1) | 97.2 (88.2) |
| 1 | B19 | + | − | 92.9 (110.4) | − | − | 96.5 (25.0) | 97.0 (23.5) | 83.1 (40.7) | 84.8 (37.2) | 98.7 (85.0) | 92.7 (73.0) | |
| 2 | 86/8/59 | + | − | 97.8 (113.4) | 19.6 (52.3) | 9.5 (29.4) | 81.8 (47.4) | 41.0 (37.9) | 89.0 (41.4) | 80.4 (66.1) | 94.1 (151.2) | 99.0 (114.4) | |
| 3 | Tulya | + | − | 97.0 (114.4) | 29.0 (67.9) | 17.0 (63.8) | 64.6 (52.8) | 66.6 (50.0) | 95.6 (62.1) | 74.6 (89.0) | 96.2 (196.3) | 92.9 (127.5) | |
| 4 | 292 | − | + | 59.2 (83.5) | 97.7 (73.6) | 96.3 (154.0) | 87.3 (59.9) | 89.6 (71.0) | 97.7 (81.3) | 78.8 (81.3) | 95.6 (40.7) | 97.0 (155.4) | |
| 5 | B3196 | − | + | 52.7 (68.5) | 94.2 (53.3) | 96.5 (54.7) | 96.4 (59.3) | 84.8 (33.7) | 98.5 (69.8) | 95.2 (71.7) | 92.7 (74.3) | 96.5 (124.1) | |
| 6 | 870 | + | − | 98.8 (121.9) | 23.1 (33.4) | 26.0 (28.6) | 98.0 (41.0) | 93.9 (34.3) | 99.1 (53.7) | 98.3 (50.0) | 97.4 (109.4) | 97.5 (101.8) | |
| 9 | C68 | − | + | 21.0 (65.0) | 93.0 (52.8) | 91.9 (43.3) | 92.5 (53.3) | 93.8 (56.7) | 98.7 (56.2) | 93.7 (58.3) | 98.9 (101.0) | 98.9 (121.9) | |
| B. melitensis | 1 | 16M | − | + | 33.1 (52.8) | 94.8 (50.0) | 95.9 (70.4) | 87.3 (54.7) | 93.0 (51.4) | 96.0 (70.4) | 83.2 (70.4) | 99.7 (133.3) | 98.6 (112.4) |
| 1 | Rev.1 | − | + | − | 58.1 (52.3) | 52.0 (56.7) | 53.3 (47.4) | 38.1 (47.0) | 69.9 (62.6) | 43.6 (101.8) | 88.0 (134.6) | 70.0 (103.7) | |
| 2 | 63/9 | + | − | 76.7 (138.2) | − | 35.4 (58.8) | 20.3 (42.2) | 37.1 (67.0) | 38.9 (68.0) | 37.8 (66.1) | 61.4 (68.8) | 74.7 (148.5) | |
| 3 | Ether | + | + | 99.0 (161.1) | − | 95.3 (58.3) | 89.9 (67.9) | 91.3 (63.8) | 96.8 (76.3) | 81.5 (117.6) | 97.4 (248.0) | 98.9 (152.6) | |
| B. suis | 1 | 1330 | + | − | 99.6 (92.2) | 21.5 (30.2) | 89.0 (25.5) | 98.0 (47.0) | 88.4 (34.0) | 99.5 (46.1) | 94.2 (61.0) | 97.7 (137.0) | 98.5 (119.7) |
| 1 | S2 | + | − | 99.3 (115.5) | − | 60.3 (42.5) | 70.1 (49.1) | 48.7 (44.9) | 91.2 (75.0) | 54.6 (114.4) | 98.2 (222.7) | 94.4 (110.4) | |
| 2 | Thomsen | + | − | 99.2 (186.0) | − | − | 89.8 (34.0) | 10.3 (23.1) | − | 89.8 (159.6) | 98.9 (276.3) | 98.4 (154.0) | |
| 2 | 93-63 | + | − | 98.2 (276.3) | − | − | 74.5 (26.0) | − | − | 81.3 (155.4) | 97.5 (245.0) | 97.0 (152.6) | |
| 2 | 94-9 | + | − | 98.4 (137.0) | − | − | 55.1 (44.1) | − | − | 75.0 (102.7) | 96.1 (189.4) | 93.5 (109.4) | |
| 2 | 94.10 | + | − | 98.6 (142.0) | − | − | 15.7 (24.1) | − | − | 87.2 (87.4) | 98.4 (198.1) | 90.3 (119.7) | |
| 2 | 94-11 | + | − | 98.9 (132.2) | 20.0 (107.5) | − | 72.7 (28.6) | − | − | 84.0 (87.4) | 98.1 (211.0) | 97.9 (115.0) | |
| 3 | 686 | + | − | 99.7 (86.6) | 14.9 (16.0) | 11.5 (22.3) | 95.9 (45.7) | 84.0 (23.9) | 74.2 (14.5) | 93.6 (57.2) | 98.4 (133.3) | 97.4 (98.2) | |
| 4 | 40 | + | + | 99.4 (72.3) | 89.1 (45.7) | 97.6 (46.6) | 97.6 (46.6) | 81.9 (38.2) | 99.0 (63.8) | 96.4 (68.5) | 99.1 (162.5) | 99.3 (144.6) | |
| 5 | 513 | − | + | 50.2 (30.8) | 95.4 (48.7) | 96.8 (47.0) | 96.0 (44.5) | 84.2 (34.9) | 95.6 (59.3) | 93.1 (61.0) | 99.3 (159.6) | 98.7 (127.5) | |
