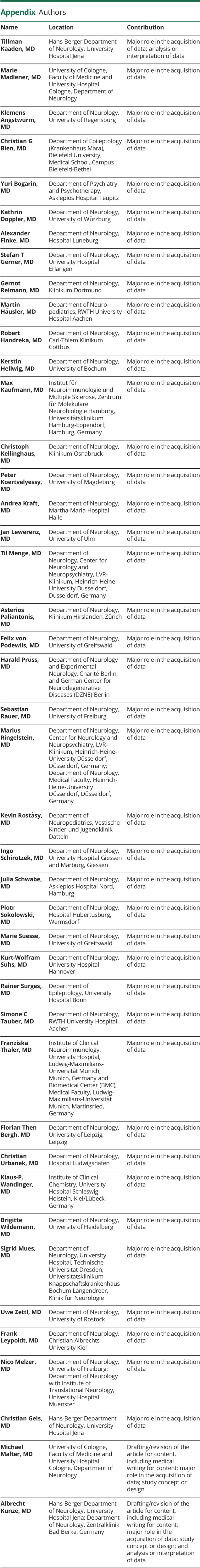
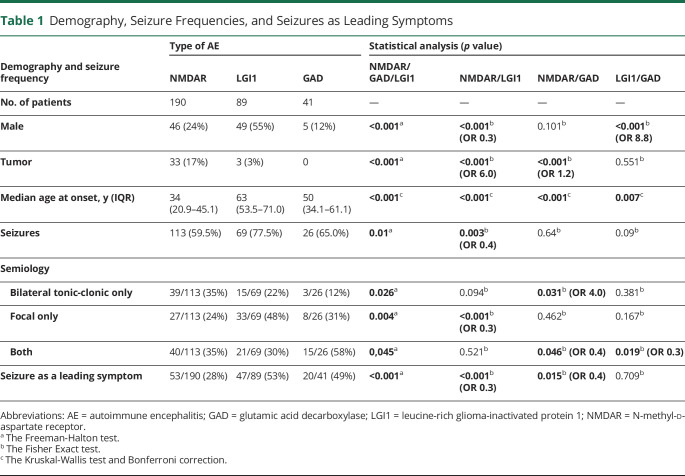
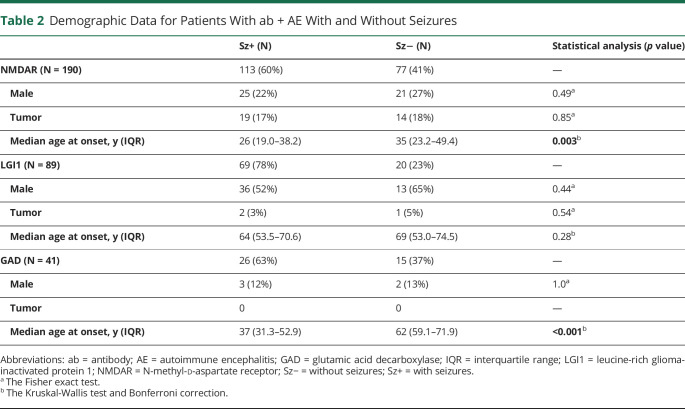
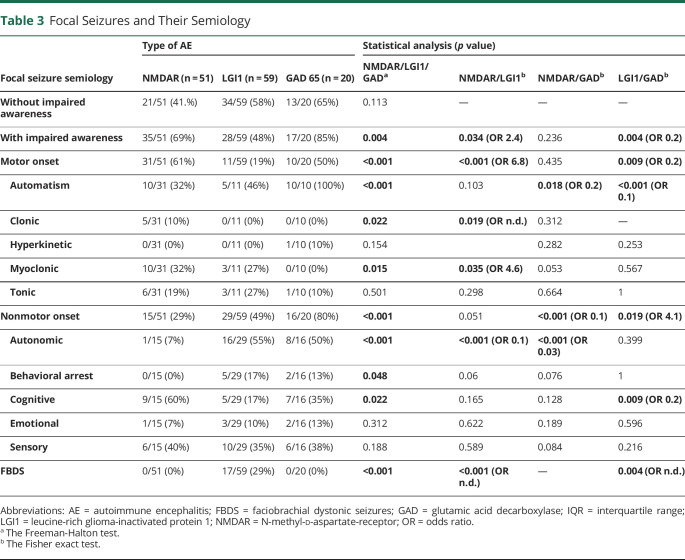
Tillman Kaaden
Tillman Kaaden, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,*,✉,
Marie Madlener
Marie Madlener, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Klemens Angstwurm
Klemens Angstwurm, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Christian G Bien
Christian G Bien, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Yuri Bogarin
Yuri Bogarin, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Kathrin Doppler
Kathrin Doppler, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Alexander Finke
Alexander Finke, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Stefan T Gerner
Stefan T Gerner, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Gernot Reimann
Gernot Reimann, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Martin Häusler
Martin Häusler, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Robert Handreka
Robert Handreka, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Kerstin Hellwig
Kerstin Hellwig, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Max Kaufmann
Max Kaufmann, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Christoph Kellinghaus
Christoph Kellinghaus, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Peter Koertvelyessy
Peter Koertvelyessy, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Andrea Kraft
Andrea Kraft, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Jan Lewerenz
Jan Lewerenz, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Til Menge
Til Menge, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Asterios Paliantonis
Asterios Paliantonis, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Felix von Podewils
Felix von Podewils, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Harald Prüss
Harald Prüss, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Sebastian Rauer
Sebastian Rauer, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Marius Ringelstein
Marius Ringelstein, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Kevin Rostásy
Kevin Rostásy, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Ingo Schirotzek
Ingo Schirotzek, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Julia Schwabe
Julia Schwabe, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Piotr Sokolowski
Piotr Sokolowski, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Marie Suesse
Marie Suesse, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Kurt-Wolfram Sühs
Kurt-Wolfram Sühs, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Rainer Surges
Rainer Surges, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Simone C Tauber
Simone C Tauber, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Franziska Thaler
Franziska Thaler, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Florian Then Bergh
Florian Then Bergh, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Christian Urbanek
Christian Urbanek, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Klaus-P Wandinger
Klaus-P Wandinger, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Brigitte Wildemann
Brigitte Wildemann, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Sigrid Mues
Sigrid Mues, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Uwe Zettl
Uwe Zettl, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Frank Leypoldt
Frank Leypoldt, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Nico Melzer
