Abstract
Background and Objectives
Intracranial artery stenosis is the predominant etiology of ischemic stroke in the Asian population. Furthermore, the presence of the RNF213 p.R4810K variant, which is a susceptibility gene for moyamoya disease, increases the risk of ischemic stroke attributable to large-artery atherosclerosis. Accordingly, we hypothesized that this genetic variant may affect the long-term outcome of intracranial artery stenosis in the East Asian population. We thus aimed to examine the effect of this variant on the long-term progression and prognosis of intracranial artery stenosis.
Methods
Using a prospective database, we identified adult patients with intracranial artery stenosis who underwent periodic MRI examinations for >5 years. We evaluated stenosis progression using a validated visual grading system. We excluded patients diagnosed with moyamoya disease at the time of initial MRI. Genotyping of RNF213 p.R4810K was performed at the end of the follow-up period.
Results
Among 52 eligible patients, 22 (42%) were carriers of the RNF213 p.R4810K variant. The median follow-up duration was 10.3 years. During the follow-up period, progression of intracranial artery stenosis was observed in 64% variant carriers and 27% noncarriers. There was a significant association of the variant with time to progression of intracranial artery stenosis (hazard ratio [HR] 3.31, 95% CI 1.38–7.90, p = 0.007), and time to the composite endpoint of symptomatic stroke and transient ischemic attack (HR 3.70, 95% CI 1.15–11.86, p = 0.028), but not to symptomatic stroke alone (HR 2.18, 95% CI 0.62–7.74, p = 0.23). Two variant carriers with progression were newly diagnosed with moyamoya disease.
Discussion
Our findings indicated that the RNF213 p.R4810K variant increases the risk of intracranial artery stenosis progression.
Intracranial artery stenosis is the predominant etiology of ischemic stroke worldwide, especially in Asia.1 There are ethnic differences in the prevalence of intracranial artery stenosis, with Asians showing a higher prevalence than Whites,2 which is suggestive of environmental and genetic susceptibility. A recent study detected the p.R4810K variant of the RNF213 gene, which is a susceptibility gene for moyamoya disease,3,4 in 20%–50% of patients with intracranial artery stenosis in East Asia.5,6 Moreover, this rare variant was detected in 1%–2% of the general population in East Asia.3 The presence of this variant increases the risk of ischemic stroke attributable to large-artery atherosclerosis.7 Accordingly, we hypothesized that this genetic variant may affect the long-term outcome of intracranial artery stenosis in the East Asian population. This study aimed to examine the association of the RNF213 p.R4810K variant with long-term progression and prognosis in patients with intracranial artery stenosis using a 15-year follow-up survey.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This longitudinal observational study was conducted at 2 comprehensive stroke centers in Osaka, Japan: Osaka University Hospital, and the National Cerebral and Cardiovascular Center (NCVC). This study was approved by the Ethics Committee for Clinical Research of Osaka University and the Ethics Committee of the NCVC. All the participants provided written informed consent.
Participants
We identified patients with asymptomatic or symptomatic intracranial artery stenosis at >1 month after the stroke onset who were enrolled in 2 prospectively collected registries (NCVC Genome Registry and Osaka Clinical Research Network for Stroke [OCR-STROKE] registry) between September 1, 2006, and October 31, 2021. We included adult patients aged older than 20 years who consented to RNF213 genetic testing and underwent periodic follow-up MRI examinations at our outpatient clinics for >5 years. We excluded patients with contraindications to MRI, patients who participated in other clinical trials, patients with dementia or intellectual disability, and patients diagnosed with moyamoya disease at the time of the initial MRI evaluation. Figure 1 shows a flow diagram of patient selection.
Figure 1. Flow Diagram of Patient Selection.
.