| B. neotomae | 5K33 | + | − | 98.7 (156.8) | 10.4 (23.1) | 12.7 (28.1) | 96.5 (87.4) | 83.0 (57.8) | 97.8 (59.3) | 77.3 (177.8) | 97.1 (276.3) | 93.8 (135.8) | |
| B. ovis | 63/290 | − | − | 19.4 (19.8) | 5.8 (14.0) | 7.4 (14.7) | 5.0 (15.5) | 5.2 (13.8) | 5.7 (14.9) | 9.3 (16.1) | 10.9 (17.5) | 5.6 (15.1) | |
| B. canis | RM6/66 | − | − | 10.8 (17.1) | 2.9 (15.5) | 2.6 (16.0) | 8.3 (13.6) | 2.6 (13.6) | 3.0 (13.9) | 4.5 (15.5) | 4.5 (16.8) | 3.3 (18.3) | |
| Y. enterocolitica O:9 | 98.9 (91.4) | 1.6 (48.7) | 4.3 (82.8) | 2.2 (25.0) | 1.0 (35.2) | 0.3 (32.0) | 94.1 (68.5) | 99.5 (88.2) | 99.2 (143.3) | ||||
Results are expressed as percentages of cells labeled and by the fluorescence intensities (f.i.) at the median (see Fig. 1). Minus sign indicates a negative result, i.e., the percentage of cells labeled is lower than that by negative control strain B. ovis 63/290 or B. canis RM6/66 and lower than that labeled by an irrelevant control MAb. The epitope specificities of the MAbs are identified in parentheses.
(ii) C epitopes.
MAbs 12G12 and 07F09, specific for the C (A=M) epitope, bound significantly in flow cytometry to most A-dominant, M-dominant, and A+M+ Brucella strains. However, the percentage of cells labeled by these MAbs varied between 15 and 95% and did not seem to depend on the A or M dominance of the strains. Indeed, both MAbs labeled a high percentage of cells (around or above 90%) whether the A or M epitope was dominant. But lower percentages of cells from the A-dominant B. melitensis strain 63/9 and the M-dominant B. melitensis strain Rev.1 were labeled (Table 1). Differences in the levels of binding by MAb 12G12 versus MAb 07F09 were also observed. In particular, whereas MAb 12G12 labeled, depending on the strain, between 15.7 and 89.9% of cells of strains of biovar 2 of B. suis, MAb 07F09 did not bind significantly to any of the same strains (Fig. 1). The differences in the binding patterns of these MAbs are probably due to differences in affinity, as was previously suggested by competitive ELISA results (54). Nevertheless, these flow cytometry results confirmed the poor expression of the C (A=M) epitope in B. suis biovar 2 strains. MAb 12B12, specific for the C (M>A) epitope, labeled a high percentage of cells of most Brucella strains tested except strains of biovar 2 of B. suis, which is in accordance with the ELISA results. A lower percentage of cells of the A-dominant B. melitensis strain 63/9 was also labeled (38.9%), as was seen with MAbs 12G12 and 07F09. But actually the (M>A) specificity of MAb 12B12 in ELISA was not observed in flow cytometry, since this MAb bound strongly in flow cytometry to all other A-dominant Brucella strains tested and the binding observed was not significantly different from that observed to M-dominant strains. As in ELISA, MAbs 12G12, 07F09, and 12B12 did not bind significantly to Y. enterocolitica O:9 in flow cytometry (Fig. 1).
