Abstract
Glutamine is the most abundant amino acid in blood and tissues, and the most important nutrient except for glucose in cancer cells. Over the past years, most studies have focused on the role of Gln metabolism in supporting energy metabolism rather than maintaining oxidative homeostasis. In fact, Gln is an important factor in maintaining oxidative homeostasis of cancer cells, especially in “Glutamine addicted” cancer cells. Here, this paper will review the recent scientific literature about the link between Gln metabolism and oxidative homeostasis, with an emphasis on the potential role of Gln metabolism in different cancers. Given that oxidative homeostasis is of critical importance in cancer, understanding the impacts of a Gln metabolism on oxidative homeostasis, gaining great insights into underlying molecular mechanisms, and developing effective therapeutic strategies are of great importance.
Keywords: Gln metabolism, oxidative homeostasis, cancer cells, ROS, health
Introduction
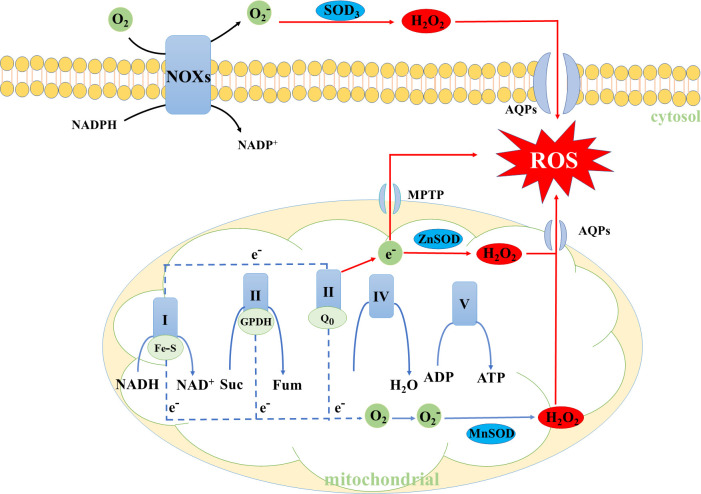
The reactive oxygen species (ROS), which mainly comes from the mitochondrial membrane as a byproduct of OXPHOS and nicotinamide adenine dinucleotide oxidases (NOXs), cannot avoid being produced in cellular metabolism (1–4). Cancer cells usually show higher levels of ROS, which acts as a signaling molecule in cancer, contributing to their growth and metastasis (5–8). Notably, when the levels of ROS in cancer cells are in excess, it will destroy oxidative homeostasis, subsequently damaging effects on macromolecules such as enzyme inactivation, DNA and protein damage ( Figure 1 ) (9, 10). Thus, maintaining oxidative homeostasis in cancer cells is of great importance and loss of balance has profound pathophysiology consequences (11).
Figure 1.
The primary generation mechanisms of intracellular ROS. SOD, Superoxide dismutase.
Glutamine (Gln), a non-essential amino acid, is essential for the survival of most cancer cells. The “Glutamine addiction” is a good description of the importance of Gln in cancer cells. When Gln is deprived of the medium, most cancer cells will be in a stagnant state or even die (12, 13). Gln metabolism, which could promote the biosynthesis of Glutathione (GSH) and nicotinamide adenine dinucleotide phosphate (NADPH), is involved in the maintenance of oxidative homeostasis in cancer cells (14). In light of the importance of Gln metabolism in oxidative homeostasis, a comprehensive understanding of the mechanics is vital for developing of tumor therapies. This review will elaborate on the functions of Gln and its products in the oxidative homeostasis of cancer cells, including roles in the biosynthesis of GSH and NADPH, and will explore the roles of Gln metabolism in different cancers via regulating oxidative homeostasis.
Gln metabolism in oxidative homeostasis
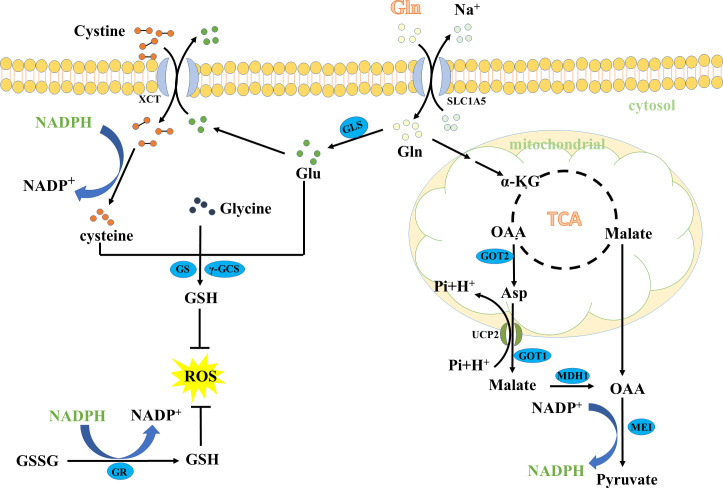
The Gln metabolism could maintain oxidative homeostasis through many pathways. One of the most important pathways is through promoting the biosynthesis of GSH. Glutamate (Glu), cysteine, and glycine are required amino acids for de novo biosynthesis of GSH (15–17). Notably, the conversion of Gln to Glu is required to maintain the large intracellular pools of Glu (13). Typically, Gln is first taken in by cancer cells through the transporters (such as ASCT2, ATB0,+, System L, System A), and then converted to Glu (18–20). The Gln-converted Glu subsequently generates GSH in two ways ( Figure 2 ). On the one hand, Glu can be polymerized with cysteine in an ATP-dependent manner to form γ-glutamylcysteine, and further condense with glycine to produce GSH (21–24). On the other hand, Glu is transported via cystine/glutamate antiporter xCT (also commonly known as SLC7A11) to the extracellular for exchanging cystine and a subsequent conversion of cystine to cysteine through a NADPH-consuming reduction reaction. The generated cysteine is subsequently used to form GSH (25, 26). GSH is a powerful reducing agent that acts as a free radical scavenger. Maintaining high levels of GSH in cancer cells can eliminate excessive ROS and detoxify xenobiotics to avoid oxidative damage.
Figure 2.
The key role of glutamine in GSH and NADPH biosynthesis. MDH1, malate dehydrogenase 1; SLC1A5, the solute carrier family 1, member 5.
