Abstract
Background.
Maintenance therapy with 13-cis-retinoic acid and immunotherapy (given after completion of intensive cytotoxic therapy) improves outcome for high-risk neuroblastoma patients. The synthetic retinoid fenretinide (4-HPR) achieved multiple complete responses in relapse/refractory neuroblastoma in early-phase clinical trials, has low systemic toxicity, and has been considered for maintenance therapy clinical trials. Difluoromethylornithine (DFMO, an irreversible inhibitor of ornithine decarboxylase with minimal single agent clinical response data) is being used for maintenance therapy of neuroblastoma. We evaluated the cytotoxic activity of DFMO and fenretinide in neuroblastoma cell lines.
Procedure.
We tested sixteen neuroblastoma cell lines in bone marrow level hypoxia (5% O2) using the DIMSCAN cytotoxicity assay. Polyamines were measured by HPLC-mass spectrometry and apoptosis by TUNEL using flow cytometry.
Results.
At clinically achievable levels (100 μM), DFMO significantly decreased (P < 0.05) polyamine putrescine and achieved modest cytotoxicity (< 1 log (90% cytotoxicity). Prolonged exposures (7 days) or culture in 2% and 20% O2 did not enhance DFMO cytotoxicity. Whereas, fenretinide (10 μM) even at a concentration lower than clinically achievable in neuroblastoma patients (20 μM), induced ≥ 1 log cell kill in 14 cell lines. The average IC90 and IC99 of fenretinide was 4.7 ± 1 μM and 9.9 ± 1.8 μM respectively. DFMO did not induce a significant increase (P > 0.05) in apoptosis (TUNEL assay). Apoptosis by fenretinide was significantly higher (P < 0.001) compared to DFMO or controls.
Conclusions.
DFMO as a single agent has minimal cytotoxic activity for neuroblastoma cell lines.
Keywords: Maintenance therapy, Difluoromethylornithine, Fenretinide, Neuroblastoma
Introduction
Neuroblastoma is a malignant sympathetic nervous system tumor that accounts for ~ 8% of childhood cancers [1]. High-risk neuroblastoma is defined by age, stage, and MYCN oncogene amplification [2], and is treated with multi-agent chemotherapy, myeloablative consolidation, followed by high-dose, pulse 13-cis-retinoic acid (13-cis-RA) to treat minimal residual disease, improved survival if utilized before progressive disease [3, 4]. A further improvement in survival was seen with the addition of the anti-GD2 antibody dinutuximab (ch14.18) + cytokines [5]. Despite these advances, many high-risk neuroblastoma patients still relapse and die from progressive disease [6]. One potential approach to improving outcome for high-risk neuroblastoma would be employing additional agents during maintenance therapy that are tolerable and attack neuroblastoma via mechanisms differing from currently employed therapies.
N-(4-hydroxyphenyl)retinamide fenretinide (4-HPR), a synthetic retinoid, has been reported to be cytotoxic and/or inhibit the growth of neuroblastoma [7–10] and other cancer cell lines [10–12]. At concentrations achievable in patients, fenretinide induced multi-log cytotoxicity in neuroblastoma cell lines resistant to all-trans retinoic acid or 13-cis-RA [7]. Fenretinide does not induce cancer cell differentiation, but is cytotoxic independent of retinoid receptor or p53 pathways through increases of reactive oxygen species [12–14] and dihydroceramides [8, 11].
The initial capsule formulation of fenretinide had low bioavailability (1–3 μM). Pediatric phase I and phase II trials with the capsule formulation showed some activity in recurrent neuroblastoma [15, 16]. These early-phase studies of capsule 4-HPR were not able to determine true maximal tolerated dose due to the bioavailability issue, suggesting that improvement in the formulation of 4-HPR could be used to enhance clinically achievable levels and anti-tumor activity. Higher fenretinide exposures (~ 40–50 μM) have been achieved without dose-limiting toxicity by continuous infusion of an intravenous emulsion formulation [17]. An oral powder formulation (4-HPR/LXS) (~10 μM), employs a lipid matrix (Lym-X-Sorb) to increase bioavailability via gut absorption into the lymphatic system, similar to that of a chylomicron [18]. Fenretinide plasma levels obtained in children with LXS fenretinide (~12 μM) were significantly higher than with the capsule formulation, out of 29 evaluable patients, four had complete responses (in patients with bone marrow or bone disease only) after 10–30+ cycles and six had stable disease after 4–27 cycles [19]. Fenretinide exposures were further increased by inhibiting fenretinide metabolism with low doses of ketoconazole (CYP3A4 inhibitor), which was demonstrated in mice [20] and shown to enhance fenretinide activity against neuroblastoma murine xenografts [21], and in children with neuroblastoma ketoconazole achieved fenretinide plasma levels of ~20 μM was well tolerated, and had clinical activity, including two complete responses [22]. Fenretinide has been shown to synergize with variety of approved and investigational agents in neuroblastoma preclinical models [9, 23–25].
Ornithine decarboxylase (ODC), encoded by ODC1, catalyzes the rate-limiting step in polyamine synthesis. ODC1 decarboxylates ornithine from the urea cycle to create putrescine, from which the other polyamines spermidine, and spermine are synthesized [26]. High ODC1 was shown to correlate with poor clinical outcome in neuroblastoma patients, and was reported to be a transcriptional target of MYCN [27–29]. Difluoromethylornithine (DFMO; Eflornithine) is an irreversible inhibitor of ODC that is FDA approved for treating Trypanosoma brucei gambiense encephalitis [26].