(iii) C/Y epitopes.
The remaining MAbs that cross-react with Y. enterocolitica O:9 (MAbs 18H08, 04F9, and 16C10) showed high levels of labeling of Y. enterocolitica O:9 cells (between 94.1 and 99.5% depending on the MAb) comparable to those observed with MAb 2C8C4, specific for the A epitope. These MAbs also bound significantly to all Brucella strains tested and were actually those that showed the highest levels of binding in flow cytometry. In particular, MAb 04F9, specific for the C/Y (A>M) epitope, showed in comparison to the other MAbs the highest level of binding in flow cytometry to all Brucella strains tested independently of the serotype. This MAb labeled more than 95% of cells in most cases and showed the highest binding fluorescence intensities, in particular to strains of biovar 2 of B. suis and to B. neotomae strain 5K33. The binding capacity of MAb 16C10, specific for the C/Y (M>A) epitope, in flow cytometry was similar to that of MAb 04F9 except that binding fluorescence intensities were generally slightly lower than those observed for MAb 04F9. This MAb, as did MAb 04F9, seemed to bind equally well to A- and M-dominant Brucella strains. Therefore, the (A>M) and (M>A) epitope specificities of MAb 04F9 and MAb 16C10, respectively, demonstrated by ELISA were not evident in flow cytometry.
DISCUSSION
Previously, seven Brucella O-PS epitope specificities have been defined according to preferential binding of MAbs in ELISA to purified S-LPS of the A-dominant B. abortus strain 99, the M-dominant B. melitensis strain Rev.1, and Y. enterocolitica O:9 (48). Competitive binding assays between the MAbs suggested that the different epitopes are probably overlapping structures (48). A number of previous studies have reported on the distribution of some of the epitopes on A- and M-dominant strains of Brucella species, but none of them really investigated the occurrence of these epitopes on representatives of all recognized Brucella species and their different biovars (6, 13, 17, 21, 22, 35, 37, 38). ELISA performed with whole Brucella cells in the present study showed that the distribution of the different O-PS epitopes on the different Brucella species and biovars is mostly in accordance with their reported epitope specificities and the serotype defined by polyclonal sera of the Brucella strain considered. However, some differences which are biovar or in some cases strain dependent were observed and in fact contribute to O-PS epitopic heterogeneity at the surface of Brucella spp. In particular, B. suis biovar 2 strains appeared to be devoid of the C (A=M) and C (M>A) epitopes. This feature appeared to be specific for B. suis biovar 2 but B. suis biovar 3 strain 686 and B. neotomae 5K33 also showed lower levels of expression of these epitopes according to the MAb titers on these strains. Some discordances were observed between ELISA and flow cytometry results. In fact, MAbs that showed low levels of binding in ELISA to some Brucella strains showed high levels of binding to the same strains in flow cytometry. For instance the MAbs defined as specific for the C/Y (A>M) and the C/Y (M>A) epitopes (MAbs 04F9 and 16C10, respectively) showed the highest levels of binding in flow cytometry to all Brucella strains studied, whether A or M-dominant. In contrast to what was observed in ELISA, A- and M-dominant strains were not distinguished by these MAbs in flow cytometry. In previous studies MAb 04F9 (C/Y [A>M] epitope) was even considered specific for the A epitope by other techniques such as immunoblotting in which this MAb showed absence of binding to M-dominant S-LPS (11, 13, 18, 20, 26). Probably depending on the technique used, the physical state and presentation of S-LPS or O-PS epitopes are different. It was also previously shown that some MAbs although O-PS specific bound well in ELISA to purified S-LPS but not to purified O-PS (48). The high level of binding of MAbs observed in flow cytometry and not in ELISA may therefore be due to a better accessibility of the epitope at the surface of bacteria maintained in suspension than on bacteria coated on microtiter plates. Or it may be that expression of coated whole cells on ELISA plates differs from that of whole cells in suspension, probably due to their alteration during binding manipulations (33). It is also interesting to note that MAb 04F9 afforded good protection in passively immunized BALB/c mice against a virulent challenge of either an A- or an M-dominant Brucella strain (11). Thus, results of binding in suspension determined by flow cytometry may be a better indication of in vivo binding capacities of MAbs and consequent protection abilities. Another discordance of interest between the ELISA and flow cytometry results was that MAb 2C8C4, specific for the A epitope and previously defined as specific for Brucella, cross-reacted with Y. enterocolitica O:9 in flow cytometry. Flow cytometry showed also that the C (A=M) epitope on B. suis biovar 2 strains is not completely absent but rather highly deficient. Nevertheless, the apparent absence of the C (M>A) on B. suis biovar 2 strains seen in ELISA was confirmed by flow cytometry.