Besides the role in the de novo biosynthesis of GSH, Gln also contributes to NADPH production. First, Gln enters the TCA cycle, and directly generates malate, or indirectly forms malate from the conversion of Asp via the Asp transporter mitochondrial uncoupling protein 2 (UCP2) and the enzymes aspartate transaminase (GOT1) and malate dehydrogenase 1 (MDH1). Then, malate crosses the mitochondrial membrane to the cytoplasm and is further catalyzed to pyruvate via the malic enzyme 1 (ME1), accompanied by reducing NADP to NADPH (27–29). Importantly, NADPH can reduce glutathione disulfide (GSSG) to GSH, an essential cofactor maintaining the reduced form of GSH (30, 31). On the other hand, NADPH can reduce cystine to cysteine for de novo biosynthesis of GSH (32, 33). Therefore, NADPH plays a role in the production of GSH, thus contributing to the maintenance of redox balance.
Overall, the Gln metabolism in this review refers to the metabolic pathway of the formation of GSH and NADPH from Gln, which could help maintain oxidative homeostasis of cancer cells and hence promote their progression.
The potential role of Gln metabolism in different cancers
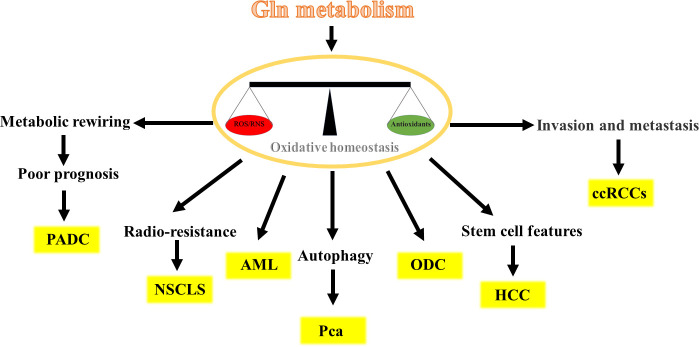
Gln metabolism has different potential roles in different cancer cells by maintaining oxidative homeostasis and is crucial for cancer development. In the following sections, we describe in detail the role of Gln metabolism in different cancer cells ( Figure 3 ).
Figure 3.
Different potential roles of Gln metabolism in different cancer cells.
Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PADC) is common malignant and poor prognosis tumors with a 5-year survival rate of approximately 10% in the USA (34–36). Multiple pieces of evidence have demonstrated that Gln metabolism implicates the progression of PADC induced by internal or external factors. For instance, Gln-metabolism is required for the hypoxia-inducible factor-2a-promoted PDAC progression (37). Moreover, the oncogenic KRAS-triggered PDAC growth is accompanied by the metabolic rewiring of Gln metabolism, which fulfills the NADPH need and balances cellular oxidative homeostasis (29). Similar increased production of Gln-derived NADPH is observed upon oxidative stress, accompanied by the survival and growth of PADC (38). These findings present us with intriguing evidence that the Gln-derived NADPH may positively associate with the poor prognosis of PDAC (39, 40). In addition, it has been demonstrated that PADC development-required NADPH strongly relies on Gln metabolism rather than on the pentose phosphate (PP) pathway. Evidence to support this hypothesis is that the Gln-derived NADPH markedly decreased after the knockdown of GOT1 or ME1 in PADC cells, which caused a significant increase in the ratio of GSSG/GSH, whereas glucose deprivation or knockdown of the limiting enzyme G6PD in the PP pathway had only a modest impact on NADPH (29, 41). Further evidence comes from the finding that the knockdown of UCP2 (the Asp transporter) decreased Gln-derived NADPH levels and increased ROS levels in PDAC cells, thus suppressing PDAC cell growth (42). Taken together, Gln-derived NADPH is required for the progression of PADC, and targeting this distinct pathway represents a novel prognostic biomarker and therapeutic target for patients with PDAC.
Acute myeloid leukemia
Several recent studies have demonstrated that Gln metabolism is implicated in the progression of acute myeloid leukemia (AML), as evidenced by exerting antileukemic effects (43–47). However, most of these studies focus on the role of Gln in supporting energy metabolism rather than maintaining oxidative homeostasis. Therefore, to better understand the role and regulatory mechanism of Gln metabolism in oxidative homeostasis of AML, one study using a FLT3-mutated AML cell model found that impaired Gln metabolism by FLT3 inhibitors could lead to depletion of GSH and accumulation of mitochondrial reactive oxygen species (mitoROS), subsequently leading to apoptosis of AML cell (48). A similar reduction of GSH levels and elevation of mitoROS and apoptosis were observed when AML cell lines were treated with the glutaminase inhibitor CB-839 for 24 h, which led to an inhibition of Gln metabolism (49).. These findings suggest that depletion of GSH is a universal consequence of inhibition of Gln metabolism in AML. In addition, inhibition of Gln metabolism makes AML cells susceptible to adjunctive drugs that further impair oxidative homeostasis. For example, combination of arsenic trioxide (ATO) and homoharringtonine (HHT) (the potent inducers of mitoROS) with CB-839 the exacerbates accumulation of mitoROS and apoptosis, which leads to complete cell death in AML cell lines, primary AML patient samples and in vivo mouse models of AML (49). Overall, Gln metabolism is implicated in promoting the development of AML, and the use of a Gln metabolism inhibitor in combination with drugs that further induces mitoROS and apoptosis may represent an effective and widely applicable therapeutic strategy for treating multiple types of AML.
Non-small cell lung cancer
In general, radiotherapy alone or in combination with chemotherapy and adjuvant durvalumab are mainly therapeutic methods for patients with locally advanced non-small cell lung cancer (NSCLC) (50, 51). However, after radiotherapy, the patient is prone to loco-regional recurrence, which remains a major clinical challenge for the cure for NSCLC (52–55). Existing evidences has linked Gln metabolism to the radio-resistance in NSCLC. For instance, a recently published article showed that the liver kinase B1-deficient NSCLC cells strongly depend on Gln-derived GSH to reduce ionizing radiation-derived ROS generation and to alleviate radiation-derived cytotoxic effects under radiotherapy. On the contrary, inhibition of Gln metabolism using knockdown of GLS could impair oxidative homeostasis, resulting in radio-sensitization of NSCLC (56). Another study also showed that the knockdown of GLS could increase response to radiotherapy of NSCLC by 30% in vitro and in vivo (57). Consistently, other studies also show that inhibition of Gln metabolism could suppress the GSH levels and enhanced radiosensitivity of NSCLC (58–60). These results indicate that NSCLC relies on Gln-derived GSH to maintain oxidative homeostasis to resist radiotherapy. All in all, inhibition of Glu metabolism may serve as a potential therapeutic strategy to cure this highly refractory subgroup of NSCLC patients.