Nico Melzer, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Christian Geis
Christian Geis, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,
Michael Malter
Michael Malter, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,†,
Albrecht Kunze
Albrecht Kunze, MD
1From the Hans-Berger Department of Neurology (T.K., C.G., Albrecht Kunze), University Hospital Jena; Department of Neurology (Marie Madlener, Michael Malter), Faculty of Medicine and University Hospital Cologne, University of Cologne; Department of Neurology (K.A.), University of Regensburg; Department of Epileptology (Krankenhaus Mara) (C.G.B.), Bielefeld University, Medical School, Campus Bielefeld-Bethel; Department of Psychiatry and Psychotherapy (Y.B.), Asklepios Hospital Teupitz; Department of Neurology (K.D.), University of Würzburg; Department of Neurology (A.F.), Hospital Lüneburg; Department of Neurology (S.T.G.), University Hospital Erlangen; Department of Neurology (G.R.), Klinikum Dortmund; Department of Neuro-pediatrics (M.H.), RWTH University Hospital Aachen; Department of Neurology (R.H.), Carl-Thiem Klinikum Cottbus; Department of Neurology (K.H.), University of Bochum; Institut für Neuroimmunologie und Multiple Sklerose (M.K.), Zentrum für Molekulare Neurobiologie Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology (C.K.), Klinikum Osnabrück; Department of Neurology (P.K.), University of Magdeburg; Department of Neurology (Andrea Kraft), Martha-Maria Hospital Halle; Department of Neurology (J.L.), University of Ulm; Department of Neurology (T.M., M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (A.P.), Klinikum Hirslanden, Zürich; Department of Neurology (F.v.P., M.S.), University of Greifswald; Department of Neurology and Experimental Neurology (H.P.), Charité Berlin, and German Center for Neurodegenerative Diseases (DZNE); Department of Neurology (S.R., N.M.), University of Freiburg; Department of Neurology (M.R.), Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany; Department of Neuropediatrics (K.R.), Vestische Kinder-und Jugendklinik Datteln; Department of Neurology (I.S.), University Hospital Giessen and Marburg, Giessen; Department of Neurology (J.S.), Asklepios Hospital Nord, Hamburg; Department of Neurology (P.S.), Hospital Hubertusburg, Wermsdorf; Department of Neurology (K.-W.S.), University Hospital Hannover; Department of Epileptology (R.S.), University Hospital Bonn; Department of Neurology (S.C.T.), RWTH University Hospital Aachen; Institute of Clinical Neuroimmunology (F.T.), University Hospital, Ludwig-Maximilians-Universität Munich, Germany and Biomedical Center (BMC), Medical Faculty, Ludwig-Maximilians-Universität Munich, Martinsried, Germany; Department of Neurology (F.T.B.), University of Leipzig; Department of Neurology (C.U.), Hospital Ludwigshafen; Institute of Clinical Chemistry (K.-P.W.), University Hospital Schleswig-Holstein, Kiel/Lübeck, Germany; Department of Neurology (B.W.), University of Heidelberg; Department of Neurology (S.M.), University Hospital, Technische Universität Dresden; Universitätsklinikum Knappschaftskrankenhaus Bochum Langendreer (S.M.), Klinik für Neurologie; Department of Neurology (U.Z.), University of Rostock; Department of Neurology (F.L.), Christian-Albrechts-University Kiel; Department of Neurology with Institute of Translational Neurology (N.M.), University Hospital Muenster; and Department of Neurology (Albrecht Kunze), Zentralklinik Bad Berka, Germany.
1,*,†,
and the Generate study group