MRI Evaluation
MRI examinations were performed using 1.5 or 3.0 T scanners at each site. Since different MRI scanners with different field strengths were used in this study, the stenosis degree was evaluated through visual inspection for 9 predefined arterial segments on MR angiography (the basilar artery, right and left intracranial internal carotid artery, right and left A1-A2 segment of the anterior cerebral artery, right and left M1-M2 segment of the middle cerebral artery, and right and left P1-P2 segment of the posterior cerebral artery) based on the Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis method.8 Each segment was classified based on the validated grading scale9 as follows: grade 1, normal or mild stenosis (0%–29% diameter stenosis); grade 2, moderate stenosis (30%–69% diameter stenosis); grade 3, severe stenosis (70%–99% diameter stenosis); and grade 4, occlusion (100% diameter stenosis). The eligibility criterion for the inclusion of stenoses was a diameter of 30%–99% (grades 2–3). Progression was defined by an increase in the stenosis degree by at least 1 grade; furthermore, marked progression was indicated by an increase in the stenosis degree by more than 1 grade or by more than 1 arterial segment. Multiple intracranial stenoses were indicated by the presence of stenoses in more than 1 arterial segment. All images were independently evaluated by 2 blinded observers (S.O. and M.O.). Interobserver reliability for the presence of progression was calculated using kappa statistics (κ = 0.75, 95% CI 0.53–0.98). Between-observer disagreements were resolved through consensus.
RNF213 Genotyping
Peripheral blood samples were obtained from all the patients. RNF213 p.R4810K genotyping was performed at the end of follow-up between 2018 and 2021 using a GTS-7000 system (Shimadzu, Kyoto, Japan) as previously described.10 Based on the results, patients with the homozygous (AA genotype) and heterozygous (GA genotype) variants for p.R4810K were defined as variant carriers, while those with the wild-type homozygous (GG genotype) variant were defined as noncarriers.
Statistical Analysis
Categorical variables are presented as numbers and percentages, with comparisons using the Fisher exact test. Continuous variables are presented as the median and interquartile range, with comparisons using the Mann-Whitney U test. The start point for the follow-up period was the initial MRI examination after registry enrollment. In the survival analysis for progression, patients were censored at the time of MRI examination, on detection of new progression, or at the last follow-up MRI examination. In the survival analysis for transient ischemic attack (TIA) and symptomatic stroke, TIA was defined as a brief episode of neurologic dysfunction resulting from focal ischemia without evidence of infarction on MRI, while symptomatic stroke was defined as a neurologic deficit with acute infarction on CT or MRI. Patients were censored at the time of TIA, symptomatic stroke event, or last follow-up MRI examination. Notably, we only included patients with a >5-year MRI follow-up period and those who underwent genetic testing at the end of follow-up. Therefore, patients who died or dropped out because of severe cerebrovascular events during the follow-up period were excluded from the study. We used the Kaplan-Meier method to generate survival curves; furthermore, the log-rank test was used to compare the curves between variant carriers and noncarriers. Univariate and multivariate Cox regression models were used to calculate the hazard ratio (HR) of the variables on survival. In the multivariate Cox regression model, since most patients with dyslipidemia were treated using statins, we set up 2 different models to examine the effect of the RNF213 p.R4810K variant on the progression of intracranial stenosis. Model 1 included age, sex, presence of dyslipidemia, and presence of the RNF213 p.R4810K variant, while model 2 included age, sex, statin use, and presence of the RNF213 p.R4810K variant. Statistical significance was set at p < 0.05. All statistical analyses were performed using JMP Pro version 16.1.0 (SAS Institute Inc., Cary, NC).
Data Availability
Anonymized data not published in this article will be made available on request from any qualified investigator.