Hepatocellular carcinoma
Liver cancer stem cells (CSCs), a subset of liver cells with stem cell features, are considered to be responsible for hepatocellular carcinoma (HCC) recurrence, metastasis, and chemoresistance (61, 62). These cells are heavily implicated in the Wnt/β-catenin pathway which is identified as one of the most frequent events occurring in CSCs (63, 64). It has been recognized that Gln metabolism is strongly correlated with Wnt/β-catenin pathway activation, contributing to liver carcinogenesis, hampering patient prognosis, and treatment stratification (65–67). Up to further investigations, the researchers found that the stemness properties in HCC were regulated by Gln metabolism through a ROS/Wnt/β-catenin signaling positive-feedback loop. More specifically, Gln metabolism could maintain low amounts of ROS and Wnt/β-catenin activation, which causes accumulation of β-catenin in the cytoplasm and then promotes the translocation of β-catenin to the nucleus. β-catenin in the nucleus activates the expression of CSC markers, such as NANOG, OCT4, KLF4, SOX2, and c-MYC and other Wnt target genes in HCC cell lines, thus promoting the progression of HCC (68). Interestingly, this study has also shown that the activated Wnt/β-catenin pathway via its agonist SKL2001 could upregulate the mRNA and protein levels of GLS1, and then promote Gln metabolism, which means that activated Wnt/β-catenin pathway could promote GLS expression with positive feedback (68). A similar study has shown that the high expression of GLS1 in HCC had a markedly shorter overall survival time than its low expression (69). Taken together, Gln metabolism can increase the stemness properties in HCC through activating ROS/Wnt/β-catenin pathway, and targeting Gln metabolism, especially GLS1, may be a therapeutic target for the elimination of CSCs.
Prostate cancer
Prostate cancer (Pca) treatments, such as radiation, chemotherapy, and hormone therapy, can induce autophagy that improves therapeutic resistance (70–72). Existing evidence has linked the Gln metabolism to autophagy through oxidative homeostasis in Pca. For instance, a recently published article showed that the radio-resistant Pca cells strongly rely on Gln metabolism to maintain oxidative homeostasis. However, Pca cells could trigger autophagy upon Gln withdrawal and do not exhibit significant radio-sensitization (73). Upon further investigations, the researchers found that the ionizing radiation-derived ROS can induce autophagy as a stress response of Pca cells, but it is neutralized by GSH and NADPH produced by Gln metabolism. When blocking Gln metabolism, Pca cells could activate the ATG -mediated autophagy as a survival strategy to withstand radiation-induced damage due to GSH depletion and ROS accumulation (73, 74). Consistently, other studies also confirmed that autophagy inhibition increases ROS production in Pca cells (75–77). Overall, Gln metabolism affects the autophagy of Pca cells by affecting the level of ROS.
Kidney cancer
Kidney cancer, the ideal model of metabolic reprogramming among all cancers, has been duly named as a “Metabolic Disease” (78–81). There is growing evidence that clear cell renal cell carcinoma cells (ccRCCs) are Gln-addicted that is reprogrammed to feed an intrinsic antioxidant system (82–84). For instance, combined proteomics and metabolomics studies have shown that the ccRCC largely uses Gln to feed the GSH/GSSG antioxidant system to attenuate oxidative stress, rather than to generate energy and cellular components through the TCA cycle (85). To further confirm the role of Gln as a source for the GSH pathway, absolute quantitative GSH and GSSG levels in cells grown with and without Gln were compared. The result showed that GSH and GSSG levels were markedly reduced in the Gln-depleted group, which confirms the necessity of Gln for maintaining oxidative homeostasis of ccRCCs (85). Similar findings were obtained in another study, showing that inhibition of Gln metabolism via CB-839 led to decreased GSH/GSSG ratio, and furtherly increased oxidative stress and ccRCCs apoptosis (86). In addition, an interesting study shows that the suppression of fatty acid metabolism by inhibition of β-oxidation lead to the RCC cells dependent on the Gln-GSH pathway to prevent lipid peroxidation and ferroptosis (87). Notably, high GSH levels have proven to be a key feature of high-grade, high-stage and metastatic ccRCCs (81, 88). All in all, these data suggest that Gln-dependent antioxidant effects may provide ccRCCs with a critical mechanism for their survival.
Oligodendroglioma
In general, Gln is an antioxidant defense only in Gln addicted cancers, but not in all cases. Oligodendroglioma cells lack Gln synthetase (a marker of Gln-addicted cancers), but are independent of extracellular Gln (thus are not Gln addicted) (89, 90). However, a previous study showed that small amounts of extracellular Gln are sufficient for oligodendroglioma cells growth. Gln starvation does not significantly affect the cell content of anaplerotic substrates, but causes a significant decrease in the intracellular content of GSH in oligodendroglioma cells (91). This result means that Gln addiction and Gln roles as antioxidants are not correlated. In addition, Gln starvation causes hindrance of the Wnt/β-catenin pathway and protein synthesis attenuation in oligodendroglioma cells, which means that Gln may stimulate Wnt/beta-catenin pathways by ROS levels to affect the activity of cells, as in HCC (68, 91).