Clinical trials of single agent DFMO as a chemopreventive agent in various cancers were inadequate [30]. In a neuroblastoma phase I trial in which single agent DFMO was given for 3 weeks followed by DFMO + etoposide, out of the eighteen toxicity-evaluable patients, none of the patients achieved a complete or partial response at clinically tolerated doses (500–1500 mg/m2/day) after one cycle of treatment with DFMO [31]. Overall, fifteen patients showed progressive disease at the end of the study, and three patients were reported to be without progression, but the contribution of etoposide to achieving stable disease also needs to be considered [31].
DFMO was reported to delay tumor growth in the TH-MYCN neuroblastoma GEM model, when the mice were treated by DFMO from birth through day 70 after birth [27, 28, 32]. DFMO as single agent was evaluated in two neuroblastoma cell line-derived xenograft models (SK-N-SH and BE2C) after tumors in mice reached ~ 75 – 175 mm3. SK-N-SH showed no significant response to DFMO, while BE2C showed delay in tumor growth for less than 10 days [32].
A single-arm trial has evaluated treating high-risk neuroblastoma patients with DFMO after they have completed myeloablative therapy and maintenance therapy with isotretinoin, dinutuximab [33, 34]. This latter study observed an apparent higher event-free and overall 2-year survival for patients receiving DFMO compared to the historical control of the COG ANBL-0032 study of dinutuximab + cytokines added to isotretinoin maintenance therapy.
Because of the use of single-agent DFMO in maintenance therapy of neuroblastoma and the consideration of fenretinide as a potential agent for use in the same setting we undertook a comparison of the anti-neuroblastoma activity of both agents using a large panel of human neuroblastoma cell lines.
Materials and Methods
Drugs
Fenretinide was supplied by the Developmental Therapeutics Program of the National Cancer Institute (Bethesda, MD), and DFMO was provided by Dr. Michael D. Hogarty.
Cell lines
The panel of sixteen neuroblastoma cell lines (Supplemental Table S1) were obtained from the Children’s Oncology Group Cell Culture and Xenograft Repository (www.COGcell.org; CHLA-15, CHLA-90, CHLA-119, CHLA-171, CHLA-253h, COG-N-269, COG-N-297, COG-N-415h, COG-N-452h, COG-N-453h, COG-N-474, COG-N-496h, COG-N-561h, FU-NB-2006, LA-N-6 and SK-N-BE(2)). Cell lines were cultured at 37oC in humidified incubators (Thermo Forma Series II Model 3130, Thermo Fisher Scientific, Marietta, OH), with oxygen tension validated by Fyrite testing (Bacharach, New Kensington, PA) and kept at bone marrow-level hypoxia (5% O2 with 5% CO2 & 90% N2) [35] except for comparator cytotoxicity experiments carried out in ambient air oxygen tension (20% O2, 5% CO2, & 75% N2) and tumor-level hypoxia (2% O2, 5% CO2, & 93% N2). Cell lines were cultured in complete Iscove’s Modified Dulbecco’s Medium (IMDM) (Thermo Scientific Hyclone, Logan, UT) supplemented with 20% FBS (Gibco – Life Technologies, Grand Island, NY), 4 mM L-glutamine (Corning Cellgro, Manassas, VA), insulin and transferrin (20 μg/ml each) and selenous acid (20 ng/mL) (ITS Culture Supplement) (BD Biosciences, San Jose, CA). To compare the variable concentrations of FBS on cytotoxicity and polyamine analysis experiments, we cultured SK-N-BE(2) cells at variable supplemental FBS conditions (5 %, 10% and 20%). CHLA-253h, COG-N-496h, COG-N-269h, COG-N-415h, COG-N-452h, COG-N-453h, and COG-N-561h were all cultured from initial establishment in culture from patient samples in 5% O2. Other cell lines were initially established in ambient air conditions. Cell line identities were verified via short tandem repeat (STR) profiling [36] validated with the database at www.COGcell.org. The cell lines were confirmed to be free of Epstein-Barr virus (EBV) by PCR as previously described [37], and were verified to be free of mycoplasma using the MycoAlert Mycoplasma Detection Kit (Lonza, Walkersville, MD).
Characterization of neuroblastoma cell line markers
Isolation of total cellular RNA employed RNeasy Mini Kit (Qiagen, Gaithersburg, MD) and genomic DNA the DNeasy Blood and Tissue Kit (Qiagen). Real-time reverse transcription PCR (qPCR) was performed using an ABI Prism 7400HT and RQ Manager 1.2 software (Life Technologies, Foster City, CA). Target genes were quantified using TaqMan primer probe sets and the TaqMan Gene Expression Master Mix (Life Technologies) in triplicate using 50°C for 2 minutes followed by 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds followed by 60°C for 60 seconds. Assay IDs of target genes were: Tyrosine hydroxylase (TH) (Hs00165941_m1) [38], glyceraldehyde 3-phosphate dehydrogenase (GAPDH – housekeeping gene control; Hs03929097_g1), MYCN copy number (Hs02718426_cn) [39], and RNaseP (copy number control; 4401631). Expression of TH (with GAPDH control), and MYCN copy number (with RNAseP control) were quantified using the ΔΔCt method. TH mRNA levels were all compared to CCD-1070Sk (human fibroblasts), assigned an expression value of 1. TH expression ≥10 was defined as positive while a value <10 was defined as negative expression. MYCN genomic amplification was defined as having ≥ 10 gene copies per diploid genome [39].