Results
Patient Characteristics
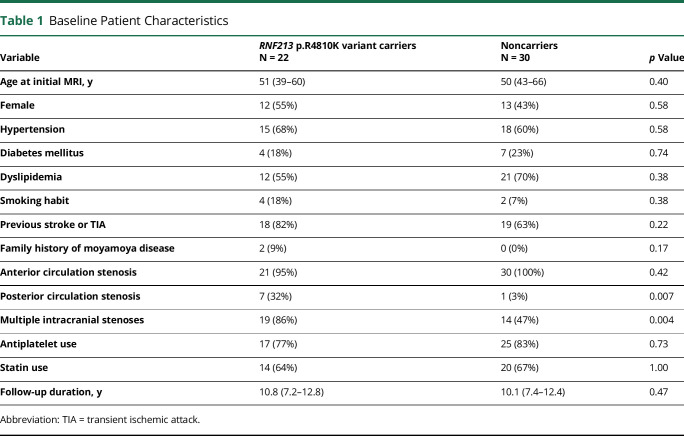
Among 52 eligible patients, 22 (42%) were carriers of the RNF213 p.R4810K variant. None of the patients were homozygous for the AA genotype. The median follow-up duration was 10.3 (interquartile range [IQR] 7.4–12.5) years, which ranged from 5.3 to 14.8 years. During the follow-up period, a median of 10 MRI scans was performed per patient (IQR: 7–13). Table 1 summarizes the baseline patient characteristics. There were no between-group differences in the age at initial MRI examination, sex, and prevalence of conventional risk factors. Two variant carriers had a family history of moyamoya disease. Most patients (98%) had anterior circulation stenosis; in addition, compared with noncarriers, variant carriers had more frequent posterior circulation stenoses. Multiple intracranial stenoses were more common among variant carriers than among noncarriers. There was no between-group difference in the follow-up duration.
Table 1.
Baseline Patient Characteristics
Progression of Intracranial Artery Stenosis
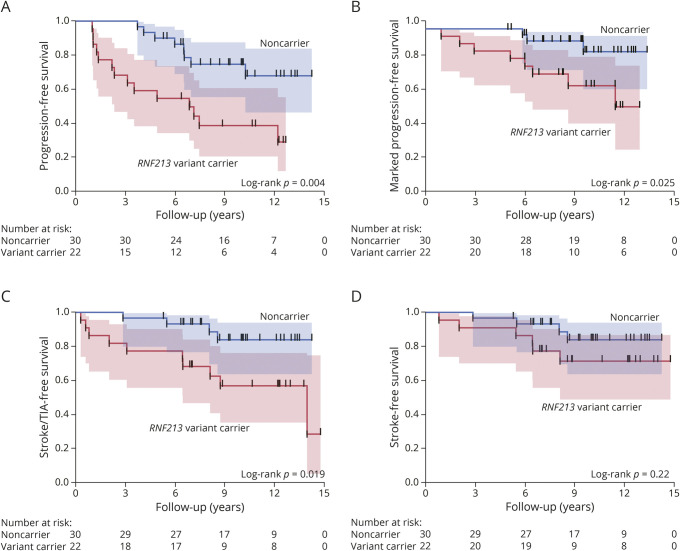
During the follow-up period, progression of intracranial artery stenosis was observed in 14 (64%) variant carriers and 8 (27%) noncarriers (odds ratio [OR] 4.81, 95% CI 1.47–15.77, p = 0.011). Marked progression was observed in 8 (36%) variant carriers and 3 (10%) noncarriers (OR 5.14, 95% CI 1.18–22.49, p = 0.037). During the follow-up period, 2 variant carriers with marked progression were newly diagnosed with moyamoya disease. Figure 2A shows the Kaplan-Meier estimates of event-free survival for the progression of intracranial artery stenosis, while Figure 2B shows those for marked progression. There was a significant association of the RNF213 p.R4810K variant with time to progression of intracranial artery stenosis (log-rank p = 0.004) and time to marked progression (log-rank p = 0.025). Univariate Cox regression analyses showed that the presence of dyslipidemia (HR 0.41, 95% CI 0.18–0.94, p = 0.035), statin use (HR 0.43, 95% CI 0.18–0.99, p = 0.047), and RNF213 p.R4810K variant carriers (HR 3.31, 95% CI 1.38–7.90, p = 0.007) were significantly associated with the progression of intracranial artery stenosis (Table 2). Statins were administered to 28 of the 33 (85%) patients with dyslipidemia. In a multivariate Cox regression model adjusted for age and sex, statin use (HR 0.25, 95% CI 0.10–0.63, p = 0.004) and the RNF213 p.R4810K variant carrier status (HR 5.11, 95% CI 1.96–13.32, p = 0.0009) were independent predictors of the progression of intracranial artery stenosis (Table 2). Figure 3A shows the Kaplan-Meier estimates of progression-free survival in patients with and without statin treatment. Progression of intracranial stenosis was significantly less frequent among patients who received statins than among patients who did not. Stratification according to the RNF213 p.R4810K variant revealed a significant risk reduction by statin treatment in variant carriers (Figure 3B; HR 0.20, 95% CI 0.06–0.63, p = 0.006) but not in noncarriers (Figure 3C; HR 0.65, 95% CI 0.16–2.63, p = 0.55). We observed a similar interaction of the RNF213 p.R4810K variant with statin treatment for the marked progression of intracranial artery stenosis (Figure 4).