ROS production and ferroptosis
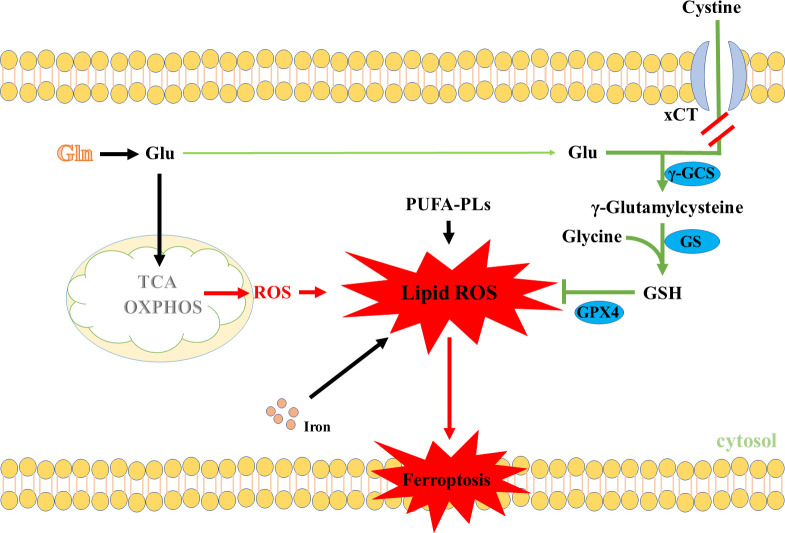
In light of the findings mentioned above, it would seem reasonable to expect that Gln metabolism plays an important role in maintaining ROS levels in cancer cells. However, we noted that most of the above-mentioned studies have mainly focused on the effects of Gln metabolism on maintaining oxidative homeostasis of cancer cells, whereas these effects were not suitable for every situation. Some studies have shown that the anaplerotic role of Gln metabolism in replenishing the TCA cycle intermediates could enhance ROS production under the blocking of GSH synthesis (92–94). For instance, a recently published article showed that Gln metabolism was crucial to maintaining cystine starvation-induced mitochondrial membrane potential (MMP) hyperpolarization, accompanied by an increase in electron transfer chain (ETC) activity and lipid ROS generation to promote ferroptosis (95). In support of this notion, data from various studies showed that inhibiting the glutaminolysis can suppress TCA cycle and MMP hyperpolarization, and reduce lipid ROS production, thus enhancing ferroptosis resistance (95–98). Similarly, various studies showed that inhibiting xCT activities could suppress Gln-derived Glu export and enhance Glu to replenish the TCA cycle intermediates (99–101). Therefore, it has been theorized that inhibition of xCT activities could promote Glu to replenish the TCA cycle intermediates, which could promote ROS production (102) ( Figure 4 ). All in all, increasing ROS levels by Gln metabolism under blocking of GSH synthesis promoted ferroptosis, which may provide a novel treatment guideline for ferroptosis-based tumor therapy.
Figure 4.
Gln metabolism promotes ROS production through the TCA cycle. PUFA-PLs, Polyunsaturated fatty acid chain(s).
Therapeutic strategies targeting Gln metabolism in cancer
The demonstration of the link between Gln metabolism and oxidative homeostasis of cancer has prompted research into strategies to target Gln metabolism to damage oxidative homeostasis of cancer. In this regard, GLS inhibitors aimed at decreasing Gln metabolism and impairing oxidative homeostasis are attracting increasing clinical interest. Many small molecules have been assayed to block GLS isoenzymes after the first attempt and failure to use 6-diazo-5-oxo-L-norleucine (DON) as an anti-cancer drug (103, 104). The bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide (BPTES) and CB-839 are the specific inhibitors most frequently (86). Notably, CB-839 is currently being administered to humans in phase 1 clinical trials for some types of cancers (49, 103–106).
However, because of the plasticity of adaptive metabolic reprogramming in cancer cells, successful single treatments against cancers are scarce (4, 107–109). Therefore, some specific inhibitor of Gln metabolism has reached better results in sensitizing cancer cells to other treatments (110). Targeting Gln metabolism combined with drugs that are strong inducers of mitochondrial ROS, is widely used for treating multiple cancers ( Table 1 ). For instance, dihydroartemisinin cooperatively induces excessive intracellular ROS resulting in profound apoptosis when combined with CB-839 in HCC (111). In a similar study, Gregory et al. demonstrated that a combination of GLS inhibition with ATO or HHT showed great activity against AML (49). Preclinical studies have also reported a benefit when combined with Gln metabolism inhibitors and radiotherapy. For example, the inhibitor CB-839 increased GSH depletion, and enhanced the radiation sensitivity of lung tumor cells xenografts in mice (57). Interestingly, one recent study showed that the combination of Gln metabolism inhibitors with radiotherapy could activate the ATG5-mediated autophagy of Prostate cancer, and proposes a strategy that a combination with autophagy inhibition and the blockade of Gln metabolism makes Pca radio-sensitization (73, 74, 122). Notably, the chemotherapy and/or radiation can also cause cellular damage in normal organs and tissues by generating free radicals (123). Antioxidants such as vitamins, minerals, and polyphenols can quench ROS activity alleviate the adverse effects of chemotherapy and/or radiotherapy (124, 125). Combining inhibition of Gln metabolism with antioxidant supplementation may enhance the chemotherapy and/or radiation sensitivity while preventing cellular damage of normal organs and tissues, which may be an effective strategy for the treatment of cancer. However, it remains controversial whether antioxidants affect treatment outcomes or whether antioxidants ameliorate adverse effects induced by chemotherapy and radiotherapy, which needs further investigations in the future (126). In conclusion, combination therapy, including inhibitors of Gln metabolism, may be a promising strategy for cancer cells.
Table 1.
Combined treatments: targeting glutaminolysis in combination with drugs that unbalance mitochondrial redox state.
| Type of cancer | Target Gln metabolism | Combined treatment | Drug mechanism | References | |
|---|---|---|---|---|---|
| Site | Type of inhibition | ||||
| AML | GLS | CB-839 | ATO; HHT | Inducing excessive ROS | (49) |
| HCC | Dihydroartemisinin | Inducing excessive ROS | (111) | ||
| NSCLC | Radiotherapy | Radiosensitization | (56) | ||
| Pca | GLS siRNA silencing | ATG5 siRNA silencing; Radiotherapy | Inhibition of autophagy; Radiosensitization | (74) | |
| PDAC | ß-lapachone | Inducing excessive ROS | (112) | ||
| GBM | ATO, H2O2 | Inducing excessive ROS | (113) | ||
| TNBC | Compound 968 | CQ | Inhibition of autophagy; inducing excessive ROS | (114) | |
| NSCLC | (115) | ||||
| LCLC | Apigenin | Inducing excessive ROS | (116) | ||
| GBM | GLS2 | GLS2 overexpression | ATO; H2O2 | Inducing excessive ROS | (113) |
| BC | SLC1A5 | V9302 | anti-PD-1 monoclonal antibody (mAb) | Enhancing antitumor immunity | (117) |
| HNSCC | Cetuximab | Dichloroacetate | Inducing excessive ROS | (118, 119) | |
| CC | SLC1A5/GDH1 | CB-839/R162 | CAI | Inducing excessive ROS | (120) |
| BC | / | Glutamine deprivation | Vorinostat | Inducing excessive ROS | (121) |
| CC | |||||
BC, Breast cancer; CAI, Carboxyamidotriazole; CQ, chloroquine; CC, Colon cancer; GBM, glioblastoma; GDH1, glutamate dehydrogenase 1; HNSCC, head and neck squamous cell carcinoma; LCLC, large cell lung carcinoma; TNBC, triple-negative breast cancer; V9302, glutamine metabolism inhibitor.