Polyamine content assay
CHLA-119, COG-N-452h and SK-N-BE(2) cell lines were cultured with polyamines added to the culture medium (10 μM each putrescine, spermidine, and spermine; Sigma-Aldrich (St Louis, MO)). Cells were treated with DFMO (100 μM) for 72 hours. Cells were collected, washed with 1X cold PBS and pelleted. Cells were suspended in 200 μL of deionized water, and extraction was carried out by adding 100 μL of 10% trichloroacetic acid (Sigma-Aldrich) (w/v) to 100 μL of aliquot. The extracts were washed twice with diethyl-ether in 5x volume of TCA-treated samples. Three μL of the aqueous layer after dilution with initial mobile phase at 1:4 ratio (v/v) was injected into the LC-MS/MS system.
Levels of putrescine, spermidine, and spermine were measured using a pH gradient-LC-MS/MS method suggested in previous study [40]. Analytes were separated on a scherzo SM-C18 column (3 μm, 50 × 2.0 mm i.d., Imtakt, Japan) with pH gradient generated from pH 5.3 to pH 2.7 with 2 mM ammonium acetate and 0.4% acetic acid in 10% acetonitrile (Sigma-Aldrich) at a flow rate of 0.2 mL/min. Detection of polyamines employed a Sciex 4000 QTRAP mass spectrometer by MRM mode using ion transitions of m/z 89.0 → 72.0 for putrescine, m/z 146.2 → 72.0 for spermidine, m/z 203.2 → 129.0 for spermine and m/z 117.1 → 100.0 for 1,6-diaminohexane (internal standard, Acros Organics). Concentrations of polyamines in all cells were normalized by the concentration of protein measured using the bicinchoninic acid assay (Thermo Fisher Scientific).
DIMSCAN cytotoxicity assay
Cytotoxicity of DFMO and fenretinide in sixteen neuroblastoma cell lines (Supplemental Table S1) was assessed with the DIMSCAN assay (BioImaging Solutions, San Diego, CA), a fluorescence based digital imaging microscopy system with a 4-log dynamic range [41]. Cell lines were seeded into 96-well plates in 150 μL of complete IMDM per well and incubated overnight. After overnight incubation, DFMO and fenretinide were added as single agents in 50 μL of complete medium, (0.01,.003,0.1,0.3,1,3,10,30,100) μM for DFMO and (0.003,0.01,.003,0.1,0.3,1,3,10,30) μM for fenretinide. Stock solution of DFMO was prepared at 100 mM in DMSO (WAK-Chemie Medical GmbHand, Siemensstr, Germany) and fenretinide at 10 mM in 100% ethanol (EMD Millipore, Billerica, MA). Six replicate wells were tested per concentration. Following 96 or 168 hours) of incubation, 50 μl of 0.5% eosin-Y + 10 μg/ml of FDA were added to the wells. After 20 min of incubation in the dark, the fluorescence of viable cells in each well was measured using the DIMSCAN system. The results were expressed as the survival fraction of treated cells for each concentration relative to the control cells. If the standard deviations for control wells exceeded 15%, the assay was repeated [42]. Data was analyzed using the DIMSCAN analyzer and the graphs were plotted using SigmaPlot (San Jose, CA).
Determination of caspase activation by immunoblotting and apoptosis by TUNEL assay
CHLA-119 and COG-N-452h were treated with DFMO (100 μM) for 72 hours and fenretinide (5 μM) for 62 hours. For caspase activation, cells were collected, washed with 1X cold PBS, and lysed in RIPA buffer (Thermo Fisher Scientific) supplemented with 1/20X protease inhibitor cocktail (Calbiochem, San Diego, CA). After 30 minutes of incubation, cells were centrifuged at 13,000 g for 25 min. Protein quantification employed the bicinchoninic acid assay. Twenty μg of protein in each sample was resolved by electrophoresis using 4–12% bis-tris gels (Thermo Fisher Scientific) and transferred to PVDF membrane (Bio-Rad, Hercules, CA). Immunoblots were probed with caspase-3, cleaved caspase-3 and beta-actin (Cell Signaling Technologies, Danvers, MA) followed by incubation with HRP-conjugated secondary antibodies. Proteins were visualized using chemiluminescence substrate [11, 13]
Apoptosis was measured using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (APO-DIRECT Kit, BD Biosciences, San Jose, CA). After treatment, cells were fixed in 1% (w/v) paraformaldehyde (Affymetrix, Santa Clara, CA) in PBS and stored in 70% ethanol at −20ºC for 24 hours. Cells were rinsed with 1X PBS and incubated with 50 μL of the DNA labeling solution for three hours at 37ºC. After incubation, cells were washed twice and suspended in 500 μL of propidium iodide/RNase staining buffer for 30 minutes of counterstaining, then analyzed with flow cytometry (BD LSR-II, BD Biosciences, San Jose, CA). Data analysis was performed using FlowJo software (Ashland, OR) and plotted using GraphPad Prism, (La Jolla, CA) [11, 43].
Statistical analysis
IC50, or IC90, or IC99 (concentrations cytotoxic and/or inhibitory of cell growth to 50%, or 90%, or 99% of control values) and the combination index (CIN) (used to assess synergy) were calculated using CalcuSyn (Biosoft, Cambridge, UK) [44]. Student’s t-test was used to determine significance for polyamines and TUNEL assay. Tests were considered significant at P < 0.05.