The Brucella O-PS structure has been described as being constituted by homopolymers of 4,6-dideoxy-4-formamido-α-d-mannopyranose residues. O-PS from A-dominant strains is a linear α-1,2-linked polymer with about 2% α-1,3 linkages, while O-PS from M-dominant strains is a linear polymer of pentasaccharide repeating units containing one α-1,3-linked and four α-1,2-linked monosaccharide residues (30). O-PS from A+M+ strains, i.e., B. melitensis biovar 3 and B. suis biovar 4, contain about 8 and 13% α-1,3 linkages, respectively (30). It was established that antibodies specific for the A epitope require five contiguous α-1,2-linked monosaccharide residues for binding (6). MAb 2C8C4, specific for the A epitope and used in the present study, showed binding characteristics in ELISA that are in agreement with this structural feature. However, its binding in flow cytometry to a significant number of cells of some M-dominant strains suggests that the distribution of the α-1,3-linked monosaccharide residues, which in principle should hinder the binding of this MAb, may not always be regular (i.e., one α-1,3-linked and four α-1,2-linked monosaccharide residues in sequence) on these strains. The binding of MAb 2C8C4 to Y. enterocolitica O:9 observed in flow cytometry seems logical since the O-PS structure of Y. enterocolitica O:9 is identical to that of Brucella but is devoid of α-1,3 linkages (9, 30). The α-1,3 linkage should be mainly involved in the structure recognized by MAbs specific for the M epitope since such MAbs fail to react with Y. enterocolitica O:9 and their preferential binding to M-dominant O-PS correlates with an increased number of α-1,3-linked monosaccharide residues. Their binding to some A-dominant strains observed in flow cytometry is consistent with the presence of some α-1,3-linked monosaccharide residues in A-dominant O-PS. The percentage of α-1,3-linked monosaccharide residues and their accessibilities on A-dominant O-PS are probably critical to allow binding of anti-M epitope MAbs. Probably the occurrence and distribution of α-1,3 linkages on the O-PS contribute largely to epitopic heterogeneity observed among Brucella strains. B. suis biovar 2 strains may be devoid of M epitopes since MAbs specific for the M epitope showed absence of binding in both ELISA and flow cytometry. Thus, the results of the present study are in agreement with the concept of simultaneous expression of A and M epitopes within the same Brucella strain except perhaps for B. suis biovar 2 strains. Yet, we do not know if α-1,3-linked monosaccharide residues may be involved in recognition by MAbs specific for the C (A=M) and C (M>A) epitopes since these MAbs also do not cross-react with Y. enterocolitica O:9. These MAbs also showed low levels of binding or the absence of binding to B. suis biovar 2 strains. Thus, determining the O-PS structure of B. suis biovar 2 strains could possibly give interesting information regarding epitope recognition of these MAbs in relation to the presence or absence of α-1,3-linked monosaccharide residues. Another possibility is that a not yet identified particular structure specific for Brucella participates in recognition by these MAbs. This may be the case in particular for MAbs recognizing the C (A=M) epitope for which binding does not correlate with the distribution of α-1,3-linked monosaccharide residues since these MAbs bound equally well to A- and M-dominant strains. MAbs specific for the C/Y epitopes probably recognize α-1,2-linked tri- or tetrasaccharides of the O-PS (6). In particular, MAb 18H08, specific for the C/Y (A=M) epitope and which showed the most invariable binding to all S Brucella strains studied and to Y. enterocolitica O:9, may recognize the smallest recognizable and most common structure of O-PS of A- and M-dominant Brucella strains and Y. enterocolitica O:9. The preferential binding observed in ELISA of MAb 04F9 specific for the C/Y (A>M) epitope to A-dominant strains may be due to a better accessibility of α-1,2-linked tetrasaccharides on A-dominant O-PS than on M-dominant O-PS. However, in flow cytometry this was apparently not the case. Interestingly, MAb 04F9 showed higher binding intensities in flow cytometry to B. suis biovar 2 strains than to the other Brucella strains studied. Perhaps the loss of the specific M, C (A=M), and C (M>A) epitopes confers a better accessibility of the epitope recognized by MAb 04F9 on the O-PS of B. suis biovar 2. The binding characteristics of MAb 16C10 specific for the C/Y (M>A) epitope are difficult to explain at this time according to the known O-PS structures since this MAb cross-reacted with Y. enterocolitica O:9 but showed preferential binding in ELISA to M-dominant Brucella strains. However, as in the case of MAb 04F9 for A-dominant strains, preferential binding of MAb 16C10 to M-dominant strains was not apparent in flow cytometry.