Conclusion
The antioxidant capacity of tumor cells is required for rapidly proliferating and aggressive cancer cells to adapt to hypoxia and excessive ROS levels. The literature reviewed here suggests that Gln has been established as an important factor in maintaining the oxidative homeostasis of cancer cells. Targeting Gln metabolism impaired oxidative homeostasis of cancer cells and may provide effective approaches for therapies against cancer. In addition, more research is urgently needed to implement multiple synergistic targeting (including Gln metabolism inhibitors) to block tumor proliferation and increase cancer cells’ sensitivity of cancer cells to other therapies. Future studies on Gln metabolism in maintaining oxidative homeostasis may provide novel and effective therapeutic strategies to treat a subset of cancer patients.
Author contributions
Conceptualization, YD, WL, and TG; writing—original draft preparation, TG, CZ, XO, JZ, JY, and SC; writing—review and editing, YD and WL; visualization, YD. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly supported by the Natural Science Foundation of the Hunan Province, China (2021JJ30335, 2021JJ20044), the National Natural Science Foundation of China (U19A2037), the Changsha Natural Science Funds for Distinguished Young Scholar (kq2009020), Young Elite Scientists Sponsorship Program by CAST (2020-2022QNRC003), the Natural Science Foundation of Guangxi Province (2020JJA130102), China Agriculture Research System of MOF and MARA (CARS-35), and the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (XDA24030204), the Scientific Research Fund of Hunan Provincial Education Department, China (21A0141).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA (2006) 103:7607–12. doi: 10.1073/pnas.0510977103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol (2004) 4:181–9. doi: 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 3. Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem (2012) 287:27255–64. doi: 10.1074/jbc.M112.374629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discovery. (2013) 12:931–47. doi: 10.1038/nrd4002 [DOI] [PubMed] [Google Scholar]
- 5. Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Disease. (2016) 7:e2253. doi: 10.1038/cddis.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxidants Redox Signaling (2014) 20:247–66. doi: 10.1089/ars.2012.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warburg O. On the origin of cancer cells. Sci (New York NY). (1956) 123:309–14. doi: 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 8. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: An overview. Ageing Res Rev (2013) 12:376–90. doi: 10.1016/j.arr.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 9. Herraiz C, Crosas-Molist E, Sanz-Moreno V. Reactive oxygen species and tumor dissemination: Allies no longer. Mol Cell Oncol (2016) 3:e1127313. doi: 10.1080/23723556.2015.1127313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maryanovich M, Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol (2013) 23:129–34. doi: 10.1016/j.tcb.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 11. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell (2020) 38:167–97. doi: 10.1016/j.ccell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kodama M, Oshikawa K, Shimizu H, Yoshioka S, Takahashi M, Izumi Y, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun (2020) 11:1320. doi: 10.1038/s41467-020-15136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene (2010) 29:313–24. doi: 10.1038/onc.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. (2016) 16:619–34. doi: 10.1038/nrc.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol aspects Med (2009) 30:86–98. doi: 10.1016/j.mam.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules (2020) 10:1429. doi: 10.3390/biom10101429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu SC. Regulation of glutathione synthesis. Mol aspects Med (2009) 30:42–59. doi: 10.1016/j.mam.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene (2016) 35:3201–8. doi: 10.1038/onc.2015.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W. Target the human Alanine/Serine/Cysteine transporter 2(ASCT2): Achievement and future for novel cancer therapy. Pharmacol Res (2020) 158:104844. doi: 10.1016/j.phrs.2020.104844 [DOI] [PubMed] [Google Scholar]
- 20. Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta (2016) 1863:2531–9. doi: 10.1016/j.bbamcr.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. C-myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature (2009) 458:762–5. doi: 10.1038/nature07823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA (2010) 107:7455–60. doi: 10.1073/pnas.1001006107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lukey MJ, Greene KS, Erickson JW, Wilson KF, Cerione RA. The oncogenic transcription factor c-jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat Commun (2016) 7:11321. doi: 10.1038/ncomms11321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA (2010) 107:7461–6. doi: 10.1073/pnas.1002459107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J (2017) 36:1302–15. doi: 10.15252/embj.201696151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (London England). (2018) 38:12. doi: 10.1186/s40880-018-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA (2007) 104:19345–50. doi: 10.1073/pnas.0709747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ying M, You D, Zhu X, Cai L, Zeng S, Hu X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol (2021) 46:102065. doi: 10.1016/j.redox.2021.102065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature (2013) 496:101–5. doi: 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moreno-Sánchez R, Gallardo-Pérez JC, Rodríguez-Enríquez S, Saavedra E, Marín-Hernández Á. Control of the NADPH supply for oxidative stress handling in cancer cells .Free Radic Biol Med. (2017) 112:149–61 doi: 10.1016/j.freeradbiomed.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 31. Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol (2018) 217:2291–8. doi: 10.1083/jcb.201804161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaganjac M, Milkovic L, Sunjic SB, Zarkovic N. The NRF2, thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants (Basel Switzerland). (2020) 9:1151. doi: 10.3390/antiox9111151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palde PB, Carroll KS. A universal entropy-driven mechanism for thioredoxin-target recognition. Proc Natl Acad Sci USA (2015) 112:7960–5. doi: 10.1073/pnas.1504376112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet (London England). (2020) 395:2008–20. doi: 10.1016/s0140-6736(20)30974-0 [DOI] [PubMed] [Google Scholar]