Results
DFMO decreased polyamine content in neuroblastoma cell lines
DFMO has been previously shown to reduce putrescine levels [32]. We evaluated the effect of DFMO on polyamines in SK-N-BE(2), COG-N-452h, and CHLA-119 neuroblastoma cell lines. In the phase I trial of DFMO (using doses of 500 to 1000 mg/m2 BID) average serum DFMO concentrations ranged from 9.5 μg/ml (52 μM) in patients receiving 500 mg/m2 to 14.7 μg/ml (80.5 μM) in patients receiving 1000 mg/m2 [31]. Thus for our studies we utilized 100 μM which is comparable to maximal levels achieved in clinical trials of DFMO as a single agent used in maintenance therapy of neuroblastoma NCT01586260 and NCT02679144. We evaluated the effect of DFMO (100 μM) on polyamines when cells were grown in varying concentrations of FBS (5%, 10% and 20%). In SK-N-BE(2), DFMO significantly reduced putrescine levels (P < 0.05) relative to controls in all the conditions (Fig. 1A).
Figure 1: DFMO decreased polyamine content in neuroblastoma cell lines.
Cell lines were cultured with polyamines (10 μM putrescine, spermidine and spermine) added to the culture media, and the cells were treated with DFMO (100 μM) for 72 hours. The bars represent concentration of polyamines normalized to the concentration of protein ± SD (n=3). (A) SK-N-BE(2) cells were cultured in varying concentrations on FBS (5%, 10% and 20%). DFMO treatment have significantly reduced putrescine levels (P < 0.05) relative to controls and the levels of spermidine and spermine were not significantly (P > 0.05, n.s) reduced. (B) In CHLA-119 and COG-N-452h (cultured in 20% FBS), DFMO treatment significantly reduced putrescine levels (P < 0.05) relative to controls and the levels of spermidine and spermine were not significantly (P > 0.05, n.s) reduced.
In all 3 cell lines that were studied, DFMO significantly reduced putrescine levels (P < 0.01 in SK-N-BE(2), P < 0.01 in COG-N-452h and P < 0.05 in CHLA-119) relative to controls. The levels of spermidine and spermine were not significantly (P > 0.05) reduced by DFMO (Fig. 1). These results are comparable to previous reports [27, 32].
DFMO is not cytotoxic to neuroblastoma cell lines
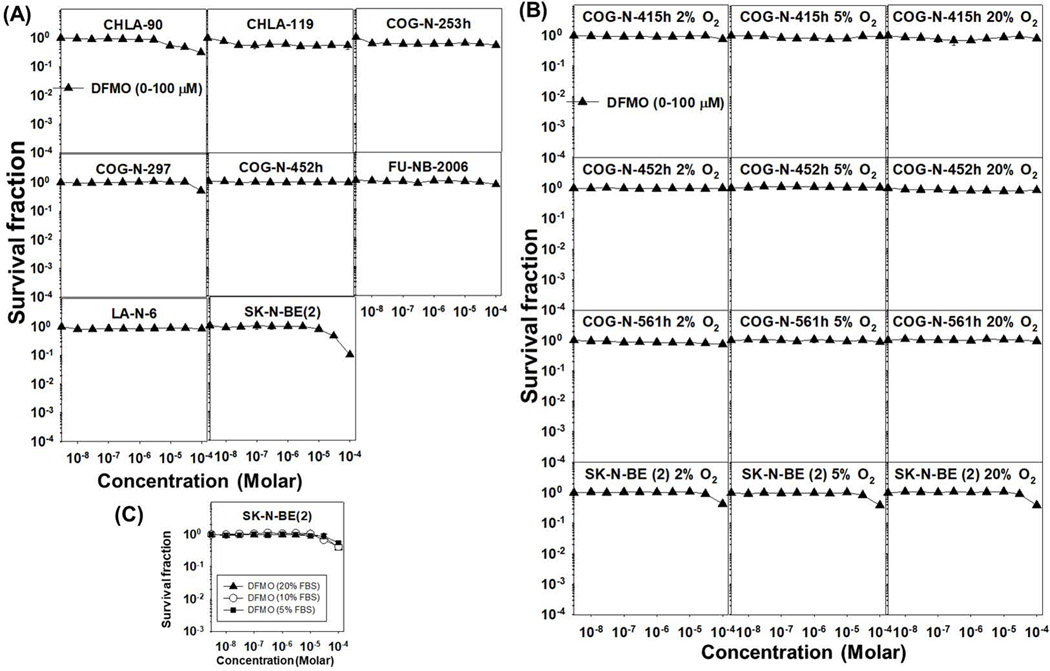
After confirming reduction of polyamines by DFMO in neuroblastoma cell lines we assessed DFMO (0.01–100 μM) cytotoxicity in a panel of sixteen neuroblastoma cell lines (Supplemental Table S1) cultured in bone marrow level hypoxia (5% O2 with 5% CO2 & 90% N2) so as to better replicate the microenvironment of the tumor cells in the patient. In none of the sixteen cell lines tested did DFMO cause significant cytotoxicity (≥ 1 log (90%) of cell kill). DFMO achieved an IC50 at 100 μM in one cell line (SK-N-BE (2)) (Fig. 2).
Figure 2: DFMO is not cytotoxic to neuroblastoma cell lines, whereas fenretinide induced multi-log cytotoxicity.