From the point of view of vaccine development against brucellosis, the results of the present study offer new perspectives. The main problem encountered in animal vaccination with the actual live attenuated S Brucella strains is that vaccinated animals cannot be clearly differentiated from infected animals by the current serological tests, which are based on detection of antibody to S-LPS (29, 52). S-LPS, and more precisely its O-PS moiety, is indeed immunodominant in S Brucella infections and is considered the best diagnostic antigen for serological diagnosis (16, 52). To overcome this problem two approaches are at present being considered. The first is to use live attenuated R Brucella strains, in particular the R B. abortus strain RB51 (25, 40, 41, 51). Since they are devoid of O-PS they should not induce antibody responses against S-LPS which would be detectable by the conventional serological tests or by an ELISA using S-LPS or O-PS as antigen. However, recent studies showed that such R strains are less protective in mice than the current live attenuated S Brucella strains (41, 51), probably because of the lack of S-LPS. S-LPS and in particular its O-PS moiety have been shown in the mouse model to be important protective antigens against S Brucella infection (11, 19, 24, 26, 28, 32, 36, 46, 49, 50). Another approach consists of identifying diagnostic protein antigens and deleting the corresponding genes in the current live attenuated S Brucella strains (4). Such constructed mutants should not induce antibody responses against the target protein, which could be used in new diagnostic tests. However, such protein should be immunodominant in infection and be able to detect a percentage of infected animals which is close to that detected by the current serological tests. Up to now antibody responses to protein antigens have been shown to be low and heterogeneous especially in infected cattle (12, 23, 27, 39, 42, 43). As shown in the present study the occurrence of Brucella strains apparently completely devoid of one specific C O-PS epitope, in particular B. suis biovar 2 strains devoid of the C (M>A) epitope, offers a new perspective, i.e., the possibility of obtaining vaccine strains devoid of a diagnostic O-PS epitope which could be used as a diagnostic antigen provided that such epitope can be isolated or synthesized. Competitive ELISA using the specific MAbs and purified Brucella S-LPS or O-PS cannot be used for such purpose since it has been shown by competitive binding assays between the MAbs that the different O-PS epitopes are probably overlapping structures on the actual O-PS or S-LPS antigens used (48). Therefore to produce appropriate serological tests, either synthetic oligosaccharides should be used (6) or another way to produce the epitopes of diagnostic interest could consist of screening with the MAbs of synthetic random peptide libraries expressed on phages (48). The vaccine strain should contain the necessary O-PS epitopes to provide sufficient S-LPS-mediated protection. As shown by passive immunization experiments with mice (11) one of the most interesting epitopes for such purpose appears to be that recognized by MAb 04F9 (C/Y [A>M] epitope). B. suis biovar 2 could be a possible vaccine candidate for this approach. The European hare (Lepus capensis) is the principal host of B. suis biovar 2 and can form a reservoir of infection from which pigs, both domestic and wild, may become infected (1). Interestingly B. suis biovar 2 is not known to be a human pathogen (1). Its immunogenic and virulence properties in laboratory and farm animals other than pigs have yet to be determined.