- 35. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 36. Park W, Chawla A, O'Reilly EM. Pancreatic cancer: A review. Jama (2021) 326:851–62. doi: 10.1001/jama.2021.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li W, Chen C, Zhao X, Ye H, Zhao Y, Fu Z, et al. HIF-2α regulates non-canonical glutamine metabolism via activation of PI3K/mTORC2 pathway in human pancreatic ductal adenocarcinoma. J Cell Mol Med (2017) 21:2896–908. doi: 10.1111/jcmm.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tong Y, Guo D, Lin SH, Liang J, Yang D, Ma C, et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell (2021) 81:2303–2316.e8. doi: 10.1016/j.molcel.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 39. Hu T, Shukla SK, Vernucci E, He C, Wang D, King RJ, et al. Metabolic rewiring by loss of Sirt5 promotes kras-induced pancreatic cancer progression. Gastroenterology (2021) 161:1584–600. doi: 10.1053/j.gastro.2021.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang YP, Zhou W, Wang J, Huang X, Zuo Y, Wang TS, et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol Cell (2016) 64:673–87. doi: 10.1016/j.molcel.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 41. Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell (2012) 149:656–70. doi: 10.1016/j.cell.2012.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raho S, Capobianco L, Malivindi R, Vozza A, Piazzolla C, De Leonardis F, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab (2020) 2:1373–81. doi: 10.1038/s42255-020-00315-1 [DOI] [PubMed] [Google Scholar]
- 43. Jacque N, Ronchetti AM, Larrue C, Meunier G, Birsen R, Willems L, et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood (2015) 126:1346–56. doi: 10.1182/blood-2015-01-621870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood (2013) 122:3521–32. doi: 10.1182/blood-2013-03-493163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gallipoli P, Giotopoulos G, Tzelepis K, Costa ASH, Vohra S, Medina-Perez P, et al. Glutaminolysis is a metabolic dependency in FLT3(ITD) acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood (2018) 131:1639–53. doi: 10.1182/blood-2017-12-820035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matre P, Velez J, Jacamo R, Qi Y, Su X, Cai T, et al. Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes. Oncotarget (2016) 7:79722–35. doi: 10.18632/oncotarget.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gregory MA, Nemkov T, Reisz JA, Zaberezhnyy V, Hansen KC, D'Alessandro A, et al. Glutaminase inhibition improves FLT3 inhibitor therapy for acute myeloid leukemia. Exp hematology. (2018) 58:52–8. doi: 10.1016/j.exphem.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gregory MA, D'Alessandro A, Alvarez-Calderon F, Kim J, Nemkov T, Adane B, et al. ATM/G6PD-driven redox metabolism promotes FLT3 inhibitor resistance in acute myeloid leukemia. Proc Natl Acad Sci USA (2016) 113:E6669–e6678. doi: 10.1073/pnas.1603876113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gregory MA, Nemkov T, Park HJ, Zaberezhnyy V, Gehrke S, Adane B, et al. Targeting glutamine metabolism and redox state for leukemia therapy. Clin Cancer Res (2019) 25:4079–90. doi: 10.1158/1078-0432.ccr-18-3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bezjak A, Temin S, Franklin G, Giaccone G, Govindan R, Johnson ML, et al. Definitive and adjuvant radiotherapy in locally advanced non-Small-Cell lung cancer: American society of clinical oncology clinical practice guideline endorsement of the American society for radiation oncology evidence-based clinical practice guideline. J Clin Oncol (2015) 33:2100–5. doi: 10.1200/jco.2014.59.2360 [DOI] [PubMed] [Google Scholar]
- 51. Cheema PK, Rothenstein J, Melosky B, Brade A, Hirsh V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol (Toronto Ont). (2019) 26:37–42. doi: 10.3747/co.25.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cassidy RJ, Zhang X, Patel PR, Shelton JW, Escott CE, Sica GL, et al. Next-generation sequencing and clinical outcomes of patients with lung adenocarcinoma treated with stereotactic body radiotherapy. Cancer (2017) 123:3681–90. doi: 10.1002/cncr.30794 [DOI] [PubMed] [Google Scholar]
- 53. Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discovery. (2017) 7:86–101. doi: 10.1158/2159-8290.cd-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gurtner K, Kryzmien Z, Koi L, Wang M, Benes CH, Hering S, et al. Radioresistance of KRAS/TP53-mutated lung cancer can be overcome by radiation dose escalation or EGFR tyrosine kinase inhibition in vivo. Int J Cancer (2020) 147:472–7. doi: 10.1002/ijc.32598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mak RH, Hermann G, Lewis JH, Aerts HJ, Baldini EH, Chen AB, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung cancer. (2015) 16:24–32. doi: 10.1016/j.cllc.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sitthideatphaiboon P, Galan-Cobo A, Negrao MV, Qu X, Poteete A, Zhang F, et al. STK11/LKB1 mutations in NSCLC are associated with KEAP1/NRF2-dependent radiotherapy resistance targetable by glutaminase inhibition. Clin Cancer Res (2021) 27:1720–33. doi: 10.1158/1078-0432.ccr-20-2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boysen G, Jamshidi-Parsian A, Davis MA, Siegel ER, Simecka CM, Kore RA, et al. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol (2019) 95:436–42. doi: 10.1080/09553002.2018.1558299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sappington DR, Siegel ER, Hiatt G, Desai A, Penney RB, Jamshidi-Parsian A, et al. Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A549 and H460 lung cancer cell lines. Biochim Biophys Acta (2016) 1860:836–43. doi: 10.1016/j.bbagen.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fujimoto M, Higashiyama R, Yasui H, Yamashita K, Inanami O. Preclinical studies for improving radiosensitivity of non-small cell lung cancer cell lines by combining glutaminase inhibition and senolysis. Trans Oncol (2022) 21:101431. doi: 10.1016/j.tranon.2022.101431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meijer TWH, Peeters WJM, Dubois LJ, van Gisbergen MW, Biemans R, Venhuizen JH, et al. Targeting glucose and glutamine metabolism combined with radiation therapy in non-small cell lung cancer. Lung Cancer (Amsterdam Netherlands). (2018) 126:32–40. doi: 10.1016/j.lungcan.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 61. Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J cancer. (2010) 126:2067–78. doi: 10.1002/ijc.24868 [DOI] [PubMed] [Google Scholar]
- 62. Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology (2007) 133:937–50. doi: 10.1053/j.gastro.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 63. Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res (2007) 67:10831–9. doi: 10.1158/0008-5472.can-07-0908 [DOI] [PubMed] [Google Scholar]