Dose response curves of DFMO (dark triangles) (0.01–100 μM) and fenretinide (4-HPR) (white circles) (0.003–30 μM) in sixteen neuroblastoma cell lines. Error bars represent standard deviation (n=6). Cell lines were cultured in bone marrow level hypoxia (5% O2). Cells were incubated with DFMO for 96 hours before DIMSCAN cytotoxicity analysis. The survival fraction was determined by mean fluorescence of the treated cells/mean fluorescence of control cells. At a concentration achievable in neuroblastoma patients, DFMO (100 μM) did not induce significant cytotoxicity (≥ 1 log (90%) of cell kill). Whereas, 4-HPR (10 μM) even at a concentration lower than clinically achievable (20 μM), induced ≥ 3 logs cells kill in three cell lines, ≥ 2 logs (99%) cells kill in eight cell lines, and ≥ 1 log in 3 cell lines.
To assess whether longer times of exposure to DFMO (0.01–100 μM) would enhance DFMO cytotoxicity we treated eight neuroblastoma cell lines (including three cells lines where DFMO at the highest concentration showed more than 30% cell kill with 4 days of incubation) with DFMO for 7 days (168 hours). Out of the eight cell lines tested DFMO induced significant cytotoxicity or growth arrest (≥ 1 log) in one cell line (SK-N-BE (2)) at the highest concentration tested. With the prolonged exposure DFMO achieved an IC50 (100 μM) in only two cell lines, SK-N-BE(2) and CHLA-90 (Fig. 3A).
Figure 3: Longer incubation, varying oxygen tensions and different FBS concentrations did not enhance DFMO cytotoxicity in neuroblastoma cell lines.
(A) Cells were incubated with DFMO for 168 hours (7 days) before DIMSCAN cytotoxicity analysis. At the highest concentration (100 μM) tested, DFMO induced significant cytotoxicity (≥ 1 log of cell kill) in one out of the eight cell lines tested. (B) Cells were cultured in 2% or in 20% O2 and incubated with DFMO (0.01–100 μM) for 96 hours before DIMSCAN cytotoxicity analysis. DFMO did not show significant cytotoxicity (≥ 1 log of cell kill) at the highest concentration (100 μM) tested. The cytotoxicity induced by DFMO was similar in all the three oxygen conditions tested. (C) The cytotoxicity induced by DFMO (0.01–100 μM) was similar at varying FBS (5, 10 and 20%) conditions tested in SK-N-BE (2) (5% O2 for 96 hours).
Varying oxygen tensions and different FBS concentrations did not enhance DFMO cytotoxicity in neuroblastoma cell lines
As we did not observe any significant cytotoxicity in neuroblastoma cell lines cultured in bone marrow level hypoxia, we determined if varying oxygen tensions might influence DFMO cytotoxicity. We assessed DFMO (0.01–100 μM) cytotoxicity in ambient air oxygen tension (20% O2, 5% CO2, & 75% N2) and tumor-level hypoxia (2% O2, 5% CO2, & 93% N2) in four neuroblastoma cell lines (after culture of the cell lines in the tested O2 concentrations for at least two passages). In all four cell lines DFMO failed to induce significant cytotoxicity (≥ 1 log cell kill) at the highest concentration (100 μM) in either 2% or in 20% O2. The modest cytotoxicity observed at 5% O2 was comparable to the other oxygen conditions tested (Fig. 3B). The cytotoxicity induced by DFMO was similar at all FBS conditions tested (Figure 3C).
Fenretinide achieved multi-log cytotoxicity in neuroblastoma cell lines
The average IC90 and IC99 of fenretinide for all sixteen neuroblastoma cell lines was 4.7 ± 1 μM and 9.9 ± 1.8 μM respectively, approximately ¼ and ½ of the clinically achievable concentration (20 μM) using oral dosing of the 4-HPR LXS formulation combined with ketoconazole [22]. Even at a lower concentration (10 μM), fenretinide induced > 3 logs (99.9%) of cell kill in three cell lines (CHLA-119, COG-N-297 and COG-N-415h), > 2 logs (99%) of cell kill in eight cell lines (CHLA-171, CHLA-253h, COG-N-269, COG-N-452h, COG-N-474, COG-N-496h and LA-N-6), and > 1 log cell kill in 3 cell lines (CHLA-15, CHLA-453h, FU-NB-2006) (Fig. 2). Fig. 4 shows images of representative DIMSCAN plates for neuroblastoma cell lines treated with DFMO or fenretinide.
Figure 4: Photomicrographs of DFMO and 4-HPR in neuroblastoma cell lines.
Photomicrographs of COG-N-452h and COG-N-297 treated with DFMO (0.01–100 μM) for 7 days and 4-HPR (0.003–30 μM) for 4 days. These photomicrographs show that the growth of surviving viable cells measured by DIMSCAN was due to growth of tumor colonies that correlated in size to the measured amount of fluorescein diacetate fluorescence.
Fenretinide induced grater apoptosis compared to DFMO in neuroblastoma cell lines
DFMO did not increase caspase cleavage or apoptosis (P > 0.05) compared to controls in COG-N-452h and CHLA-119 (Fig. 5). Fenretinide showed higher cleaved capase-3 and greater reduction in full length caspase-3 compared to DFMO in CHLA-119 and COG-N-452h (Fig. 5A). DFMO did not significantly increase apoptotic cells compared to controls (P > 0.05). Fenretinide caused apoptosis (TUNEL assay) in CHLA-119 (57.6% ± 0.5) and COG-N-452h (42.4% ± 2.6) both significantly higher (P < 0.001) compared to DFMO or to controls (Fig. 5B).
Figure 5. Fenretinide (4-HPR) induced grater apoptosis compared to DFMO in neuroblastoma cell lines.