ACKNOWLEDGMENT
We thank S. Bernard for assistance in flow cytometry experiments.
REFERENCES
- 1.Alton G G. Brucella suis. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 411–422. [Google Scholar]
- 2.Alton G G, Jones L M, Angus R D, Verger J M. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 3.Blasco J M. Brucella ovis. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 351–378. [Google Scholar]
- 4.Boschiroli M L, Cravero S L, Arese A I, Campos E, Rossetti O L. Protection against infection in mice vaccinated with a Brucella abortus mutant. Infect Immun. 1997;65:798–800. doi: 10.1128/iai.65.2.798-800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden R A, Cloeckaert A, Zygmunt M S, Bernard S, Dubray G. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun. 1995;63:3945–3952. doi: 10.1128/iai.63.10.3945-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundle D R, Cherwonogrodzky J W, Gidney M A J, Meikle P J, Perry M B, Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989;57:2829–2836. doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundle D R, Gidney M A J, Perry M B, Duncan J R, Cherwonogrodzky J W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 antigens by monoclonal antibodies. Infect Immun. 1984;46:389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael L E. Brucella canis. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 335–350. [Google Scholar]
- 9.Caroff M, Bundle D R, Perry M B. Structure of the O-chain of the phenol-phase soluble lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984;139:195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- 10.Cloeckaert A, de Wergifosse P, Dubray G, Limet J N. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect Immun. 1990;58:3980–3987. doi: 10.1128/iai.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloeckaert A, Jacques I, de Wergifosse P, Dubray G, Limet J N. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect Immun. 1992;60:312–315. doi: 10.1128/iai.60.1.312-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloeckaert A, Kerkhofs P, Limet J N. Antibody response to Brucella outer membrane proteins in bovine brucellosis: immunoblot analysis and competitive enzyme-linked immunosorbent assay using monoclonal antibodies. J Clin Microbiol. 1992;30:3168–3174. doi: 10.1128/jcm.30.12.3168-3174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloeckaert A, Zygmunt M S, Dubray G, Limet J N. Characterization of O-polysaccharide specific monoclonal antibodies derived from mice infected with the rough Brucella melitensis strain B115. J Gen Microbiol. 1993;139:1551–1556. doi: 10.1099/00221287-139-7-1551. [DOI] [PubMed] [Google Scholar]
- 14.Corbel M J. Recent advances in the study of Brucella antigens and their serological cross-reactions. Vet Bull. 1985;55:927–942. [Google Scholar]
- 15.Corbel M J, Brinley-Morgan W J. Genus Brucella Meyer and Shaw 1920, 173AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 377–388. [Google Scholar]
- 16.Diaz-Aparicio E, Aragon V, Marin C, Alonso B, Font M, Moreno E, Perez-Ortiz S, Blasco J M, Diaz R, Moriyon I. Comparative analysis of Brucella serotype A and M and Yersinia enterocolitica O:9 polysaccharides for serological diagnosis of brucellosis in cattle, sheep, and goats. J Clin Microbiol. 1993;31:3136–3141. doi: 10.1128/jcm.31.12.3136-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas J T, Palmer D A. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J Clin Microbiol. 1988;26:1353–1356. doi: 10.1128/jcm.26.7.1353-1356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubray G, Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987;138:27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- 19.Elzer P H, Jacobson R H, Jones S M, Nielsen K H, Douglas J T, Winter A J. Antibody-mediated protection against Brucella abortus in BALB/c mice at successive periods after infection: variation between virulent strain 2308 and attenuated vaccine strain 19. Immunology. 1994;82:651–658. [PMC free article] [PubMed] [Google Scholar]