- 64. Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res (2008) 68:4287–95. doi: 10.1158/0008-5472.can-07-6691 [DOI] [PubMed] [Google Scholar]
- 65. Cadoret A, Ovejero C, Terris B, Souil E, Lévy L, Lamers WH, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene (2002) 21:8293–301. doi: 10.1038/sj.onc.1206118 [DOI] [PubMed] [Google Scholar]
- 66. Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatol (Baltimore Md). (2006) 43:817–25. doi: 10.1002/hep.21131 [DOI] [PubMed] [Google Scholar]
- 67. Liao J, Liu PP, Hou G, Shao J, Yang J, Liu K, et al. Regulation of stem-like cancer cells by glutamine through β-catenin pathway mediated by redox signaling. Mol cancer. (2017) 16:51. doi: 10.1186/s12943-017-0623-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li B, Cao Y, Meng G, Qian L, Xu T, Yan C, et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing wnt/beta-catenin pathway. EBioMedicine (2019) 39:239–54. doi: 10.1016/j.ebiom.2018.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu D, Shi X, Meng G, Chen J, Yan C, Jiang Y, et al. Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget (2015) 6:7619–31. doi: 10.18632/oncotarget.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urology. (2014) 11:508–16. doi: 10.1038/nrurol.2014.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ashrafizadeh M, Paskeh MDA, Mirzaei S, Gholami MH, Zarrabi A, Hashemi F, et al. Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response. J Exp Clin Cancer Res CR. (2022) 41:105. doi: 10.1186/s13046-022-02293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luo S, Shao L, Chen Z, Hu D, Jiang L, Tang W. NPRL2 promotes docetaxel chemoresistance in castration resistant prostate cancer cells by regulating autophagy through the mTOR pathway. Exp Cell Res (2020) 390:111981. doi: 10.1016/j.yexcr.2020.111981 [DOI] [PubMed] [Google Scholar]
- 73. Mukha A, Kahya U, Linge A, Chen O, Löck S, Lukiyanchuk V, et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics (2021) 11:7844–68. doi: 10.7150/thno.58655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mukha A, Kahya U, Dubrovska A. Targeting glutamine metabolism and autophagy: the combination for prostate cancer radiosensitization. Autophagy (2021) 17:3879–81. doi: 10.1080/15548627.2021.1962682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim KY, Park KI, Kim SH, Yu SN, Park SG, Kim YW, et al. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci (2017) 18:1088. doi: 10.3390/ijms18051088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jangamreddy JR, Ghavami S, Grabarek J, Kratz G, Wiechec E, Fredriksson BA, et al. Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: differences between primary and cancer cells. Biochim Biophys Acta (2013) 1833:2057–69. doi: 10.1016/j.bbamcr.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 77. Kim KY, Yu SN, Lee SY, Chun SS, Choi YL, Park YM, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun (2011) 413:80–6. doi: 10.1016/j.bbrc.2011.08.054 [DOI] [PubMed] [Google Scholar]
- 78. Rathmell WK, Rathmell JC, Linehan WM. Metabolic pathways in kidney cancer: current therapies and future directions. J Clin Oncol (2018) JCO2018792309. doi: 10.1200/JCO.2018.79.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weiss RH. Metabolomics and metabolic reprogramming in kidney cancer. Semin Nephrol (2018) 38(2):175–82. doi: 10.1016/j.semnephrol.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wettersten HI. Reprogramming of metabolism in kidney cancer. Semin Nephrol (2020) 40(1):2–13. doi: 10.1016/j.semnephrol.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 81. Wettersten HI, Aboud OA, Lara PN, Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol. (2017) 13:410–9. doi: 10.1038/nrneph.2017.59 [DOI] [PubMed] [Google Scholar]
- 82. Shroff EH, Eberlin LS, Dang VM, Gouw AM, Gabay M, Adam SJ, et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc Natl Acad Sci USA (2015) 112:6539–44. doi: 10.1073/pnas.1507228112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gameiro PA, Yang J, Metelo AM, Pérez-Carro R, Baker R, Wang Z, et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab (2013) 17:372–85. doi: 10.1016/j.cmet.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chakraborty S, Balan M, Sabarwal A, Choueiri TK, Pal S. Metabolic reprogramming in renal cancer: Events of a metabolic disease. Biochim Biophys Acta Rev cancer. (2021) 1876:188559. doi: 10.1016/j.bbcan.2021.188559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wettersten HI, Hakimi AA, Morin D, Bianchi C, Johnstone ME, Donohoe DR, et al. Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res (2015) 75:2541–52. doi: 10.1158/0008-5472.can-14-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abu Aboud O, Habib SL, Trott J, Stewart B, Liang S, Chaudhari AJ, et al. Glutamine addiction in kidney cancer suppresses oxidative stress and can be exploited for real-time imaging. Cancer Res (2017) 77:6746–58. doi: 10.1158/0008-5472.can-17-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miess H, Dankworth B, Gouw AM, Rosenfeldt M, Schmitz W, Jiang M, et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene (2018) 37:5435–50. doi: 10.1038/s41388-018-0315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A, et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell (2016) 29:104–16. doi: 10.1016/j.ccell.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhuang Z, Qi M, Li J, Okamoto H, Xu DS, Iyer RR, et al. Proteomic identification of glutamine synthetase as a differential marker for oligodendrogliomas and astrocytomas. J Neurosurg (2011) 115:789–95. doi: 10.3171/2011.5.jns11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pilkington GJ, Lantos PL. The role of glutamine synthetase in the diagnosis of cerebral tumours. Neuropathol Appl Neurobiol (1982) 8:227–36. doi: 10.1111/j.1365-2990.1982.tb00277.x [DOI] [PubMed] [Google Scholar]
- 91. Chiu M, Taurino G, Bianchi MG, Ottaviani L, Andreoli R, Ciociola T, et al. Oligodendroglioma cells lack glutamine synthetase and are auxotrophic for glutamine, but do not depend on glutamine anaplerosis for growth. Int J Mol Sci (2018) 19:1099. doi: 10.3390/ijms19041099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell (2015) 59:298–308. doi: 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for kras-mediated tumorigenicity. Proc Natl Acad Sci USA (2010) 107:8788–93. doi: 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anderson NM, Mucka P, Kern JG, Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell (2018) 9:216–37. doi: 10.1007/s13238-017-0451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell (2019) 73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shin D, Lee J, You JH, Kim D, Roh JL. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol (2020) 30:101418. doi: 10.1016/j.redox.2019.101418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. (2018) 25:1457–72. doi: 10.1038/s41418-017-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang K, Wu L, Zhang P, Luo M, Du J, Gao T, et al. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol carcinogenesis. (2018) 57:1566–76. doi: 10.1002/mc.22878 [DOI] [PubMed] [Google Scholar]