Figure 2. Kaplan-Meier Curves for Events in Patients With and Without the RNF213 p.R4810K Variant.
Figures represent the Kaplan-Meier estimates for the progression of intracranial artery stenosis (A), marked progression of intracranial artery stenosis (B), composite endpoint of symptomatic stroke and TIA (C), and symptomatic stroke (D) based on the presence of RNF213 p.R4810K variant. TIA = transient ischemic attack.
Table 2.
Univariate and Multivariate Cox Regression Analyses for Progression-Free Survival
Figure 3. Kaplan-Meier Curves for the Progression of Intracranial Artery Stenosis in Patients With and Without Statin Use.
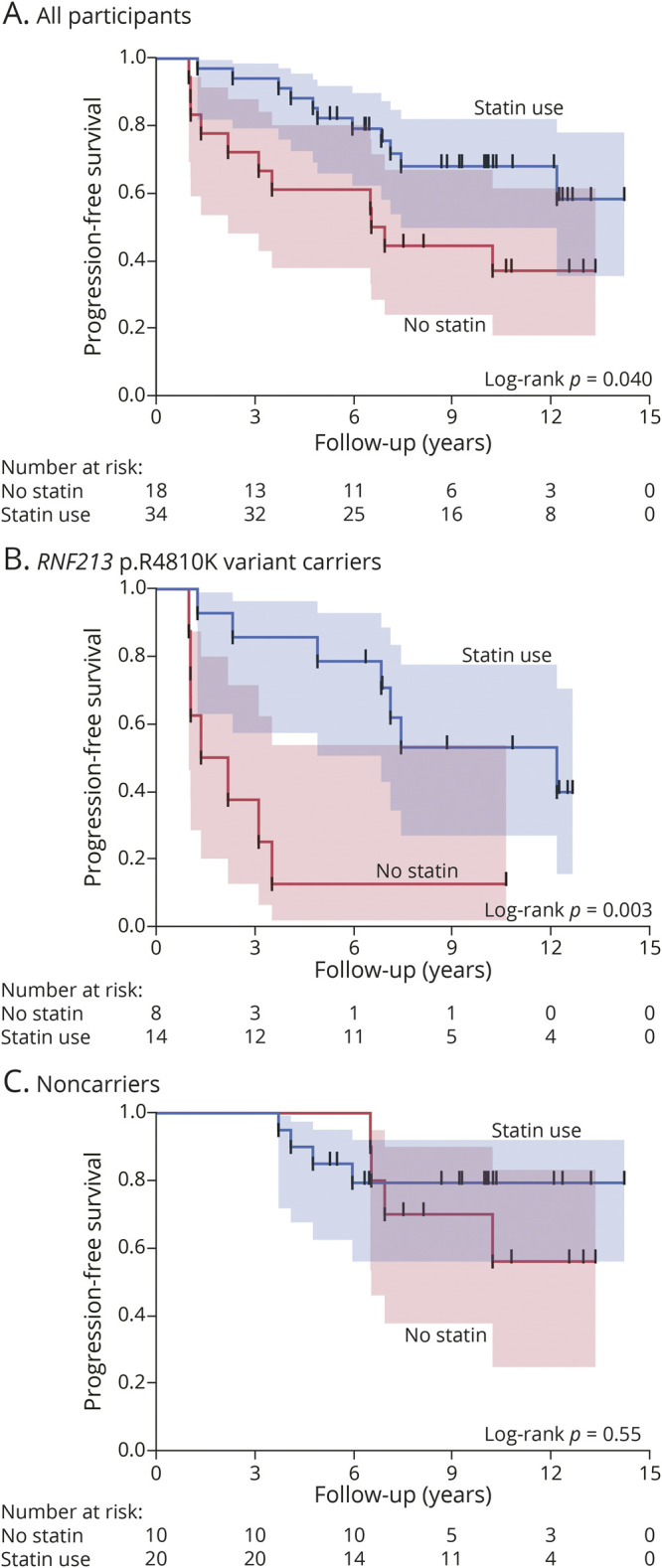
Kaplan-Meier estimates for the progression of intracranial artery stenosis according to the use of statins (A). Subgroup analyses of RNF213 p.R4810K variant carriers (B) and noncarriers (C).
Figure 4. Kaplan-Meier Curves for the Marked Progression of Intracranial Artery Stenosis in Patients With and Without Statin Use.
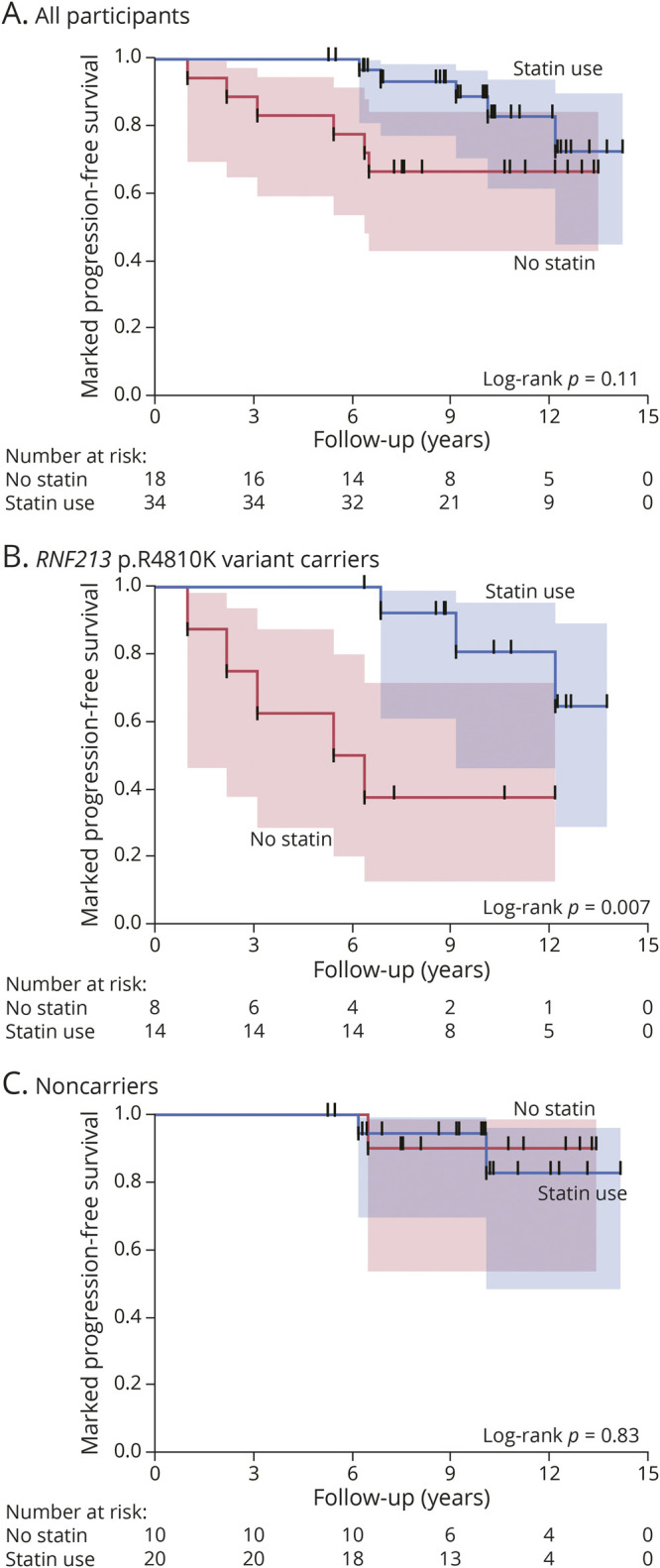
Kaplan-Meier estimates for the progression of intracranial artery stenosis according to the use of statins (A). Subgroup analyses of RNF213 p.R4810K variant carriers (B) and noncarriers (C).
Cerebrovascular Events