(A) Immunoblot of capase-3 and cleaved capase-3 proteins. CHLA-119 and COG-N-452h were treated with DFMO (100 μM) for 72 hours and 4-HPR (5 μM) for 62 hours. The cell pellets were collected and immunoblotting was performed. Beta-actin served as a loading control. 4-HPR showed higher caspase cleavage compared to DFMO. (B) TUNEL assay was performed to detect apoptosis. The bars represent the mean percentage of apoptotic cells ± SD (n=3). DFMO did not significantly increase apoptotic cells compared to controls (P > 0.05, n.s). The percentage of apoptotic cells were significantly greater (P < 0.001) with 4-HPR compared to DFMO and controls in CHLA-119 and COG-N-452h.
Combining DFMO with fenretinide
We determined if DFMO and fenretinide had any cytotoxic interaction if combined (additivity, synergism, or antagonism) in neuroblastoma cell lines. Fenretinide and DFMO in combination showed no difference in cytotoxicity compared with fenretinide as a single agent in COG-N-415h and FU-NB-2006 (CIN > 1) (Fig. 6).
Figure 6: Combining DFMO with fenretinide.
Dose response curves of DFMO (dark triangles) (0.01–100 μM), 4-HPR (white circles) (0.003–30 μM) and DFMO + 4-HPR (dark squares) in COG-LL-415h and FU-NB-2006 in 5% O2 at 96 hours. Fenretinide and DFMO in combination showed no difference in cytotoxicity compared with fenretinide as a single agent (CIN > 1).
Discussion
This study was undertaken to compare the direct anti-neuroblastoma effect of two well-tolerated agents (DFMO and fenretinide) that have been considered for addition to the maintenance phase of therapy for high-risk neuroblastoma. The panel of neuroblastoma cell lines we employed represent high-risk neuroblastoma and included cell lines that have MYCN genomic amplification [45], the alternate lengthening of telomeres (ALT) phenotype [39], and anaplastic lymphoma kinase (ALK) mutations [46, 47]. DFMO was used to treat neuroblastoma cell lines at a concentration comparable to maximal levels achieved in a neuroblastoma maintenance therapy clinical trial (NCT01586260 and NCT02679144). DFMO decreased putrescine levels, both in a cell line which showed significant cytotoxicity or growth arrest (> 1 log) (SK-N-BE (2)), and also in cell lines which DFMO did not achieve significant cytotoxicity or growth arrest (COG-N-452h and CHLA-119). In spite of the effect on putrescine, DFMO showed minimal cytotoxicity across a large panel of neuroblastoma cell lines. Comparable to the cytotoxicity data, we did not observe caspase activation or an increase in apoptotic cells by DFMO compared to controls in neuroblastoma cells. Examination of images from the experiments confirmed that the surviving viable cells after DFMO treatment were due to outgrowth of tumor colonies.
Though ODC was shown to be a direct target of MYCN [27, 28], we did not see any significant single agent DFMO activity in the 10 MYCN amplified cell lines that we tested. Our results are comparable to the modest cytotoxicity of DFMO as a single agent in three neuroblastoma cell lines (SMS-KCNR, SH-SY5Y and BE (2)-C) reported by others [48].
In contrast to the minimal cytotoxicity observed with DFMO, fenretinide induced multi-log cytotoxicity in most of the cell lines tested. Comparable to the cytotoxicity data, we fenretinide activated caspases and significantly increased apoptotic cells compared to controls or to cells treated with DFMO. The multi-log cytotoxicity of fenretinide observed in some neuroblastoma cell lines is consistent with the multiple objective complete responses observed with fenretinide in neuroblastoma clinical trials [15, 16, 19, 22].
A limitation of this study is that it only employs cell lines in vitro, but this approach has the advantage of allowing testing across a well-controlled range of concentrations. Cell lines were tested in physiological hypoxia, minimizing the impact of non-physiological culture conditions (hyperoxia) common to most cell culture cytotoxicity testing. As the goal was to compare two well-tolerated agents that are both being considered for addition to maintenance therapy for high-risk neuroblastoma it would be ideal to have carried out the study also using animal models of neuroblastoma minimal residual disease, but such animal models have yet to be developed. We note that anti-neuroblastoma activity of fenretinide is not just limited to cell cultures but has also been demonstrated in vivo in multiple neuroblastoma xenograft models, including with patient derived xenografts (PDXs) [9, 18, 21, 23] and in clinical trials [15, 16, 19, 22].
This study is limited to assessing the activity of single agent DFMO at a concentration comparable to that used in an ongoing maintenance therapy clinical trial. The lack of cytotoxicity of DFMO does not preclude it being able to enhance cytotoxicity from other drugs used in combination, though we did not find any enhancement of fenretinide cytotoxicity. Also, DFMO has been postulated to potentially enhance immunological activity against neuroblastoma [26], as has fenretinide [24], and this study is not capable of assessing potential immunomodulatory effects.
We conclude that, single agent DFMO was not cytotoxic or cytostatic to a majority of neuroblastoma cell lines even at prolonged exposures and testing in various oxygen conditions. In contrast to the results with DFMO, fenretinide induced multi-log cytotoxicity in all neuroblastoma cell lines tested. These results are consistent with clinical studies of these agents in patients with neuroblastoma, where no clinical responses have been reported for single-agent DFMO [30, 31] and multiple objective clinical responses have been observed with fenretinide [15, 16, 19, 22]. In considering use of additional agents during the maintenance phase of neuroblastoma therapy, the sum of preclinical and clinical data should be employed to inform decisions. As maintenance therapy of neuroblastoma involves treating minimal residual disease, for which no objective response assessment is possible, the only definitive assessment can come from conducting well-controlled prospective randomized clinical trials.