- 20.Garin-Bastuji B, Bowden R A, Dubray G, Limet J N. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of smooth-lipopolysaccharide heterogeneity among Brucella biovars related to A and M specificities. J Clin Microbiol. 1990;28:2169–2174. doi: 10.1128/jcm.28.10.2169-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greiser-Wilke I, Moennig V. Monoclonal antibodies and characterization of epitopes of smooth Brucella lipopolysaccharides. Ann Inst Pasteur Microbiol. 1987;138:549–560. doi: 10.1016/0769-2609(87)90040-8. [DOI] [PubMed] [Google Scholar]
- 22.Greiser-Wilke I, Moennig V, Thon D, Rauter K. Characterization of monoclonal antibodies against Brucella melitensis. Zentbl VetMed Reihe B. 1985;32:616–627. doi: 10.1111/j.1439-0450.1985.tb02002.x. [DOI] [PubMed] [Google Scholar]
- 23.Hemmen F, Weynants V, Scarcez T, Letesson J J, Saman E. Cloning and sequence analysis of a newly identified Brucella abortus gene and serological evaluation of the 17-kilodalton antigen that it encodes. Clin Diagn Lab Immunol. 1995;2:263–267. doi: 10.1128/cdli.2.3.263-267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacques I, Olivier-Bernardin V, Dubray G. Induction of antibody and protective responses in mice by Brucella O-polysaccharide-BSA conjugate. Vaccine. 1991;9:896–900. doi: 10.1016/0264-410x(91)90010-4. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez de Bagüés M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limet J N, Bosseray N, Garin-Bastuji B, Dubray G, Plommet M. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J Med Microbiol. 1989;30:37–43. doi: 10.1099/00222615-30-1-37. [DOI] [PubMed] [Google Scholar]
- 27.Limet J N, Cloeckaert A, Bezard G, Van Broeck J, Dubray G. Antibody response to the 89-kDa outer membrane protein of Brucella in bovine brucellosis. J Med Microbiol. 1993;39:403–407. doi: 10.1099/00222615-39-6-403. [DOI] [PubMed] [Google Scholar]
- 28.Limet J, Plommet A-M, Dubray G, Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987;138:417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan A. Conventional serological tests. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 153–197. [Google Scholar]
- 30.Meikle P J, Perry M B, Cherwonogrodzky J W, Bundle D R. Fine structure of A and M antigens from Brucella biovars. Infect Immun. 1989;57:2820–2828. doi: 10.1128/iai.57.9.2820-2828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer M E. Current concepts in the taxonomy of the genus Brucella. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 1–17. [Google Scholar]
- 32.Montaraz J A, Winter A J, Hunter D M, Sowa B A, Wu A M, Adams L G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986;51:961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson D, Neil W, Poxton I R. A comparison of immunoblotting, flow cytometry and ELISA to monitor the binding of anti-lipopolysaccharide monoclonal antibodies. J Immunol Methods. 1990;133:227–233. doi: 10.1016/0022-1759(90)90363-z. [DOI] [PubMed] [Google Scholar]
- 34.Nicoletti P. Vaccination. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 283–299. [Google Scholar]
- 35.Palmer D A, Douglas J T. Analysis of Brucella lipopolysaccharide with specific and cross-reacting monoclonal antibodies. J Clin Microbiol. 1989;27:2331–2337. doi: 10.1128/jcm.27.10.2331-2337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips M, Deyoe B L, Canning P C. Protection of mice against Brucella abortus infection by inoculation with monoclonal antibodies recognizing Brucella O-antigen. Am J Vet Res. 1989;50:2158–2161. [PubMed] [Google Scholar]
- 37.Quinn R, Campbell A M, Phillips A P. A monoclonal antibody specific for the A antigen of Brucella spp. J Gen Microbiol. 1984;130:2285–2289. doi: 10.1099/00221287-130-9-2285. [DOI] [PubMed] [Google Scholar]