- 99. Huang Y, Dai Z, Barbacioru C, Sadée W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res (2005) 65:7446–54. doi: 10.1158/0008-5472.can-04-4267 [DOI] [PubMed] [Google Scholar]
- 100. Lim JKM, Delaidelli A, Minaker SW, Zhang HF, Colovic M, Yang H, et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci USA (2019) 116:9433–42. doi: 10.1073/pnas.1821323116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell (2019) 35:177–190.e8. doi: 10.1016/j.ccell.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 102. Yao X, Li W, Fang D, Xiao C, Wu X, Li M, et al. Emerging roles of energy metabolism in ferroptosis regulation of tumor cells. Adv. Sci (Weinheim Baden-Wurttemberg Germany). (2021) 8:e2100997. doi: 10.1002/advs.202100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matés JM, Campos-Sandoval JA, de Los Santos-Jiménez J, Segura JA, Alonso FJ, Márquez J. Metabolic reprogramming of cancer by chemicals that target glutaminase isoenzymes. Curr Med. Chem (2020) 27:5317–39. doi: 10.2174/0929867326666190416165004 [DOI] [PubMed] [Google Scholar]
- 104. Matés JM, Di Paola FJ, Campos-Sandoval JA, Mazurek S, Márquez J. Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer. Semin Cell Dev Biol (2020) 98:34–43. doi: 10.1016/j.semcdb.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 105. Lee P, Malik D, Perkons N, Huangyang P, Khare S, Rhoades S, et al. Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat Commun (2020) 11:498. doi: 10.1038/s41467-020-14374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Meric-Bernstam F, Tannir NM, Mier JW, DeMichele A, Telli ML, Fan AC, et al. Phase 1 study of CB-839, a small molecule inhibitor of glutaminase (GLS), alone and in combination with everolimus (E) in patients (pts) with renal cell cancer (RCC). Am Soc Clin Oncol (2016) 69:12–13. doi: 10.1016/S0959-8049(16)32626-0 [DOI] [Google Scholar]
- 107. Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxidants Redox Signaling (2009) 11:3013–69. doi: 10.1089/ars.2009.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discovery (2009) 8:579–91. doi: 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- 109. Kalyanaraman B, Cheng G, Hardy M, Ouari O, Bennett B, Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol (2018) 15:347–62. doi: 10.1016/j.redox.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Matés JM, Campos-Sandoval JA, Santos-Jiménez JL, Márquez J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer letters. (2019) 467:29–39. doi: 10.1016/j.canlet.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 111. Wang D, Meng G, Zheng M, Zhang Y, Chen A, Wu J, et al. The glutaminase-1 inhibitor 968 enhances dihydroartemisinin-mediated antitumor efficacy in hepatocellular carcinoma cells. PloS One (2016) 11:e0166423. doi: 10.1371/journal.pone.0166423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chakrabarti G, Moore ZR, Luo X, Ilcheva M, Ali A, Padanad M, et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by ß-lapachone. Cancer Metab (2015) 3:12. doi: 10.1186/s40170-015-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martín-Rufián M, Nascimento-Gomes R, Higuero A, Crisma AR, Campos-Sandoval JA, Gómez-García MC, et al. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J Mol Med (Berl). (2014) 92:277–90. doi: 10.1007/s00109-013-1105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Halama A, Kulinski M, Dib SS, Zaghlool SB, Siveen KS, Iskandarani A, et al. Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis. Cancer letters. (2018) 430:133–47. doi: 10.1016/j.canlet.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 115. Han T, Guo M, Zhang T, Gan M, Xie C, Wang JB. A novel glutaminase inhibitor-968 inhibits the migration and proliferation of non-small cell lung cancer cells by targeting EGFR/ERK signaling pathway. Oncotarget (2017) 8:28063–73. doi: 10.18632/oncotarget.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee YM, Lee G, Oh TI, Kim BM, Shim DW, Lee KH, et al. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol (2016) 48:399–408. doi: 10.3892/ijo.2015.3243 [DOI] [PubMed] [Google Scholar]
- 117. Li Q, Zhong X, Yao W, Yu J, Wang C, Li Z, et al. Inhibitor of glutamine metabolism V9302 promotes ROS-induced autophagic degradation of B7H3 to enhance antitumor immunity. J Biol Chem (2022) 298:101753. doi: 10.1016/j.jbc.2022.101753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tao X, Lu Y, Qiu S, Wang Y, Qin J, Fan Z. AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer letters. (2017) 408:33–42. doi: 10.1016/j.canlet.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lu H, Lu Y, Xie Y, Qiu S, Li X, Fan Z. Rational combination with PDK1 inhibition overcomes cetuximab resistance in head and neck squamous cell carcinoma. JCI Insight (2019) 4:e131106. doi: 10.1172/jci.insight.131106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shi J, Ju R, Gao H, Huang Y, Guo L, Zhang D. Targeting glutamine utilization to block metabolic adaptation of tumor cells under the stress of carboxyamidotriazole-induced nutrients unavailability. Acta Pharm Sin B (2022) 12:759–73. doi: 10.1016/j.apsb.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miyamoto K, Watanabe M, Boku S, Sukeno M, Morita M, Kondo H, et al. xCT inhibition increases sensitivity to vorinostat in a ROS-dependent manner. Cancers (Basel). (2020) 12:827. doi: 10.3390/cancers12040827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res (2013) 19:6741–50. doi: 10.1158/1078-0432.ccr-13-1787 [DOI] [PubMed] [Google Scholar]
- 123. Ziech D, Franco R, Georgakilas AG, Georgakila S, Malamou-Mitsi V, Schoneveld O, et al. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact (2010) 188:334–9. doi: 10.1016/j.cbi.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 124. Fuchs-Tarlovsky V. Role of antioxidants in cancer therapy. Nutrition (2013) 29:15–21. doi: 10.1016/j.nut.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 125. Lamson DW, Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev (1999) 4:304–29. [PubMed] [Google Scholar]
- 126. Yasueda A, Urushima H, Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr Cancer Ther (2016) 15:17–39. doi: 10.1177/1534735415610427 [DOI] [PMC free article] [PubMed] [Google Scholar]