Supplementary Material
Supplemental Table S1: Cell lines used in this study.
Acknowledgements
The authors thank Dr. Michael Hogarty for providing DFMO, the Alex’s Lemonade Stand/Children’s Oncology Group (COG) Childhood Cancer Repository (www.CCcells.org) for providing cell lines, TTUHSC Cancer Center cell culture core staff, Brian Rodriguez, and Dr. Michael Song for performing STR and EBV assays, determining TH status, MYCN amplification, mycoplasma testing, and technical support.
This work was presented previously in part at the 49th Annual Congress of the International Society of Pediatric Oncology (SIOP) conference in 2017 October, Washington DC, USA.
Abbreviations
- DFMO
Difluoromethylornithine
- 4-HPR
N-(4-hydroxyphenyl)retinamide or fenretenide
- 13-cis-RA
13-cis-retinoic acid
- ODC
Ornithine decarboxylase
- STR
Short tandem repeat
- EBV
Epstein-Barr virus
- TH
Tyrosine hydroxylase
- DIMSCAN
Semi-automatic digital image microscopy
- IC50
Reduction of cell survival to 50% of the control value
- IC90
Reduction of cell survival to 90% of the control value
- IC99
Reduction of cell survival to 99% of the control value
- CIN
Combination Index Number
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- ALT
Alternative lengthening of telomeres
- ALK
Anaplastic lymphoma kinase
Footnotes
Disclosures of Potential Conflicts of Interest
C. P. Reynolds report being co-inventor on issued patents for intravenous and oral formulations of fenretinide with financial interests through institutional intellectual property revenue sharing agreements, and is also consultant to, and owns stock in, CerRx, Inc., that licenses this technology. Dr. Kang is a consultant for CerRx, Inc. There are no other potential conflicts of interests.
Reference List
- 1.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematology/oncology clinics of North America 2010:24:65–86. [DOI] [PubMed] [Google Scholar]
- 2.Park JR, Bagatell R, London WB, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013:60:985–993. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. New England Journal of Medicine 1999:341:1165–1173. [DOI] [PubMed] [Google Scholar]
- 4.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. Journal of clinical oncology 2009:27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New England Journal of Medicine 2010:363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. Journal of clinical oncology 2007:25:1054–1060. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds CP, Wang Y, Melton LJ, et al. Retinoic-acid-resistant neuroblastoma cell lines show altered MYC regulation and high sensitivity to fenretinide. Medical and pediatric oncology 2000:35:597–602. [DOI] [PubMed] [Google Scholar]
- 8.Maurer BJ, Metelitsa LS, Seeger RC, et al. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst 1999:91:1138–1146. [DOI] [PubMed] [Google Scholar]
- 9.Chen NE, Maldonado V, Khankaldyyan V, et al. Reactive oxygen species mediates the synergistic activity of fenretinide combined with the microtubule Inhibitor ABT-751 against multi-drug resistant recurrent neuroblastoma xenografts. Mol Cancer Ther 2016:15:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Song MM, Makena MR, Hindle A, et al. Comparison of the cytotoxicity and increase of reactive oxygen species and dihydroceramides of fenretinide to its major metabolites (4-oxo-and 4-methoxyphenyl fenretinide) in T-cell lymphoid malignancy, neuroblastoma, and ovarian cancer cell lines. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research 2015:75(15 Suppl):Abstract nr 2616. [Google Scholar]
- 11.Holliday MW Jr., Cox SB, Kang MH, Maurer BJ. C22:0- and C24:0-dihydroceramides confer mixed cytotoxicity in T-cell acute lymphoblastic leukemia cell lines. PLoS One 2013:8:e74768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makena MR, Koneru B, Nguyen TH, et al. Reactive Oxygen Species-Mediated Synergism of Fenretinide and Romidepsin in Preclinical Models of T-cell Lymphoid Malignancies. Molecular Cancer Therapeutics 2017:16:649–661. [DOI] [PubMed] [Google Scholar]
- 13.Kang MH, Wan Z, Kang YH, et al. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst 2008:100:580–595. [DOI] [PubMed] [Google Scholar]
- 14.Corazzari M, Lovat PE, Oliverio S, et al. Fenretinide: a p53-independent way to kill cancer cells. Biochemical and biophysical research communications 2005:331:810–815. [DOI] [PubMed] [Google Scholar]
- 15.Villablanca JG, Krailo MD, Ames MM, et al. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children’s Oncology Group (CCG 09709). Journal of clinical oncology 2006:24:3423–3430. [DOI] [PubMed] [Google Scholar]
- 16.Villablanca JG, London WB, Naranjo A, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children’s Oncology Group. Clin Cancer Res 2011:17:6858–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohrbacher AM, Yang AS, Groshen S, et al. Phase I Study of Fenretinide Delivered Intravenously in Patients with Relapsed or Refractory Hematologic Malignancies: A California Cancer Consortium Trial. Clin Cancer Res 2017:23:4550–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer BJ, Kalous O, Yesair DW, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res 2007:13:3079–3086. [DOI] [PubMed] [Google Scholar]
- 19.Maurer BJ, Kang MH, Villablanca JG, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatric blood & cancer 2013:60:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper JP, Hwang K, Singh H, et al. Fenretinide metabolism in humans and mice: utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br J Pharmacol 2011:163:1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Barcons L, Maurer BJ, Kang MH, Reynolds CP. P450 inhibitor ketoconazole increased the intratumor drug levels and antitumor activity of fenretinide in human neuroblastoma xenograft models. Int J Cancer 2017:141:405–413. [DOI] [PubMed] [Google Scholar]