The composite endpoint of symptomatic stroke and TIA was observed in 45% and 13% of the variant carriers and noncarriers, respectively (OR 5.52, 95% CI 1.41–20.82, p = 0.013), while symptomatic stroke was observed in 27% and 13% of variant carriers and noncarriers, respectively (OR 2.44, 95% CI 0.60–9.99, p = 0.29). The RNF213 p.R4810K variant increased the risk of the composite endpoint of stroke and TIA (Figure 2C, log-rank p = 0.019), but not symptomatic stroke alone (Figure 2D, log-rank p = 0.215). Univariate Cox regression analyses revealed that the RNF213 variant carrier was associated with the time to the composite endpoint of symptomatic stroke and TIA (HR 3.70, 95% CI 1.15–11.86, p = 0.028), but not to symptomatic stroke alone (HR 2.18, 95% CI 0.62–7.74, p = 0.23).
Discussion
Our findings demonstrated that the RNF213 p.R4810K variant significantly increased the risk of intracranial artery stenosis progression; furthermore, this variant increased the composite risk of symptomatic stroke and TIA, but not symptomatic stroke alone.
A study on patients with moyamoya disease reported an association between the RNF213 p.R4810K variant and the progression of intracranial artery stenosis. The RNF213 p.R4810K variant was associated with contralateral progression in patients with unilateral moyamoya disease.11 Furthermore, bilateral involvement was observed in 83% and 52% of p.R4810K-positive and p.R4810K-negative patients with moyamoya disease, respectively.12 Moreover, the p.R4810K variant was associated with the recurrence of stroke or TIA in patients with symptomatic intracranial artery stenosis, including moyamoya disease.13 Taken together, these findings indicated continuity between moyamoya disease and intracranial artery stenosis, which is termed RNF213-related vasculopathy.7,14 Although the biological function of RNF213 protein remains unclear, it is a known key regulator of lipid metabolism, inflammation, and vascular stability.15 Vascular vulnerability caused by the RNF213 variant may promote the progression of intracranial artery stenosis.
Notably, we observed that the presence of dyslipidemia significantly prevented the progression of intracranial artery stenosis. Since 85% of our patients with dyslipidemia received statins, this negative correlation might be suggestive of the beneficial effects of statin therapy for preventing the progression of intracranial artery stenosis. There have been consistent reports regarding the protective effects of statins against intracranial artery stenosis2,16,17 and moyamoya disease18 in East Asian countries. Our findings demonstrated a significant risk reduction by statin treatment mainly in variant carriers. A recent experimental study reported a relationship between RNF213 protein and lipid metabolism mediated by the regulation of cytoplasmic lipid droplets.19 Based on these results, aggressive lipid lowering treatment is a possible target for future research in RNF213 variant carriers with intracranial artery stenosis.