- 38.Rojas N, Freer E, Weintraub A, Ramirez M, Lind S, Moreno E. Immunochemical identification of Brucella abortus lipopolysaccharide epitopes. Clin Diagn Lab Immunol. 1994;1:206–213. doi: 10.1128/cdli.1.2.206-213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossetti O L, Arese A I, Boschiroli M L, Cravero S L. Cloning of a Brucella abortus gene and characterization of expressed 26-kilodalton periplasmic protein: potential use for diagnosis. J Clin Microbiol. 1996;34:165–169. doi: 10.1128/jcm.34.1.165-169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 41.Stevens M G, Olsen S C, Pugh G W, Jr, Brees D. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella abortus 19 or RB51. Infect Immun. 1995;63:264–270. doi: 10.1128/iai.63.1.264-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabatabai L B, Hennager S G. Cattle serologically positive for Brucella abortus have antibodies to B. abortus Cu-Zn superoxide dismutase. Clin Diagn Lab Immunol. 1994;1:506–510. doi: 10.1128/cdli.1.5.506-510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tibor A, Saman E, de Wergifosse P, Cloeckaert A, Limet J N, Letesson J-J. Molecular characterization, occurrence, and immunogenicity in infected sheep and cattle of two minor outer membrane proteins of Brucella abortus. Infect Immun. 1996;64:100–107. doi: 10.1128/iai.64.1.100-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verger J-M, Grayon M, Zundel E, Lechopier P, Olivier-Bernardin V. Comparison of the efficacy of Brucella suis strain 2 and Brucella melitensis Rev.1 live vaccines against a Brucella melitensis experimental infection in pregnant ewes. Vaccine. 1995;13:191–196. doi: 10.1016/0264-410x(95)93135-v. [DOI] [PubMed] [Google Scholar]
- 45.Vizcaino N, Chordi A, Fernandez-Lago L. Characterization of smooth Brucella lipopolysaccharides and polysaccharides by monoclonal antibodies. Res Microbiol. 1991;142:971–978. doi: 10.1016/0923-2508(91)90007-w. [DOI] [PubMed] [Google Scholar]
- 46.Vizcaino N, Fernandez-Lago L. Protection and suppression of the humoral immune response in mice mediated by a monoclonal antibody against the M epitope of Brucella. FEMS Immunol Med Microbiol. 1994;8:133–140. doi: 10.1111/j.1574-695X.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 47.Weynants V, Gilson D, Cloeckaert A, Denoel P A, Tibor A, Thiange P, Limet J N, Letesson J-J. Characterization of a monoclonal antibody specific for Brucella smooth lipopolysaccharide and development of a competitive enzyme-linked immunosorbent assay to improve the serological diagnosis of brucellosis. Clin Diagn Lab Immunol. 1996;3:309–314. doi: 10.1128/cdli.3.3.309-314.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weynants V, Gilson D, Cloeckaert A, Tibor A, Denoel P A, Godfroid F, Limet J N, Letesson J-J. Characterization of smooth lipopolysaccharides and O polysaccharides of Brucella species by competition binding assays with monoclonal antibodies. Infect Immun. 1997;65:1939–1943. doi: 10.1128/iai.65.5.1939-1943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winter A J, Duncan J R, Santisteban C G, Douglas J T, Adams L G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989;57:3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winter A J, Rowe G E, Duncan J R, Eis M J, Widom J, Ganem B, Morein B. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect Immun. 1988;56:2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter A J, Schurig G G, Boyle S M, Sriranganathan N, Bevins J S, Enright F M, Elzer P H, Kopec J D. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am J Vet Res. 1996;57:677–683. [PubMed] [Google Scholar]
- 52.Wright P F, Nielsen K H, Kelly W A. Primary binding techniques for the serodiagnosis of bovine brucellosis: enzyme immunoassay. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 199–235. [Google Scholar]
- 53.Xie X. Orally administrable brucellosis vaccine: Brucella suis strain 2 vaccine. Vaccine. 1986;4:212–216. doi: 10.1016/0264-410x(86)90131-3. [DOI] [PubMed] [Google Scholar]
- 54.Zygmunt M S, Cloeckaert A, Dubray G. Brucella melitensis cell envelope protein and lipopolysaccharide epitopes involved in humoral immune responses of naturally and experimentally infected sheep. J Clin Microbiol. 1994;32:2514–2522. doi: 10.1128/jcm.32.10.2514-2522.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]