- 22.Maurer BJ, Glade Bender JL, Kang MH, et al. Fenretinide (4-HPR)/Lym-X-Sorb (LXS) oral powder plus ketoconazole in patients with high-risk (HR) recurrent or resistant neuroblastoma: A New Approach to Neuroblastoma Therapy (NANT) Consortium trial. In: 2014. p ASCO Annual Meeting Proceedings. Vol. 32. No. 15_suppl. 10071. [Google Scholar]
- 23.Fang H, Harned TM, Kalous O, et al. Synergistic activity of fenretinide and the Bcl-2 family protein inhibitor ABT-737 against human neuroblastoma. Clin Cancer Res 2011:17:7093–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibina A, Seidel D, Somanchi SS, et al. Fenretinide sensitizes multidrug-resistant human neuroblastoma cells to antibody-independent and ch14. 18-mediated NK cell cytotoxicity. Journal of Molecular Medicine 2013:91:459–472. [DOI] [PubMed] [Google Scholar]
- 25.Cheung BB, Tan O, Koach J, et al. Thymosin-ß4 is a determinant of drug sensitivity for Fenretinide and Vorinostat combination therapy in neuroblastoma. Molecular oncology 2015:9:1484–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassiri H, Benavides A, Haber M, et al. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Translational pediatrics 2015:4:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogarty MD, Norris MD, Davis K, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Research 2008:68:9735–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rounbehler RJ, Li W, Hall MA, et al. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Research 2009:69:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geerts D, Koster J, Albert D, et al. The polyamine metabolism genes ornithine decarboxylase and antizyme 2 predict aggressive behavior in neuroblastomas with and without MYCN amplification. International Journal of Cancer 2010:126:2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyskens FL, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clinical Cancer Research 1999:5:945–951. [PubMed] [Google Scholar]
- 31.Sholler GLS, Gerner EW, Bergendahl G, et al. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PloS one 2015:10:e0127246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evageliou NF, Haber M, Vu A, et al. Polyamine antagonist therapies inhibit neuroblastoma initiation and progression. Clinical Cancer Research 2016:22(17); 4391–404. [DOI] [PubMed] [Google Scholar]
- 33.Saulnier-Sholler G, Ferguson W, Bergendahl G, et al. Maintenance DFMO increases survival in high risk neuroblastoma. Pediatr Blood Cancer 2016:63 Suppl 3:S5–S321 [Abstract no. PD-087]. [Google Scholar]
- 34.Sholler G, Ferguson W, Bergendahl G, et al. DFMO maintains remission and increases overall survival in high risk neuroblastoma: results of a phase II prevention trial. In: Proceedings of the Advances in Neuroblastoma Research; 2016. p 19th-23rd June, Queensland, Australia, [Abstract no. 88]. [Google Scholar]
- 35.Grigoryan R, Keshelava N, Anderson C, Reynolds CP. In vitro testing of chemosensitivity in physiological hypoxia. Methods Mol Med 2005:110:87–100. [DOI] [PubMed] [Google Scholar]
- 36.Barallon R, Bauer SR, Butler J, et al. Recommendation of short tandem repeat profiling for authenticating human cell lines, stem cells, and tissues. In Vitro Cell Dev Biol Anim 2010:46:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai X, Hosler G, Rogers BB, et al. Quantitative polymerase chain reaction for human herpesvirus diagnosis and measurement of Epstein-Barr virus burden in posttransplant lymphoproliferative disorder. Clin Chem 1997:43:1843–1849. [PubMed] [Google Scholar]
- 38.Trager C, Kogner P, Lindskog M, et al. Quantitative analysis of tyrosine hydroxylase mRNA for sensitive detection of neuroblastoma cells in blood and bone marrow. Clinical chemistry 2003:49:104–112. [DOI] [PubMed] [Google Scholar]
- 39.Farooqi AS, Dagg RA, Choi LMR, et al. Alternative lengthening of telomeres in neuroblastoma cell lines is associated with a lack of MYCN genomic amplification and with p53 pathway aberrations. Journal of neuro-oncology 2014:119:17–26. [DOI] [PubMed] [Google Scholar]
- 40.Cho HE, Kang MH. pH gradient-liquid chromatography tandem mass spectrometric assay for determination of underivatized polyamines in cancer cells. J Chromatogr B Analyt Technol Biomed Life Sci 2018:1085:21–29. [DOI] [PubMed] [Google Scholar]
- 41.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther 2007:6:886–897. [DOI] [PubMed] [Google Scholar]
- 42.Kang MH, Smith MA, Morton CL, et al. National Cancer Institute pediatric preclinical testing program: model description for in vitro cytotoxicity testing. Pediatr Blood Cancer 2011:56:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tagde A, Singh H, Kang MH, Reynolds CP. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J 2014:4:e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med 2005:110:173–183. [DOI] [PubMed] [Google Scholar]
- 45.Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harbor perspectives in medicine 2013:3:a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenter EL, Mosse YP. Targeting ALK in neuroblastoma−preclinical and clinical advancements. Nature reviews Clinical oncology 2012:9:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krytska K, Ryles HT, Sano R, et al. Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin Cancer Res 2016:22:948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samal K, Zhao P, Kendzicky A, et al. AMXT-1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. International Journal of Cancer 2013:133:1323–1333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Cell lines used in this study.