The strengths of this study include its long follow-up period and the high ratio of completed periodic MRI examinations. However, this study has some limitations, including its small sample size and population stratification bias due to the study design. According to our results, the RNF213 p.R4810K variant carriers developed cerebrovascular events more often than noncarriers. It is possible that the excluded patients, who died or dropped out during the follow-up period, included a large number of variant carriers, which may result in an underestimation of the effect of this genetic variant. In addition, we excluded patients diagnosed with moyamoya disease, 80%–90% of whom have this genetic variant.3 Patients with moyamoya disease develop cerebrovascular events with an annual incidence of 2.4%–5.7%.20 This exclusion criterion may also lead to an underestimation of the risk of the RNF213 p.R4810K variant. Moreover, due to advances in MRI scanners, we could not standardize the MRI protocol and field strength. Accordingly, this resulted in relatively low interobserver reliability regarding the presence of progression. Further validation studies using high-resolution MRI with vessel wall imaging are warranted to confirm these findings. Finally, the RNF213 p.R4810K variant was found only in East Asians, but it was extremely rare in Whites (maximum allele frequency: 0.0006) and other ethnic groups.7,21 Therefore, our findings cannot be generalized to ethnic groups outside East Asian countries.
In conclusion, our findings demonstrated that the RNF213 p.R4810K variant is a strong predictor of the progression of intracranial artery stenosis. Our results indicate the importance of genetic testing for RNF213 in patients with intracranial stenosis for predicting prognosis. Targeted prevention and management strategies, including aggressive lipid lowering therapy, are warranted for RNF213 p.R4810K variant carriers with intracranial artery stenosis.
Acknowledgment
We are grateful to Dr. Yumi Yamamoto and Ms. Natsuki Hanada for their technical assistance with RNF213 genotyping. We thank the OCR-STROKE investigators and NCVC Genome Registry investigators for their participation in data collection. A full list of investigators is provided in Appendix 2.
Glossary
- IQR
interquartile range
- OR
odds ratio
- TIA
transient ischemic attack
Appendix 1. Authors

Appendix 2. Coinvestigators
Contributor Information
Takeshi Yoshimoto, Email: yoshimototakeshi1982@ncvc.go.jp.
Mariko Ohara, Email: m.ohara@neurol.med.osaka-u.ac.jp.
Masatoshi Takagaki, Email: m-takagaki@nsurg.med.osaka-u.ac.jp.
Hajime Nakamura, Email: hajime@nsurg.med.osaka-u.ac.jp.
Kotaro Watanabe, Email: watawata@ommc-hp.jp.
Yasufumi Gon, Email: gon@neurol.med.osaka-u.ac.jp.
Kenichi Todo, Email: ktodo@neurol.med.osaka-u.ac.jp.
Tsutomu Sasaki, Email: sasaki@neurol.med.osaka-u.ac.jp.
Hiroyuki Araki, Email: araki@jbcrg.jp.
Tomomi Yamada, Email: tomomi.yamada@dmi.med.osaka-u.ac.jp.
Shirou Manabe, Email: manabe@hp-info.med.osaka-u.ac.jp.
Haruhiko Kishima, Email: hkishima@nsurg.med.osaka-u.ac.jp.
Masafumi Ihara, Email: ihara@ncvc.go.jp.
Hideki Mochizuki, Email: hmochizuki@neurol.med.osaka-u.ac.jp.
Study Funding
This work was supported by JSPS KAKENHI (grant number JP19K06922) and the Japan Agency for Medical Research and Development (grant number JP21ek0210120).
Disclosure
S. Okazaki was funded by JSPS KAKENHI (grant number JP19K06922). T. Yoshimoto, M. Ohara, M. Takagaki, H. Nakamura, K. Watanabe, Y. Gon, K. Todo, T. Sasaki, H. Araki, T. Yamada, S. Manabe, and H. Kishima report no disclosures. M. Ihara was funded by the Japan Agency for Medical Research and Development (grant number JP21ek0210120). H. Mochizuki reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396-2399. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res. 2017;120(3):502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Morito D, Takashima S, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6(7):e22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada F, Aoki Y, Narisawa A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011;56(1):34-40. [DOI] [PubMed] [Google Scholar]
- 5.Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N. Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke. 2012;43(12):3371-3374. [DOI] [PubMed] [Google Scholar]
- 6.Bang OY, Chung JW, Cha J, et al. A polymorphism in RNF213 is a susceptibility gene for intracranial atherosclerosis. Plos One. 2016;11(6):e0156607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki S, Morimoto T, Kamatani Y, et al. Moyamoya disease susceptibility variant RNF213 p.R4810K increases the risk of ischemic stroke attributable to large-artery atherosclerosis. Circulation. 2019;139(2):295-298. [DOI] [PubMed] [Google Scholar]
- 8.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643-646. [PMC free article] [PubMed] [Google Scholar]
- 9.Wong KS, Lam WW, Liang E, Huang YN, Chan YL, Kay R. Variability of magnetic resonance angiography and computed tomography angiography in grading middle cerebral artery stenosis. Stroke. 1996;27(6):1084-1087. [DOI] [PubMed] [Google Scholar]
- 10.Ohara M, Yoshimoto T, Okazaki S, et al. RNF213 p.R4810K variant carriers with intracranial arterial stenosis have a low atherosclerotic burden. J Atheroscler Thromb. Published online December 25, 2021. doi: 10.5551/jat.63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mineharu Y, Takagi Y, Koizumi A, et al. Genetic and nongenetic factors for contralateral progression of unilateral moyamoya disease: the first report from the SUPRA Japan Study Group. J Neurosurg. 2021;136(4):1005-1014. [DOI] [PubMed] [Google Scholar]
- 12.Moteki Y, Onda H, Kasuya H, et al. Systematic validation of RNF213 coding variants in Japanese patients with moyamoya disease. J Am Heart Assoc. 2015;4(5):e001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Choi EH, Chung JW, et al. Luminal and wall changes in intracranial arterial lesions for predicting stroke occurrence. Stroke. 2020;51(8):2495-2504. [DOI] [PubMed] [Google Scholar]
- 14.Bang OY, Chung JW, Kim DH, et al. Moyamoya disease and spectrums of RNF213 vasculopathy. Transl Stroke Res. 2020;11(4):580-589. [DOI] [PubMed] [Google Scholar]
- 15.Mineharu Y, Miyamoto S. RNF213 and GUCY1A3 in moyamoya disease: key regulators of metabolism, inflammation, and vascular stability. Front Neurol. 2021;12:687088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JW, Cha J, Lee MJ, et al. Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study (STAMINA-MRI Study). J Neurol Neurosurg Psychiatry. 2020;91(2):204-211. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Lu Z, Gao P, et al. Efficacy and safety of intensive statin therapy in Chinese patients with atherosclerotic intracranial arterial stenosis: a single-center, randomized, single-blind, parallel-group study with one-year follow-up. Clin Neurol Neurosurg. 2014;120:6-13. [DOI] [PubMed] [Google Scholar]
- 18.Wang QN, Bao XY, Zou ZX, et al. The role of atorvastatin in collateral circulation formation induced by encephaloduroarteriosynangiosis: a prospective trial. Neurosurg Focus. 2021;51(3):E9. [DOI] [PubMed] [Google Scholar]
- 19.Sugihara M, Morito D, Ainuki S, et al. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J Cell Biol. 2019;218(3):949-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ihara M, Yamamoto Y, Hattori Y, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 2022;21(8):747-758. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Senevirathna ST, Hitomi T, et al. Genomewide association study identifies no major founder variant in Caucasian moyamoya disease. J Genet. 2013;92(3):605-609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published in this article will be made available on request from any qualified investigator.